Abstract
Culture-based methods used for microbial detection and identification are simple to use, relatively inexpensive, and sensitive. However, culture-based methods are too time-consuming for high-throughput testing and too tedious for analysis of samples with multiple organisms and provide little clinical information regarding the pathogen (e.g., antibiotic resistance genes, virulence factors, or strain subtype). DNA-based methods, such as polymerase chain reaction (PCR), overcome some these limitations since they are generally faster and can provide more information than culture-based methods. One limitation of traditional PCR-based methods is that they are normally limited to the analysis of a single pathogen, a small group of related pathogens, or a small number of relevant genes. Microarray technology enables a significant expansion of the capability of DNA-based methods in terms of the number of DNA sequences that can be analyzed simultaneously, enabling molecular identification and characterization of multiple pathogens and many genes in a single array assay. Microarray analysis of microbial pathogens has potential uses in research, food safety, medical, agricultural, regulatory, public health, and industrial settings. In this article, we describe the main technical elements of microarray technology and the application and potential use of DNA microarrays for food microbial analysis.
Introduction
Microarrays are arrays of recognition ligands, such as oligonucleotide, cDNA, protein, peptide, antibody, carbohydrate, tissue, or aptamer, immobilized (chemically bonded) in discrete locations on a solid matrix. The target molecule to be analyzed, such as DNA or protein, is labeled and hybridized to recognition probes on the array. The signal generated by the bound labeled target on the array allows identification based on the known locations of the probes. Oligonucleotide microarrays are widely used for analysis of gene expression, but the same technology can also be used for genotyping and for analysis of microbial pathogens for food safety, medical, and environmental applications (Sergeev et al., 2006b). Microarray technology permits molecular identification and characterization of multiple target sequences in a single array assay, enabling thousands of identification assays to be conducted in parallel, where each probe represents a specific small section of a genome or a sequence common to multiple genomes. For microbial analysis, this provides the capability for obtaining detailed genomic information on the pathogen, including relevant identification, typing, and clinical information regarding virulence factors and antibiotic resistance. Other important technologies for food microbial analysis include various biosensors which are mainly used for protein analysis (Rasooly and Herold, 2006).
All array technologies share three main features: multi-target analysis, specific binding or hybridization of the target, and labeling of the target molecules. The main steps in the design and implementation of a DNA microarray experiment are i) probe development; ii) array fabrication; iii) sample preparation; iv) assay; v) detection; and vi) data analysis as shown in Fig. 1. A significant challenge in many microbial detection applications is the need to detect and characterize small numbers of microorganisms in a large sample volume, often with a background of similar organisms and interfering matrix material. Thus, for many microarray applications one of the initial steps is amplification of the specific target sequence to increase sensitivity.
FIG. 1.
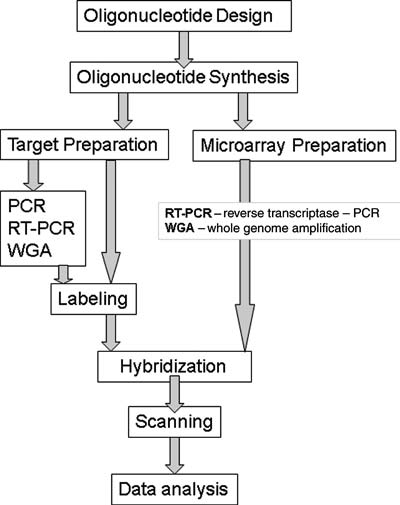
Microarray design and fabrication for microbial analysis. The main steps for design and fabrication of microarrays for food microbial analysis are oligonucleotide design for spotting and primer design for target amplification (if needed); array spotting and target labeling; and hybridization/washing followed by microarray scanning and data analysis.
The most common DNA microarray technology is based on spatially ordered immobilization of short single-stranded oligonucleotide probes (oligoprobes) on a solid matrix such as glass. Recently, an alternative bead array technology emerged where, unlike the traditional technology, the capture probes are immobilized on beads. The following elements define microarray technology:
• The format of the array (beads or spatially ordered array)
• The design and length of the oligoucleotide probes on the array, which determine their specificity and binding affinity
• The surface matrix and the technology of immobilization of the oligonucleotides
• The target molecule to be analyzed (cDNA, polymerase chain reaction [PCR] amplicon, or amplified whole genome)
• The type of target labeling
• The detector and data analysis
The choice of a microarray technology for a specific application depends on the details of the application. In particular, the size of the array (i.e., the number of individual recognition elements or probes) has a major impact on cost.
Spatially ordered microarrays
Spatially ordered arrays incorporate probes immobilized (chemically bonded) in discrete locations on a solid matrix. During hybridization, the labeled target molecules are brought into contact with the array and bind to a specific probe, in a specific position on the array, which enables identification of the target sequence. The two main approaches for spatially ordered array production are robotic spotting of pre-synthesized probes, used primarily for low density arrays, and in situ synthesis of probes on the chip surface, used commercially for production of high density arrays. Table 1 summarizes common characteristics of these microarray platforms. Many microbial diagnostic applications involve less than a few hundred probes allowing custom printing of low density arrays for such applications.
Table 1.
Common Manufacturing Approaches for Microarray Production
| Method of manufacturing | Probe length, nt | Maximum array probes or spots | Array production notes | Ligand | Development cost | Cost per array |
|---|---|---|---|---|---|---|
| Bead technology | Any | Less than 100 per well | Use 96-well plate | Oligonucleotide, cDNA, PCR products, protein, peptide, antibody, carbohydrate, or tissue. | High | High |
| Photolitography | 20–25 | 500,000 | Automated production allows high volume applications | DNA | Very high | High due to amortization of development costs |
| Spotted arrays | Any | 100,000 (practically several thousands) | Slow printing process, primarily for low-volume applications | Oligonucleotide, cDNA, PCR products, protein, peptide, antibody, carbohydrate, or tissue. | High | Low |
Bead array technology
Microarray bead technology involves attaching probes to identifiable beads, each labeled with a unique ratio of two spectrally distinct fluorophores (Yang et al., 2001; Summerbell et al., 2005; Deregt et al., 2006; Diaz et al., 2006; Porschewski et al., 2006; Schmitt et al., 2006). Each bead generates a unique signature (variable intensity of the two fluorophores) that provides ∼ 100 spectrally unique beads/signals that can be identified by flow cytometry. The probes (e.g., oligonucleotide or antibody) are bound to the surface of the beads enabling capture of the fluorescently labeled target (e.g., DNA or protein). A bead array assay is typically performed in a 96-well plate with up to 100 probes in each well. Bead technology assays can be performed in less time than a spatially ordered microarray experiment due to favorable solution phase kinetics. On the other hand, spatially ordered arrays allow a larger number of assays per experiment. Thus, bead technology may be preferred when a large number of samples must be assayed for a smaller number of features (maximum 100 per well) while spatially ordered arrays are preferred when a large number of features (up to hundreds of thousands) are being assayed from each sample.
Bead technology has been used for microbial analysis applications, including detection of fecal indicator bacteria in river samples (the probes used 16S and 23S rRNA genes) (Baums et al., 2007) and ascomycetous yeasts in clinical specimens (probe based on the large subunit of the rRNA gene) (Page and Kurtzman, 2005; Page et al., 2006). Bead technology for food microbial analysis is relatively new; however, these applications, which often involve an assay for a relatively small number of features, show great promise. The short assay time is advantageous, particularly in critical applications such as clinical medicine and public safety. Although most of the food microbial pathogen assays described in this article are based on spatially ordered microarrays, all appear to be adaptable to a bead array format and such applications are expected to grow in number in the coming years.
DNA Microarrays for Microbial Analysis
The power of microarray technology is the ability for simultaneous analysis of a large number of DNA sequences in a sample and a large number of samples in a compact and relatively economical format. The most common applications of the technology are i) analysis of gene expression and ii) genotyping. Gene expression experiments are used primarily to study the fundamental biology of an organism while genotyping has a wide range of varied uses revolving around the ability to confirm the presence of a particular sequence or a set of sequences in the target sample.
Microarrays for microbial gene expression analysis
The most common application of microarray technology is differential gene expression analysis. In a differential expression analysis, RNA from two samples to be compared is isolated, converted to a form suitable for hybridization (e.g., cDNA by reverse transcriptase), labeled with a dye, and hybridized to a microarray. If hybridization is to a single array, different dyes are needed for each sample. In a typical experiment, the signal level for each gene is analyzed and the expression patterns from the two samples are compared. In eukaryotic systems, mRNA isolation is followed by generation of cDNA using oligo(dT) binding to the eukaryotic mRNA poly(A) tails. In contrast, bacterial mRNA amplification and labeling is more complicated due to the lack of the poly(A) tails. To purify microbial mRNA, 16S and 23S rRNA (which is 80% or more of bacterial RNA) must be removed. Capture oligonucleotides which bind to bacterial 16S and 23S rRNAs have been used for this purpose (Pang et al., 2004; Lloyd et al., 2005). For generating bacterial cDNA, random primers are commonly used. Unlike poly-T priming used for eukaryotic mRNA, random priming generates cDNA with a more balanced representation of the transcripts and, in particular, it is not biased toward the 3′ end. However, random priming is less efficient than poly-T priming, yielding less cDNA.
When bacterial RNA is mixed with host RNA (e.g., analysis of infected tissues), avoiding host contamination requires specific host cell lysis followed by elimination of the host mRNA (Di Cello et al., 2005). This can be accomplished by centrifugation where the intact bacterial cells are easily separated, followed by RNA extraction and microarray analysis.
Microarrays for microbial genotyping
Microarray genotyping can be used to determine whether a specific sequence is present in a genomic sample. The analysis can cover an entire genome, a subset of genes from a single genome, a set of genes from several genomes, and/or multiple alleles of a single gene (e.g., single nucleotide polymorphisms). The use of microarray technology for microbial pathogen analysis is an emerging application for microarrays (Sergeev et al., 2006b).
Microbial genotyping using microarrays is based on the ability of the microarray to identify sequences present in the target molecule. It is technically feasible to use a high density microarray with coverage of an entire microbial genome. However, a more common use of microarray genotyping for food microbial analysis is to identify a limited number of signature sequences in a target for the purpose of species identification or differentiation between strains.
