Abstract
Background
Mild hand tremor occurs in most normal adults. There are no surveys of the prevalence or clinical correlates of such tremor among children.
Methods
A cross-sectional study of tics, tremor and other neurological disorders was conducted in Spanish children; thus, 819 schoolchildren in Burgos, Spain, drew Archimedes spirals with each hand. Tremor in spirals was rated (0–2) by a blinded neurologist and an overall tremor rating (0–4) was assigned.
Results
The mean age was 10.9 ± 3.1 years. A tremor rating of 1 (mild tremor) was present in either hand in 424 (51.7%) children, and in both hands in 88 (10.7%) children. Higher tremor ratings were very uncommon. The overall tremor rating was higher in boys than girls (1.31 ± 0.41 vs. 1.22 ± 0.34, p = 0.002) and correlated weakly yet significantly with age (ρ = 0.09, p = 0.01). Within subjects, the left hand spiral rating was greater than the right (p < 0.001).
Conclusions
In this cross-sectional study of 819 Spanish schoolchildren, mild tremor was commonly observed. As in adults, males had more tremor than females, tremor scores increased with age, and tremor scores were higher in the left than right arm, demonstrating that these clinical correlations seem to be more broadly generalizable to children. The functional significance of tremor in children, particularly as it relates to handwriting proficiency, deserves additional scrutiny.
Copyright © 2011 S. Karger AG, Basel
Key Words: Tremor, children; Tremor, gender and age; Hand tremor; Essential tremor; Schoolchildren, cross-sectional study
Introduction
Essential tremor (ET) is highly prevalent in human populations [1,2,3,4,5]. Milder action tremor of the hands is also widespread and is far more common than ET [6]. These kinds of tremor have mainly been written about as maladies of the elderly [7]. Yet tremors may occur in children as well. ET has been reported in children of all ages [8]. By contrast, the extent to which children exhibit mild action tremor is not really known. There have been no surveys of the prevalence of mild action tremor in children. A cross-sectional study of tics and other neurological disorders was conducted in Spanish children [9], and we used this opportunity to study tremor in more than 800 randomly selected schoolchildren in the community of Burgos, Spain. Each child drew spirals with each hand, and tremor was systematically quantified using a clinical rating scale.
Methods
Study Sample
Participants were Spanish schoolchildren enrolled between March 2007 and December 2009 in a cross-sectional study of neurological disorders. The study was conducted by investigators in the Department of Neurology, General Yagüe Hospital, Burgos, Spain. Although the neurological disorder of major interest was tics, tremor was briefly but systematically assessed as well.
At the start of the project, investigators received a computerized roster from the Burgos school district of all 28,706 students in primary and secondary education (aged 6–16 years). Using a random digit table, four of 161 mainstream (i.e. not special education) schools were selected to participate. Each of the four mainstream schools included elementary school students (grades 1–6); two also included students in middle school and high school.
The study protocol was approved by the ethical review board of the Burgos Hospital and the School Government District (Consejeria de Educación de Castilla y León). Written consent from a parent/guardian was obtained in all participants.
819 (89.8%) of 912 students consented and participated; 93 did not. The 819 participants were similar to the 93 non-participants in gender (482 (58.9%) vs. 48 (51.6%) boys, χ2 = 1.80, p = 0.18) but were on average 0.7 years older (10.9 ± 3.1 vs. 10.2 ± 2.7 years, t = 2.09, p = 0.04).
