Abstract
Toluene diisocyanate (TDI) is an industrially important polymer cross-linker used in the production of polyurethane. Workplace exposure to TDI and other diisocyanates is reported to be a leading cause of low molecular weight–induced occupational asthma (OA). Currently we have a limited understanding of the pathogenesis of OA. Monoclonal antibodies (MAbs) that recognize TDI bound proteins would be valuable tools/reagents, both in exposure monitoring and in TDI-induced asthma research. We sought to develop toluene diisocyanate (TDI)-specific MAbs for potential use in the development of standardized immunoassays for exposure and biomarker assessments. Mice were exposed 4 h/day for 12 consecutive weekdays to 50 ppb, 2,4;2,6 TDI vapor (80/20 mixture). Splenocytes were isolated 24 h after the last exposure for hybridoma production. Hybridomas were screened in a solid-phase indirect enzyme-linked immunosorbent assay (ELISA) against a 2,4 TDI–human serum albumin (2,4 TDI-HSA) protein conjugate. Three hybridomas producing 2,4 TDI-HSA reactive IgM MAbs were obtained. The properties of these MAbs (isotype and reactivity to various protein-isocyanate conjugate epitopes) were characterized using ELISA, dot blot, and Western blot analyses. Western blot analyses demonstrated that some TDI conjugates form inter- and intra-molecular links, resulting in multimers and a change in the electrophoretic mobility of the conjugate. These antibodies may be useful tools for the isolation of endogenous diisocyanate-modified proteins after natural or experimental exposures and for characterization of the toxicity of specific dNCOs.
Introduction
Diisocyanates (dNCO) are a leading cause of occupational asthma in industrialized countries.(1) Clinical similarities between diisocyanate-induced asthma and asthma caused by protein allergens suggest similar immunopathogenic mechanisms may be involved; however the association between anti-dNCO IgE and dNCO allergy and asthma is poor.(2,3) Attempts to elucidate the pathogenic mechanisms of diisocyanate-induced asthma are hampered by the extreme reactivity of these chemicals, the lack of dNCO-specific immunoreagents, and questions concerning appropriateness of present animal models. Conjugation (haptenation) of diisocyanate to human proteins after exposure is commonly accepted as the primary event in the development of dNCO-induced allergic sensitization and asthma.(4,5) Diisocyanates have been shown to bind skin and lung proteins, and major adducts found in the blood are hemoglobin and albumin.(6) TDI-conjugated lung proteins have been suggested to include keratin, tubulin, laminin, and actin.(6–9) Diisocyanates have also been shown to bind to airway epithelial cell proteins in vitro(10) and TDI has been co-localized with tubulin on cilia of exposed differentiated human airway epithelial cells.(8) Although TDI seems to form conjugates with a select group of proteins, the actual target proteins and the type(s) of chemical linkages involved in dNCO pathogenesis remain to be identified.
The antigenic epitopes of TDI-conjugated proteins and the subsequent steps leading to respiratory disease are poorly understood.(11) The dNCO-HSA conjugates are commonly used as the antigen of choice for dNCO-specific antibody measurements from exposed workers. Diisocyanates can self-polymerize, they can form inter- and intramolecular cross-links between themselves and proteins, and they can haptenate proteins at single or multiple sites.(12,13) This complex chemistry presents a major difficulty in the preparation of the most relevant antigens for in vitro antibody measurements and the development of standardized screening assays for dNCO exposure using patients' sera.(14)
Polyclonal antibodies against hexamethylene diisocyanate (HDI)–albumin conjugates have been previously produced and were proposed to be useful reagents for biomonitoring HDI exposures.(15) To our knowledge, no attempts have previously been made to produce MAbs from mice exposed to TDI vapor. In this article, we describe the production and characterization of monoclonal antibodies from TDI vapor–exposed mice.
Material and Methods
Conjugation of diisocyanates, monoisocyanates, or diisothiocyanates to proteins
Keyhole limpet hemocyanin (KLH, hemocyanin from Megathura crenulata), mouse serum albumin (MSA, fraction V), human serum albumin (HSA, fraction V), lysozyme (chicken egg white), keratin protein (derived from hair, wool, horn, nails, or other similar tissues in animals), and collagen (calf skin type 1 species) were obtained from Sigma Aldrich (St. Louis, MO). Dimethyl phenyl isocyanates (DMPI) (2,3 DMPI, 3,5 DMPI, and 2,5 DMPI) were obtained from Alfa Aesar (Wade Hill, MA). 2,4 toluene diisocyanate (2,4 TDI), 2,6 toluene diisocyanate (2,6 TDI), hexamethylene diisocyanate (HDI), o-toluene isocyanate (OTI), p-toluene isocyanate (PTI), phenyl isocyanate (PI), toluene diisothiocyanate phenyl isocyanates (2,4 TITC and 2,6 TITC), and methylene bis-cyclohexylisocyanate (MDI) were obtained from Sigma Aldrich.