Genotyping analyses typically start with purification of genomic DNA followed by amplification using PCR or whole genome amplification, and labeling with a fluorescent dye. For such an analysis, only one dye is needed because the assay is designed to answer the question of whether or not a sequence is present in the sample. However, a second dye can be used effectively for quality control (QC) purposes (Volokhov et al., 2002, 2003a; Sergeev et al., 2004b) where a specific QC oligonucleotide, labeled with a different fluorescent dye, is mixed with the target DNA. For the analysis, each spot of the microarray is printed with a mixture of the probe and the QC oligonucleotide. This mixed sample is hybridized and, following washing, the scanned image has a signal at every spot on the microarray for the QC dye (Fig. 2). This QC feature verifies proper printing and hybridization of the microarray. For food safety analysis microbial genotyping enables microbial identification, mutational analysis, antibiotic resistance determination, and virulence factor identification with potential applications in research, clinical, regulatory, public health (for epidemiological investigations), and industrial settings.
FIG. 2.
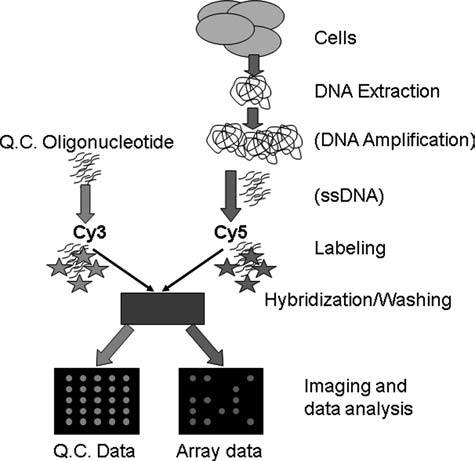
Microarray microbial genotyping analysis. Genomic target DNA is extracted from a cell, amplified (if needed), and converted to single-stranded DNA (if needed). The DNA can be labeled, during or after amplification, with a fluorescent dye (e.g., Cy5). In some applications, the labeled target DNA is mixed with a quality control (QC) oligonucleotide (complementary to a QC oligonucleotide printed in each spot) which is labeled with a different fluorescent dye (e.g., Cy3). Target DNA (or the mixed DNA sample) is hybridized to the microarray followed by washing, scanning and data analysis. A QC scan, with signals at every spot on the array, can be used to verify proper printing and hybridization of the microarray. The data scan will typically have signal at only a fraction of the spots, assuming the target molecule carries only a subset of the sequences complementary to the probes on the array.
DNA Target Sequences for Microbial Analysis
For microbial microarray genotyping analysis, two approaches are used in designing the probes: i) whole genome microarrays, which represent all the DNA sequences of a microorganism, and ii) signature sequence microarrays with probes for a subset of sequences from the genome (signature sequences). Such signature sequences typically represent genomic characteristics such as genes coding for rRNA, toxins, virulence factors, antibiotic resistance determinants, or shared conserved indels (insertions/deletions) (Gupta and Johari, 1998; Karlin and Brocchieri, 1998; Gupta and Griffiths, 2002; Gupta 2004). These signature sequences provide information regarding the identification of the microorganism or important characteristics. To generate signature sequences, genomic searches and multiple alignment analysis are performed to identify both conserved and unique sequences among the organisms of interest. Conserved sequences are useful in primer design for PCR amplification and unique sequences are useful for microbial identification.
The design of the signature sequences requires identification of target sequences and the design of oligoprobes to represent these sequences on the array. This bioinformatics task relies on computer programs developed for such analysis which are numerous with varying capabilities (Google, 2008a). A program developed by us (Sergeev et al., 2006b) for microarray design support is Oligo Design (http://www.enme.umd.edu/bioengineering). This program has several tools for the analysis of oligonucleotide sequences for development of probes and primers for oligonucleotide microarrays (Herold and Rasooly, 2003; Sergeev et al., 2005). This freely available program was used to develop a microarray for detection and identification of bacteria based on known short sequences of DNA (Sergeev et al., 2006a). The software automates several design aspects that enable the improved selection of oligonucleotides for use with microarrays for these applications. Major features of the program include i) a flexible interface for basic melting temperature calculations and sequence editing including various search algorithms; ii) Internet capabilities including sequence download from public databases; iii) a tiling algorithm for the design of short overlapping temperature-matched oligonucleotides of variable length, which are useful for the analysis of single nucleotide polymorphisms; iv) a set of tools for the analysis of multiple alignments of gene families and related short DNA sequences, which allow for the identification of conserved DNA sequences for PCR primer selection and variable DNA sequences for the selection of unique probes for identification; and v) a primer selection tool that guides primer selection and identifies potential problems with primer pairs.
Ribosomal sequences for bacterial identification
Ribosomal RNA and corresponding gene (rrn) polymorphisms can be used for bacterial identification and classification. In prokaryotes, the rrn locus typically contains the genes for all three ribosomal RNA sequences (16S, 23S, and 5S), which are highly conserved but separated by highly variable spacer regions (Ludwig and Schliefer, 1994). Although the 16S rRNA is the most common ribosomal sequence used (Anthony et al., 2000), it has been suggested that the 16S rRNA gene does not typically allow resolution below the species level (Kakinuma et al., 2003; Bodrossy and Sessitsch, 2004).
The rrn locus is used for bacterial identification because of several characteristics (Olsen et al., 1986): i) ribosomal RNAs show significant structural and functional conservation, even between divergent species; ii) ribosomal RNAs are abundant (making up as much as 80% of RNA in the cell), and are relatively readily isolated and easily identified; iii) the rrn sequence contains both conserved regions, facilitating amplification by PCR (using primers designed for the conserved regions), and variable regions, allowing for differentiation of species; and iv) the rrn sequences of many species are publicly available. One example application is the Affymetrix (Santa Clara, CA) GeneChip with over 30,000 microbial 16S rDNA oligoprobes which was used to accurately identify 17 of 19 populations of airborne bacteria (Wilson et al., 2002a, 2002b). 23S rDNA was used for microarray analysis of blood pathogens (Anthony et al., 2000) and for bacteria causing infertility and abortions in mares (Mitterer et al., 2004). The 16S–23S rDNA spacer region has also been used for microbial classification. Examples of use include identification of Bacillus anthracis (Nubel et al., 2004), targeting the 16S rRNA and 16S–23S rRNA intergenic region, and for Campylobacter identification (Keramas et al., 2003). Although the variability of 23S rDNA is similar to that of the 16S–23S rDNA spacer region (Anthony et al., 2000), the 23S rDNA variable region is longer than that of the 16S–23S rDNA spacer region so it provides more information for bacterial identification.
Bacterial virulence factors and antibiotic resistance determinants
Beyond species and strain identification, microarrays can be used for characterization of specific features of microbial pathogens. This capability includes analysis of microbial virulence factors and antibiotic resistance genes using signature sequences and characteristic genes. Such characterization has clinical importance as well as epidemiological applications. Genes for these features can be found on plasmids as well as on the chromosome. Genes found on plasmids are readily acquired through horizontal gene transfer, which can be monitored using an appropriate microarray system. Examples of the use of microarrays for microbial characterization are included later in this article.
Target DNA Preparation
Although hybridization with long double-stranded DNA targets (e.g., PCR amplicons of a few hundred base pairs) is possible, the hybridization efficiency for this approach is low generating a low microarray signal. To increase signal strength, at the cost of an additional step in the procedure, DNA can be either fragmented or converted to single-stranded DNA (ssDNA) prior to hybridization. In addition, target DNA can be amplified by PCR or, more recently, by whole genome amplification (WGA) to increase sensitivity (Kwon and Cox, 2004; Uda et al., 2007). For mRNA analysis (gene expression), a common approach is to use the RNA to make labeled cDNA for hybridization to the array. Labeled RNA can also be used directly but, due to the complementary nature of the RNA/cDNA conversion, the probes for these two approaches are complementary pairs. The choice of sense or antisense strand probes may be governed by commercially available arrays.
PCR amplification of target DNA
PCR amplification is a practical and effective method for microbial DNA target preparation for microarray applications (Chizhikov et al., 2001; Al-Khaldi et al., 2002, 2004; Volokhov et al., 2002, 2003b, 2004; Johnson et al., 2004; Sergeev et al. 2004a, 2006a; Tang et al. 2005). PCR typically yields >100,000 fold amplification of target sequences, even in the presence of much larger amounts of nonspecific DNA, enabling detection of a low number of bacterial cells in complex samples. PCR primer design is a critical element for PCR amplification which can be done systematically using programs such as Oligo Design (Herold and Rasooly, 2003; Sergeev et al., 2005). The most significant pitfall in application of PCR amplification is the possibility of amplification of non-target sequences through unexpected primer sequence matches. Thus an important part of primer design is checking the sequence against all known sequences (e.g., using BLAST [NCBI, 2008]) to ensure target specificity.
PCR amplicons are typically double-stranded DNA which can be used for hybridization on microarrays but the signal strength is often poor. Signal strength can be improved somewhat by fragmentation of the amplicon by sonication (Chizhikov et al., 2001). Single-stranded target DNA (or RNA) is a more efficient target for hybridization especially for use with short oligoprobes. ssDNA can be produced directly by asymmetric PCR (Tang et al., 2005), from PCR amplicons by a primer extension reaction (Volokhov et al., 2002), by strand separation (applying biotinylated primer and streptavidin-coated magnetic beads), or by in vitro transcription by RNA polymerase utilizing a T7 promoter tag attached to the 5′ end of the primer, serving as a recognition site for RNA polymerase (Volokhov et al., 2003a).
Whole genome amplification
There are three WGA approaches that are useful for target preparation for detection and identification of bacterial pathogens. WGA is based on extension of random primers (hexamers to octamers) annealed randomly along the genome, yielding amplification of 10–10,000 fold. The three commonly used methods are
• Degenerate oligonucleotide primed PCR (Barbaux et al., 2001), which utilizes random hexamers or octamers flanked with specific primer sequences. The random sections provide an approach to uniform amplification of the entire genome and the specific sequences allow subsequent amplification cycles to be done efficiently.
• OmniPlex is a method which starts with genome fragmentation, followed by adapter ligation and generation of a genomic DNA library which can be amplified by PCR (Barker et al., 2004a,b). In this case, the uniformity of genome coverage is primarily dependent on the success of the fragmentation step.
• Multiple-displacement amplification is an isothermal amplification reaction utilizing the highly processive bacteriophage Phi29 DNA polymerase and random exonuclease-resistant primers (Luthra and Medeiros, 2004; Paez et al., 2004; Gadkar and Rillig, 2005). The strand-displacement synthesis with the Phi29 polymerase (a polymerase with high proofreading activity) generates long DNA products, >10 kb in length, which is a benefit to many downstream technologies such as sequence identification because it implies fewer breaks in the sequence of interest.
Target DNA Labeling
Most microarray detection is based on fluorescent labeling in which the target molecule is modified with a fluorophore. However colorimetric and densitometric methods (e.g., methods based on horseradish peroxidase, alkaline phosphatase, or gold–silver staining) have also been used in a few applications. In general these alternative labeling techniques enable only a single color analysis while fluorescent labeling enables multiple color analysis on the same microarray, providing additional features such as enabling differential gene expression analysis.
