Abstract
Objectives
Some large population-based studies have reported a dose-related increased risk of cataracts and glaucoma associated with use of inhaled corticosteroids (ICS) in patients with asthma or chronic obstructive pulmonary disease (COPD). We evaluated the association between use of ICS-containing products, specifically fluticasone propionate/salmeterol fixed-dose combination (FSC), and incidence of cataracts and glaucoma among patients with COPD in a large electronic medical record database in the United Kingdom.
Methods
We identified a cohort of patients aged 45 years and over with COPD in the General Practice Research Database (GPRD) between 2003 and 2006. Cases of incident cataracts or glaucoma were defined based on diagnosis and procedure codes and matched to controls from the risk set to estimate odds ratios (OR) and 95% confidence intervals (CI). The association with FSC or ICS exposure was modeled using conditional logistic regression. Medication exposure was assessed with respect to recency, duration, and number of prescriptions prior to the index date. Average daily dose was defined as none, low (1–250 mcg), medium (251–500 mcg), high (501–1000 mcg), or very high (1001+ mcg) using fluticasone propionate (FP) equivalents.
Results
We identified 2941 incident cataract cases and 327 incident glaucoma cases in the COPD cohort (n = 53,191). FSC or ICS prescriptions were not associated with risk of incident cataracts or glaucoma for any exposure category, after adjusting for confounders. We observed a lack of a dose response in all analyses, where low dose was the reference group. The odds of cataracts associated with FSC dose were medium OR: 1.1 (95% CI: 0.9–1.4); high OR: 1.2 (95% CI: 0.9–1.5); and very high OR: 1.2 (95% CI: 0.9–1.7). The odds of glaucoma associated with FSC dose: medium OR: 1.0 (95% CI: 0.5–2.1); high OR: 1.0 (95% CI: 0.5–2.0); and very high OR: 1.0 (95% CI: 0.4–2.8).
Conclusions
FSC or other ICS exposure was not associated with an increased odds of cataracts or glaucoma, nor was a dose–response relationship observed in this population-based nested case-control study of COPD patients in the United Kingdom.
Keywords: inhaled corticosteroids, fluticasone propionate/salmeterol, cataracts, glaucoma, risk
Introduction
Inhaled corticosteroids (ICS) have been established as first-line therapy for the management of moderate to severe asthma due to their favorable benefit to risk profile and the low systemic absorption of corticosteroids via topical application to the lung. Over the past 25 years, the number of community prescriptions administered for ICS has increased substantially in the United Kingdom.1,2 These medications are typically added to long-acting bronchodilators, and their use is often a marker of chronic obstructive pulmonary disease (COPD) severity. Patients treated with ICS have demonstrated improvements in baseline lung function, reduction in chronic symptoms and exacerbations, and improvements in quality of life.3
Despite the known benefits of corticosteroids for approved indications, oral corticosteroid (OCS) use has been associated with increased intraocular pressure, open angle glaucoma, and cataracts in the published literature. Corticosteroids are believed to increase resistance to aqueous humor outflow in the eye potentially increasing intraocular pressure.4,5 Moreover, these medications may increase the rate of opacification of the crystalline lens leading to accelerated formation of cataracts. Ophthalmic glucocorticoids have been associated with cataract formation as well as increased intraocular pressure or glaucoma; however, studies on the effect of ICS show inconsistent effect estimates in the published literature.
Two large population-based studies examined the association between ICS use and increased intraocular pressure or glaucoma. The Blue Mountain Eye Study (BMES) reported the odds of intraocular pressure or glaucoma among patients aged at least 49 years old with a family history of glaucoma were 2.5 times higher among ICS users compared to nonusers. A dose–response relationship was observed, yet the precision of estimates was poor.6 A second study in a cohort of elderly patients using respiratory medication reported no association between current use of ICS, including high doses of ICS for at least 3 months, and incident ocular hypertension or glaucoma.7
No effect of ICS use on risk of cataracts was observed in four smaller studies of asthmatics.8–11 However, four large population-based case-control studies reported a dose–response relationship with increased odds of incident cataracts with increasing daily dose of ICS.12–15 In one large UK-based study, ICS use was associated with a 25% increase in odds of cataract with every increase of 1000 μg daily dose of beclomethasone dipropionate equivalencies relative to no ICS use.16 Considering subtypes of cataracts, posterior subcapsular cataracts appear to have the strongest association with ever long-term use of ICS.17
Studies to date assessing the association between ICS use and these ocular events demonstrate evidence of an inconsistent, weak, or null association. The purpose of the current study is to quantify the relationship between use of fluticasone propionate/salmeterol fixed-dose combination (FSC) and other ICS and glaucoma and cataracts among patients with COPD in a large health care database. This study considers various time windows of ICS exposure, duration of exposure, as well as cumulative dose.