All proteins were prepared at 5 mg/mL in phosphate-buffered saline (PBS, pH 7.4). Five μL aliquots of each dNCO, mono-isocyanate (NCO), or dithioisocyanate (dNCS) were added to 450 μL dry HPLC grade acetone (Sigma) and infused with rapid stirring into the protein solution at a rate of 1.2 mL/h at room temperature (RT) using a syringe pump (model 100; KD Scientific, Holliston, MA) until a molar ratio of 1:40 protein-dNCO (NCO or dNCS) was achieved. The resulting conjugates were centrifuged at 300 g for 10 min, then dialyzed with 3× buffer changes in 1× PBS buffer at 4°C using molecular porous membrane tubing (MWCO, 12–14,000 kDa; Spectrum Laboratories Rancho Dominguez, CA). HSA was acylated with acetic anhydride to block all available primary amines prior to reaction with 2,4 TDI in a separate preparation. The conjugates were filtered through 0.45 μm syringe filters (Millipore, Billerica, MA) and stored in aliquots at −20°C. The full list of conjugates is shown in Table 1.
Table 1.
Monoclonal Antibody Reactivity Studies
| |
|
|
Optical density (405 nm, 30 min) |
||
|---|---|---|---|---|---|
| Chemical name | Structure | Test antigen (5μg/mL) | 16F4 (10 ng/mL) | 29E5 (80 ng/mL) | 56F9 (29 ng/mL) |
| Human serum albumin | HSA | 0.078 ± 0.047 | 0.065 ± 0.004 | 0.089 ± 0.022 | |
| 2,4 toluene diisocyanate | 2.4-TDI-HSA | 2.512 ± 0.121a | 1.274 ± 0.128a | 2.140 ± 0.109a | |
| 2.4-TDI-KLH | 0.578 ± 0.037a | 3.635 ± 0.164a | 0.881 ± 0.088a | ||
| 2.4-TDI-MSA | 0.398 ± 0.071a | 0.236 ± 0.067a | 0.443 ± 0.043a | ||
| 2.4-TDI-keratin | −0.012 ± 0.005 | −0.004 ± 0.004 | 0.031 ± 0.015 | ||
| 2,4-TDI-lysozyme | −0.019 ± 0.01 | 0.022 ± 0.005 | 0.011 ± 0.005 | ||
| 2,4-TDI-collagen | −0.010 ± 0.018 | −0.015 ± 0.025 | 0.005 ± 0.064 | ||
| 2,6 toluene diisocyanate | 2.6-TDI-HSA | 2.356 ± 0.039a | 1.824 ± 0.180a | 1.596 ± 0.02a | |
| 2.6-TDI-KLH | 0.047 ± 0.028 | 0.267 ± 0.013a | 0.142 ± 0.02 | ||
| 2.6-TDI-MSA | 0.279 ± 0.027a | 0.092 ± 0.028 | 0.367 ± 0.066a | ||
| 2.6-TDI-keratin | −0.002 ± 0.005 | 0.016 ± 0.011 | 0.039 ± 0.011 | ||
| 2.6-TDI-lysozyme | −0.008 ± 0.002 | −0.013 ± 0.007 | 0.012 ± 0.012 | ||
| 2,4 TDI-collagen | −0.016 ± 1.009 | 0.010 ± 0.005 | 0.005 ± 0.012 | ||
| 2,4;2,6 TDI (industrial mix) | 2,4;2,6 TDI-HSA | 0.723 ± 0.038a | 0.954 ± 0.08a | 0.149 ± 0.036 | |
| 4,4 methylene diphenyl diisocyanate | MDI-HSA | 0.491 ± 0.029a | 0.370 ± 0.017a | 0.091 ± 0.017 | |
| Hexamethylene diisocyanate | HDI-HSA | 0.858 ± 0.023a | 1.590 ± 0.132a | 0.765 ± 0.022a | |
| 2,5 dimethyl phenylisocyanate | 2,5-DMPI-HSA | 0.832 ± 0.097a | 0.150 ± 0.046 | 0.174 ± 0.028 | |
| 3,4 dimethyl phenylisocyanate | 3,4-DMPI-HSA | 0.633 ± 0.03a | 0.132 ± 0.015 | 0.226 ± 0.032 | |
| 4 toluene isocyanate | PTI-HSA | 0.015 ± 0.021 | 0.025 ± 0.191 | 0.020 ± 0.091 | |
| 2 toluene isocyanate | 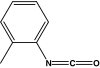 |
OTI-HSA | 0.070 ± 0.037 | 0.009 ± 0.015 | 0.087 ± 0.01 |
| Phenyl isocyanate | 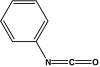 |
PI-HSA | −0.004 ± 0.014 | 0.011 ± 0.012 | 0.007 ± 0.006 |
| 2,4 toluene diisothiocyanate | 2,4-TITC-HSA | 0.096 ± 0.018 | 0.119 ± 0.009 | 0.227 ± 0.017 | |
| 2,6 toluene diisothiocyanate | 2,6-TITC-HSA | 0.006 ± 0.041 | −0.016 ± 0.035 | 0.135 ± 0.05 | |
Numbers with a mean OD405 value of four replicates three times greater than the mean OD405 value of HSA control.