Study Assessment
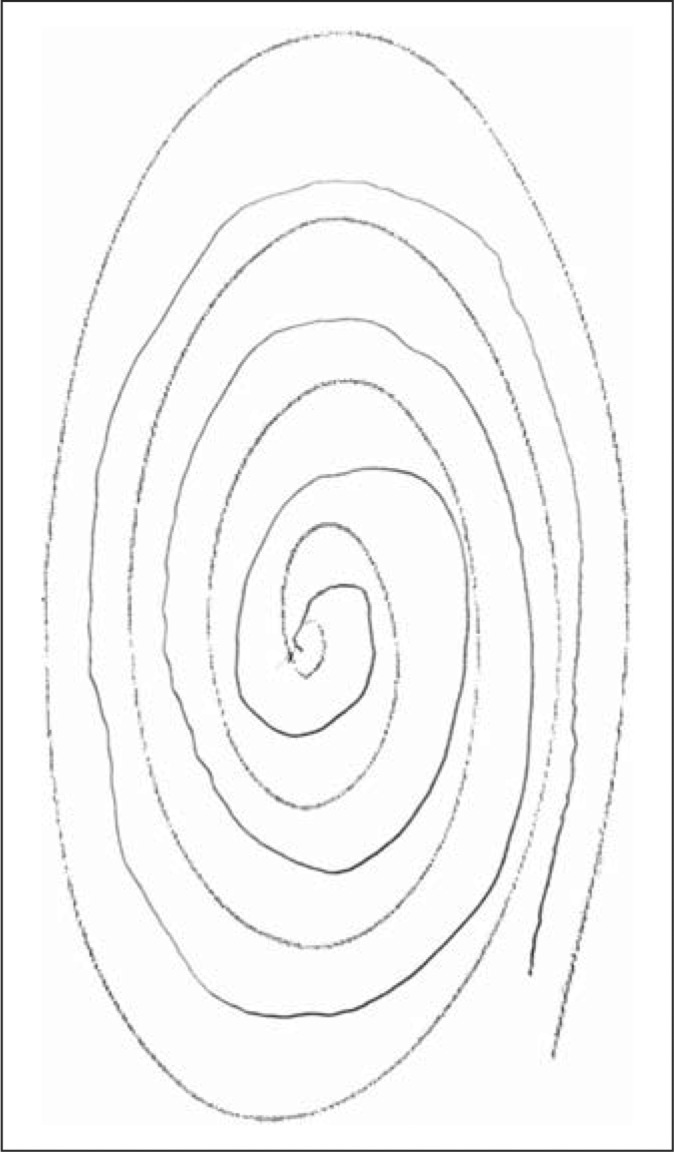
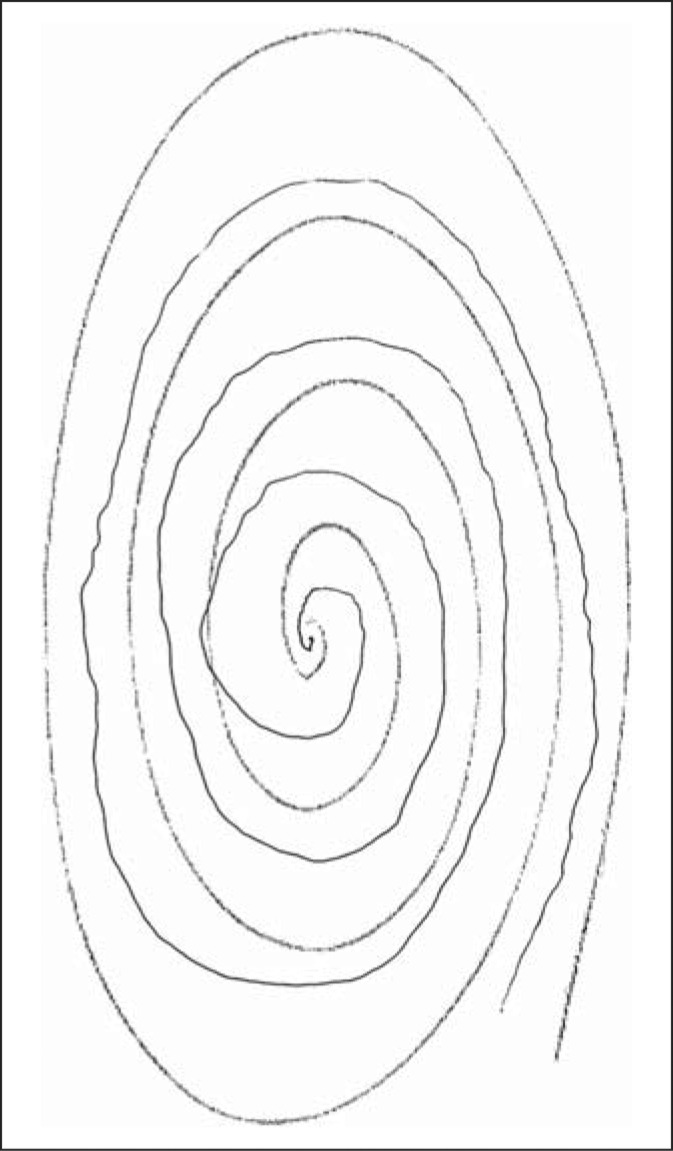
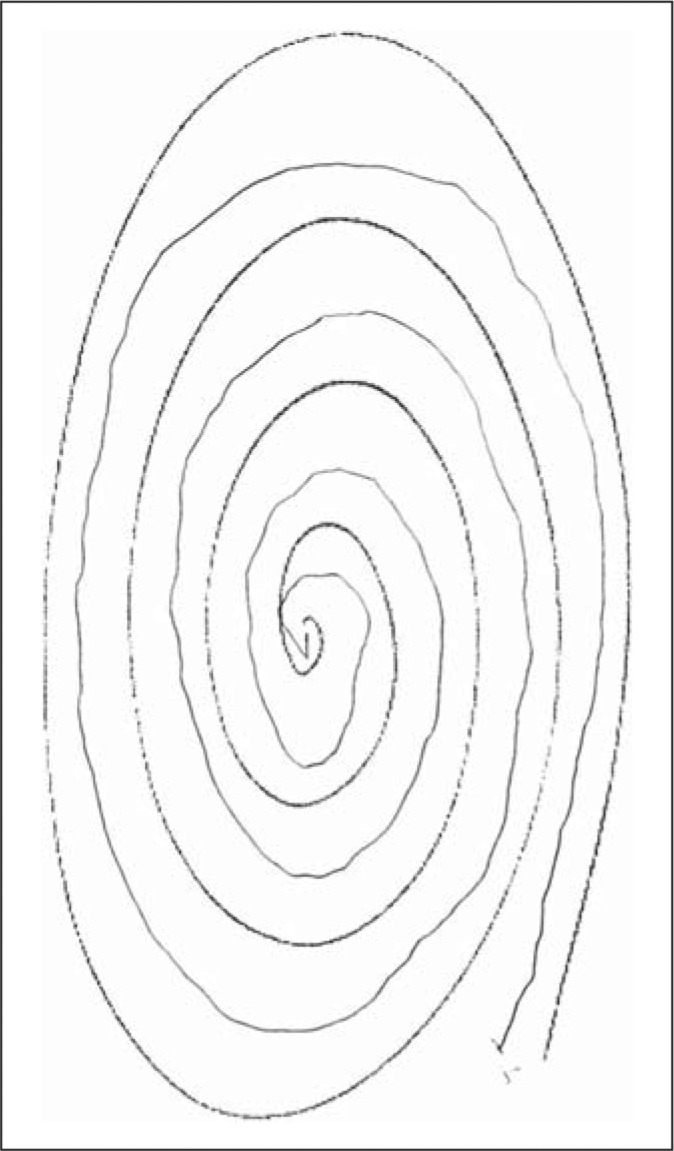
Each participating child underwent a basic assessment that included demographic data (age, gender), a standardized tic screen [9], and two spiral drawings. Thus, each participant was asked to draw two spirals, as described previously [10]. In doing so, each enrolled participant was asked to first use their right hand to draw an Archimedes spiral. Spirals were drawn on a standard 8.5 × 11 inch sheet of paper using a pen or pencil while the participant was seated at a table. The paper was centered at right angles directly in front of them and held down by their other hand. The drawing hand was not allowed to rest or be supported when the spiral was being drawn. Participants started at the center of the page, without lifting their pen/pencil. This was repeated with the left hand, yielding two spirals [10]. In contrast to the prior study [10], spirals were not drawn free-hand on a blank sheet of paper, but rather, were drawn in between the lines of a standardized, pre-drawn, photocopied, spiral (fig. 1, 2, 3, 4).
Fig. 1.