Methods
Data source
The General Practice Research Database (GPRD) contains computerized medical records from general practitioners (GPs) in the United Kingdom. The database provides anonymized longitudinal data that centralizes information from primary care physicians as well as specialist referrals and hospital admissions. Information collected in this database includes demographics, prescription details, clinical events, preventive care provided, specialist referrals, hospital admissions, and other major health outcomes. This database was previously used in descriptive epidemiological studies of respiratory disease and has been described elsewhere.18–21
Study design
We used a case-control design nested within a cohort of patients with COPD in the GPRD between the years 2003 and 2006. Patients were included in the cohort if they were at least 45 years old, had at least 1 year of data in the database, did not have a diagnosis of cystic fibrosis or prevalent lung cancer, and had a diagnosis of COPD, chronic bronchitis, chronic obstructive airway disease, or emphysema. Oxford Medical Information System (OXMIS) and READ codes defining COPD have been previously validated.22
Cases
The first new case of the event of interest (cataract or glaucoma) was ascertained during follow-up from OXMIS or READ codes. Patients with a history of cataract or glaucoma in the year prior to the COPD event were excluded, and patients were censored at the time of the first event during the follow-up. Cases of cataracts, ascertained from diagnosis and procedure codes, included all combined subtypes, since it was difficult to discern coding for site-specific analyses. Incident cases of glaucoma were ascertained from a glaucoma diagnosis, an increase in ocular pressure, or a relevant prescription for treatment of these symptoms recorded in GPRD.
Controls
Controls were selected from the risk set at the time in which an incident case of either cataracts or glaucoma occurred. This date was referred to as the ‘index date’. The risk set included those patients in the cohort who were at risk for developing either end point, yet did not meet the database criteria for a case in the cohort at the time the case of cataract or glaucoma occurred. Cases were matched to controls up to a 1:2 ratio, respectively, on age within 2 years, gender, general practice, and number of years in the cohort prior to the ocular event.
Exposure
Exposure to either FSC or other ICS medications was ascertained by reviewing the prescription history of the patient in the 1-year period preceding the case of glaucoma or cataract (or matched index date for controls). Exposure to these medications was classified according to ever use, recency of use, duration of use, and number of prescriptions prescribed. Ever use was defined as having at least one prescription of the medication of interest in the 1-year exposure window and coded as yes or no. Recency of use was defined as the number of days from the date of the ocular event (or index date for controls) in which the last prescription for the medication of interest was prescribed. Duration of medication exposure was quantified by calculating the sum of the days supplied for each prescription of interest in the year preceding the event. Number of prescriptions was defined as the sum of prescriptions for each medication of interest in the preceding 1-year exposure window. Finally, average daily dose was calculated for FSC and other ICS medications separately in the 12 months preceding the index date.
Overall cumulative exposure to the medication of interest was converted into fluticasone propionate equivalents and divided by a 12-month time window to obtain the average daily dose.
Covariates
We considered several variables as potential confounders of the association between FSC or other ICS use and incident glaucoma or cataract among patients with COPD. Information on age, gender, body mass index (BMI), smoking status, number of COPD visits, number of COPD hospitalizations, comedications (statin use, oxygen use, nebulizer use, or OCS), comorbidities (depression, diabetes, rheumatoid arthritis, or hypertension), and count of antibiotic prescriptions was collected in the 1-year period prior to cohort entry.
Statistical analysis
Two separate models were generated to estimate the effects of FSC/ICS use and cataract and glaucoma outcomes. Both crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) estimating the association between FSC use or ICS use and incident cataract and glaucoma were estimated using conditional logistic regression models. These models allowed estimation of effect estimates while adjusting for the confounding structure imposed by matching. Covariates included in the model were those that were considered a priori to confound the association between FSC/ICS use and the outcome. ORs and their respective 95% CIs were also calculated within strata of age and OCS use. However, data were sparse in these strata, resulting in poor precision of these stratified effect estimates.
Recency of exposure, duration of exposure, and number of prescriptions prescribed were modeled as categorical variables using quartiles from the frequency distribution in the controls of each variable with no use as the referent group. Average daily dose was modeled using four categories with low dose as the referent group (low: 1–250 FP, medium: 251–500 FP, high: 501–1000 FP, and very high: 1001+ FP). These categories reflect dose ranges commonly used in clinical practice. We were able to detect an OR of 1.65 for glaucoma and 1.20 for cataracts with 80% power based on 273 glaucoma cases and 2404 cataract cases with an exposure frequency of 16%.