Experimental animals
Female specific-pathogen-free inbred C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) at 5 to 6 weeks of age. Upon arrival, the mice were quarantined for 2 weeks and acclimated to a 12-h light/dark cycle. Animals were housed in ventilated microisolator cages under environmentally controlled conditions at the NIOSH animal facility in compliance with AAALAC approved guidelines and an approved IACUC protocol. The animal rooms were monitored for specific pathogens through a disease surveillance and sentinel animal program. Food and water were provided ad libitum.
TDI exposure system
Toluene diisocyanate was purchased from Bayer Corporation (TDI, Mondur TD80 Grade A, 80/20 mixture of 2,4 and 2,6 isomers, respectively; Pittsburgh, PA). The TDI exposure system has been described in detail previously.(16) Briefly, mice were exposed in a 1200 L stainless steel live-in chamber (Unifab Corp., Kalamazoo, MI) supplied with HEPA purified and conditioned air supply providing nine air changes per hour and maintaining temperature and humidity at 23 ± 2°C and 50 ± 5%, respectively. Mice were housed individually in hanging stainless steel mesh cages and remained in the chamber on weekdays and returned to ventilated shoebox style cages on weekends. Generation of a TDI vapor atmosphere was achieved by passing dried HEPA filtered air over a 50 cm2 surface of liquid TDI followed by dilution to the desired concentration with HEPA filtered and humidified air. The TDI concentration (50 ± 5 ppb) in the chamber was continuously monitored using a Remote Intelligent Sensor (RIS) TDI analyzer (Scott Safety and Health, Monroe, NC). The RIS units were calibrated against a fluorescamine assay with a detection limit of 10 ng/mL, as previously described.(16) Five mice were exposed to TDI vapor for 4 h/day, for 12 consecutive work days. Lymph nodes and spleens were collected 24 h following the final exposure.
Monoclonal antibody production
Spleens and lymph nodes were removed aseptically and lymphocytes harvested after lysing red blood cells by osmotic shock. Hybridomas were produced following standard techniques as previously described(17) using SP2/0-AG14 myelomas (ATCC# CRL-1581) as fusion partners and polyethylene glycol (molecular weight 1500 kDa) as the fusogen.
Hybridoma cultures were maintained in Dulbecco's modified Eagle medium (Life Technologies, Rockville, MD), supplemented with 1 mM pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin, 0.292 mg/mL L-glutamine, 100 μM sodium hypoxanthine, 16 μM thymidine, 10% fetal calf serum (HyClone, Logan, UT), and 100 U/mL IL-6 (Boehringer Mannheim, Germany). Culture supernatant fluid from wells with cell growth were screened using an indirect ELISA using 2,4 TDI-HSA as the solid-phase antigen. Aliquots of stable TDI-specific hybridomas were frozen in a mixture of 10% (v/v) dimethylsulfoxide and 90% fetal calf serum for storage in liquid nitrogen.
Enzyme-linked immunosorbent assay
Format for hybridoma screening
Hybridoma screening tests were carried out using an indirect ELISA according to the method of Schmechel and colleagues.(18) MaxiSorp ELISA plate wells (Nalge Nunc International, Naperville, IL) were coated with 5 μg/mL of 2,4 TDI-HSA and incubated overnight at room temperature (RT). The plates were kept in a plastic box containing moist filter paper. Following overnight incubation and all subsequent ELISA steps, wells were washed three times with 200 μL PBS with 0.05% Tween-20 (PBST)/well. The plates were blocked by incubating for 1 h at RT in 200 μL/well PBST containing 1% non-fat dry milk powder (PBSTM). Initial hybridoma culture supernatant (CSN) fluids were diluted 1:5 in PBSTM and 100 μL/well were incubated for 1 h at 37°C. Bound antibodies were labeled by incubation with 100 μL/well of Biotin-SP-conjugated Affinity Pure goat anti-mouse IgG + IgM secondary antibody (diluted 1:5000 in PBSTM; Jackson ImmunoResearch) for 1 h at 37°C. One hundred μL of alkaline phosphatase-conjugated streptavidin (dilution of 1:5000 in PBSTM; Jackson ImmunoResearch) was added and incubated for 1 h at 37°C. The ELISA was developed by incubating 100 μL p-nitrophenyl phosphate-containing buffer/well (0.5 mg/mL) of buffer (97 mL diethanolamine, 100 mg MgCl2 diluted in one liter distilled water, pH 9.8) at RT for 30 min. The optical density (OD) was determined spectrophotometrically at 405 nm using an UltraMicroplate Reader, Model ELx800 (Bio-Tek Instruments, Winooski, VT). Assay background controls were processed in parallel and contained plain cell-free culture medium instead of MAb supernatant fluid. Optical densities greater than three times the OD405 of the background controls were considered to be positive.
