Abstract
Mounting evidence suggests that σB and PrfA coregulate transcription of multiple genes in Listeria monocytogenes, therefore, the relative contributions of σB and PrfA to transcript levels of genes identified previously as differentially regulated by PrfA were measured. Group I genes are recognized virulence genes that are positively regulated by PrfA; group II genes were reported previously as negatively regulated by PrfA; and multiple group III genes were proposed to be coregulated by σB and PrfA. Transcript levels for selected genes were measured by quantitative reverse transcriptase polymerase chain reaction (RT-PCR) in L. monocytogenes 10403S as well as in otherwise isogenic ΔsigB, ΔprfA, and ΔsigBΔprfA strains grown under conditions demonstrated to induce either PrfA activity (0.2% activated charcoal) or both PrfA and σB activity (stationary phase). Although the Group I gene plcA was positively regulated by PrfA, transcript levels for the group II genes lmo0278 and lmo0178 were not affected by the prfA deletion. While the sigB deletion significantly affected transcript levels for the selected group III genes (i.e., lmo0596, lmo0654, bsh, and opuCA), with lower transcript levels in the ΔsigB strains under all conditions tested, transcript levels for these genes were not significantly affected by the prfA deletion. Our results suggest that the regulatory interactions between PrfA and σB contribute to PrfA's predominant role as a direct regulator of virulence genes critical for invasion and intracellular survival in L. monocytogenes 10403S, while σB regulates a wider range of virulence and stress response genes.
Introduction
The gram-positive bacterium Listeria monocytogenes, which can be found in many natural environments, can cause severe invasive disease in humans and many other animal species (Gellin and Broome, 1989; Mead et al., 1999; Vazquez-Boland et al., 2001; Roberts and Wiedmann, 2003). To fully understand how foodborne pathogens such as L. monocytogenes are transmitted through food systems, and hence, to enable development of effective control strategies for these pathogens, bacterial mechanisms that enable the switch between survival in the environment and survival in a host must be identified and characterized. In L. monocytogenes, mounting evidence suggests that the transformation between saphrophyte and pathogen is mediated through complex regulatory pathways that modulate the expression of multiple genes, including virulence factors, in response to environmental signals (Gray et al., 2006). While initial data (Kazmierczak et al., 2003; Kim et al., 2005; McGann et al., 2007) suggest that two regulators, positive regulatory factor A (PrfA) and the alternative sigma factor σB, are critical for this switch, our understanding of their interactions is still limited.
A number of L. monocytogenes virulence genes and their specific functions in the intracellular infection process have been identified and characterized (Portnoy et al., 1992; Vazquez-Boland et al., 2001). One of these virulence genes, designated prfA, encodes PrfA. PrfA plays a central role in regulating the expression of many virulence genes that are critical for L. monocytogenes entry into host cells and its spread into neighboring cells (Leimeister-Wachter et al., 1990; Chakraborty et al., 1992; Ireton et al., 1999). PrfA shares sequence and structural similarities with the cAMP receptor protein (CRP) in Escherichia coli (Lampidis et al., 1994). Specifically, the helix-turn-helix DNA binding domains are highly conserved between CRP and PrfA (Sheehan et al., 1996; Kreft and Vazquez-Boland, 2001). PrfA recognizes and binds to a 14 bp palindromic DNA sequence called the PrfA box, which is typically located ∼41 bp upstream of the transcriptional start site of PrfA-regulated genes (Mengaud et al., 1991; Freitag et al., 1992, 1993; Sheehan et al., 1996). Post-transcriptional modifications of the wildtype PrfA protein have been shown to affect transcriptional activity of this protein in a growth medium-dependent manner (Ripio et al., 1996; Renzoni et al., 1997).
A whole genome macroarray-based characterization of gene expression patterns in L. monocytogenes strains EGDe, P14, P14-A (which contains a prfA* allele that encodes a constitutively active PrfA*) and their isogenic ΔprfA derivatives, grown under PrfA-inducing or –re-pressing conditions, resulted in identification of 73 putative PrfA-regulated genes, which were then classified into three groups (Milohanic et al., 2003). Group I contains 12 genes that are positively regulated by PrfA, including 10 recognized virulence genes; transcription of group I genes was activated by charcoal and repressed by cellobiose, conditions previously reported to activate or inhibit PrfA activity, respectively (Ripio et al., 1996; Renzoni et al., 1997). The eight group II genes appeared to be negatively regulated by PrfA, suggesting the possibility of a transcriptional repressor function for PrfA. While the majority of the 53 group III genes were reported to be positively regulated by both PrfA and PrfA*, PrfA-dependent transcription of many group III genes was neither repressed by cellobiose nor induced by the presence of activated charcoal. Interestingly, putative σB-dependent promoters were identified upstream of a number of group III genes, suggesting the possibility of σB-PrfA coregulation of these genes in L. monocytogenes (Milohanic et al., 2003).
The alternative general stress response sigma factor σB directs transcription of genes contributing to survival under multiple environmental stress conditions in gram-positive bacteria (Hecker and Völker, 2001). In L. monocytogenes, expression of σB-dependent genes is induced by exposure to a number of conditions, including low pH and elevated osmolarity, which mimic conditions encountered during gastrointestinal infection (Sue et al., 2004; Garner et al., 2006). Previous studies have shown that L. monocytogenes σB also contributes to virulence (Wiedmann et al., 1998; Nadon et al., 2002; Kazmierczak et al., 2003, 2006; Kim et al., 2004) by regulating transcription of PrfA-dependent genes (Kazmierczak et al., 2003, 2006; Kim et al., 2004,2005) as well as of prfA itself (Nadon et al., 2002; Schwab et al., 2005). Multiple lines of evidence thus suggest that (i) both PrfA and σB contribute to transcriptional regulation of numerous genes, including those contributing directly to L. monocytogenes virulence, and (ii) PrfA and σB are part of a regulatory network that is important for virulence and transmission of L. monocytogenes (Kazmierczak et al., 2003).
While the σB and PrfA regulons in L. monocytogenes have been investigated previously using macro- and microarray-based approaches, these studies compared transcript levels only between a parent strain and either an isogenic ΔsigB strain (Kazmierczak et al., 2003) or an isogenic ΔprfA strain (Milohanic et al., 2003). The objective of the present study was to examine the regulatory network between PrfA and σB by using quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) to measure relative transcript levels for genes previously classified into the PrfA regulon in isogenic ΔsigB, ΔprfA, and ΔsigBΔprfA strains grown under conditions that either activate both σB and PrfA or PrfA alone.