Direct incorporation
For microarray analysis, a simple labeling method is direct incorporation of fluorescently labeled cyanine dye–modified (often Cy3 or Cy5) dCTP during reverse transcription, RT-PCR, or PCR amplification. A limitation of this approach is that the incorporation of cyanine-labeled dCTP is normally less efficient than unlabeled dCTP, reducing the yield of the reactions. Two-color analyses are complicated by the fact that Cy5 is incorporated less effectively than Cy3 but the extinction coefficient of Cy5 is higher than Cy3. These differences may introduce intensity bias in two-color experiments.
Indirect labeling
Indirect labeling of nucleic acids involves incorporation of an amino-allyl-modified dCTP during cDNA synthesis or PCR, followed by reaction of the resulting nucleic acids with an active ester of the dye. Indirect labeling overcomes the incorporation efficiency limitation of direct labeling (Manduchi et al., 2002). Because of this advantage, indirect labeling has become much more common than direct labeling.
Chemical labeling
Chemical processes can be used to add fluorescent cyanine labels directly to target molecules (Badiee et al., 2003) after synthesis. A method for direct chemical labeling of DNA with Cy3/Cy5-bearing alkylation agents was described by Goswami et al. (2004). An advantage of this method is that the labeling can be performed at any step of the assay before the hybridization.
Microarray Printing
Most microarrays commercially available today are designed for gene expression analysis studies. Such microarrays typically have a high density of probes, are relatively high in cost, and are typically focused on a particular organism. Thus, most commercially available microarrays do not address the needs for a specialized application such as food microbial analysis. Such specialized applications normally require only a small number of probes for each of multiple organisms and can be produced in-house using equipment with a modest cost. Contact printing (Fig. 3) of pre-synthesized oligonucleotides is practical for many small laboratories, particularly if the cost of the equipment can be amortized over several microarray projects. Custom arrays can be ordered from microarray companies but the cost can be prohibitive. For microarrays comprising a larger number of probes, several methods based on in situ synthesis of DNA ligands are available. These methods involve synthesis of oligonucleotides on the surface of the array in multiple cycles where one base (nucleotide) is added in each cycle.
FIG. 3.
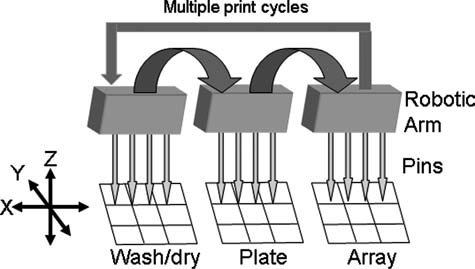
Microarray robotic contact spotting. The pins on the printing head can be programmed to move in three dimensions (X, Y, and Z). This allows the pins to be dipped into the probe reservoir and the washing stations and then to be directed to a precise location on the array surface.
Microarray contact printing
Contact printing (or spotting) uses pre-made DNA probes (or other ligands) that are transferred from a reservoir (e.g., a microtiter plate) to the surface of the solid substrate using a pin on a robotic arm (Fig. 3). The most commonly used substrates are glass microscope slides that have been coated with a binding layer. During printing, the pins take up a small quantity of the probe solution from the reservoir and then deposit a small droplet to the array surface creating a circular “spot.” Spotting of oligonucleotides is followed by irreversible bonding of the DNA to the binding layer (details depend on the immobilization chemistry) (Brown, 2008; Google, 2008b).
The robotic spotting system, the arrayer (Fig. 3), is relatively simple and inexpensive (on the order of $40,000 or more depending on features). The pins that carry the DNA from the microtiter plate to positions on the slides are moved in three dimensions (X, Y, and Z) by computer-controlled stepper motors with positional accuracy of ± 2.5 μm. The printing heads can carry multiple pins which allow increased printing speed. After printing a spot, the pins are moved to washing and drying stations before returning to the reservoir to minimize cross-contamination of the printed oligonucleotides. The primary advantage of this type of microarray printing is the ability to create a custom microarray at reasonable cost. However the minimum spot size is larger as compared to in situ synthesis technologies and the printing is slower, implying that contact printing is only practical for low density arrays.
Several types of substrates have been developed for covalent attachment of modified oligonucleotides to pre-activated solid supports including glass (Beier and Hoheisel, 1999; Rogers et al., 1999), oxidized silicon (Chrisey et al., 1996), gold-coated surfaces (Csaki et al., 2001), optical fibers (Healey et al., 1997), plastics [e.g., poly(methylmethacrylate)] (Fixe et al., 2004; Soper et al., 2005; Xu et al., 2007), three-dimensional matrices such as nitrocellulose or nylon membranes (Stillman and Tonkinson, 2000; Wang et al., 2002; Wrobel et al., 2003; Mao et al., 2006), polyacrylamide gel pads (Guschin et al. 1997a,b), and agarose films (Stillman and Tonkinson, 2000). Several surface chemistry approaches are available for attachment of the DNA probes to the substrate including amine, aldehyde, epoxy, and polylysine groups, and dendrimers polyamidoamine (PAMAM). Amine-modified glass, probably the most widely used technology, adsorbs nucleic acids through nonspecific electrostatic binding along the DNA backbone followed by inter- and intrastrand cross-linking via UV activation to covalently bind the DNA.
Hybridization and Washing
During hybridization the DNA probes bound to the microarray substrate and the complementary labeled DNA (or RNA) targets anneal to form a double-stranded molecule. Following annealing the unbound target is then washed off the array leaving only the labeled target bound to the surface at positions indexed to known probe sequences.
Hybridization and washing kinetics depend on many factors including the DNA probe surface density, the length of the immobilized DNA probes, and the stringency of the hybridization/washing conditions including temperature, salt concentration, formamide concentration, target concentration, hybridization chamber configuration, and time.
Array Scanning
After washing, the labeled target bound to each element on the array is detected by optically scanning the surface. Most scanners used for array analysis are optical devices that image the array surface and record signal intensity and the position of the signal on the array. Among several types of scanners available (Sergeev et al., 2006b), the most common types used for microarray detection are confocal scanning devices. However CCD cameras and flatbed scanners also can be used. In many fluorescent detection systems a laser is used to excite the fluorophore and then fluorescent emission is measured. Confocal scanning provides high resolution imaging by scanning a very narrow depth of focus, limiting background artifacts, with 5–10 μm resolution. Most systems utilize two lasers, a green laser (for Cy3, excitation wavelength is 550 nm and emission wavelength is 581 nm) and a red laser (for Cy5, excitation wavelength is 649 nm and emission wavelength is 670 nm). Some systems collect both signals in a single pass and others scan the array separately for each color. A charge-coupled device (CCD)-based scanner is less expensive and the continuous wavelength light sources (e.g., arc lamps) typically used may enable the detection of more dyes, eliminating the need for multiple lasers. Several CCD-based microarray analyses were reported (Lee et al., 2005; Glasenapp et al., 2007; Hansen et al., 2008). CCD-based imaging typically provides detection of a larger portion of the slide, reducing the complexity of the scanning system, simplifying instrument design, and reducing cost. However, CCD scanners have lower spatial resolution (i.e., approximately 20–50 μm) and broader depth of focus compared to a confocal system, which can increase the background signal.
A simpler approach for microarray imaging is the use of an off-the-shelf desktop flatbed scanner. Most flatbed scanner systems utilize colorimetric detection labels (e.g., products of horseradish peroxidase, alkaline phosphatase, or gold–silver staining) so only one color can be used. Although flat bed scanners have spatial resolution as good as 5 μm, the effective resolution for the proposed application is in the range 20–50 μm due to noise and scattering.
A flatbed scanner application was reported for analysis of cDNA labeled with gold nanoparticles with sensitivity of < 2 pg of DNA molecules captured on the array surface (Sun et al., 2007). Lai et al. (2005) used a flatbed scanner for microarray-based quantitative gene expression analysis. In another study utilizing a flatbed scanner, the target cDNA was labeled with biotin and was detected by streptavidin-conjugated alkaline phosphatase staining (Petersen et al., 2007). One of the barriers to the application of microarray technology is the equipment cost. However, inexpensive flatbed scanners and CCD-based detectors open new opportunities, even for small laboratories, to develop clinical and environmental applications for microarray technology.
Applications of Microarray Technologies for Food Safety Analysis
Food microbial pathogen identification and characterization
The initial application that pioneered the use of microarray technology for food microbial characterization was done at the U.S. Food and Drug Administration laboratories with the aim of identification of enteric food pathogens based on their various virulence factors (Chizhikov et al., 2001). The basic technology used in this and other related work was the amplification of clinically relevant target sequences, such as virulence factors, by PCR followed by identification via hybridization to a microarray.
Microarray analysis of enteric food pathogens
The presence of six genes encoding antigenic determinants and virulence factors (eaeA, slt-I, slt-II, fliC, rfbE, and ipaH) of enteric bacteria was determined by amplification using multiplex PCR followed by hybridization of denatured PCR products to gene-specific oligonucleotide probes on a microarray (Loy et al., 2005). The assay was able to detect these virulence factors in 15 Salmonella, Shigella, and E. coli strains. The results from this work demonstrate that microarray analysis of microbial virulence factors might be useful for automated identification and characterization of bacterial pathogens.
A similar approach was used for detecting and genotyping Escherichia coli O157:H7, a major enteric food pathogen. Amplicons obtained by multiplex PCR of four virulence loci (intimin, Shiga-like toxins I and II, and hemolysin A) were hybridized to the array which was shown to be 32-fold more sensitive than gel electrophoresis and capable of detecting amplification products from <1 cell equivalent of genomic DNA (1 fg) in pure microbial culture (Call et al., 2001). Immunomagnetic capture followed by PCR and microarray hybridization were subsequently used to detect 55 colony-forming units (CFU)/mL (E. coli O157:H7) from chicken rinsate without pre-enrichment (Call et al., 2001).
The main conventional method for typing enteric bacteria is serotyping targeting O and H antigens. A serotype-specific DNA microarray was developed for identification of some Shigella species and pathogenic E. coli strains targeting O serotype–specific genes and was used to detect 15 serotypes of Shigella and E. coli (Li et al., 2006). The array was tested with 186 representative strains of all Shigella and E. coli serotypes, and the sensitivity was 50 ng genomic DNA or 104 CFU/mL in mock stool specimens.
A bead array application for detection of enteric bacteria was based on a fiber-optic substrate using microsphere-immobilized oligonucleotide probes specific for the Salmonella invA and spvB genes (Ahn and Walt, 2005). Hybridization of the probe-functionalized microspheres to target DNA from Salmonella was performed and visualized using Cy3-labeled secondary probes in a sandwich assay format. This system was capable of detection of 103–104 CFU/mL after 1-hour hybridization without amplification.