Antibody isotyping and quantification
Antibodies produced by TDI-specific hybridomas were isotyped using a mouse monoclonal isotyping reagent kit (Jackson ImmunoResearch) according to the manufacturer's instructions. In brief, plates were coated with antigen (2,4 TDI-HSA), blocked, and washed as described for the screening ELISA. To determine the isotype, bound MAbs were incubated with 100 μL of Biotin-SP-conjugated goat anti-mouse isotype-specific secondary antibodies (IgM, IgG1, IgG2a, IgG2b, or IgG3).
The monoclonal antibodies were quantified using isotype-specific ELISA kits (Jackson ImmunoResearch) according to the manufacturer's instructions. The amount of specific antibody in the supernatant was determined from a standard curve generated with specific antibodies of known concentrations. The standards and supernatant were assayed in parallel.
ELISA Format for MAb reactivity
We tested the MAb's reactivity toward proteins conjugated to several different dNCOs, NCOs, and dNCSs using an alkaline phosphatase-mediated indirect ELISA. PolySorp ELISA plate wells (Nalge Nunc) were coated with 5 μg/mL of test antigen (see Table 1) by overnight incubation at RT. The plates were processed as previously described for the antibody screening ELISA, except Biotin-SP-conjugated Affinity Pure goat anti-mouse IgM (dilution of 1:5000; Jackson ImmunoResearch) was used as secondary antibody. The results represent the average OD405 of four ELISA well replicates, which were corrected by subtracting the average OD405 of four ELISA background control wells.
Western blot analysis of MAb reactivity
Protein-isocyanate conjugates were separated by SDS-PAGE (5 μg/lane, 6% separation gels) and transferred overnight at 15 mA to nitrocellulose membranes (0.2 μm, Bio-Rad, Hercules, CA). The membranes were blocked with 3% bovine serum albumin (BSA) in PBST for 1 h before being reacted with a 1:10 dilution of MAb CSNs for 1 h. After washing, the blot was incubated with Biotin-SP-conjugated Affinity Pure goat anti-mouse IgM secondary antibody (dilution of 1:5000 in 3% BSA in PBST; Jackson ImmunoResearch) for 1 h at 37°C. Immune complexes were labeled with alkaline phosphatase-conjugated streptavidin (Jackson ImmunoResearch) by incubating a 1:5000 dilution in 3% BSA in PBST for 1 h at 37°C. Protein bands were visualized using a nitroblue tetrazolium/bromo-chloro-indolyl phosphate substrate reagent kit (NBT/BCIP, Promega, Madison, WI). Color was allowed to develop for 5 min and stopped by washing the membranes with distilled/deionized H2O.
Dot blot analysis
A dot blot analysis was carried out to evaluate the MAb reactivity towards native and denatured protein conjugates. Native TDI-conjugates (4 μL of 5 μg/mL of antigen per spot) were spotted onto a nitrocellulose membrane (0.2 μm, Bio-Rad) and allowed to dry overnight. The dot blots were repeated using denatured TDI conjugates that were treated with 2-mercaptoethanol (final concentration 25 mL/L) at 100°C for 10 min prior to spotting. All the other steps were identical to the Western blot protocol with regard to incubation times and color development.
Results
Conjugate analysis
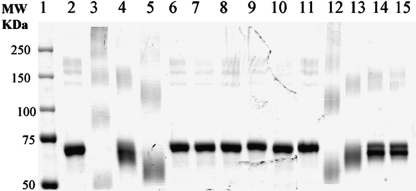
Figure 1 is a representative SDS-PAGE Coomassie blue stained protein gel of diisocyanate-, monoisocyanate-, and diisothiocyanate-conjugated HSA.The protein stain showed extensive cross-linking of the 2,4 TDI-HSA and the other diisocyanates. Minor higher MW protein bands were observed in the unconjugated HSA standard (lane 2). There are upward shifts in the dNCO-conjugated HSA in lanes 3, 4, 5, 12, and 13. This indicates the formation of polymers through intermolecular cross-linking. It is also apparent that inter-molecular cross-linking of dNCS haptenated protein (lanes 14 and 15) was not as extensive as that seen with dNCO. The apparent lower molecular weight of some dNCO-HSA conjugates compared to unconjugated HSA may be due to intra-molecular cross-linking that prevents complete denaturation and an increase in electophoretic mobility of the conjugates. The monoisocyanates are not capable of cross-linking nucleophilic moieties within or between proteins (lanes 6–10).
FIG. 1.
SDS-PAGE Coomassie blue stained protein gel of antigens used to characterize anti-TDI MAb reactivity. Protein-isocyanate conjugates were separated by SDS-PAGE (5 μg/lane, 6% separation gel). The protein bands were visualized by staining the gel with Coomassie blue (Bio-Rad). Lane 1, Precision Plus Protein standard (Bio-Rad); lane 2, HSA; lane 3, 2,4 TDI-HSA; lane 4, 2,6 TDI-HSA; lane 5, 2,4;2,6 TDI-HSA; lane 6, 2,3 DMPI-HSA; lane 7, 2,5 DMPI-HSA; lane 8, 3,4 DMPI-HSA; lane 9, OTI-HSA; lane 10, PTI-HSA; lane 11, HSA; lane 12, MDI-HSA; lane 13, HDI-HSA; lane 14, 2,4 TITC-HAS; and lane 15, 2,6 TITC-HSA.