Materials and Methods
Bacterial strains
L. monocytogenes 10403S and its derivatives were used throughout this study (Table 1). Stock cultures were stored at −80°C in brain heart infusion (BHI; Difco Laboratories, Sparks, MD) broth with 15% glycerol. Prior to each experiment, stock cultures were streaked onto BHI plates and incubated for 24 hours at 37°C to obtain isolated colonies for subsequent experiments.
Table 1.
Strains Used in This Study
Growth to log and stationary phase
For each experimental culture, an isolated colony was used to inoculate 5 mL of BHI broth, which was then incubated overnight at 37°C with shaking (220 rpm); overnight (12 hours) cultures were then inoculated 1:100 into 5mL fresh BHI broth, followed by growth at 37°C with shaking (220 rpm) to an optical density representing log-phase cells (OD600 = 0.4). To further synchronize cells, the log-phase culture was diluted again (1:100) into a side arm flask containing 50 mL fresh BHI that had been pre-warmed to 37°C. When cells reached OD600 = 0.4, 4 mL of culture were added to 8 mL of RNAprotect™ (Qiagen, Valencia, CA) to preserve cells for RNA isolation as described below. The remainder of the culture was incubated until cells reached stationary phase (defined as an OD600 = 2.0) followed by RNAprotect™ treatment as described above.
Exposure of L. monocytogenes to activated charcoal
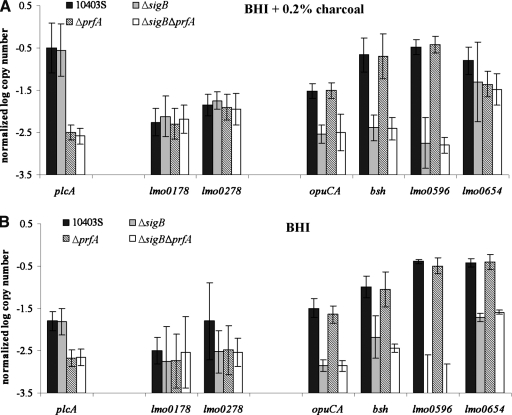
Previous studies have shown that PrfA can be present in two functional states (weakly or highly active [Renzoni et al., 1997]) and that exposure of L. monocytogenes cultures to activated charcoal results in increased transcription of PrfA-regulated virulence genes (Ripio et al., 1996). Therefore, to activate PrfA-mediated transcription, in one set of experiments, L. monocytogenes 10403S and its isogenic mutants (ΔsigB, ΔprfA, and ΔsigBΔprfA) were grown to log phase and then exposed to a final concentration of 0.2% activated charcoal for 2 hours at 37°C as previously validated and reported (McGann et al., 2007). Briefly, BHI containing 0.4% (w/v) activated charcoal was prepared by adding 160 μLof a sterile 5% aqueous activated charcoal stock solution into 2 mL sterile BHI. Cells were grown to log phase (OD600 = 0.4) in 50 mL fresh prewarmed BHI as described above and 2 mL of log phase cultures were added to either 2 mL fresh, prewarmed BHI or to 2 mL prewarmed BHI containing 0.4% activated charcoal (to yield a final concentration of 0.2% activated charcoal). After 2 hours at 37°C with shaking, 8mL of RNAprotect™ was added to each tube to stabilize the RNA for subsequent RNA purification, as detailed below. Quantitative RT-PCR analysis of transcript levels for plcA, which is positively regulated by PrfA (Mengaud et al., 1991; Bohne et al., 1994; Sheehan et al., 1995; Lalic-Multhaler et al., 2001), validated that this charcoal exposure protocol increased PrfA-dependent transcription by >1 log (Fig. 1).
FIG. 1.
Transcript levels for selected group I, II, and III genes in the L. monocytogenes parent strain 10403S and in isogenic ΔsigB, ΔprfA, and ΔsigBΔprfA strains exposed to either (A) BHI + 0.2% activated charcoal or (B) BHI alone. Normalized log copy numbers shown on the Y-axis represent log-transformed transcript levels for a given target gene normalized to the geometric mean of the transcript levels for the housekeeping genes rpoB and gap. Genes shown on the X-axis are, from left to right, the group I gene (plcA), group II genes (lmo0178, lmo0278), and group III genes (opuCA, bsh, lmo0596, and lmo0654). Data represent mean values from qRT-PCR data collected from three or four independent RNA extractions (except for transcript levels for lmo0654 and lmo0596 from cells grown in BHI + 0.2% charcoal, which are represented by two replicates); error bars represent one standard deviation.
RNA purification
Following addition of RNAprotect™, the culture was held for 5 minutes at room temperature, then cells were collected by centrifugation at 5000 g for 5 minutes. Total RNA was extracted as previously described (Kazmierczak et al., 2006) except that contaminating DNA was removed using Turbo DNase (Ambion Inc., Austin, TX) according to the manufacturer's instructions. Optical densities (OD) at 230, 260, and 280 nm were determined for the purified RNA using the NanoDrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). Purity of extracted RNA was evaluated using OD260/OD280 nm and OD260/OD230 nm ratios, which assess protein and organic substance contamination, respectively. RNA concentrations were calculated based on OD260nm measurements. All purified RNA preparations were precipitated using sodium acetate and ethanol and stored at −80°C.
TaqMan qRT-PCR
All primers and probes used for qRT-PCR were designed using Primer Express software (Applied Biosystems, Foster City, CA; Table 2). Probes with a Minor Grove Binder (MGB) and a nonfluorescent quencher (NFQ) were synthesized by Applied Biosystems; probes with QSY7 quencher dye were synthesized by MegaBases Inc. (Evanston, IL). Quantitative RT-PCR was performed in 25 μL reactions as described previously (Sue et al., 2004). All reactions were performed in duplicate on at least three independent RNA preparations. A gene-specific genomic DNA standard curve was generated to allow for absolute quantification of mRNA levels (Sue et al., 2004).
Table 2.