Microarray analysis of staphylococcal enterotoxins
The heat-stable staphylococcal enterotoxins (SEs) consist of a family of 18 major serological types of toxins (SEA through SEV). Microarray analysis demonstrated that many S. aureus strains contain multiple enterotoxin genes (Sergeev et al., 2004a). In this work, a combination PCR–microarray assay for detection and identification of ent genes was used. The analysis is based on PCR amplification of a variable region of almost all known enterotoxin genes using a single set of degenerate primers corresponding to the flanking highly conserved regions of these genes. The enterotoxin microarray was prepared by immobilizing four unique ∼ 25 base oligonucleotide probes for each of the 16 genes, where each gene is represented by four unique sequences to increase the reliability of the assay. The four probes for each toxin gene were arranged in a row for each gene as shown in Fig. 4. The left-hand block of the array contained probes for eight enterotoxins (sea–sei) and the right-hand block contained probes for the other eight (sej–seq). The QC scan of the array is shown in Fig. 4A. In our QC procedure (Volokhov et al., 2002, 2003a; Sergeev et al., 2004b), each spot was printed with a QC oligonucleotide in addition to the gene specific oligoprobe. Each Cy5-labeled target ssDNA was spiked with Cy3-labeled ssDNA complementary to the QC oligonucleotide. The Cy3 QC scan provides a control image of the entire microarray, allowing validation of the array printing and hybridization steps (Fig. 4A).
FIG. 4.
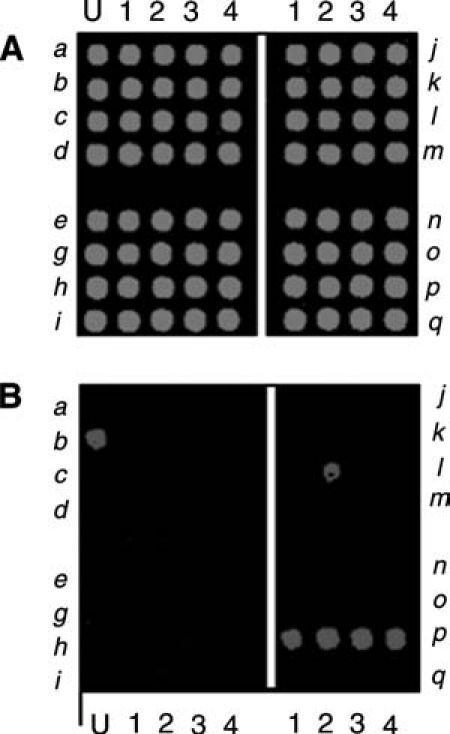
Staphylococcal enterotoxin microarray. This microarray was designed to detect 16 S. aureus enterotoxin genes (sea-seq). The microarray is composed of four sections, each representing four enterotoxin genes (each row is one gene). Each gene is detected by four unique probes (the four spots in each row). The first column labeled U is printed with a spotting solution that provides an indicator of the microarray position on the substrate. The upper section (A) is an image of the signal from the quality control (QC) probes and the lower section (B) is an image of the signal from the data probes for the enterotoxin genes. The spots for the sea–sei genes are in the left block and the sej–seq genes are in the right block, with each gene represented by four unique spots. (A) The QC scan of the array in which each spot of the array shows a signal because each spot contains a small amount of a QC oligoprobe, which hybridizes with a Cy3-labeled QC oligonucleotide spiked in the target mixture. The QC scan is excited using the 543 nm laser. (B) The data scan for a particular sample generated by amplifying genomic DNA from S. aureus strain N315 using primers specific for enterotoxin p (sep). The PCR product was labeled with Cy5. The data scan was done using 632 nm excitation.
For testing of the SE microarray, DNA from multiple S. aureus strains were amplified using enterotoxin gene specific primers and fluorescently labeled ssDNA was synthesized from the amplicons using primer extension of the PCR products. The microarray accurately detected each toxin gene. For example, the assay for strain N315, which is known to carry the sep gene, is shown in Fig. 4B. It is important to note that occasional cross-reacting spots are observed, such as the single sel spot (no. l2; Fig. 4B) that cross-reacted with sep amplicons because of sequence similarity. However, since four unique oligoprobes were used to detect each gene, a small number of cross-reacting spots did not interfere with toxin identification. Figure 4 is a representative SE assay which demonstrates clearly the detection of sep in the strain tested. The column labeled U was printed with a visible dye, called a spotting solution, that allows the user to see the location of the printed array in visible light, in addition to the QC oligo. It was occasionally observed that target molecules would bind to the spots in column U, as in Fig. 4B row b, but these results are assumed to be due to nonspecific binding and are not considered significant.
An important observation from this work is that that many of the isolates contain previously unreported SE genes and that multiple SE genes were found in the majority (∼92%) of S. aureus strains tested (Sergeev et al., 2004a, 2004b). For example as shown in Fig. 5 (using the same array format as in Fig. 4), strain N315 contains multiple enterotoxin genes (Fig. 5A, I). Strain ATCC 14458 (Fig. 5A, II) known to possess seb, was found to also contain the recently discovered sek and seq genes. Similarly, strain NTCC 10656 was found to encode the seg, sei, sej, sem, sen, and seo genes, in addition to the already known sed gene (Fig. 5B, I). The seq gene was unexpectedly found in S. aureus ATCC 27664 (Fig. 5B, II).
FIG. 5.
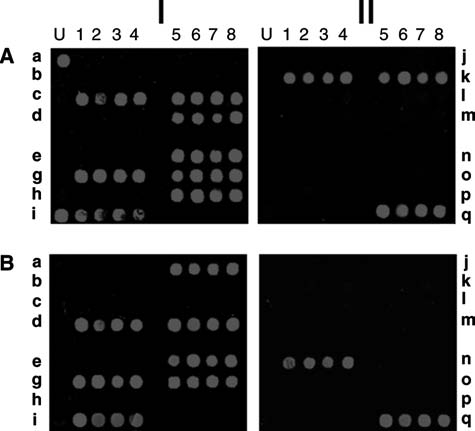
Analysis of previously analyzed S. aureus strains for new enterotoxins. Genomic DNA from four previously analyzed strains (strains N315, ATCC 14458, NTCC 10656, and ATCC 27664 were amplified using a mixture of enterotoxin gene universal primers supplemented with the specific primer for seh. The resulting polymerase chain reaction (PCR) amplicons were subjected to primer extension followed by Cy5 chemical labeling and then hybridized to the enterotoxin gene microarray. The resulting image was scanned using the 632 nm laser. The four results are presented in a 2×2 matrix composite image. AI, strain N315; BI, NTCC 10656; AII, ATCC 14458 and BII, ATCC 27664.
Microarray analysis of Bacillus cereus group
Several important virulence factors of B. cereus, B. thuringiensis, and B. anthracis, including enterotoxins, phospholipases, and exotoxins, were analyzed using a microarray (Volokhov et al., 2004; Sergeev et al., 2006a). For B. anthracis, toxin genes used on the microarray included pagA, lef, and cya. The assay involved an initial multiplex PCR amplification step, followed by identification of the PCR amplicons by hybridization to an oligonucleotide microarray containing genes for all three types of Bacillus virulence factors. This work demonstrated that virulence factors were present in several combinations in the strains analyzed.
Beyond microbial diagnostics, microarrays have been used for forensic fingerprinting of Bacillus isolates (Chandler et al., 2006). A combined microarray used virulence factor genes of B. anthracis (pag, lef, and cap), the variable number tandem repeat region of the B. anthracis vrrA gene, the 16S–23S rRNA intergenic transcribed spacer region, and the pleiotropic regulator regions of the Bacillus cereus subgroup (Burton et al., 2006). The array was used for differential identification of B. anthracis from environmental Bacillus species mixed-culture. The identification of B. anthracis was unambiguous when based on the pag, lef, and cap genes. However, cross-reactions were observed between B. anthracis and other Bacillus probes for the other genes on the array. This demonstrated the utility of this microarray as an effective assay for the identification of B. anthracis from mixed-culture environmental samples without the false-positive problems that have been observed with conventional PCR assays.
Campylobacter species
An oligonucleotide microarray was developed and used for the analysis of four thermophilic Campylobacter spp., C. jejuni, C. coli, C. lari, and C. upsaliensis (Volokhov et al., 2003a). The assay relies on PCR amplification of specific regions in five target genes (fur, glyA, cdtABC, ceuB-C, and fliY) as a first step, followed by microarray-based analysis of amplified DNA. The array was developed and validated by using 51 previously characterized Campylobacter isolates. All isolates were unambiguously identified on the basis of hybridization patterns with 72 individual species-specific oligoprobes.
In another example, a multiplex PCR DNA microarray assay targeting the 16S rRNA and the 16S–23S rRNA intergenic region was used to detect Campylobacter directly from fecal cloacal swabs (Keramas et al., 2003) with sensitivity of 3–30 genome equivalents (6–60 fg DNA) of Campylobacter within 3 hours. Closely related Campylobacter species, C. jejuni and C. coli, could be detected and differentiated directly from chicken feces.
Microarrays for analysis of Listeria spp
Six species of the Listeria genus: L. monocytogenes, L. ivanovii, L. innocua, L. welshimeri, L. seeligeri, and L. grayi were analyzed by multiplex PCR amplification of six target bacterial virulence factor genes (iap, hly, inlB, plcA, plcB, and clpE) followed by hybridization to a microarray. The microarray consisted of multiple individual oligonucleotide probes specific for each Listeria species immobilized on a glass surface (Volokhov et al., 2002). Results of the microarray analysis of 53 reference and clinical isolates of Listeria spp. demonstrated that this method allowed unambiguous identification of all six Listeria species based on sequence differences in the iap gene while another virulence factor gene, hly, enabled the detection and genotyping of only L. monocytogenes, L. ivanovii, and 8 of 11 L. seeligeri isolates since other members of the genus Listeria and three L. seeligeri isolates did not contain the hly gene.
Differential gene expression analysis was performed before and after hydrostatic pressure processing (HPP) to understand how L. monocytogenes responds to mechanical stress injury (Bowman et al., 2008). The microarray data suggested that HPP induced increased expression of genes associated with i) DNA repair mechanisms, ii) transcription and translation protein complexes such as the septal ring, iii) the general protein translocase system, iv) flagella assembly and chemotaxis, and v) lipid and peptidoglycan biosynthetic pathways. HPP also appeared to suppress a wide range of energy production and conversion systems, carbohydrate metabolism, and virulence-associated genes, particularly a strong suppression of the SigB and PrfA regulons.