Results
Descriptive statistics
Cohort
The cohort included 53,191 patients with COPD. We matched 2404 of the 2941 cataract cases to 5621 controls by age, gender, general practice, and length of time in the cohort. The additional 537 cases had no suitable controls in the risk set, so these patients were excluded from the case control analyses. Patients with cataracts included in the analysis were older compared to excluded cases. Ninety-seven percent of included cases were above the age of 50 compared to 91% of the excluded cases.
Approximately 50% of the cases and controls were aged 70 years or older when the cataract was detected. The distribution of BMI, smoking status, and comorbidities were similar across both groups (Table 1). Thirty-three percent of the cases used FSC in the year prior to the index date compared to 29% of the controls; other ICS use was similar between cases and controls (52% and 50%, respectively).
Table 1.
Demographic characteristics of cases and controls by analysis
| Cataract
|
Glaucoma
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cases (N = 2404) | Controls (N = 5621) | Cases (N = 273) | Controls (N = 703) | |||||
| N | (%) | N | (%) | N | (%) | N | (%) | |
| Age at indexa | ||||||||
| 45–49 | 0 | 0 | 2 | (0.04) | – | – | – | – |
| 50–54 | 8 | (0.3) | 22 | (0.4) | 3 | (1.1) | 8 | (1.1) |
| 55–59 | 54 | (2.3) | 129 | (2.3) | 9 | (3.3) | 20 | (2.8) |
| 60–64 | 122 | (5.1) | 329 | (5.9) | 24 | (8.8) | 54 | (7.7) |
| 65–69 | 284 | (11.8) | 781 | (13.9) | 44 | (16.1) | 126 | (17.9) |
| 70–74 | 472 | (19.6) | 1220 | (21.7) | 61 | (22.3) | 159 | (22.6) |
| 75–79 | 697 | (29) | 1674 | (29.8) | 66 | (24.2) | 179 | (25.5) |
| 80–85 | 653 | (27.2) | 1274 | (22.7) | 54 | (19.8) | 138 | (19.6) |
| ≥86 | 114 | (4.7) | 190 | (3.4) | 12 | (4.4) | 19 | (2.7) |
| Gender | ||||||||
| Female | 1235 | (51.4) | 2793 | (49.7) | 143 | (52.4) | 357 | (50.8) |
| Male | 1169 | (48.6) | 2828 | (50.3) | 130 | (47.6) | 346 | (49.2) |
| BMI | ||||||||
| Missing | 1203 | (50.0) | 2958 | (52.6) | 138 | (50.6) | 366 | (52.1) |
| 10–18.49 | 82 | (3.4) | 185 | (3.3) | 4 | (1.5) | 23 | (3.3) |
| 18.50–24.99 | 435 | (18.1) | 963 | (17.1) | 56 | (20.5) | 111 | (15.7) |
| 25–29.99 | 382 | (15.9) | 881 | (15.7) | 39 | (14.3) | 109 | (15.5) |
| 30–70 | 302 | (12.6) | 634 | (11.3) | 36 | (13.2) | 94 | (13.4) |
| Smoking status | ||||||||
| Nonsmoker | 348 | (14.5) | 743 | (13.2) | 31 | (11.4) | 91 | (12.9) |
| Ex-smoker | 1112 | (46.3) | 2437 | (43.4) | 120 | (44) | 292 | (41.5) |
| Smoker | 493 | (20.5) | 1324 | (23.6) | 69 | (25.3) | 186 | (26.5) |
| Unknown | 451 | (18.8) | 1117 | (19.9) | 53 | (19.4) | 134 | (19.1) |
| Comorbid conditions | ||||||||
| Vertebral fracture | 11 | (0.5) | 24 | (0.4) | 2 | (0.7) | 3 | (0.4) |
| Depression | 120 | (5) | 228 | (4.1) | 12 | (4.4) | 38 | (5.4) |
| Diabetes | 350 | (14.6) | 538 | (9.6) | 23 | (8.4) | 76 | (10.8) |
| Rheumatoid arthritis | 11 | (0.5) | 40 | (0.7) | 0 | 0 | 5 | (0.7) |
| Asthma | 276 | (11.5) | 625 | (11.1) | 35 | (12.8) | 75 | (10.7) |
| Osteoporosis | 53 | (2.2) | 112 | (1.2) | 7 | (2.6) | 11 | (1.6) |