TaqMan Primers and Probes Used in This Study
| Gene | Forward primer (5′-3′) | Taqman probe (5′-3′)a | Reverse primer (5′-3′) | Reference |
|---|---|---|---|---|
| rpoB | TGTAAAATATGGACGGCATCGT | CTGATTCGCGCAAAACTTCTACGCG-QSY7 | GCTGTTTGAATCTCAATTAAGTTTGG | Sue et al.,2004 |
| gap | AAAGCTGGCGCTAAAAAAGTTG | TTCATGGTTTACATTGTAAAGCATTG-QSY7 | ATCTCCGCTCCAGCAACTGGCGATAT | Schwab et al.,2005 |
| plcA | GATTTATTTACGACGCACATTAGTTT | CCCATTAGGCGGAAAAGCATATTCGC-QSY7 | GAGTTCTTTATTGGCTTATTCCAGTTATT | Kazmierczak et al.,2006 |
| lmo0178 | ATTTACAGCAAGAAGGCATTA | AGGTGCCGAAACAT-MGB-NFQ | GCACGTCTGCCTCCAGTAGAT | This study |
| lmo0278 | GAATTCATCGTTTTTGTAGGACCAT | TGGTTGTGGTAAATCTA-MGB-NFQ | CCAGCAATCATACGCAAAGTTG | This study |
| lmo0596 | TTCTGGTATTTTCCACACAATCTCTT | TTTTCAGACAGAAAATCACAAA-MGB-NFQ | GTCAGCTAGTACCCAGCCAGAAA | This study |
| lmo0654 | TTGCGACAAACATATCAAGAGGTAA | CAGCGAAACAAGAGAGT-MGB-NFQ | TTTGGAGTTGTGGGTCTTCA | This study |
| opuCA | ACATCGATAAAGGAGAATTTGTTTGTT | CGTTTTCCCACAACCACTTGGACCG-QSY7 | GCCGGTTAATCATCTTCATTGTT | Sue et al.,2004 |
| bsh | GGCCTTAGTATGGCAGGACTCA | CCTTGTAATCCGCATTTCCTGAGAA-QSY7 | CTCATTGTCCTTACCTTCTGCAAA | Sue et al.,2003 |
All probes have a 6-FAM reporter dye at the 5′ end. Taqman probes were synthesized with either the QSY7 dark quencher or a Minor Groove Binder (MGB) and a non-fluorescent quencher (NFQ) at the 3′ end.
Statistical analysis
mRNA transcript level data were log10 transformed to approximate normality. Prior to our final statistical analysis, absolute mRNA transcript levels for our target genes were normalized to the geometric mean of the transcript levels for the two housekeeping genes, rpoB and gap, as previously described (Chaturongakul and Boor, 2006; Kazmierczak et al., 2006). For statistical analysis, transcript levels measured in cells grown in the presence or absence of activated charcoal were separately analyzed from transcript levels measured in cells grown to different growth phases. To assess the effects of growth condition and strain on transcript levels observed for a given gene and to measure statistical interactions among data obtained under different conditions, ANOVA was performed on the log-transformed normalized copy number of each gene with strain and growth condition as main effects using the following linear model: transcript levels = μ + strain + growth condition + strain*growth condition + RNA extraction date + ɛ (factor “strain” includes 10403S, ΔsigB, ΔprfA, and ΔsigBΔprfA, factor “growth condition” includes growth with or without charcoal or growth to log or stationary phase). A second ANOVA was subsequently performed to assess the effects of (i) the sigB deletion, (ii) the prfA deletion, and (iii) the interactions between the sigB and the prfA deletions on the transcript levels for a given gene in L. monocytogenes grown under a given condition using the following linear model: transcript levels = μ + sigB + prfA + sigB*prfA + RNA extraction date + ɛ (factor “sigB” represents presence or absence of sigB, factor “prfA” represents presence or absence of prfA). RNA extraction date was included in all models to control for contributing variation. All effects were treated as fixed effects. All statistical analyses were performed in S-Plus 6.2 (Insightful Corp., Seattle, WA).
Results and Discussion
Pathogenesis of listeriosis typically includes a gastrointestinal stage followed by a systemic stage, which is characterized by intracellular infection of a number of different host cell types. PrfA has long been recognized as a transcriptional activator of virulence genes that are important during the intracellular stage of infection, in particular the virulence genes located in the prfA virulence gene island (i.e., actA, hly, plcA, plcB, and mpl). The dual observations of coregulation of some virulence genes (i.e., inlA, inlB) by σB and PrfA (Kim et al., 2005; McGann et al., 2007) as well as the observation that σB also directly regulates transcription of prfA (Kazmierczak et al., 2006; Nadon et al., 2002; Rauch et al., 2005) suggests that a regulatory network involving σB and PrfA is important for L. monocytogenes virulence. Therefore, the focus of this study was to quantitatively characterize relative contributions of PrfA and σB to transcription of genes previously characterized as members of the PrfA operon in isogenic sigB and prfA single and double mutants. We thus used qRT-PCR to measure transcript levels for genes selected for this study on the basis of their regulatory elements (Table 3) and preliminary PrfA regulon classification (Milohanic et al., 2003). Transcript levels were measured in L. monocytogenes 10403S and isogenic ΔsigB, ΔprfA, and ΔsigBΔprfA strains grown under conditions demonstrated to induce σB and PrfA activity. A qRT-PCR approach was chosen rather than a microarray-based strategy to allow for absolute transcript quantification and thus, comparison of transcript levels for bacteria grown under multiple conditions. Our data indicate that (i) transcription of L. monocytogenes group II genes is PrfA-independent, at least under a number of conditions that induce PrfA activity, and (ii) selected group III genes show σB-dependent, but PrfA-independent, transcription under a number conditions that induce PrfA activity.
Table 3.
Genes Characterized in This Study
| Gene | Groupa | Function | Regulatory Elements |
|---|---|---|---|
| plcA | I | Lecithinase | PrfA boxb |
| lmo0178 | II | Similar to xylose repressor | PrfA boxc |
| lmo0278 | II | Similar to sugar ABC transporter, ATP-binding protein | PrfA boxc |
| opuCA | III | Similar to glycine/ choline ABC transporter | σB promoterd |
| lmo0654 | III | Unknown | σB promoterd |
| bsh | III | Bile salt hydrolase | PrfA boxc;σB promoterd |
| lmo0596 | III | Unknown | PrfA boxc overlapping −35 region of σB promoterd |
Groups I, II, and III as defined by Milohanic et al. (2003).
Identified by primer extension (Vazquez-Boland et al., 1992).
Identified by sequence analysis (Glaser et al., 2001).
Identified by HMM; σB -dependent promoters for opuCA and bsh were also confirmed by 5′ Rapid Amplification of cDNA ends (RACE) (Kazmierczak et al., 2003).