Hybridoma production
A total of 10 hybridomas were obtained and subsequent isotyping showed that they all secreted IgMs (data not shown). All hybridomas produced κ light chain antibodies; their concentrations in the culture supernant fluid ranged from 82 to 467 ng/mL. Although most MAbs strongly reacted with dNCO-HSA conjugates, they also showed significant cross-reactivity with dNCO-unconjugated HSA (data not shown). Only three MAbs, designated as 16F4, 29E5, and 56F9, were found to preferentially react with conjugated HSA and were selected for further characterization (Table 1).
Reactivity studies
ELISA
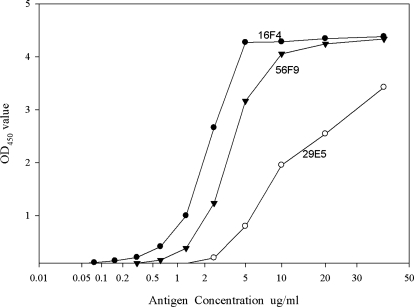
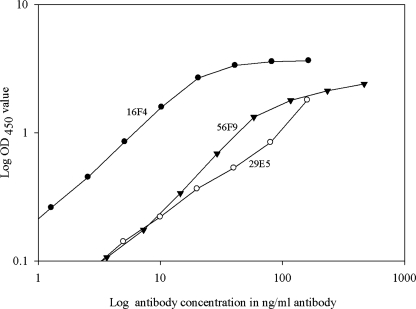
Preliminary ELISAs were performed to optimize the antigen concentration and to select the linear range of the MAb reactivity against 2,4 TDI-HSA. Based on the reactivity shown in Figure 2, an antigen concentration of 5 μg/mL was selected for the analysis of all three MAbs. Each antibody was titrated as shown in Figure 3, and MAbs 16F4, 29E5, and 56F9 were used at a concentration of 10 ng/mL, 80 ng/mL, and 30 ng/mL, respectively, to ensure an antibody concentration in the linear range of reactivity.
FIG. 2.
Optimization of coating concentration of 2,4 TDI-HSA. Each value represents the mean of duplicate wells. The mean control OD405 was 0.02.
FIG. 3.
Representative antibody titration. MAb titration was performed to identify the MAb concentration to be used in subsequent reactivity studies. Log-linear responses were found for MAb up to 100 ng/mL under the present ELISA conditions.
Table 1 provides a summary of the reactivity of the MAbs toward proteins conjugated with dNCOs, NCOs, and NCSs. It can be seen that all three MAbs react with 2,4 TDI-HSA, 2,6 TDI-HSA, HDI-HSA, 2,4 TDI-MSA, and 2,4 TDI-KLH. MAbs 16F4 and 29E5 also react with 2,4;2,6 TDI-HSA conjugates and MDI-HSA. MAbs 16F4 and 59F9 also react with 2,6 TDI-MSA. 16F4 was the only MAb to react with 2,3 DMPI and 2,5 DMPI while 29E5 was the only MAb to react with 2,6 TDI-KLH. None of the other conjugates were recognized by the MAbs in the ELISA format.
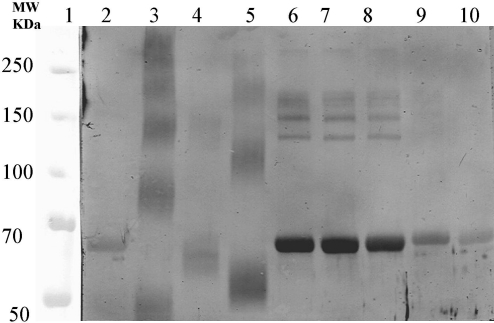
Western blot analyses
The representative Western blot analysis for MAb 56F9 (Fig. 4) showed that it reacted with the denatured TDI-conjugated proteins. The pattern of reactivity of all three MAbs was similar, varying only in intensity of the reaction (data not shown). The MAbs bound to TDI-conjugated proteins that contained both intra- and intermolecular TDI-mediated cross-links. There was also some reactivity in the Western blot analyses that was not seen in the ELISA assay format. All the MAbs reacted with the methyl substituted monoisocyanates in Western blot, yet only 16F4 was reactive in ELISA (see Table 1). The OTI-HSA and PTI-HSA conjugates had greater reactivity in Western blot analyses than in ELISA for all three MAbs.
FIG. 4.
Western blot analyses of MAb 56F9. Lane 1, Precision Plus Protein standard (Bio-Rad); lane 2, HSA; lane 3, 2,4 TDI-HSA; lane 4, 2,6 TDI-HSA; lane 5, 2,4;2,6 TDI-HSA; lane 6, 2,3 DMPI-HSA; lane 7, 2,5 DMPI-HSA; lane 8, 3,4 DMPI-HSA; lane 9, OTI-HSA; and lane 10, PTI-HSA.