| Stroke | 38 | (1.6) | 74 | (1.3) | 4 | (1.5) | 10 | (1.4) |
| Anemia | 90 | (3.7) | 184 | (3.3) | 5 | (1.8) | 20 | (2.8) |
| Osteopenia | 13 | (0.5) | 26 | (0.5) | 1 | (0.4) | 5 | (0.7) |
| Back pain | 307 | (12.8) | 627 | (11.2) | 40 | (14.7) | 86 | (12.2) |
| Falls | 137 | (5.7) | 247 | (4.4) | 14 | (5.1) | 31 | (4.4) |
| Hyperparathyroid | 4 | (0.2) | 4 | (0.1) | 0 | 0 | 1 | (0.14) |
| Dementia | 9 | (0.4) | 28 | (0.5) | 2 | (0.7) | 7 | (1.0) |
| Hypertension | 364 | (15.1) | 946 | (16.8) | 47 | (17.2) | 115 | (16.4) |
| Dyslipidemia | 114 | (4.7) | 243 | (4.3) | 15 | (5.5) | 20 | (2.84) |
| Obesity | 306 | (12.7) | 639 | (11.4) | 36 | (13.2) | 95 | (13.5) |
| Antibiotic: number of scripts in the previous year | ||||||||
| None | 723 | (30.1) | 1907 | (34) | 83 | (30.4) | 245 | (34.9) |
| 1 | 440 | (18.3) | 1257 | (22.4) | 63 | (23.1) | 154 | (21.9) |
| 2 | 344 | (114.3) | 832 | (14.8) | 37 | (13.6) | 100 | (14.2) |
| 3–4 | 434 | (18.1) | 876 | (15.6) | 41 | (15.0) | 125 | (17.8) |
| ≥5 | 463 | (19.3) | 749 | (13.3) | 49 | (18) | 79 | (11.2) |
| Number of COPD visits | ||||||||
| None | 549 | (22.9) | 1366 | (24.3) | 75 | (27.5) | 185 | (26.3) |
| 1 | 990 | (41.2) | 2385 | (42.4) | 104 | (38.1) | 277 | (39.4) |
| 2 | 386 | (16.1) | 968 | (17.2) | 47 | (17.2) | 113 | (16.1) |
| 3+ | 479 | (19.9) | 902 | (16.1) | 47 | (17.2) | 128 | (18.2) |
| Number of COPD hospitalizations | ||||||||
| None | 2189 | (91.1) | 5306 | (94.4) | 254 | (93.0) | 662 | (94.2) |
| 1 | 167 | (7) | 252 | (4.5) | 15 | (5.5) | 33 | (4.7) |
| 2+ | 48 | (2.0) | 63 | (1.1) | 4 | (1.5) | 8 | (1.1) |
| Comedications | ||||||||
| FSC | 799 | (33.2) | 1655 | (29.4) | 78 | (28.6) | 226 | (32.2) |
| ICS-containing product | 1823 | (75.8) | 3961 | (70.5) | 197 | (72.2) | 510 | (72.6) |
| BDP-containing product | 847 | (35.2) | 1990 | (35.4) | 90 | (33) | 223 | (31.7) |
| BUD-containing product | 350 | (14.6) | 663 | (11.8) | 43 | (15.8) | 95 | (13.5) |
| FP-containing product | 898 | (37.4) | 1864 | (33.2) | 88 | (32.2) | 253 | (36) |
| Oral corticosteroids | 1098 | (45.7) | 2128 | (37.9) | 119 | (43.6) | 264 | (37.6) |
| Antibiotic | 1681 | (69.9) | 3714 | (66.1) | 190 | (69.6) | 458 | (65.2) |
| Statin | 850 | (35.4) | 1763 | (31.4) | 76 | (27.8) | 210 | (29.9) |
| Nebulizer | 476 | (19.8) | 761 | (13.5) | 51 | (18.7) | 92 | (13.1) |
| Other respiratory product | 1369 | (56.9) | 2827 | (50.3) | 146 | (53.5) | 359 | (51.1) |
| Oxygen | 160 | (6.7) | 268 | (4.8) | 15 | (5.5) | 45 | (6.4) |
| Tiotropium-only product | 616 | (25.6) | 1224 | (21.8) | 57 | (20.9) | 164 | (23.3) |
| SABD-containing product | 2156 | (89.7) | 4898 | (87.1) | 229 | (83.9) | 603 | (85.8) |
| SABD with ICS product | 0 | 0 | 3 | (0.1) | NA | NA | NA | NA |
| ICS-containing product excluding FSC | 1260 | (52.4) | 2799 | (49.8) | 136 | (49.8) | 338 | (48.1) |
Notes: Population includes patients with COPD in the GPRD between 2003 and 2006. Controls matched to cases on gender, age within 2 years, general practice, and number of years in the cohort prior to the event of interest.