Transcription of group II genes lmo0178 and lmo0278 is PrfA-independent
Macroarray studies by Milohanic et al. (2003) identified eight group II genes (including one seven-gene operon [lmo0178-lmo0184] as well as a single gene [lmo0278]), which were negatively regulated by PrfA, but not differentially regulated by the constitutively active PrfA*. To evaluate transcriptional regulation of these group II genes, we designed qRT-PCR probes for lmo0278 as well as for the first gene of the group II operon (i.e., lmo0178; Table 2). When lmo0178 and lmo0278 transcript levels were determined for L. monocytogenes grown in the presence or absence of charcoal, transcription of both genes was significantly affected by the addition of activated charcoal (ANOVA using data for all four strains; p = 0.0013 and 0.0103 for lmo0178 and lmo0278, respectively; Suppl. Table S1 and Suppl. Fig. S1 [to view supplementary material, go to www.liebertpub.com/fpd]);both genes generally showed higher transcript levels in cells exposed to activated charcoal than in cells not exposed to charcoal. lmo0178 and lmo0278 transcript levels were not significantly affected by a prfA deletion, however (Table 4, Suppl. Fig. S1), indicating that increased transcription of these genes in cells exposed to charcoal is not mediated by PrfA. In contrast, the known PrfA-dependent plcA (which served as a control gene) not only showed increased transcript levels in cells grown in the presence of charcoal (Suppl. Table S1, Suppl. Fig. S2), but also clearly showed PrfA-dependent transcription (Table 4; Fig. 1). Overall, our results on the effects of charcoal on lmo0178 and lmo0278 transcript levels are consistent with an increasing body of data indicating that activated charcoal not only increases PrfA activity, but may also interfere with other, not yet identified, environmental signals that affect PrfA-independent transcription (Andre et al., 2003; McGann et al., 2007). While L. monocytogenes cultures supplemented with activated charcoal have been used as a tool to increase PrfA-mediated transcription (Geoffroy et al., 1989; Ripio et al., 1996; Milohanic et al., 2003), pleiotropic effects of charcoal thus may complicate interpretation of transcriptional data aimed at understanding PrfA-dependent gene regulation.
Table 4.
Results from Statistical Analyses of the Effects of sigB and prfA Null Mutations on Transcript Levels for Selected Group I, II, and III Target Genes in L. monocytogenes Grown Under Different Conditions
| |
|
Effect of sigB deletion |
Effect of prfA deletion |
||
|---|---|---|---|---|---|
| Gene | Condition | Difference in marginal means (in log normalized copies)a | p-valueb | Difference in marginal means (in log normalized copies)a | p-valueb |
| plcA | Charcoal | — | NS | −2.02 | <0.0001 |
| No charcoal | — | NS | −0.86 | <0.0001 | |
| Log phase | — | NS | −0.62 | <0.0001 | |
| Stationary phase | — | NS | −0.89 | <0.0001 | |
| lmo0178 | Charcoal | +0.12 | 0.0221 | — | NS |
| No charcoal | — | NS | — | NS | |
| Log phase | — | NS | — | NS | |
| Stationary phase | −0.61 | 0.0512 | — | NS | |
| opuCA | Charcoal | −1.01 | <0.0001 | — | NS |
| No charcoal | −1.29 | <0.0001 | — | NS | |
| Log phase | −1.03 | 0.0012 | — | NS | |
| Stationary phase | −0.83 | 0.0004 | — | NS | |
| bsh | Charcoal | −1.72 | <0.0001 | — | NS |
| No charcoal | −1.29 | <0.0001 | — | NS | |
| Log phase | −0.91 | 0.0005 | — | NS | |
| Stationary phase | −1.45 | <0.0001 | — | NS | |
| lmo0596 | Charcoal | −2.33 | <0.0001 | — | NS |
| No charcoal | −3.67 | 0.0099 | — | NS | |
| Log phase | −2.16 | 0.0009 | — | NS | |
| Stationary phase | −2.69 | <0.0001 | — | NS | |
| lmo0654 | Charcoal | — | NS | — | NS |
| No charcoal | −1.24 | 0.0006 | — | NS | |
| Log phase | −0.73 | 0.0078 | — | NS | |
| Stationary phase | −0.82 | 0.0020 | — | NS | |
“Difference in marginal means” refers to the difference in log normalized transcript copies for a given gene between strains that have a given gene (e.g., for sigB, the parent strain 10403S and the ΔprfA strain) and the strains that lack a given gene (e.g., for sigB, the ΔsigB and the ΔsigBΔprfA strains); negative values indicate lower transcript levels in the mutant strains. Differences in marginal means are only reported for gene/condition combinations with p < 0.1.
Actual p-values are given for p-values between 0.0001 and 0.10; NS represents a p-value > 0.1; no significant effects of either the prfA or the sigB deletion on lmo0278 transcript levels were observed; therefore, data from this gene were not included in this table. Interactions between the sigB deletion and the prfA deletion were not statistically significant for any of the genes under any of the conditions tested.
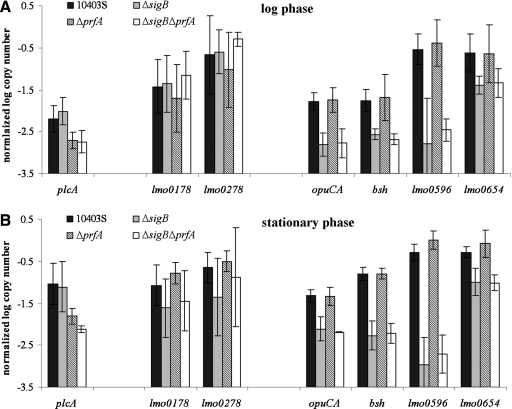
While it has been well documented that σB activity increases in L. monocytogenes stationary phase cells (Ferreira et al., 2001), transcription of PrfA-dependent genes and PrfA activity also appear to be higher in stationary phase cells as compared to log phase cells (Ermolaeva et al., 2004; Kazmierczak et al., 2006). Transcript levels for the PrfA-dependent plcA were higher in stationary phase cells as compared to log phase cells in the experiments reported here (Table 4, Suppl. Fig. S2). Similar to the data for L. monocytogenes exposed to charcoal, the prfA deletion did not affect lmo0178 and lmo0278 transcript levels in bacteria grown to either stationary phase or log phase (Table 4; Fig. 2), further supporting PrfA-independent transcription of the group II genes lmo0178 and lmo0278 under different conditions, including conditions that yielded high transcript levels for the PrfA-dependent control gene plcA (Suppl. Fig. S2). While putative PrfA boxes have been identified upstream of lmo0178 and lmo0278 (Glaser et al., 2001), these PrfA boxes differ from the PrfA consensus sequence by 2 nt (Fig. 3), a difference that has been shown to greatly reduce efficient PrfA binding (Sheehan et al., 1995). These observations suggest that PrfA binding to these putative PrfA sites (thus possibly resulting in negative regulation) may only occur under specific environmental conditions and/or in the presence of high concentrations of active PrfA. Overall, however, our findings are consistent with in vitro transcription results by Rauch et al. (2005), which suggested PrfA-independent transcription of lmo0178 and lmo0278, with no evidence for direct PrfA-dependent transcriptional repression of these genes, as well as with microarray findings by Marr et al. (2006), who reported that lmo0178, lmo0182, lmo0183, and lmo0278 were not differentially regulated by PrfA.
