Abstract
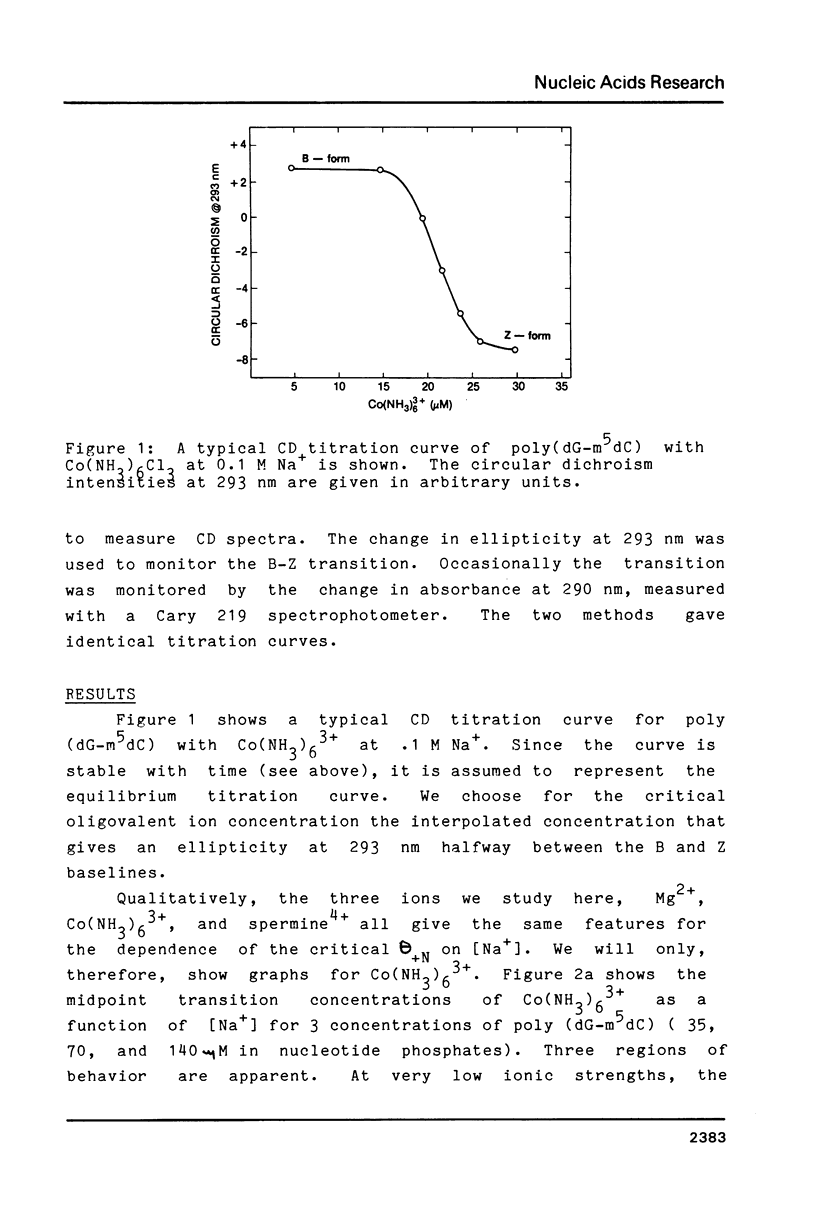
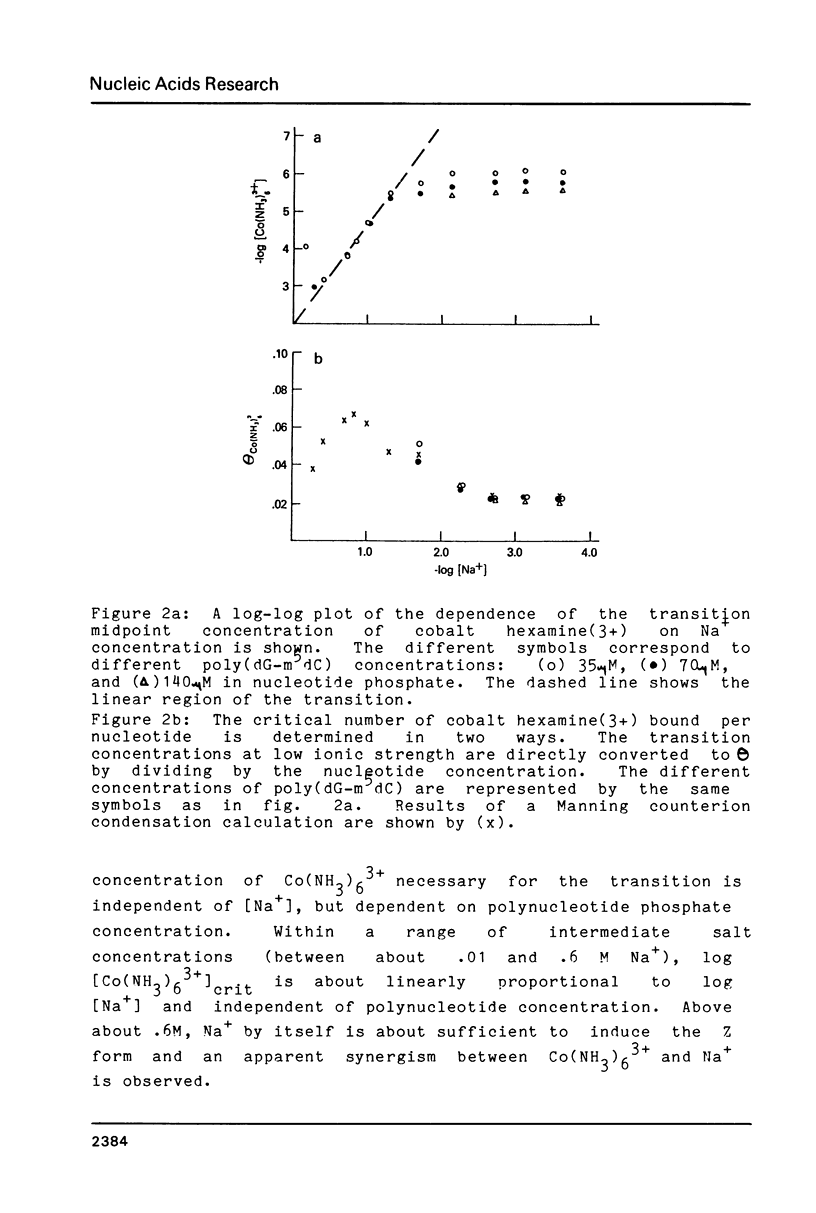
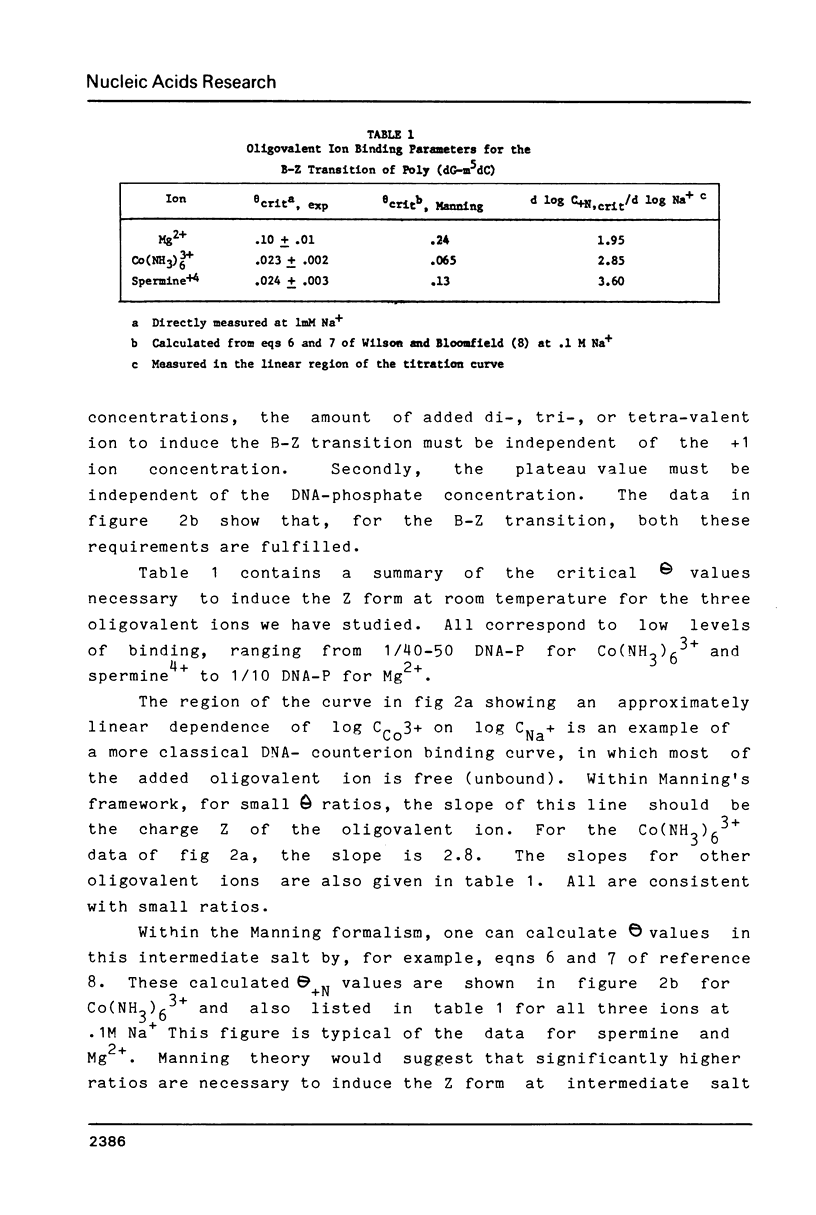
By working at very low Na+ concentrations (1mM and less), the number of bound Mg(2+), cobalt hexamine (3+), and spermine (4+) necessary to induce the B-Z transition of poly (dG-m5dC) has been directly measured. The results show that if as little as 1 cobalt hexamine(3+) or spermine(4+) is bound per 40-50 nucleotides the transition will occur. A greater fraction of bound Mg(2+) is required, 1 bound Mg(2+)/10 nucleotides. The dependence of the transition midpoint concentrations of oligovalent ions on Na+ concentrations suggests that specific ion binding energies, not included in counterion condensation theory, are responsible for the transition.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot J., Kearns D. R. Interactions of DNA with divalent metal ions. III. Extent of metal binding: experiment and theory. Biopolymers. 