Microarray detection of pathogenic Vibrio spp
Multiplex PCR of 10 characteristic marker sequences of Vibrio vulnificus, V. cholerae, and V. parahaemolyticus followed by hybridization of the amplicons to microarray was used to ensure that total and pathogenic strains could be detected and discriminated (Panicker et al., 2004). The detection sensitivity for pure cultures without enrichment was 102 to 103 CFU/mL, and the specificity was 100%. Using 5 hours of sample enrichment followed by DNA extraction enabled the detection of 1 CFU in 1 g of oyster tissue homogenate. Application of the DNA microarray methodology to natural oysters revealed the presence of V. vulnificus (100%) and V. parahaemolyticus (83%) but not V. cholerae.
Microarray for monitoring the production of mycotoxins
A microarray that covered most of the known relevant food mycotoxin biosynthesis genes, including the primary pathway genes for fumonisin, aflatoxin, ochratoxin, trichothecene (type A and B), and patulin, was developed. The microarray was used to study the expression kinetics of mycotoxins (Schmidt-Heydt and Geisen, 2007).
Multi-pathogen microarrays
The microarrays described above were used for the analysis of individual or related pathogens. Several such pathogen-specific microarrays were integrated into a single DNA chip (Sergeev et al., 2004a) for simultaneous analysis of multiple virulence factors of different pathogens, including S. aureus enterotoxin genes, Listeria spp., Campylobacter spp., and Clostridium perfringens. Similarly, multi-pathogen microarrays have been used for detection of 18 potential biowarfare agents (Wilson et al., 2002b). A larger microarray with over 53,000 oligoprobes was used for multi-pathogen (142 unique diagnostic regions of 11 bacteria, five RNA viruses, and two eukaryotes) analysis (Wilson et al., 2002a).
An oligonucleotide microarray for the detection of foodborne bacterial pathogens based on the 16S rRNA gene was developed (Wang et al., 2007). The system employed universal PCR primers to amplify a variable region of the bacterial 16S rRNA gene, followed by hybridization of the products to species-specific oligonucleotide probes for 23 species of food microbial pathogens including Salmonella spp., Shigella spp., E. coli, S. aureus, L. monocytogenes, Vibrio parahaemolyticus, Campylobacter jejuni, Vibrio cholerae, Clostridium botulinum, and C. perfringens. The array demonstrated discrimination of 115 strains of bacteria isolated from foods with 97.4% correct identification with sensitivity of 102 CFU/mL.
Analysis of antibiotic-resistant bacteria
Antibiotic resistance in foodborne pathogens has direct implications for food safety. For the purposes of administering appropriate antibiotics and monitoring the spread of antibiotic-resistant microorganisms, it is very important to determine the antibiotic resistance profile of a pathogen. Traditional culture-based antibiotic resistance determination is slow, especially for bacteria such as Mycobacterium tuberculosis, and may not provide information about the mechanism of the resistance. Microarrays can provide molecular information on antibiotic resistance determinants. Several microarrays have been developed to provide such an analysis for bacteria, yeasts, and other pathogenic microorganisms. In general, such analyses can be divided into two main groups: i) microarrays for genotyping of antibiotic resistance determinants and ii) microarrays for studying the mechanisms of antibiotic resistance.
Microarray analysis of antibiotic resistance in S. aureus.
Several microarrays for analysis of antibiotic resistance in S. aureus have been developed. One system involved analysis of S. aureus erythromycin resistance determinants (Volokhov et al., 2003b). The microarray contained six oligoprobes representing each of the six genes (ermA, ermB, ermC, ereA, ereB, msrA/B) that account for more than 98% of erythromycin (and the related macrolide-lincosamide-streptogramin [MLS]) resistance. In general, these antibiotics are effective for gram-positive and some gram-negative bacteria. The microarray was tested with reference and clinical S. aureus and Streptococcus pyrogenes strains. The target genes from the samples were amplified by multiplex PCR and simultaneously fluorescently labeled. The results showed that of the 18 S. aureus clinical strains tested, 11 isolates carried MLS determinants with 8 (72%) of the 11 strains carrying two or three MLS resistance genes. These results demonstrated that microarray technology can provide considerable insight into the genetic profile of these aggressive pathogens which may be useful in designing therapies and strategies for public health. Other microarrays were developed for the detection of 10 antibiotic resistance genes and mutations in these S. aureus genes [mecA, aacA-aphD, tetK, tetM, vat(A), vat(B), vat(C), erm(A), erm(C), grlA-mutation] (Strommenger et al., 2007).
A DNA microarray was developed for simultaneous detection of genes leading to penicillin, methicillin, aminoglycoside, macrolide, lincosamide, and streptogramin B [MLS(B)] resistance in staphylococcal isolates (Zhu et al., 2007). The array contained specific conserved regions of the resistance determinants for these antibiotics and the variable sequence region of the 16S rRNA gene to broadly differentiate between Staphylococcus aureus and other coagulase-negative staphylococci. The performance of the microarray was validated with a total of 178 clinically important S. aureus and 237 coagulase-negative staphylococci isolates. There were correlations of 100% for S. aureus discrimination and greater than 90% for antibiotic resistance comparing microarray analysis versus the phenotype determined by standard methods of species identification and susceptibility testing. The assay was fast (≤5 hours) and enabled accurate and sensitive analysis of antibiotic resistance genes from a single colony. For isolates that did not correlate, antibiotic resistance genes were detected by the microarray but the bacteria were found to be susceptible to the corresponding antibiotic.
Analysis of antibiotic resistance in enteric bacteria
Microarray technology was used for the analysis of multiple tetracycline (tet) resistance genes and β-lactamase blaTEM-1 genes in E. coli (Call et al., 2003). Isolates of Salmonella from cases of human infection and from food production animals were screened using a microarray to determine the number and spectra of resistance genes. Resistance to streptomycin, trimethoprim, and sulfonamides was usually encoded by only one resistance gene in animal isolates, but human isolates often carried more than one gene encoding resistance to the same class of antimicrobial (Hopkins et al., 2007).
An oligonucleotide microarray targeting 38 commonly found genes related to antimicrobial resistance for tetracycline, erythromycin, and clindamycin was developed and used to study the impact of feed supplementation with antimicrobial agents on growth performance of broiler chickens (Diarra et al., 2007). Analysis of the incidence and distribution of antibiotic resistance in 197 commensal E. coli isolates from broiler chickens demonstrated that multi-antibiotic–resistant E. coli isolates can be found in broiler chickens regardless of the antimicrobial growth promoters used, although the phenotype and the distribution of resistance determinants in E. coli can be modulated by feed supplementation with some of the antimicrobial agents used in broiler chicken production.
In another study (Chen et al., 2005), a microarray was used for analysis of multiple antimicrobial resistance genes (aadA, tetA, and sulI) which were most commonly detected in bacteria resistant to streptomycin, tetracycline, and sulfonamide. The array also included the blaCMY-2 and blaTEM-1 genes, conferring resistance to third generation cephalosporins in Salmonella and E. coli. In addition to the gene analysis described above, single nucleotide polymorphism (SNP) mutations which confer extended-spectrum beta-lactamase–mediated resistance or inhibitor-resistant TEM (IRT) phenotype (causing resistance to extended-spectrum cephalosporins, monobactams, and beta-lactamase inhibitors) were analyzed by a SNP microarray for E. coli, Enterobacter cloacae, and Klebsiella pneumoniae (Grimm et al., 2004). In another work, a DNA microarray consisting of approximately 300 oligonucleotide probes representing seven classes of antibiotic resistance genes was used for molecular characterization of intrinsic and acquired antibiotic resistance in lactic acid bacteria and bifidobacteria (Ammor et al., 2008).
A disposable microarray for detection of up to 90 antibiotic resistance genes in gram-positive bacteria was tested against 36 strains and enabled the detection of multi-drug–resistant strains of Enterococcus faecalis, E. faecium, Lactococcus lactis, Staphylococcus haemolyticus, Clostridium perfringens, and an avirulent strain of B. anthracis harboring the broad-host-range resistance plasmid (Perreten et al., 2005). Hybridization with DNA from two field strains allowed the detection of 12 different antibiotic resistance genes in a Staphylococcus haemolyticus strain isolated from milk from cows with mastitis (Perreten et al., 2005). These publications demonstrate that microarray technology has a large potential for application in basic and applied food safety research, and in surveillance programs for antimicrobial resistance.
Sensitivity of microarray detection
Conventional culturing methods can have a detection limit as low as one cell per 25 g of sample after enrichment (Liu-Stratton et al., 2004). For microarray analysis, PCR amplification is often used prior to hybridization to increase sensitivity of detection. The detection level depends on many parameters such as the quality of DNA, the primers used, the size of the amplicons, the size of the probe, labeling technology, hybridization conditions, the scanner used, and other technical aspects. To provide a representative sensitivity range, several examples are provided. It is noted that these examples span a broad range of experimental conditions.
DNA microarrays with oligoprobes complementary to four E. coli O157:H7 virulence loci (intimin, Shiga-like toxins I and II, and hemolyxin A) were used to detect amplification products from <1 cell equivalent of genomic DNA (1 fg) in culture (Call et al., 2001). Immunomagnetic capture, PCR, and a microarray enabled detection of 55 CFU/mL (E. coli O157:H7) from chicken rinsate without pre-enrichment.
Using a DNA microarray employing 10 functional genes as detection targets, the sensitivity of the microarray was determined to be approximately 1.0 μg of E. coli genomic DNA, or 2 × 108 copies of the target gene. The sensitivity of the microarray was enhanced by approximately six orders of magnitude when the target 23S rRNA gene sequences were PCR amplified with a novel universal primer set followed by hybridization to 24 species-specific oligonucleotide probes. The minimum detection limit was estimated to be about 100 fg of E. coli genomic DNA or 1.4 × 102 copies of the 23S rRNA gene (Lee et al., 2006).
Microarray analysis of mixed microbial samples
The identification of pathogenic bacteria in a background of non-pathogens is a challenge, especially for the detection and identification of low-abundance pathogens mixed within a complex microbial population. To overcome this challenge, a diagnostic microarray based on gyrB as the marker gene was used (Fukushima et al., 2003; Kakinuma et al., 2003; Gonzalez et al., 2004; Kostic et al., 2007) with a single nucleotide extension labeling assay for specific detection of a broad range of pathogenic bacteria. A microarray with 35 oligonucleotide probes (Kostic et al., 2007) targeting E. coli, Shigella spp., Salmonella spp., Aeromonas hydrophila, V. cholerae, Mycobacterium avium, M. tuberculosis, Helicobacter pylori, Proteus mirabilis, Yersinia enterocolitica, and C. jejuni was developed. In this assay, the introduction of competitive oligonucleotides (exact matches to sequences in the microbial community exhibiting false-positive signals on the microarray in preliminary tests) in the labeling reaction successfully suppressed cross-reaction by closely related sequences, significantly improving the performance of the assay. In environmental and veterinary samples harboring complex microbial communities, the detection sensitivity of target DNA in the range of 0.1% of total DNA in the sample was demonstrated, far below the 5% detection limit typical of microarray assays applied to such complex samples. Developing microarrays for multi-organism analysis opens new applications in the analysis of microbial communities.