FIG. 2.
Transcript levels for the selected group I, II, and III genes in the L. monocytogenes parent strain 10403S and in isogenic ΔsigB, ΔprfA, and ΔsigB ΔprfA strains grown to either (A) log phase (OD600 = 0.4) or (B) stationary phase (OD600 = 2.0). Normalized log copy numbers shown on the Y-axis represent log-transformed transcript levels for a given target gene normalized to the geometric mean of the transcript levels for the housekeeping genes rpoB and gap. Genes shown on the X-axis are, from left to right, the group I gene (plcA), group II genes (lmo0178, lmo0278), and group III genes (opuCA, bsh, lmo0596, and lmo0654). Data represent mean values from qRT-PCR data collected from three or four independent RNA extractions; error bars represent one standard deviation. To enable comparisons between transcript levels for a given gene in a given mutant strain, the data presented in Figs. 1 and 2 were also rearranged by strain and are presented in Suppl. Figs. S1 through S3.
FIG. 3.
L. monocytogenes 10403S promoter sequences of confirmed (i.e., plcA, actA, hly) and putative PrfA-regulated genes (i.e. lmo0178, lmo0278, bsh, lmo0596, lmo0654, opuCA). DNA sequences were obtained from the genome sequence for strain 10403S (Listeria monocytogenes Sequencing Project. Broad Institute of Harvard and MIT; http://www.broad.mit.edu). Promoter sequences are underlined and PrfA boxes are italicized. Deviations from the PrfA binding site consensus sequence are shown by lower case letters. The PrfA boxes and σA-dependent −10 and −35 sites for plcA, hly, and actA (Mengaud et al., 1989; Vazquez-Boland et al., 1992); the PrfA boxes for bsh, lmo0178, lmo0278, and lmo0596 (Glaser et al., 2001; Dussurget et al., 2002); and the σB-dependent −10 and −35 sites for bsh, lmo0596, and opuCA (Kazmierczak et al., 2003) have all been reported previously. For bsh, a putative −10 site has been described 19 nt downstream of the PrfA box (Dussurget et al., 2002) however, this promoter has not been confirmed experimentally and, therefore, was not included in this figure. The putative PrfA boxes for lmo0596, lmo0178, and lmo0278 are shown as described by Glaser et al. (2003). The indicated σA-dependent −10 promoter site for lmo0178 was mapped by primer extension (Luo et al., 2004).
Transcript levels for lmo0278 and lmo0178 were not significantly affected by the prfA deletion in cells exposed to activated charcoal, however, deletion of sigB significantly affected lmo0178 transcript levels (ANOVA; p = 0.0221, Table 4), with slightly (0.12 log) higher transcript levels in the L. monocytogenes strains with sigB deletions. Interestingly, in stationary-phase cells, lmo0178 and lmo0278 transcripts were lower in the sigB deletion strains as compared to the strains with intact sigB (0.61 logs and 0.55 logs, respectively; Table 4 and data not shown, respectively); however, the effect of the sigB deletion on lmo0178 transcript levels was only borderline significant (ANOVA; p = 0.051; Table 4) and the effect on lmo0278 transcript levels was not significant. No significant interaction effects between the sigB and the prfA deletions were observed on either lmo0178 or lmo0278 transcript levels. Application of a Hidden Markov Model that had been trained with 33 confirmed σB-dependent promoter sequences (Raengpra-dub et al., 2007) did not identify putative σB-dependent promoter sequences upstream of lmo0178 or lmo0278, suggesting that possible effects of the sigB deletion on transcript levels for these genes is indirect. Additionally, neither the lmo0178-lmo0184 operon nor lmo0278 were found to be σB dependent in a full genome microarray study of the L. monocytogenes σB regulon (Raengpradub et al., 2007), further supporting that group II genes are only weakly or inconsistently regulated by either σB or PrfA.
Transcript levels for the group III genes lmo0596, lmo0654, bsh, and opuCA are σB-dependent and PrfA-independent, even under PrfA inducing conditions
A total of 53 of the 73 genes identified as members of the PrfA regulon had been classified as group III genes by Milohanic et al. (2003), who reported that transcript levels for all group III genes were positively regulated by PrfA, with the majority of genes also positively regulated by the constitutively active PrfA*. While PrfA-dependent transcription of L. monocytogenes virulence genes (i.e., group I genes) appeared to increase in cells grown in the presence of activated charcoal and to decrease in cells grown in the presence of cellobiose, PrfA-dependent transcription of most group III genes was not affected by cellobiose (Milohanic et al., 2003). In addition, only one of the group III genes showed evidence of PrfA-dependent transcription in L. monocytogenes EGD-e cells grown in the presence of charcoal (Milohanic et al., 2003), suggesting that the group III genes identified by Milohanic et al. (2003) do not follow the classical PrfA-dependent transcription patterns of group I genes. Group III genes also show diverse promoter configurations; a number of group III genes have putative σB dependent and/or putative PrfA-dependent promoter sites (Milohanic et al., 2003; Fig. 3). To evaluate σB - and PrfA-dependent transcription of different group III genes, we designed qRT-PCR primers and probes for four group III genes, including lmo0596, bsh, opuCA, and lmo0654 (Table 2). All four of these genes have upstream confirmed (bsh, opuCA) (Fraser et al., 2003; Kazmierczak et al., 2003) or putative σB-dependent promoters. bsh also has an upstream promoter with a PrfA box that was previously mapped by primer extension (Fig. 3) (Dussurget et al., 2002) and lmo0596 has an upstream PrfA box, which overlaps with the −35 region of the putative σB-dependent promoter (Fig. 3). Interestingly, there was no significant effect of the prfA deletion on transcript levels for any of the four group III genes tested (Table 4), indicating that transcription of these genes was PrfA independent under the conditions tested. In addition, while growth in the presence of activated charcoal led to a clear PrfA-dependent increase in transcript levels for the PrfA-regulated plcA (Suppl. Fig. S2), the tested group III genes did not show consistent patterns of higher transcript levels in cells grown in the presence of charcoal (Suppl. Fig. S3), consistent with the findings by Milohanic et al. (2003), who also found that the four group III genes tested here do not show PrfA-dependent transcript levels in EGD-e grown in the presence of charcoal. The PrfA boxes identified upstream of lmo0596 and bsh differ from the PrfA binding site consensus by 2 nt; in addition, putative −10 promoter sequences were 12 and 86 nt downstream from the PrfA binding sites for lmo0596 and bsh, respectively; closer and further, respectively, than the optimal 22–23 nt distance (Luo et al., 2005) between a PrfA box and the promoter's −10 region (Fig. 3). Similar to our results, microarray data reported by Marr et al. (2006) also did not find evidence of PrfA-mediated differential regulation for lmo0596 and bsh. Previous in vitro studies also reported PrfA-independent transcription of lmo0596 and bsh with no evidence for direct PrfA-dependent activation of these genes (Rauch et al., 2005). Overall, these data support that group III genes are weakly or inconsistently regulated by PrfA, as proposed by Scortti et al. (2007).