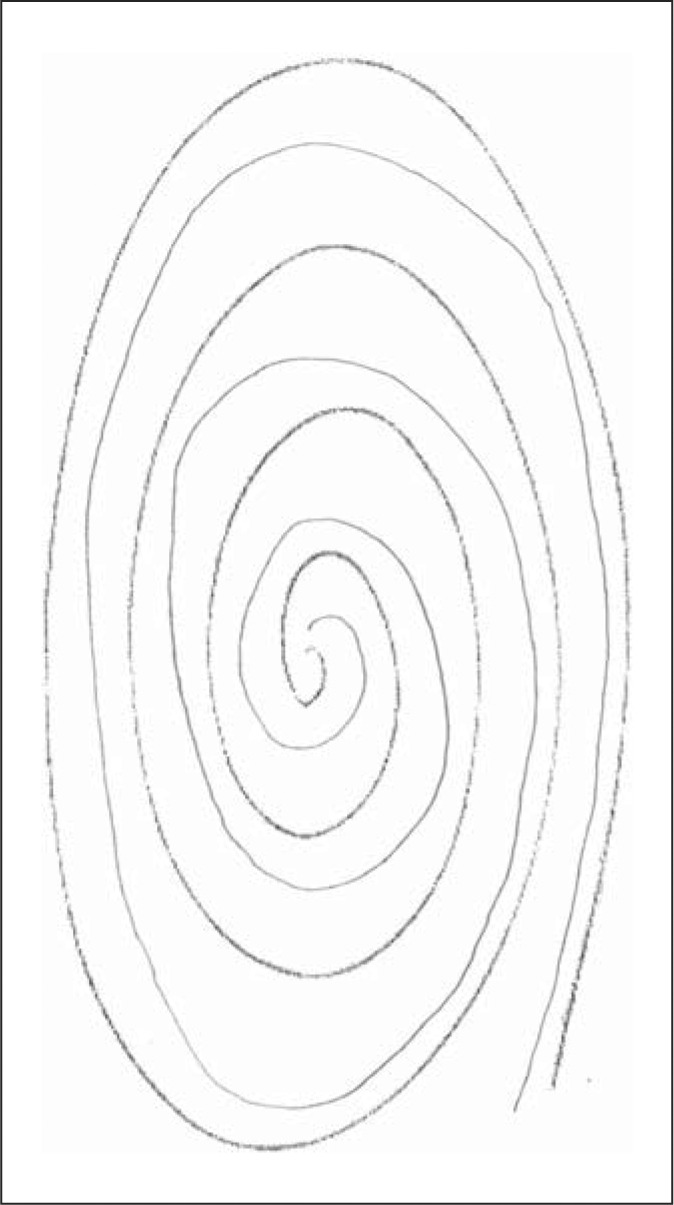
The patient drew the spiral (lighter lines) in between the lines of a standardized, pre-drawn, photocopied spiral (darker lines). The patient's spiral received a rating of 0.
Fig. 2.
This spiral received a rating of 0.5.
Fig. 3.
This spiral received a rating of 1.
Fig. 4.
This spiral received a rating of 1.5. Areas of tremor (i.e. oscillations) are interspersed with areas of sloppiness and spatial errors.
Tremor in these spirals was later rated by a neurologist specializing in movement disorders (E.D.L.) who was blinded to clinical information. Tremor ratings for each spiral were: 0 (no tremor), 0.5 (subtle, low amplitude oscillations are present in a few spots but are not consistently present throughout the spiral), 1.0 (low amplitude oscillations are present in multiple places), 1.5 (low amplitude oscillations are present in multiple places and oscillations can at times reach moderate amplitude), 2 (moderate amplitude oscillations present throughout the spiral) (see examples of drawings assigned ratings of 0, 0.5, 1, 1.5 and 2 in a prior population-based study [11] and in the current study (fig. 1, 2, 3, 4)). On both spirals, the neurologist was careful to distinguish clear, regular, oscillations from sloppiness, spatial errors, and other irregularities or movement dysfluencies (present in many spirals from younger children) that were not strictly oscillatory. The overall tremor rating (range 0–4) was the sum of the right (0–2) and left (0–2) tremor ratings for each participant.