Summary and Future Trends
Microarray technology plays a significant and growing role in identification and analysis of food microbial pathogens. The ability to identify pathogens at the genomic level, using signature sequences, provides a capability that is not available in the traditional culture-based identification methods. This capability expands the analysis possibilities beyond species identification to include virulence factor and antibiotic resistance determinants that are known to participate in horizontal transfer. In comparison to culture-based methods, microarray assays are much faster. This speed, in combination with comparable accuracy and more informative results explains why microarray assays are being explored as alternatives to culture-based methods in food microbial analysis.
Typically, food microbial microarray applications involve less than a few hundred probes. This is in contrast to gene expression microarray applications that often involve many thousands of probes. Most commercial microarrays are designed to meet the needs of gene expression applications. These high density arrays require special manufacturing techniques and they are relatively expensive. The low density arrays typical of food applications have attracted less attention from the microarray vendors. Instead, these arrays are normally custom fabricated by the researcher using a robotic spotting system. Combining the robotic spotting system with a low cost scanner puts the technology in reach of many laboratories. Such available technologies open new opportunities, even to smaller laboratories, to develop food safety applications for microarray technology that may offer improvements in foodborne pathogen identification and characterization protocols compared to the traditional culture and immunological methodologies.
Microarray technologies have undergone rapid development and innovation continues today. Commercial printing technologies can be expected to continue to improve, lowering the cost of higher density arrays. However, the number of probes in most commercial arrays is higher than needed for typical food applications. As the demand for robotic spotters and low-end scanning systems increases, cost would also be expected to decrease. This market is also served by bead arrays, and it is not clear which of these technologies will find greater application for food testing. Genomic-based identification of pathogens provides information needed to more fully understand the occurrence of horizontal gene transfer in foodborne pathogens, as well as how pathogens co-exist with non-pathogenic species within microbial communities. Microarray technology is one of the tools bringing about a new era of genomics-based technologies, which will provide a greater understanding of both foodborne pathogens, as well as help to ensure a higher level of safety in the food chain.
References
- Ahn S. Walt DR. Detection of Salmonella spp. using microsphere-based, fiber-optic DNA microarrays. Anal. Chem. 2005;77(15):5041–5047. doi: 10.1021/ac0505270. [DOI] [PubMed] [Google Scholar]
- Al-Khaldi SF. Martin SA. Rasooly A. Evans JD. DNA microarray technology used for studying foodborne pathogens and microbial habitats: minireview. J. AOAC Int. 2002;85(4):906–910. [PubMed] [Google Scholar]
- Al-Khaldi SF. Villanueva D. Chizhikov V. Identification and characterization of Clostridium perfringens using single target DNA microarray chip. Int. J. Food Microbiol. 2004;91(3):289–296. doi: 10.1016/j.ijfoodmicro.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Ammor MS. Florez AB. van Hoek AH. de Los Reyes-Gavilan CG. Aarts HJ. Margolles A. Mayo B. Molecular characterization of intrinsic and acquired antibiotic resistance in lactic acid bacteria and bifidobacteria. J. Mol. Microbiol. Biotechnol. 2008;14(1–3):6–15. doi: 10.1159/000106077. [DOI] [PubMed] [Google Scholar]
- Anthony RM. Brown TJ. French GL. Rapid diagnosis of bacteremia by universal amplification of 23S ribosomal DNA followed by hybridization to an oligonucleotide array. J. Clin. Microbiol. 2000;38(2):781–788. doi: 10.1128/jcm.38.2.781-788.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiee A. Eiken HG. Steen VM. Lovlie R. Evaluation of five different cDNA labeling methods for microarrays using spike controls. BMC Biotechnol. 2003;3(1):23. doi: 10.1186/1472-6750-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaux S. Poirier O. Cambien F. Use of degenerate oligonucleotide primed PCR (DOP-PCR) for the genotyping of low-concentration DNA samples. J. Mol. Med. 2001;79(5–6):329–332. doi: 10.1007/s001090100214. [DOI] [PubMed] [Google Scholar]
- Barker DL. Hansen MS. Faruqi AF. Giannola D. Irsula OR. Lasken RS. Latterich M. Makarov V. Oliphant A. Pinter JH. Shen R. Sleptsova I. Ziehler W. Lai E. Two methods of whole-genome amplification enable accurate genotyping across a 2320-SNP linkage panel. Genome Res. 2004a;14(5):901–907. doi: 10.1101/gr.1949704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker KS. Crisp S. Wiederhold N. Lewis RE. Bareither B. Eckstein J. Barbuch R. Bard M. Rogers PD. Genome-wide expression profiling reveals genes associated with amphotericin B and fluconazole resistance in experimentally induced antifungal resistant isolates of Candida albicans. J. Antimicrob. Chemother. 2004b;54(2):376–385. doi: 10.1093/jac/dkh336. [DOI] [PubMed] [Google Scholar]
- Baums IB. Goodwin KD. Kiesling T. Wanless D. Diaz MR. Fell JW. Luminex detection of fecal indicators in river samples, marine recreational water, and beach sand. Mar. Pollut. Bull. 2007;54(5):521–536. doi: 10.1016/j.marpolbul.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier M. Hoheisel JD. Versatile derivatisation of solid support media for covalent bonding on DNA-microchips. Nucleic Acids Res. 1999;27(9):1970–1977. doi: 10.1093/nar/27.9.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodrossy L. Sessitsch A. Oligonucleotide microarrays in microbial diagnostics. Curr. Opin. Microbiol. 2004;7(3):245–254. doi: 10.1016/j.mib.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Bowman JP. Bittencourt CR. Ross T. Differential gene expression of Listeria monocytogenes during high hydrostatic pressure processing. Microbiology. 2008;154(Pt 2):462–475. doi: 10.1099/mic.0.2007/010314-0. [DOI] [PubMed] [Google Scholar]
- Brown P. Palo Alto, CA: Stanford University; 2008. [Apr 1;2008 ]. The Brown Lab's complete guide to microarraying for the molecular biologist. [Google Scholar]
- Burton JE. Oshota OJ. Silman NJ. Differential identification of Bacillus anthracis from environmental Bacillus species using microarray analysis. J. Appl. Microbiol. 2006;101(4):754–763. doi: 10.1111/j.1365-2672.2006.02991.x. [DOI] [PubMed] [Google Scholar]
- Call DR. Bakko MK. Krug MJ. Roberts MC. Identifying antimicrobial resistance genes with DNA microarrays. Antimicrob. Agents Chemother. 2003;47(10):3290–3295. doi: 10.1128/AAC.47.10.3290-3295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call DR. Brockman FJ. Chandler DP. Detecting and genotyping Escherichia coli O157:H7 using multiplexed PCR and nucleic acid microarrays. Int. J. Food Microbiol. 2001;67(1–2):71–80. doi: 10.1016/s0168-1605(01)00437-8. [DOI] [PubMed] [Google Scholar]
- Chandler DP. Alferov O. Chernov B. Daly DS. Golova J. Perov A. Protic M. Robison R. Schipma M. White A. Willse A. Diagnostic oligonucleotide microarray fingerprinting of Bacillus isolates. J. Clin. Microbiol. 2006;44(1):244–250. doi: 10.1128/JCM.44.1.244-250.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Zhao S. McDermott PF. Schroeder CM. White DG. Meng J. A DNA microarray for identification of virulence and antimicrobial resistance genes in Salmonella serovars and Escherichia coli. Mol. Cell. Probes. 2005;19(3):195–201. doi: 10.1016/j.mcp.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Chizhikov V. Rasooly A. Chumakov K. Levy DD. Microarray analysis of microbial virulence factors. Appl. Environ. Microbiol. 2001;67(7):3258–3263. doi: 10.1128/AEM.67.7.3258-3263.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisey LA. O'Ferrall CE. Spargo BJ. Dulcey CS. Calvert JM. Fabrication of patterned DNA surfaces. Nucleic Acids Res. 1996;24(15):3040–3047. doi: 10.1093/nar/24.15.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csaki A. Moller R. Straube W. Kohler JM. Fritzsche W. DNA monolayer on gold substrates characterized by nanoparticle labeling and scanning force microscopy. Nucleic Acids Res. 2001;29(16):E81. doi: 10.1093/nar/29.16.e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deregt D. Gilbert SA. Dudas S. Pasick J. Baxi S. Burton KM. Baxi MK. A multiplex DNA suspension microarray for simultaneous detection and differentiation of classical swine fever virus and other pestiviruses. J. Virol. Methods. 2006;136(1–2):17–23. doi: 10.1016/j.jviromet.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Diarra MS. Silversides FG. Diarrassouba F. Pritchard J. Masson L. Brousseau R. Bonnet C. Delaquis P. Bach S. Skura BJ. Topp E. Impact of feed supplementation with antimicrobial agents on growth performance of broiler chickens, Clostridium perfringens and enterococcus counts, and antibiotic resistance phenotypes and distribution of antimicrobial resistance determinants in Escherichia coli isolates. Appl. Environ. Microbiol. 2007;73(20):6566–6576. doi: 10.1128/AEM.01086-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MR. Boekhout T. Theelen B. Bovers M. Cabanes FJ. Fell JW. Microcoding and flow cytometry as a high-throughput fungal identification system for Malassezia species. J. Med. Microbiol. 2006;55(Pt 9):1197–1209. doi: 10.1099/jmm.0.46630-0. [DOI] [PubMed] [Google Scholar]
- Di Cello F. Xie Y. Paul-Satyaseela M. Kim KS. Approaches to bacterial RNA isolation and purification for microarray analysis of Escherichia coli K1 interaction with human brain microvascular endothelial cells. J. Clin. Microbiol. 2005;43(8):4197–4199. doi: 10.1128/JCM.43.8.4197-4199.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fixe F. Dufva M. Telleman P. Christensen CB. One-step immobilization of aminated and thiolated DNA onto poly(methylmethacrylate) (PMMA) substrates. Lab. Chip. 2004;4(3):191–195. doi: 10.1039/b316616c. [DOI] [PubMed] [Google Scholar]
- Fukushima M. Kakinuma K. Hayashi H. Nagai H. Ito K. Kawaguchi R. Detection and identification of Mycobacterium species isolates by DNA microarray. J. Clin. Microbiol. 2003;41(6):2605–2615. doi: 10.1128/JCM.41.6.2605-2615.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadkar V. Rillig MC. Application of Phi29 DNA polymerase mediated whole genome amplification on single spores of arbuscular mycorrhizal (AM) fungi. FEMS Microbiol. Lett. 2005;242(1):65–71. doi: 10.1016/j.femsle.2004.10.041. [DOI] [PubMed] [Google Scholar]
- Glasenapp C. Monch W. Krause H. Zappe H. Biochip reader with dynamic holographic excitation and hyperspectral fluorescence detection. J. Biomed. Opt. 2007;12(1):014038. doi: 10.1117/1.2437143. [DOI] [PubMed] [Google Scholar]
- Gonzalez SF. Krug MJ. Nielsen ME. Santos Y. Call DR. Simultaneous detection of marine fish pathogens by using multiplex PCR and a DNA microarray. J. Clin. Microbiol. 2004;42(4):1414–1419. doi: 10.1128/JCM.42.4.1414-1419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Google. Google Directory>Science>Biology>Bioinformatics>Software. http://www.google.com. 2008a. http://www.google.com.