1982 Jan;21(1):219–232. doi: 10.1002/bip.360210117. [DOI] [PubMed] [Google Scholar]
- Krakauer H. The binding of Mg++ ions to polyadenylate, polyuridylate, and their complexes. Biopolymers. 1971;10(12):2459–2490. doi: 10.1002/bip.360101209. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Mercado C. M., Tomasz M. Circular dichroism of mitomycin-DNA complexes. Evidence for a conformational change in DNA. Biochemistry. 1977 May 3;16(9):2040–2046. doi: 10.1021/bi00628a044. [DOI] [PubMed] [Google Scholar]
- Rose D. M., Bleam M. L., Record M. T., Bryant R. G. Mg NMR in DNA solutions: Dominance of site binding effects. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6289–6292. doi: 10.1073/pnas.77.11.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. L. Spermidine-Deoxyribonucleic acid interaction in vitro and in Escherichia coli. J Bacteriol. 1977 Feb;129(2):916–925. doi: 10.1128/jb.129.2.916-925.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santella R. M., Grunberger D., Weinstein I. B., Rich A. Induction of the Z conformation in poly(dG-dC).poly(dG-dC) by binding of N-2-acetylaminofluorene to guanine residues. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1451–1455. doi: 10.1073/pnas.78.3.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. W., Bloomfield V. A. Counterion-induced condesation of deoxyribonucleic acid. a light-scattering study. Biochemistry. 1979 May 29;18(11):2192–2196. doi: 10.1021/bi00578a009. [DOI] [PubMed] [Google Scholar]
- Wilson R. W., Rau D. C., Bloomfield V. A. Comparison of polyelectrolyte theories of the binding of cations to DNA. Biophys J. 1980 May;30(2):317–325. doi: 10.1016/S0006-3495(80)85097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]