As the focus of the main study was tics, each child who screened positive for tics (n = 179) and an approximately equal number of gender-matched controls (n = 145) also underwent a supplementary evaluation, including additional medical history (a more detailed assessment of tics, history of premature delivery, birth weight, history of perinatal problems, history of epilepsy), and current usage of all medications including oral medication and inhalers. Given the age of the study participants, coffee, cigarettes and ethanol exposures were not assessed. This supplementary evaluation thereby provided us a subsample of children among whom we could assess the possible confounding effects of other medical conditions and use of medications on their tremor.
Statistical Analyses
Age and tremor ratings were not normally distributed (Kolmogorov-Smirnov p < 0.001 for each); therefore, non-parametric tests (Mann-Whitney, Spearman's ρ) were used when assessing these variables. The one exception was the within-subject comparison of right versus left spiral scores, for which a paired sample t test was used. A small number of schoolchildren (n = 2, 0.2%) had had to repeat grades and these 2 were >18 years old (i.e. 19 and 21). Sub-analyses, in which these 2 participants were excluded, did not differ from those in which they were included. Pen versus pencil use did not influence the assigned tremor ratings; hence, data were not presented separately based on choice of writing implement. As hand dominance was assessed in only 257 of 819 children, data were presented in the form of right and left hand rather than dominant and non-dominant hand. In one sub-analysis of the 257 children with known hand dominance, we presented data stratified by handedness. All statistical analyses were performed by E.D.L.
Results
There were 819 children (mean ± SD (median) age 10.9 ± 3.1 (10) years, range 5–21), including 482 (58.9%) boys and 337 (41.1%) girls. Boys (10.9 ± 3.0 (10) years) and girls (10.9 ± 3.1 (10) years) did not differ by age (p = 0.73).
A tremor rating of 1 (mild but consistent tremor; fig. 3) was present in either hand in 424 (51.7%) children, in the right hand in 137 (16.7%) children, in the left hand in 287 (35.0%) children, and in both hands in 88 (10.7%) children. A higher rating (1.5, mild to moderate tremor) was present in either hand in 17 (2.1%) children, in the right hand in 6 (0.7%) children, in the left hand in 11 (1.3%) children, and in both hands in 2 (0.2%) children.
The overall tremor rating was the sum of the right (0–2) and left (0–2) tremor ratings for each participant. The overall tremor rating was 1.28 ± 0.39 (1). Boys had higher right hand tremor ratings than girls (0.61 ± 0.22 (0.5) vs. 0.56 ± 0.18 (0.5), p < 0.001) and marginally higher left hand tremor ratings than girls (0.70 ± 0.27 (0.5) vs. 0.67 ± 0.24 (0.5), p = 0.06), and the overall tremor rating was higher in boys than girls (1.31 ± 0.41 (1) vs. 1.22 ± 0.34 (1), p = 0.002). The distribution of the overall tremor rating is shown for boys vs. girls (table 1); the two distributions differed (χ2 test = 11.42, p = 0.04).
Table 1.
Overall tremor ratings in boys versus girls
| Overall tremor rating | Boys (n = 482) | Girls (n = 337) |
|---|---|---|
| 0 | 0 (0.0) | 0 (0.0) |
| 0.5 | 3 (0.6) | 3 (0.9) |
| 1 | 260 (53.9) | 213 (63.2) |
| 1.5 | 146 (30.3) | 91 (27.0) |
| 2 | 62 (12.9) | 28 (8.3) |
| 2.5 | 9 (1.9) | 2 (0.6) |
| 3 | 2 (0.4) | 0 (0.0) |
| 3.5 | 0 (0.0) | 0 (0.0) |
| 4 | 0 (0.0) | 0 (0.0) |
Overall tremor rating may range from 0 to 4 and was the sum of the right (0–2) and left (0–2) tremor ratings for each participant. Values in parentheses are column percentages, χ2 test = 11.42, p = 0.04.
The overall tremor rating correlated weakly but significantly with age (ρ = 0.09, p = 0.01). Participants were stratified based on the median age of the entire sample (≤10 vs. >10 years) into a younger (8.4 ± 1.2 (9) years) vs. older (13.5 ± 2.1 (13) years) age group; overall spiral rating was higher in the older children than younger children (1.33 ± 0.42 (1) vs. 1.22 ± 0.34 (1), p < 0.001).