While the prfA deletion had no significant effects on transcript levels for group III genes, there was a significant effect of the sigB deletion on transcript levels for all four group III genes tested (Figs. 1 and 2, Table 4). This significant effect was observed for bacteria grown under all four conditions (log and stationary phase; with and without charcoal) (Suppl. Fig. S3); except for lmo0654 transcript levels, which were not significantly affected by the sigB deletion in cells grown in the presence of charcoal (Table 4). Interactions between the sigB and the prfA deletions did not have a significant effect on transcript levels for any of the four group III genes tested. While bsh and opuCA transcription has previously been reported to be σB-dependent (Fraser et al., 2003; Kazmierczak et al., 2003), lmo0596 and lmo0654 have not previously been reported as σB-dependent. Recent full genome microarray studies using only a parent and a ΔsigB strain have confirmed lmo0596 and lmo0654 as σB-dependent (Raengpradub et al., 2007). Among all 53 group III genes, a total of 33 were initially predicted by Milohanic et al. (2003) to be preceded by a σB-dependent promoter (either directly upstream or upstream of the first gene of an operon); recent Hidden Markov Model searches identified putative σB-dependent promoters upstream of an additional eight genes within this group (Raengpradub et al., 2007). Not only are the majority of group III genes preceded by putative (or confirmed, e.g., for opuCA, bsh) σB-dependent promoters, 50 of these 53 group III genes were also recently confirmed as positively regulated by σB in microarray experiments comparing transcript levels between the L. monocytogenes 10403S parent strain and an isogenic ΔsigB strain (Raengpradub et al., 2007). Our data provide specific evidence that the tested L. monocytogenes group III genes, which were previously reported to be part of the PrfA regulon, actually appear to be predominantly regulated by σB with weak or inconsistent contributions by PrfA. Although both Milohanic et al. (2003) and Marr et al. (2006) found considerable numbers of L. monocytogenes genes (i.e., 73 and 201, respectively) to be differentially regulated by PrfA, including genes that are up- or down-regulated by PrfA, only a few PrfA-regulated genes, representing predominantly the group I virulence genes, were identified by both groups. A number of PrfA-dependent genes, including many of those classified by Milohanic et al. (2003) into groups II and III, appear to be weakly or inconsistently regulated by PrfA (Scortti et al., 2007). On the other hand, σB appears to play a major and robust role in the regulation of the group III genes identified by Milohanic et al. (2003).
Conclusions
While interactions between σB and PrfA clearly are important for transcription of a number of virulence genes, as supported by the observations that σB regulates prfA transcription (Nadon et al., 2002) and that inlA and inlB transcription are affected by both σB and PrfA (McGann et al., 2007), σB and PrfA coregulation of selected group III genes were not confirmed under the conditions used in this study. Our data support that PrfA has a role in regulating transcription of specific virulence genes critical for intracellular survival, while σB broadly contributes to transcription of stress response as well as some virulence genes. Interactions between σB and PrfA in a regulatory network certainly appear to be critical for appropriate expression of virulence genes important for early stages of listerial infection (e.g., inlA, inlB). It is possible that additional regulatory interactions between these two proteins may be enhanced only under conditions found within the host. If this is true, these interactions may not be easily detectable under many of the laboratory growth conditions typically used to characterize L. monocytogenes stress response and virulence gene expression. Further studies designed to investigate physical interactions between σB and PrfA at targeted promoter sites will be necessary to obtain additional insight into specific interactions between these two regulatory proteins.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health Award No. R01-AI052151- 01A1 (to K.J.B.).