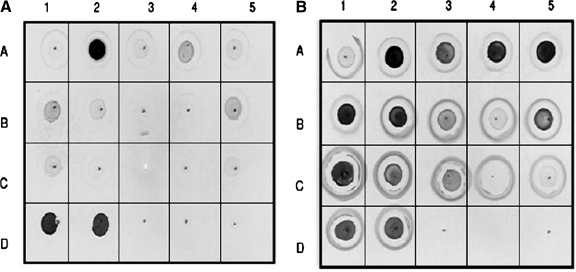
Dot blot analysis
Figure 5A and B are representative dot blots showing MAb 56F9 reactivity to native and denatured protein conjugates, respectively, using MAb 56F9. MAbs 16F4 and 56F9 had similar reactivity towards both the native and denatured forms of the isocyanate protein conjugates. All the MAbs showed the strongest reactivity towards 2,4 TDI-HSA, 2,4 TDI-KLH, and 2,6 TDI-KLH and reacted the least with 2,4;2,6 TDI-HSA (mix). MAb 29E5 had no other unique reactivity while MAbs 16F4 and 56F9 also reacted with 2,6 TDI-HSA, the methyl substituted monoisocyanates, HDI-HSA and MDI-HSA. The pattern of reactivity of the MAbs for denatured conjugated proteins observed in the dot blots (Fig. 5B) was consistent with that observed with the Western blot analyses.
FIG. 5.
(A, B) Dot blot analysis of MAb 56F9. (A) Native proteins; (B) denatured proteins. The protein lay out is identical in both panels. Lane A1, HSA; lane A2, 2,4 TDI-HSA; lane A3, 2,6 TDI-HSA; lane A4, 2,4;2,6 TDI (industrial mix)-HSA; lane A5, 2,5 DMPI-HSA; lane B1, 3,4 DMPI-HSA; lane B2, 2,4 DMPI-HSA; lane B3, OTI-HSA; lane B4, PTI-HSA; lane B5, MDI-HSA; lane C1, HDI-MSA; lane C2, 2,4 TITC-HSA, lane C3, 2,6 TITC-HSA, lane C4, PI-HSA; lane C5, KLH; lane D1, 2,4 TDI-KLH; lane D2, 2,6 TDI-KLH; lane D3–D5, negative controls.
Discussion
Toluene diisocyanate is a high production chemical with major uses in the production of flexible polyurethane foams, elastomers, and coatings. Exposure to diisocyanates can occur in both polyurethane production and during the use of the diisocyanate containing products by the consumer. Both aromatic and aliphatic diisocyanates are etiological agents of hypersensitivity diseases including asthma, rhinitis, allergic contact dermatitis, and hypersensitivity pneumonitis.(16,19–21)
In vivo conjugation of TDI to proteins in the human airways is thought to be a primary event in TDI exposure.(4) However, the characterization of dNCO haptenated proteins is complicated by the ability of the dNCOs to haptenate multiple proteins to self-polymerize and form both intra- and intermolecular cross-links with diverse proteins and non-protein species.
The antigens used in the characterization of the MAbs produced in the present study can be divided according to (1) the chemical nature of the carrier protein as basic (lysozyme), acidic (HSA), or insoluble (keratin); (2) the origin of the carrier protein as animal or human; and (3) the chemical nature of the isocyanate adduct. The TDI-adduct formation was modified by changing the position of the NCO group on the benzene ring, removing one NCO, substituting one NCO with a methyl group, or substituting dNCO groups with dNCS groups.
The isocyanate functional groups in TDI can potentially react with a hydroxyl group (in hydrophobic pockets) to form a urethane linkage, a thiol group to form a thiourea, or an amine group to form a urea. 2,4 TDI is an asymmetrical molecule and thus has two isocyanate groups of different reactivity. The 4 position is more reactive than the 2 position because it is more accessible. 2,6 TDI is a symmetrical molecule and thus has two isocyanate groups of similar reactivity, similar to the 2 position of 2,4 TDI. Reaction of one isocyanate group will cause a change in the reactivity of the second isocyanate group since both isocyanate groups are attached to the same aromatic ring. The first NCO of a diisocyanate may form a urea linkage with a primary amine of a protein while the second NCO may be hydrolyzed to an amine with the potential to react with additional NCO groups to undergo intra- or intermolecular cross-linking with other proteins resulting in dimers, trimers, and so forth.(12) Intra- and intermolecular crossing can also be mediated by a single dNCO molecule reacting to two separate amino acids. All MAbs produced herein reacted with proteins that contained intra- and intermolecular TDI-mediated cross-linking, suggesting that their reactivity was not impaired by the presence of cross-linking.