Within subjects, the left hand spiral rating was greater than the right (0.69 ± 0.26 (0.5) vs. 0.59 ± 0.21 (0.5), p < 0.001). The left hand spiral rating was greater than the right hand spiral rating in 210 (25.6%) participants versus only 46 (5.6%) participants in whom the right was greater than the left; the right and left were equal in 563 (68.7%).
In 257 children, hand dominance was known. In the 234 right hand children, left hand spiral rating was greater than the right (0.73 ± 0.27 (0.5) vs. 0.59 ± 0.21 (0.5), p < 0.001). In the 16 left-handed children, the tremor in the two hands was equal (0.72 ± 0.26 (0.5) vs. 0.72 ± 0.26 (0.5), p = 0.38). Seven remaining children were ambidextrous.
Each child who screened positive for tics (n = 179) and an approximately equal number of gender-matched controls (n = 145) also underwent a supplementary evaluation. The overall tremor rating in these 324 children (1.28 ± 0.39 (1)) was similar to that of the larger group of 819. The overall tremor rating did not differ with regards to any of the additional clinical variables, i.e. presence of tics (present in 102 children or 31.5% of a subsample of 324), history of premature delivery (26 children), history of perinatal problems (15 children), history of epilepsy (4 children), usage of medications (21 children), and birth weight (data for these six comparisons not shown, but all p > 0.05), indicating that these factors were not likely to have been the underlying cause of the tremor.
Discussion
Mild action tremor is a widespread human condition, yet the focus thus far has almost exclusively been on the adult population. Curiously, there have been virtually no surveys of the prevalence, features or correlates of mild action tremor in children. There was one accelerometric study of tremor in 287 British children aged 2–16 years; the study focused on the effects of age on tremor frequency [12]. Tremor amplitude was not assessed nor were gender differences [12]. Only the right hand was assessed, therefore side-side differences could not be assessed [12]. In 2000, nearly 1 in 5 (i.e. 18.9%) of all people in the United States were aged 6–17 years, making the health of this age group, which also forms the core of the future adult population, of considerable importance.
We present data on tremor in 819 boys and girls attending school in Burgos, Spain. Higher tremor rating (1.5, mild to moderate tremor) was present in both hands in few children (2, 0.2%). However, milder tremor (rating of 1) was present in both hands in approximately 1 in 10 children (i.e. 88 or 10.7%), indicating that there is some tremor in this age group. It is important to note that the mild tremor that was observed in most children was likely to be normal or enhanced physiological tremor rather than a pathological condition such as ET. Physiological tremor of the hands has been extensively studied and two main mechanisms have been shown to drive this tremor: a mechanical resonance of the oscillating limb (i.e. motor units firing coincidentally at a resonant frequency, causing the arm to oscillate) and a central drive originating from hypothesized oscillators within the central nervous system [13].
What might be the functional significance of mild tremor in children? Ours was the broad survey of a large sample of children and was not designed to evaluate functional correlates. Yet a study of 48 normal children (aged 7.6–11.0 years) in elementary schools in the Netherlands showed that tremor is a major distinguishing feature between poor writers and their counterparts who are proficient [14]. The authors concluded that tremor in children is an unwanted movement component that contributes to inaccuracies and greater functional difficulty [14]. To our knowledge, there are no other published data. Further studies of the functional correlates of tremor in children seem warranted, however tremor may be an additional feature of an immature and developing motor-coordination/cerebellar system.
The tremor scores reported in this study of normal children are higher than some scores previously reported in normal adults. Previously, tremor on spirals was rated in 2,524 adults aged 18–60 years in Bangladesh using a similar rating scale, however the results are difficult to compare because the Bangladeshi spirals were drawn free-hand whereas the children in this study were asked to draw within the careful confines of a pre-drawn spiral. Previous work has shown that spirals drawn between the lines tend to be more severe than those that are drawn free-hand [15]. Having rigidly fixed task goals places more pressure on the subject, which worsens their tremor [15]. Also, tremor on spirals was previously rated in a study of 273 normal adults in New York [16] but these results are difficult to compare again because spirals were drawn free-hand and furthermore, a rating of 0.5 was not used in that study, which likely resulted in lower tremor scores in that study. The other possibility, alluded to above, is that tremor may indeed be more common in children than previously suspected and this could be an additional feature of an immature motor-coordination/cerebellar system.