References
- Andre P. Bilger S. Remy P. Bettinger S. Vidon DJ. Effects of iron and oxygen species scavengers on Listeria spp. chemiluminescence. Biochem. Biophys. Res. Commun. 2003;304:807–811. doi: 10.1016/s0006-291x(03)00671-5. [DOI] [PubMed] [Google Scholar]
- Bishop DK. Hinrichs DJ. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- Bohne J. Sokolovic Z. Goebel W. Transcriptional regulation of prfA and PrfA-regulated virulence genes in Listeria monocytogenes. Mol. Microbiol. 1994;11:1141–1150. doi: 10.1111/j.1365-2958.1994.tb00390.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty T. Leimeister-Wachter M. Domann E. Hartl M. Goebel W. Nichterlein T. Notermans S. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J. Bacteriol. 1992;174:568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturongakul S. Boor KJ. σB activation under environmental and energy stress conditions in Listeria monocytogenes. Appl. Environ. Microbiol. 2006;72:5197–5203. doi: 10.1128/AEM.03058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussurget O. Cabanes D. Dehoux P. Lecuit M. Buchrieser C. Glaser P. Cossart P. Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol. Microbiol. 2002;45:1095–1106. doi: 10.1046/j.1365-2958.2002.03080.x. [DOI] [PubMed] [Google Scholar]
- Ermolaeva S. Novella S. Vega Y. Ripio MT. Scortti M. Vazquez-Boland JA. Negative control of Listeria monocytogenes virulence genes by a diffusible autorepressor. Mol. Microbiol. 2004;52:601–611. doi: 10.1111/j.1365-2958.2004.04003.x. [DOI] [PubMed] [Google Scholar]
- Ferreira A. O'Byrne CP. Boor KJ. Role of σB in heat, ethanol, acid, and oxidative stress resistance and during carbon starvation in Listeria monocytogenes. Appl. Environ. Microbiol. 2001;67:4454–4457. doi: 10.1128/AEM.67.10.4454-4457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser KR. Sue D. Wiedmann M. Boor K. O'Byrne CP. Role of σB in regulating the compatible solute uptake systems of Listeria monocytogenes: osmotic induction of opuC is σB dependent. Appl. Environ. Microbiol. 2003;69:2015–2022. doi: 10.1128/AEM.69.4.2015-2022.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag NE. Rong L. Portnoy DA. Regulation of the prfA transcriptional activator of Listeria monocytogenes: multiple promoter elements contribute to intracellular growth and cell-to-cell spread. Infect. Immun. 1993;61:2537–2544. doi: 10.1128/iai.61.6.2537-2544.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag NE. Youngman P. Portnoy DA. Transcriptional activation of the Listeria monocytogenes hemolysin gene in Bacillus subtilis. J. Bacteriol. 1992;174:1293–1298. doi: 10.1128/jb.174.4.1293-1298.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner MR. Njaa BL. Wiedmann M. Boor KJ. σB contributes to Listeria monocytogenes gastrointestinal infection but not to systemic spread in the guinea pig infection model. Infect. Immun. 2006;74:876–886. doi: 10.1128/IAI.74.2.876-886.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellin BG. Broome CV. Listeriosis. JAMA. 1989;261:1313–1320. [PubMed] [Google Scholar]
- Geoffroy C. Gaillard JL. Alouf JE. Berche P. Production of thiol-dependent haemolysins by Listeria monocytogenes and related species. J. Gen. Microbiol. 1989;135:481–487. doi: 10.1099/00221287-135-3-481. [DOI] [PubMed] [Google Scholar]
- Glaser P. Frangeul L. Buchrieser C. Rusniok C. Amend A. Baquero F. Berche P. Bloecker H. Brandt P. Chakraborty T. Charbit A. Chetouani F. Couve E. de Daruvar A. Dehoux P. Domann E. Dominguez-Bernal G. Duchaud E. Durant L. Dussurget O. Entian KD. Fsihi H. Garcia-del Portillo F. Garrido P. Gautier L. Goebel W. Gomez-Lopez N. Hain T. Hauf J. Jackson D. Jones LM. Kaerst U. Kreft J. Kuhn M. Kunst F. Kurapkat G. Madueno E. Maitournam A. Vicente JM. Ng E. Nedjari H. Nordsiek G. Novella S. de Pablos B. Perez-Diaz JC. Purcell R. Remmel B. Rose M. Schlueter T. Simoes N. Tierrez A. Vazquez-Boland JA. Voss H. Wehland J. Cossart P. Comparative genomics of Listeria species. Science. 2001;294:849–852. doi: 10.1126/science.1063447. [DOI] [PubMed] [Google Scholar]
- Gray MJ. Freitag NE. Boor KJ. How the bacterial pathogen Listeria monocytogenes mediates the switch from environmental Dr. Jekyll to pathogenic Mr. Hyde. Infect. Immun. 2006;74:2505–2512. doi: 10.1128/IAI.74.5.2505-2512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M. Völker U. General stress response of Bacillus subtilis and other bacteria. Adv. Microb. Physiol. 2001;44:35–91. doi: 10.1016/s0065-2911(01)44011-2. [DOI] [PubMed] [Google Scholar]
- Ireton K. Payrastre B. Cossart P. The Listeria monocytogenes protein InlB is an agonist of mammalian phosphoinositide 3-kinase. J. Biol. Chem. 1999;274:17025–17032. doi: 10.1074/jbc.274.24.17025. [DOI] [PubMed] [Google Scholar]
- Kazmierczak MJ. Mithoe SC. Boor KJ. Wiedmann M. Listeria monocytogenes σB regulates stress response and virulence functions. J. Bacteriol. 2003;185:5722–5734. doi: 10.1128/JB.185.19.5722-5734.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak MJ. Wiedmann M. Boor KJ. Contributions of Listeria monocytogenes σB and PrfA to expression of virulence and stress response genes during extra- and intracellular growth. Microbiology. 2006;152:1827–1838. doi: 10.1099/mic.0.28758-0. [DOI] [PubMed] [Google Scholar]
- Kim H. Boor KJ. Marquis H. Listeria monocytogenes σB contributes to invasion of human intestinal epithelial cells. Infect. Immun. 2004;72:7374–7378. doi: 10.1128/IAI.72.12.7374-7378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Marquis H. Boor KJ. σB contributes to Listeria monocytogenes invasion by controlling expression of inlA and inlB. Microbiology. 2005;151:3215–3222. doi: 10.1099/mic.0.28070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft J. Vazquez-Boland JA. Regulation of virulence genes in Listeria. Int. J. Med. Microbiol. 2001;291:145–157. doi: 10.1078/1438-4221-00111. [DOI] [PubMed] [Google Scholar]
- Lalic-Multhaler M. Bohne J. Goebel W. In vitro transcription of PrfA-dependent and -independent genes of Listeria monocytogenes. Mol. Microbiol. 2001;42:111–120. doi: 10.1046/j.1365-2958.2001.02607.x. [DOI] [PubMed] [Google Scholar]
- Lampidis R. Gross R. Sokolovic Z. Goebel W. Kreft J. The virulence regulator protein of Listeria ivanovii is highly homologous to PrfA from Listeria monocytogenes and both belong to the Crp-Fnr family of transcription regulators. Mol. Microbiol. 1994;13:141–151. doi: 10.1111/j.1365-2958.1994.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Leimeister-Wächter M. Haffner C. Domann E. Goebel W. Chakraborty T. Identification of a gene that positively regulates expression of listeriolysin, the major virulence factor of Listeria monocytogenes. Proc. Natl. Acad. Sci. U S A. 1990;87:8336–8340. doi: 10.1073/pnas.87.21.8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q. Herler M. Müller-Altrock S. Goebel W. Supportive and inhibitory elements of a putative PrfA-dependent promoter in Listeria monocytogenes. Mol. Microbiol. 2005;55:986–997. doi: 10.1111/j.1365-2958.2005.04417.x. [DOI] [PubMed] [Google Scholar]