- Google. Google Directory>Science>Biology>Biochemistry and Molecular Biology>Methods and Techniques>Arrays. http://www.google.com. 2008b. http://www.google.com.
- Goswami S. Wang W. Wyckoff JB. Condeelis JS. Breast cancer cells isolated by chemotaxis from primary tumors show increased survival and resistance to chemotherapy. Cancer Res. 2004;64(21):7664–7667. doi: 10.1158/0008-5472.CAN-04-2027. [DOI] [PubMed] [Google Scholar]
- Grimm V. Ezaki S. Susa M. Knabbe C. Schmid RD. Bachmann TT. Use of DNA microarrays for rapid genotyping of TEM beta-lactamases that confer resistance. J. Clin. Microbiol. 2004;42(8):3766–3774. doi: 10.1128/JCM.42.8.3766-3774.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RS. The phylogeny and signature sequences characteristics of Fibrobacteres, Chlorobi, and Bacteroidetes. Crit. Rev. Microbiol. 2004;30(2):123–143. doi: 10.1080/10408410490435133. [DOI] [PubMed] [Google Scholar]
- Gupta RS. Griffiths E. Critical issues in bacterial phylogeny. Theor. Popul. Biol. 2002;61(4):423–434. doi: 10.1006/tpbi.2002.1589. [DOI] [PubMed] [Google Scholar]
- Gupta RS. Johari V. Signature sequences in diverse proteins provide evidence of a close evolutionary relationship between the Deinococcus-thermus group and cyanobacteria. J. Mol. Evol. 1998;46(6):716–720. doi: 10.1007/pl00006352. [DOI] [PubMed] [Google Scholar]
- Guschin D. Yershov G. Zaslavsky A. Gemmell A. Shick V. Proudnikov D. Arenkov P. Mirzabekov A. Manual manufacturing of oligonucleotide, DNA, and protein microchips. Anal. Biochem. 1997a;250(2):203–211. doi: 10.1006/abio.1997.2209. [DOI] [PubMed] [Google Scholar]
- Guschin DY. Mobarry BK. Proudnikov D. Stahl DA. Rittmann BE. Mirzabekov AD. Oligonucleotide microchips as genosensors for determinative and environmental studies in microbiology. Appl. Environ. Microbiol. 1997b;63(6):2397–2402. doi: 10.1128/aem.63.6.2397-2402.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RR. Sikes HD. Bowman CN. Visual detection of labeled oligonucleotides using visible-light-polymerization-based amplification. Biomacromolecules. 2008;9(1):355–362. doi: 10.1021/bm700672z. [DOI] [PubMed] [Google Scholar]
- Healey BG. Matson RS. Walt DR. Fiberoptic DNA sensor array capable of detecting point mutations. Anal. Biochem. 1997;251(2):270–279. doi: 10.1006/abio.1997.2254. [DOI] [PubMed] [Google Scholar]
- Herold KE. Rasooly A. Oligo Design: a computer program for development of probes for oligonucleotide microarrays. Biotechniques. 2003;35(6):1216–1221. doi: 10.2144/03356bc02. [DOI] [PubMed] [Google Scholar]
- Hopkins KL. Batchelor MJ. Anjum M. Davies RH. Threlfall EJ. Comparison of antimicrobial resistance genes in nontyphoidal salmonellae of serotypes Enteritidis, Hadar, and Virchow from humans and food-producing animals in England and Wales. Microb. Drug Resist. 2007;13(4):281–288. doi: 10.1089/mdr.2007.779. [DOI] [PubMed] [Google Scholar]
- Johnson J. Jinneman K. Stelma G. Smith BG. Lye D. Messer J. Ulaszek J. Evsen L. Gendel S. Bennett RW. Swaminathan B. Pruckler J. Steigerwalt A. Kathariou S. Yildirim S. Volokhov D. Rasooly A. Chizhikov V. Wiedmann M. Fortes E. Duvall RE. Hitchins AD. Natural atypical Listeria innocua strains with Listeria monocytogenes pathogenicity island 1 genes. Appl. Environ. Microbiol. 2004;70(7):4256–4266. doi: 10.1128/AEM.70.7.4256-4266.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma K. Fukushima M. Kawaguchi R. Detection and identification of Escherichia coli, Shigella, and Salmonella by microarrays using the gyrB gene. Biotechnol. Bioeng. 2003;83(6):721–728. doi: 10.1002/bit.10709. [DOI] [PubMed] [Google Scholar]
- Karlin S. Brocchieri L. Heat shock protein 70 family: multiple sequence comparisons, function, and evolution. J. Mol. Evol. 1998;47(5):565–577. doi: 10.1007/pl00006413. [DOI] [PubMed] [Google Scholar]
- Keramas G. Bang DD. Lund M. Madsen M. Rasmussen SE. Bunkenborg H. Telleman P. Christensen CB. Development of a sensitive DNA microarray suitable for rapid detection of Campylobacter. spp. Mol. Cell. Probes. 2003;17(4):187–196. doi: 10.1016/s0890-8508(03)00052-5. [DOI] [PubMed] [Google Scholar]
- Kostic T. Weilharter A. Rubino S. Delogu G. Uzzau S. Rudi K. Sessitsch A. Bodrossy L. A microbial diagnostic microarray technique for the sensitive detection and identification of pathogenic bacteria in a background of nonpathogens. Anal. Biochem. 2007;360(2):244–254. doi: 10.1016/j.ab.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Kwon YM. Cox MM. Improved efficacy of whole genome amplification from bacterial cells. Biotechniques. 2004;37(1):40. doi: 10.2144/04371BM03. ,42,44. [DOI] [PubMed] [Google Scholar]
- Lai HS. Chen Y. Lin WH. Chen CN. Wu HC. Chang CJ. Lee PH. Chang KJ. Chen WJ. Quantitative gene expression analysis by cDNA microarray during liver regeneration after partial hepatectomy in rats. Surg. Today. 2005;35(5):396–403. doi: 10.1007/s00595-004-2962-7. [DOI] [PubMed] [Google Scholar]
- Lee DY. Shannon K. Beaudette LA. Detection of bacterial pathogens in municipal wastewater using an oligonucleotide microarray and real-time quantitative PCR. J. Microbiol. Methods. 2006;65(3):453–467. doi: 10.1016/j.mimet.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Lee JH. Mitchell RJ. Kim BC. Cullen DC. Gu MB. A cell array biosensor for environmental toxicity analysis. Biosens. Bioelectron. 2005;21(3):500–507. doi: 10.1016/j.bios.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Li Y. Liu D. Cao B. Han W. Liu Y. Liu F. Guo X. Bastin DA. Feng L. Wang L. Development of a serotype-specific DNA microarray for identification of some Shigella and pathogenic Escherichia coli strains. J. Clin. Microbiol. 2006;44(12):4376–4383. doi: 10.1128/JCM.01389-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Stratton Y. Roy S. Sen CK. DNA microarray technology in nutraceutical and food safety. Toxicol. Lett. 2004;150(1):29–42. doi: 10.1016/j.toxlet.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Lloyd AL. Marshall BJ. Mee BJ. Identifying cloned Helicobacter pylori promoters by primer extension using a FAM-labelled primer and GeneScan analysis. J. Microbiol. Methods. 2005;60(3):291–298. doi: 10.1016/j.mimet.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Loy A. Schulz C. Lucker S. Schopfer-Wendels A. Stoecker K. Baranyi C. Lehner A. Wagner M. 16S rRNA gene-based oligonucleotide microarray for environmental monitoring of the betaproteobacterial order Rhodocyclales. Appl. Environ. Microbiol. 2005;71(3):1373–1386. doi: 10.1128/AEM.71.3.1373-1386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig W. Schleifer KH. Bacterial phylogeny based on 16S and 23S rRNA sequence analysis. FEMS Microbiol. Rev. 1994;15(2–3):155–173. doi: 10.1111/j.1574-6976.1994.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Luthra R. Medeiros LJ. Isothermal multiple displacement amplification: a highly reliable approach for generating unlimited high molecular weight genomic DNA from clinical specimens. J. Mol. Diagn. 2004;6(3):236–242. doi: 10.1016/S1525-1578(10)60516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manduchi E. Scearce LM. Brestelli JE. Grant GR. Kaestner KH. Stoeckert CJ., Jr Comparison of different labeling methods for two-channel high-density microarray experiments. Physiol. Genomics. 2002;10(3):169–179. doi: 10.1152/physiolgenomics.00120.2001. [DOI] [PubMed] [Google Scholar]
- Mao H. Wang H. Zhang D. Mao H. Zhao J. Shi J. Cui Z. Study of hepatitis B virus gene mutations with enzymatic colorimetry-based DNA microarray. Clin. Biochem. 2006;39(1):67–73. doi: 10.1016/j.clinbiochem.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Mitterer G. Huber M. Leidinger E. Kirisits C. Lubitz W. Mueller MW. Schmidt WM. Microarray-based identification of bacteria in clinical samples by solid-phase PCR amplification of 23S ribosomal DNA sequences. J. Clin. Microbiol. 2004;42(3):1048–1057. doi: 10.1128/JCM.42.3.1048-1057.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [NCBI] National Center for Biotechnology Information. Bethesda, MD: NCBI; 2008. BLAST—Basic local alignment and search tool. [Google Scholar]
- Nubel U. Schmidt PM. Reiss E. Bier F. Beyer W. Naumann D. Oligonucleotide microarray for identification of Bacillus anthracis based on intergenic transcribed spacers in ribosomal DNA. FEMS Microbiol. Lett. 2004;240(2):215–223. doi: 10.1016/j.femsle.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Olsen GJ. Lane DJ. Giovannoni SJ. Pace NR. Stahl DA. Microbial ecology and evolution: a ribosomal RNA approach. Annu. Rev. Microbiol. 1986;40:337–365. doi: 10.1146/annurev.mi.40.100186.002005. [DOI] [PubMed] [Google Scholar]