Boys had higher tremor scores than girls. There are no other data in children but there are similar data in adults. A clinical study of 273 normal adults in New York (age 65.7 ± 11.5 years, range 18–92) similarly reported that tremor scores in males were greater than those in females [16]. An accelerometric study of 117 normal German adults aged 20–94 noted a trend for tremor amplitude to be higher in males than females but this trend did not reach statistical significance [13]. The explanation for this gender difference is not apparent, although one proposed explanation is that hand volume could play a role in tremor mechanics [13].
Aside from gender differences, we also examined other clinical correlates of hand tremor in children. We found that tremor scores in the left hand were higher than those in the right (usually dominant) hand. A study of 273 normal adults in New York similarly reported that tremor scores in the left hand were higher than those in the right hand [16]. Motor control, in general, is superior in the dominant than non-dominant arm, and this is one possible explanation.
We also reported a mild increase in tremor scores with age among these children. Tremor scores seem to increase across the age spectrum. A study of 2,524 normal adults, aged 18–60, reported a weak but significant correlation between age and higher tremor scores [17]. Similarly, studies in the elderly continue to show mild increases in tremor scores as age progresses [18]. That the increase with age is apparent even in children seems to indicate this is a phenomenon of aging rather than simply one of advanced aging or senescence. The biological basis is unclear.
This study had limitations. First, we recognize that tremor was assessed using a clinical scale rather than accelerometry. Unfortunately, accelerometry is not feasible as a large-scale, epidemiological, screening tool, as was required here. Second, while we were able to assess medication use and several other factors, we recognize that we were unable to assess all causes of tremor in these children. Thus, caffeinated soda and exposure to toxins (e.g. lead) could have acted as antecedent variables, resulting in the tremor we observed, and we cannot assess the role these could have played here. Nevertheless, this issue relates to the underlying cause of the tremor rather than the empiric observation that tremor was present. Third, we realize that our assessment was limited to two handwriting samples. What is the sensitivity of this method? Our data in other studies [6] indicated that spiral drawings are a reasonably sensitive measure of tremor, as 97.0% of individuals with mild or greater tremor on a more detailed tremor examination exhibited tremor ratings ≥0.5 in one or more hands during spiral drawing. Fourth, the study was cross-sectional rather than longitudinal; additional follow-up studies are needed. Finally, to precisely evaluate tremor, the rater assigned ratings of 0.5 to distinguish children with subtle tremor from those with no detectible tremor. These were empiric observations; hence, we do not think the added precision falsely elevated tremor ratings. The study also had considerable strengths. This is the only clinical study that we are aware of to assess tremor in a large group of school-aged children and to furthermore examine the correlates of that tremor.
In this cross-sectional study of 819 Spanish schoolchildren, mild tremor was observed in both hands in approximately 1 in 10 children, indicating that it was not rare. More marked tremor was rare. Interestingly, as in adults, males had more tremor than females, tremor scores increased with age, and tremor scores were higher in the left than right arm, demonstrating that these clinical correlations seem to be more broadly generalizable to children. The functional significance of tremor in children, particularly as it relates to handwriting proficiency, deserves additional scrutiny.
Disclosure Statement
The authors declare that there are no conflicts of interest and no competing financial interests.
Acknowledgements
This research was supported by National Institutes of Health Grants R01 NS39422 and Sanidad Castilla y León (SACYL), Biomedicine Project GRS 157-A, Health Research Grant PI 070846, and European General Development Cofunding.