Examination of the MAbs reactivity towards monoisocyanate-haptenated proteins was used to assess the need of molecular cross-linking on the ability of the MAbs to recognize TDI-haptenated proteins. OTI and PTI are monoisocyanates with the second isocyanate group removed from the ortho and para positions relative to the methyl group. 2,3 DMPI, 2,5 DMPI, and 3,5 DMPI are monoisocyanates with the ortho and para positions substituted with methyl groups. PI has only one isocyanate and no methyl group attached to the phenyl ring. All monoclonal antibodies recognized monoisocyanate haptenated albumin, suggesting that the cross-linking was not critical to immune recognition. The assay format, however, was found to influence the MAb reactivity with the various haptenated species. Having an isocyanate or a substitution at the ortho position on the tolyl group was critical to MAb recognition in the ELISA format. This was true for all three MAbs, suggesting a possible influence of ELISA plate chemistry in the interactions between the MAbs and haptenized protein. The MAbs recognized haptenated albumin in which there was substitution of one isocyanate on the tolyl group (in the Western blot and dot blot formats) but not simple removal of the isocyanate. Interestingly, higher molecular weight proteins than ∼66.5 kDa in the stock HSA as observed in the protein blot were also haptenated by TDI and recognized by the MAbs (Figs. 1 and 4). These MAb recognized MDI- and/or HDI-conjugated HSA, in addition to the TDI-HSA. This suggests that the resultant bond formation following reaction of the dNCO to protein was important in the recognition of TDI-haptenated proteins by the MAbs.
The reactivity of the antibodies may also be influenced by the choice of the experimental carrier protein. The MAbs reacted with 2,4 TDI bound to HSA or MSA and also to KLH, which is a non-mammalian protein, indicating the importance of the dNCO-protein bond rather than the source species of the carrier protein. Although lysozyme and keratin were demonstrated by matrix-assisted laser desorption ionization and/or loss of primary amines (data not shown) to be haptenated, none of the MAbs in the present study showed reactivity toward their conjugates. This may be due to poor binding of the conjugates to the ELISA plate surface, lower haptenation rates, or steric inaccessibility of the TDI group on these carrier proteins.
A total of 10 MAbs (all IgMs) generated from TDI vapor-exposed mice were isolated during the initial screening against TDI-HSA; however, only three MAbs showed no or very low reactivity to unconjugated HSA. The reason for the high reactivity of the other MAbs with HSA is not known but we were unable to block this binding with either milk or BSA. It is possible that haptenation of proteins may also lead to recognition of the self protein by the immune system as seen with exposure to aldehydes(22) although this has not been investigated for isocyanate exposures. Interestingly, these TDI vapor-sensitized mice had IgG anti-2,4 TDI-MSA titers of 18,106 ± 7,994 (sera dilution to reach an ELISA OD >0.1). IgM anti-2,4 TDI-MSA was not assessed in these mice; however, anti-2,4 TDI-MSA IgM was not observed in the sera of another group of mice exposed to an identical inhalation protocol (data not shown).
Future potential applications of these MAbs include the isolation of endogenous carrier proteins that are conjugated after natural or experimental exposure episodes. The purified proteins can then be identified and their structure and chemical linkages determined by mass spectrometry. We recently demonstrated the potential for such methods for isocyanate-peptide adducts using tandem mass spectrometry.(23) The MAbs may also be useful reagents for the development of immunoassays designed for dNCO exposure assessments.
In summary, we have successfully developed monoclonal antibodies that recognize dNCO protein adducts from TDI vapor-exposed mice. The specificity of these antibodies was demonstrated by ELISA, Western blot, and dot blot analyses. The antibodies may be useful tools for the identification of endogenous dNCO-modified proteins and the characterization of the importance of the type of chemical linkage in terms of isocyanate toxicity.
Acknowledgments
This work was supported in part by NIEHS/NIOSH Inter Agency Agreement (Y1-ES-0001). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- 1.Liu Q. Wisnewski AV. Recent developments in diisocyanate asthma. Ann Allergy Asthma Immunol. 2003;90:35–41. doi: 10.1016/s1081-1206(10)61647-x. [DOI] [PubMed] [Google Scholar]
- 2.Park HS. Lee SK. Kim HY. Nahm DH. Kim SS. Specific immunoglobulin E and immunoglobulin G antibodies to toluene diisocyanate-human serum albumin conjugate: useful markers for predicting long-term prognosis in toluene diisocyanate-induced asthma. Clin Exp Allergy. 2002;32:551–555. doi: 10.1046/j.0954-7894.2002.01349.x. [DOI] [PubMed] [Google Scholar]