- Paez JG. Lin M. Beroukhim R. Lee JC. Zhao X. Richter DJ. Gabriel S. Herman P. Sasaki H. Altshuler D. Li C. Meyerson M. Sellers WR. Genome coverage and sequence fidelity of phi29 polymerase-based multiple strand displacement whole genome amplification. Nucleic Acids Res. 2004;32(9):e71. doi: 10.1093/nar/gnh069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page BT. Kurtzman CP. Rapid identification of Candida species and other clinically important yeast species by flow cytometry. J. Clin. Microbiol. 2005;43(9):4507–4514. doi: 10.1128/JCM.43.9.4507-4514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page BT. Shields CE. Merz WG. Kurtzman CP. Rapid identification of ascomycetous yeasts from clinical specimens by a molecular method based on flow cytometry and comparison with identifications from phenotypic assays. J. Clin. Microbiol. 2006;44(9):3167–3171. doi: 10.1128/JCM.00390-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X. Zhou D. Song Y. Pei D. Wang J. Guo Z. Yang R. Bacterial mRNA purification by magnetic capture-hybridization method. Microbiol. Immunol. 2004;48(2):91–96. doi: 10.1111/j.1348-0421.2004.tb03493.x. [DOI] [PubMed] [Google Scholar]
- Panicker G. Call DR. Krug MJ. Bej AK. Detection of pathogenic Vibrio spp. in shellfish by using multiplex PCR and DNA microarrays. Appl. Environ. Microbiol. 2004;70(12):7436–7444. doi: 10.1128/AEM.70.12.7436-7444.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreten V. Vorlet-Fawer L. Slickers P. Ehricht R. Kuhnert P. Frey J. Microarray-based detection of 90 antibiotic resistance genes of gram-positive bacteria. J. Clin. Microbiol. 2005;43(5):2291–2302. doi: 10.1128/JCM.43.5.2291-2302.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J. Stangegaard M. Birgens H. Dufva M. Detection of mutations in the beta-globin gene by colorimetric staining of DNA microarrays visualized by a flatbed scanner. Anal. Biochem. 2007;360(1):169–171. doi: 10.1016/j.ab.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Porschewski P. Grattinger MA. Klenzke K. Erpenbach A. Blind MR. Schafer F. Using aptamers as capture reagents in bead-based assay systems for diagnostics and hit identification. J. Biomol. Screen. 2006;11(7):773–781. doi: 10.1177/1087057106292138. [DOI] [PubMed] [Google Scholar]
- Rasooly A. Herold KE. Biosensors for the analysis of food and waterborne pathogens and their toxins. J. AOAC Int. 2006;89(3):873–883. [PubMed] [Google Scholar]
- Rogers YH. Jiang-Baucom P. Huang ZJ. Bogdanov V. Anderson S. Boyce-Jacino MT. Immobilization of oligonucleotides onto a glass support via disulfide bonds: a method for preparation of DNA microarrays. Anal. Biochem. 1999;266(1):23–30. doi: 10.1006/abio.1998.2857. [DOI] [PubMed] [Google Scholar]
- Schmidt-Heydt M. Geisen R. A microarray for monitoring the production of mycotoxins in food. Int. J. Food Microbiol. 2007;117(2):131–140. doi: 10.1016/j.ijfoodmicro.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Schmitt M. Bravo IG. Snijders PJ. Gissmann L. Pawlita M. Waterboer T. Bead-based multiplex genotyping of human papillomaviruses. J. Clin. Microbiol. 2006;44(2):504–512. doi: 10.1128/JCM.44.2.504-512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeev N. Distler M. Courtney S. Al-Khaldi SF. Volokhov D. Chizhikov V. Rasooly A. Multipathogen oligonucleotide microarray for environmental and biodefense applications. Biosens. Bioelectron. 2004a;20(4):684–698. doi: 10.1016/j.bios.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Sergeev N. Distler M. Vargas M. Chizhikov V. Herold KE. Rasooly A. Microarray analysis of Bacillus cereus group virulence factors. J. Microbiol. Methods. 2006a;65(3):488–502. doi: 10.1016/j.mimet.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Sergeev N. Herold KE. Rasooly A. Encyclopedia of Rapid Microbiological Methods. Volume 3. DHI Publishing; River Grove, IL: 2006b. Microarray analysis of microbial pathogens; pp. 363–405. [Google Scholar]
- Sergeev N. Rasooly A. Herold KE. Encyclopedia of Medical Genomics and Proteomics. Volume 2. Informa Healthcare; New York: 2005. Oligonucleotide design for PCR primers and microarray probes; pp. 938–942. [Google Scholar]
- Sergeev N. Volokhov D. Chizhikov V. Rasooly A. Simultaneous analysis of multiple staphylococcal enterotoxin genes by an oligonucleotide microarray assay. J. Clin. Microbiol. 2004b;42(5):2134–2143. doi: 10.1128/JCM.42.5.2134-2143.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper SA. Hashimoto M. Situma C. Murphy MC. McCarley RL. Cheng YW. Barany F. Fabrication of DNA microarrays onto polymer substrates using UV modification protocols with integration into microfluidic platforms for the sensing of low-abundant DNA point mutations. Methods. 2005;37(1):103–113. doi: 10.1016/j.ymeth.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Stillman BA. Tonkinson JL. FAST slides: a novel surface for microarrays. Biotechniques. 2000;29(3):630–635. doi: 10.2144/00293pf01. [DOI] [PubMed] [Google Scholar]
- Strommenger B. Schmidt C. Werner G. Roessle-Lorch B. Bachmann TT. Witte W. DNA microarray for the detection of therapeutically relevant antibiotic resistance determinants in clinical isolates of Staphylococcus aureus. Mol. Cell. Probes. 2007;21(3):161–170. doi: 10.1016/j.mcp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Summerbell RC. Levesque CA. Seifert KA. Bovers M. Fell JW. Diaz MR. Boekhout T. de Hoog GS. Stalpers J. Crous PW. Microcoding: the second step in DNA bar-coding. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360(1462):1897–1903. doi: 10.1098/rstb.2005.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. Jacobson KB. Golovlev V. Label-free detection of biomolecules on microarrays using surface-colloid interaction. Anal. Biochem. 2007;361(2):244–252. doi: 10.1016/j.ab.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X. Morris SL. Langone JJ. Bockstahler LE. Microarray and allele specific PCR detection of point mutations in Mycobacterium tuberculosis genes associated with drug resistance. J. Microbiol. Methods. 2005;63(3):318–330. doi: 10.1016/j.mimet.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Uda A. Tanabayashi K. Fujita O. Hotta A. Yamamoto Y. Yamada A. Comparison of whole genome amplification methods for detecting pathogenic bacterial genomic DNA using microarray. Jpn. J. Infect. Dis. 2007;60(6):355–361. [PubMed] [Google Scholar]
- Volokhov D. Chizhikov V. Chumakov K. Rasooly A. Microarray-based identification of thermophilic Campylobacter jejuni, C. coli, C. lari, and C. upsaliensis. J. Clin. Microbiol. 2003a;41(9):4071–4080. doi: 10.1128/JCM.41.9.4071-4080.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokhov D. Chizhikov V. Chumakov K. Rasooly A. Microarray analysis of erythromycin resistance determinants. J. Appl. Microbiol. 2003b;95(4):787–798. doi: 10.1046/j.1365-2672.2003.02046.x. [DOI] [PubMed] [Google Scholar]
- Volokhov D. Pomerantsev A. Kivovich V. Rasooly A. Chizhikov V. Identification of Bacillus anthracis by multiprobe microarray hybridization. Diagn. Microbiol. Infect. Dis. 2004;49(3):163–171. doi: 10.1016/j.diagmicrobio.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Volokhov D. Rasooly A. Chumakov K. Chizhikov V. Identification of Listeria species by microarray-based assay. J. Clin. Microbiol. 2002;40(12):4720–4728. doi: 10.1128/JCM.40.12.4720-4728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RF. Kim SJ. Robertson LH. Cerniglia CE. Development of a membrane-array method for the detection of human intestinal bacteria in fecal samples. Mol. Cell. Probes. 2002;16(5):341–350. doi: 10.1006/mcpr.2002.0432. [DOI] [PubMed] [Google Scholar]
- Wang XW. Zhang L. Jin LQ. Jin M. Shen ZQ. An S. Chao FH. Li JW. Development and application of an oligonucleotide microarray for the detection of food-borne bacterial pathogens. Appl. Microbiol. Biotechnol. 2007;76(1):225–233. doi: 10.1007/s00253-007-0993-x. [DOI] [PubMed] [Google Scholar]
- Wilson KH. Wilson WJ. Radosevich JL. DeSantis TZ. Viswanathan VS. Kuczmarski TA. Andersen GL. High-density microarray of small-subunit ribosomal DNA probes. Appl. Environ. Microbiol. 2002a;68(5):2535–2541. doi: 10.1128/AEM.68.5.2535-2541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WJ. Strout CL. DeSantis TZ. Stilwell JL. Carrano AV. Andersen GL. Sequence-specific identification of 18 pathogenic microorganisms using microarray technology. Mol. Cell. Probes. 2002b;16(2):119–127. doi: 10.1006/mcpr.2001.0397. [DOI] [PubMed] [Google Scholar]
- Wrobel G. Schlingemann J. Hummerich L. Kramer H. Lichter P. Hahn M. Optimization of high-density cDNA-microarray protocols by ‘design of experiments'. Nucleic Acids Res. 2003;31(12):e67. doi: 10.1093/nar/gng067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F. Datta P. Wang H. Gurung S. Hashimoto M. Wei S. Goettert J. McCarley RL. Soper SA. Polymer microfluidic chips with integrated waveguides for reading microarrays. Anal Chem. 2007;79(23):9007–9013. doi: 10.1021/ac7016597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. Tran DK. Wang X. BADGE, Beads Array for the Detection of Gene Expression, a high-throughput diagnostic bioassay. Genome Res. 2001;11(11):1888–1898. doi: 10.1101/gr.190901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LX. Zhang ZW. Wang C. Yang HW. Jiang D. Zhang Q. Mitchelson K. Cheng J. Use of a DNA microarray for simultaneous detection of antibiotic resistance genes among staphylococcal clinical isolates. J. Clin. Microbiol. 2007;45(11):3514–3521. doi: 10.1128/JCM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