References
- 1.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:435–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 2.Benito-Leon J. Essential tremor: from a monosymptomatic disorder to a more complex entity. Neuroepidemiology. 2008;31:191–192. doi: 10.1159/000154933. [DOI] [PubMed] [Google Scholar]
- 3.Bermejo-Pareja F, Louis ED, Benito-Leon J. Risk of incident dementia in essential tremor: a population-based study. Mov Disord. 2007;22:1573–1580. doi: 10.1002/mds.21553. [DOI] [PubMed] [Google Scholar]
- 4.Dogu O, Sevim S, Camdeviren H, Sasmaz T, Bugdayci R, Aral M, Kaleagasi H, Un S, Louis ED. Prevalence of essential tremor: door-to-door neurologic exams in Mersin Province, Turkey. Neurology. 2003;61:1804–1806. doi: 10.1212/01.wnl.0000099075.19951.8c. [DOI] [PubMed] [Google Scholar]
- 5.Dogu O, Louis ED, Sevim S, Kaleagasi H, Aral M. Clinical characteristics of essential tremor in Mersin, Turkey – a population-based door-to-door study. J Neurol. 2005;252:570–574. doi: 10.1007/s00415-005-0700-8. [DOI] [PubMed] [Google Scholar]
- 6.Louis ED, Ford B, Pullman S, Baron K. How normal is ‘normal’? Mild tremor in a multiethnic cohort of normal subjects. Arch Neurol. 1998;55:222–227. doi: 10.1001/archneur.55.2.222. [DOI] [PubMed] [Google Scholar]
- 7.Benito-Leon J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of Central Spain. Mov Disord. 2003;18:389–394. doi: 10.1002/mds.10376. [DOI] [PubMed] [Google Scholar]
- 8.Louis ED, Dure L, Pullman S. Essential tremor in childhood: a series of nineteen cases. Mov Disord. 2001;16:921–923. doi: 10.1002/mds.1182. [DOI] [PubMed] [Google Scholar]
- 9.Cubo E, Saez Velasco S, Delgado Benito V, Ausin Villaverde V, Maria Trejo Gabriel YGJ, Martin Santidrian A, Macarron Vicente J, Cordero Guevara J, Louis ED, Benito-Leon J. Validation of screening instruments for neuroepidemiological surveys of tic disorders. Mov Disord. 2011;26:520–526. doi: 10.1002/mds.23460. [DOI] [PubMed] [Google Scholar]
- 10.Hafeman D, Ahsan H, Islam T, Louis E. Betel quid: its tremor-producing effects in residents of Araihazar, Bangladesh. Mov Disord. 2006;21:567–571. doi: 10.1002/mds.20754. [DOI] [PubMed] [Google Scholar]
- 11.Louis ED, Thawani SP, Andrews HF. Prevalence of essential tremor in a multiethnic, community-based study in Northern Manhattan, New York, N.Y. Neuroepidemiology. 2009;32:208–214. doi: 10.1159/000195691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall J. Physiological tremor in children. J Neurol Neurosurg Psychiatry. 1959;22:33–35. doi: 10.1136/jnnp.22.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raethjen J, Pawlas F, Lindemann M, Wenzelburger R, Deuschl G. Determinants of physiologic tremor in a large normal population. Clin Neurophysiol. 2000;111:1825–1837. doi: 10.1016/s1388-2457(00)00384-9. [DOI] [PubMed] [Google Scholar]
- 14.Smits-Engelsman BC, Van Galen GP. Dysgraphia in children: Lasting psychomotor deficiency or transient developmental delay? J Exp Child Psychol. 1997;67:164–184. doi: 10.1006/jecp.1997.2400. [DOI] [PubMed] [Google Scholar]
- 15.Ondo WG, Wang A, Thomas M, Vuong KD. Evaluating factors that can influence spirography ratings in patients with essential tremor. Parkinsonism Relat Disord. 2005;11:45–48. doi: 10.1016/j.parkreldis.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Louis ED. Kinetic tremor: differences between smokers and non-smokers. Neurotoxicology. 2007;28:569–575. doi: 10.1016/j.neuro.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louis ED, Hafeman D, Parvez F, Liu X, Alcalay RN, Islam T, Ahmed A, Siddique AB, Patwary TI, Melkonian S, Argos M, Levy D, Ahsan H. Tremor severity and age: a cross-sectional, population-based study of 2,524 young and midlife normal adults. Mov Disord. 2011 doi: 10.1002/mds.23674. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louis ED, Wendt KJ, Ford B. Senile tremor. What is the prevalence and severity of tremor in older adults? Gerontology. 2000;46:12–16. doi: 10.1159/000022127. [DOI] [PubMed] [Google Scholar]