- 3.Son M. Lee M. Kim YT. Youn JK. Park H. Heterogeneity of IgE response to TDI-HSA conjugates by ELISA in toluene diisocyanate (TDI)-induced occupational asthma (OA) patients. J Korean Med Sci. 1998;13:147–152. doi: 10.3346/jkms.1998.13.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wisnewski AV. Redlich CA. Recent developments in diisocyanate asthma. Curr Opin Allergy Clin Immunol. 2001;1:169–175. doi: 10.1097/01.all.0000011003.36723.d8. [DOI] [PubMed] [Google Scholar]
- 5.Wisnewski AV. Developments in laboratory diagnostics for isocyanate asthma. Curr Opin Allergy Clin Immunol. 2007;7:138–145. doi: 10.1097/ACI.0b013e3280895d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mraz J. Bouskova S. 2,4-toluenediisocyanate and hexamethylene-diisocyanate adducts with blood proteins: assessment of reactivity of amino acid residues in vitro. Chem Biol Interact. 1999;117:173–186. doi: 10.1016/s0009-2797(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 7.Wisnewski AV. Srivastava R. Herick C. Xu L. Lemus R. Cain H. Magoski NM. Karol HM. Bottomly K. Redlich CA. Identification of human lung and skin proteins conjugated with hexamethylene diisocyanate in vitro and in vivo. Am J Respir Crit Care Med. 2000;162:2330–2336. doi: 10.1164/ajrccm.162.6.2002086. [DOI] [PubMed] [Google Scholar]
- 8.Lange RW. Lantz RC. Stolz DB. Watkins SC. Sundareshan P. Lemus R. Karol MH. Toluene diisocyanate colocalizes with tubulin on cilia of differentiated human airway epithelial cells. Toxicol Sci. 1999;50:64–71. doi: 10.1093/toxsci/50.1.64. [DOI] [PubMed] [Google Scholar]
- 9.Johannesson G. Sennbro CJ. Willix P. Lindh CH. Jonsson BA. Identification and characterisation of adducts between serum albumin and 4,4′-methylenediphenyl diisocyanate (MDI) in human plasma. Arch Toxicol. 2004;78:378–383. doi: 10.1007/s00204-004-0555-2. [DOI] [PubMed] [Google Scholar]
- 10.Wisnewski AV. Liu Q. Liu J. Redlich CA. Glutathione protects human airway proteins and epithelial cells from isocyanates. Clin Exp Allergy. 2005;35:352–357. doi: 10.1111/j.1365-2222.2005.02185.x. [DOI] [PubMed] [Google Scholar]
- 11.Ott MG. Jolly AT. Burkert AL. Brown WE. Issues in diisocyanate antibody testing. Crit Rev Toxicol. 2007;37:567–585. doi: 10.1080/10408440701419553. [DOI] [PubMed] [Google Scholar]
- 12.Allport DC. Gilbert DS. Outterside SM. MDI, TDI: Safety, Health, the Environment: A Source Book and Practical Guide. John Wiley; New York: 2003. [Google Scholar]
- 13.Chadwick DH, editor; Cleveland TH, editor. Kirk-Othmer: Isocyanates, Organic. Kirk-Othmer Encyclopedia of Chemical Technology. Wiley-Interscience; Hoboken, NJ: 2001. pp. 902–931. [Google Scholar]
- 14.Campo P. Wisnewski AV. Lummus Z. Cartier A. Malo JL. Boulet LP. Bernstein DI. Diisocyanate conjugate and immunoassay characteristics influence detection of specific antibodies in HDI-exposed workers. Clin Exp Allergy. 2007;37:1095–1102. doi: 10.1111/j.1365-2222.2007.02745.x. [DOI] [PubMed] [Google Scholar]
- 15.Lemus R. Lukinskeine L. Bier ME. Wisnewski AV. Redlich CA. Karol MH. Development of immunoassays for biomonitoring of hexamethylene diisocyanate exposure. Environ Health Perspect. 2001;109:1103–1108. doi: 10.1289/ehp.011091103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson VJ. Yucesoy B. Reynolds JS. Fluharty K. Wang W. Richardson D. Luster MI. Inhalation of toluene diisocyanate vapor induces allergic rhinitis in mice. J Immunol. 2007;179:1864–1871. doi: 10.4049/jimmunol.179.3.1864. [DOI] [PubMed] [Google Scholar]
- 17.Harlow E. Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1988. [Google Scholar]
- 18.Schmechel D. Simpson JP. Beezhold D. Lewis DM. The development of species-specific immunodiagnostics for Stachybotrys chartarum: the role of cross-reactivity. J Immunol Methods. 2006;309:150–159. doi: 10.1016/j.jim.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Matheson JM. Johnson VJ. Vallyathan V. Luster MI. Exposure and immunological determinants in a murine model for toluene diisocyanate (TDI) asthma. Toxicol Sci. 2005;84:88–98. doi: 10.1093/toxsci/kfi050. [DOI] [PubMed] [Google Scholar]
- 20.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 21.Maestrelli P. Boschetto P. Fabbri LM. Mapp CE. Mechanisms of occupational asthma. J Allergy Clin Immunol. 2009;123:531–542. doi: 10.1016/j.jaci.2009.01.057. [DOI] [PubMed] [Google Scholar]
- 22.Sandberg E. Bergenholtz G. Kahu H. Dahlgren UI. Low HEMA conjugation induces high autoantibody titer in mice. J Dent Res. 2005;84:537–541. doi: 10.1177/154405910508400610. [DOI] [PubMed] [Google Scholar]
- 23.Hettick JM. Ruwona TB. Siegel PD. Structural elucidation of isocyanate-peptide adducts using tandem mass spectrometry. J Am Soc Mass Spectrom. 2009;20:1567–1575. doi: 10.1016/j.jasms.2009.04.016. [DOI] [PubMed] [Google Scholar]