- Luo Q. Rauch M. Marr AK. Müller-Altrock S. Goebel W. In vitro transcription of the Listeria monocytogenes virulence genes inlC and mpl reveals overlapping PrfA-dependent and -independent promoters that are differentially activated by GTP. Mol. Microbiol. 2004;52:39–52. doi: 10.1111/j.1365-2958.2003.03960.x. [DOI] [PubMed] [Google Scholar]
- Marr AK. Joseph B. Mertins S. Ecke R. Müller-Altrock S. Goebel W. Overexpression of PrfA leads to growth inhibition of Listeria monocytogenes in glucose-containing culture media by interfering with glucose uptake. J. Bacteriol. 2006;188:3887–3901. doi: 10.1128/JB.01978-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann P. Wiedmann M. Boor KJ. The alternative sigma factor σB and the virulence gene regulator PrfA both regulate transcription of Listeria monocytogenes internalins. Appl. Environ. Microbiol. 2007;73:2919–2930. doi: 10.1128/AEM.02664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead PS. Slutsker L. Dietz V. McCaig LF. Bresee JS. Shapiro C. Griffin PM. Tauxe RV. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengaud J. Dramsi S. Gouin E. Vazquez-Boland JA. Milon G. Cossart P. Pleiotropic control of Listeria monocytogenes virulence factors by a gene that is auto-regulated. Mol. Microbiol. 1991;5:2273–2283. doi: 10.1111/j.1365-2958.1991.tb02158.x. [DOI] [PubMed] [Google Scholar]
- Mengaud J. Vicente MF. Cossart P. Transcriptional mapping and nucleotide sequence of the Listeria monocytogenes hlyA region reveal structural features that may be involved in regulation. Infect. Immun. 1989;57:3695–3701. doi: 10.1128/iai.57.12.3695-3701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milohanic E. Glaser P. Coppee JY. Frangeul L. Vega Y. Vazquez-Boland JA. Kunst F. Cossart P. Buchrieser C. Transcriptome analysis of Listeria monocytogenes identifies three groups of genes differently regulated by PrfA. Mol. Microbiol. 2003;47:1613–1625. doi: 10.1046/j.1365-2958.2003.03413.x. [DOI] [PubMed] [Google Scholar]
- Nadon CA. Bowen BM. Wiedmann M. Boor KJ. σB contributes to PrfA-mediated virulence in Listeria monocytogenes. Infect. Immun. 2002;70:3948–3952. doi: 10.1128/IAI.70.7.3948-3952.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy DA. Chakraborty T. Goebel W. Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect. Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raengpradub S. Wiedmann M. Boor KJ. Comparative analysis of the σB -dependent stress response in Listeria monocytogenes and Listeria innocua exposed to selected stress conditions. Appl. Environ. Microbiol. 2007. Nov 16; [Epub ahead of print] doi:10.1128/AEM.00951–07. [DOI] [PMC free article] [PubMed]
- Rauch M. Luo Q. Müller-Altrock S. Goebel W. σB-dependent in vitro transcription of prfA and some newly identified genes of Listeria monocytogenes whose expression is affected by PrfA in vivo. J. Bacteriol. 2005;187:800–804. doi: 10.1128/JB.187.2.800-804.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzoni A. Klarsfeld A. Dramsi S. Cossart P. Evidence that PrfA, the pleiotropic activator of virulence genes in Listeria monocytogenes, can be present but inactive. Infect. Immun. 1997;65:1515–1518. doi: 10.1128/iai.65.4.1515-1518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripio MT. Dominguez-Bernal G. Suarez M. Brehm K. Berche P. Vazquez-Boland JA. Transcriptional activation of virulence genes in wild-type strains of Listeria monocytogenes in response to a change in the extracellular medium composition. Res. Microbiol. 1996;147:371–384. doi: 10.1016/0923-2508(96)84712-7. [DOI] [PubMed] [Google Scholar]
- Roberts AJ. Wiedmann M. Pathogen, host and environmental factors contributing to the pathogenesis of listeriosis. Cell Mol. Life Sci. 2003;60:904–918. doi: 10.1007/s00018-003-2225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab U. Bowen B. Nadon C. Wiedmann M. Boor KJ. The Listeria monocytogenes prfAP2 promoter is regulated by σB in a growth phase dependent manner. FEMS Microbiol. Lett. 2005;245:329–336. doi: 10.1016/j.femsle.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Scortti M. Monzó HJ. Lacharme-Lora L. Lewis DA. Vazquez-Boland JA. The PrfA virulence regulon. Microbes Infect. 2007;9:1196–1207. doi: 10.1016/j.micinf.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Sheehan B. Klarsfeld A. Ebright R. Cossart P. A single substitution in the putative helix-turn-helix motif of the pleiotropic activator PrfA attenuates Listeria monocytogenes virulence. Mol. Microbiol. 1996;20:785–797. doi: 10.1111/j.1365-2958.1996.tb02517.x. [DOI] [PubMed] [Google Scholar]
- Sheehan B. Klarsfeld A. Msadek T. Cossart P. Differential activation of virulence gene expression by PrfA, the Listeria monocytogenes virulence regulator. J. Bacteriol. 1995;177:6469–6476. doi: 10.1128/jb.177.22.6469-6476.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sue D. Boor KJ. Wiedmann M. σB-dependent expression patterns of compatible solute transporter genes opuCA and lmo1421 and the conjugated bile salt hydrolase gene bsh in Listeria monocytogenes. Microbiology. 2003;149:3247–3256. doi: 10.1099/mic.0.26526-0. [DOI] [PubMed] [Google Scholar]
- Sue D. Fink D. Wiedmann M. Boor KJ. σB-dependent gene induction and expression in Listeria monocytogenes during osmotic and acid stress conditions simulating the intestinal environment. Microbiology. 2004;150:3843–3855. doi: 10.1099/mic.0.27257-0. [DOI] [PubMed] [Google Scholar]
- Vazquez-Boland JA. Kocks C. Dramsi S. Ohayon H. Geoffroy C. Mengaud J. Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect. Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Boland JA. Kuhn M. Berche P. Chakraborty T. Dominguez-Bernal G. Goebel W. Gonzalez-Zorn B. Wehland J. Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann M. Arvik TJ. Hurley RJ. Boor KJ. General stress transcription factor σB and its role in acid tolerance and virulence of Listeria monocytogenes. J. Bacteriol. 1998;180:3650–3656. doi: 10.1128/jb.180.14.3650-3656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KK. Freitag NE. A novel mutation within the central Listeria monocytogenes regulator PrfA that results in constitutive expression of virulence gene products. J. Bacteriol. 2004;186:6265–6276. doi: 10.1128/JB.186.18.6265-6276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.