Index date is the date of the incident case of cataract or glaucoma and matched control.
Abbreviations: BDP, beclomethasone dipropionate; BMI, body mass index; BUD, budesonide; FSC, fluticasone propionate/salmeterol fixed-close combination; FP, fluticasone propionate; GPRD, General Practice Research Data base; SABD, short-acting bronchodilator; NA, not applicable.
Of the 327 glaucoma cases, 273 were matched by age, gender, general practice, and length of time in the cohort to 703 controls. Excluded glaucoma cases (16.7%) were four times more likely to be between the ages of 44 and 49 years compared to cases included (4.4%) in the analysis. Smoking status appeared to be equally distributed between cases and controls; however, data were missing on smoking status of 19% of the cases and controls. Approximately 50% of cases and controls were aged above 65, and 25% were smokers. FSC use in the year prior to the index date (29% and 32%) and prior use of an ICS-containing prescription (50% and 48%) were similar between cases and controls, respectively.
Multivariate models
Cataracts
We estimated the ORs and 95% CIs for the association between FSC or other ICS use and cataracts adjusting for potential confounding variables (COPD hospitalizations, BMI, smoking status, statin use, depression, diabetes, rheumatoid arthritis, hypertension, oxygen use, nebulizer use, and count of antibiotic prescriptions). Exposure to FSC and other ICS was categorized using multiple exposure criteria with the reference group being ‘no use in the year prior to the index date’ for all analyses.
The unadjusted odds of incident cataracts associated with ever use of ICS in the last year (2006) was 1.32 (95% CI: 1.18–1.47) relative to no ICS use in the last 12 months (Table 3). An increased odds (OR: 1.19; 95% CI: 1.08–1.32) of lesser magnitude was also observed for the unadjusted odds of cataracts among ever users of FSC in the last year (Table 2). The unadjusted odds of cataracts increased with number of prescriptions in the last year as well as duration of use compared to no use for users of both FSC and other ICS medications. However, no association between FSC or other ICS use and the odds of cataract after adjusting for potential confounding variables was observed.
Table 3.
Crude and adjusted OR and 95% CI of the association between ICS use and incident cataracts between 2003 and 2006
| ICS1 | Crude OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Ever use in the last year | ||||
| No use | Reference | – | Reference | – |
| Ever use | 1.32 | (1.18–1.47) | 1.10 | (0.97, 1.24) |
| Last use prior to index date (days) | ||||
| No use | Reference | – | Reference | – |
| 18 days | 1.19 | (1.03–1.37) | 1.19 | (1.00, 1.41) |
| 19–38 days | 1.07 | (0.91–1.25) | 1.09 | (0.90, 1.31) |
| 39–100 days | 1.06 | (0.91–1.23) | 1.08 | (0.90, 1.28) |
| 101–365 days | 1.12 | (0.97–1.3) | 1.06 | (0.90, 1.25) |
| Duration of use (days) | ||||
| No use in last year | Reference | – | Reference | – |
| 1–120 | 1.15 | (0.99–1.33) | 1.11 | (0.94, 1.32) |
| 121–230 | 0.99 | (0.85–1.15) | 0.99 | (0.83, 1.18) |
| 231–380 | 1.14 | (0.98–1.32) | 1.15 | (0.97, 1.37) |
| ≥381 | 1.17 | (1.01–1.36) | 1.22 | (1.02, 1.46) |
| Number of prescriptions | ||||
| No prescriptions in last year | Reference | – | Reference | – |
| 1–3 | 1.06 | (0.93–1.22) | 1.07 | (0.92, 1.25) |
| 4–5 | 1.02 | (0.87, 1.21) | 1.02 | (0.84, 1.23) |
| 6–8 | 1.10 | (0.95–1.28) | 1.14 | (0.95, 1.36) |
| ≥9 | 1.27 | (1.09–1.48) | 1.33 | (1.10, 1.60) |
Notes: Population includes patients with COPD in the GPRD between 2003 and 2006. Controls matched to cases on gender, age within 2 years, general practice, and number of years in the cohort prior to the event of interest. Models adjusted for FSC, asthma, long-acting beta agonists, short-acting bronchodilators – containing product, oral corticosteroids, COPD hospitalizations, BMI, smoking status, statin use, depression, diabetes, rheumatoid arthritis, hypertension, oxygen use, nebulizer use, and count of antibiotic prescriptions.
ICS use excluding FSC.
Abbreviations: BMI, body mass index; CI, confidence interval; ICS, inhaled corticosteroids; GPRD, General Practice Research Database; OR, odds ratio.
Table 2.
Crude and adjusted ORs and 95% CIs for FSC use and incident cataracts between 2003 and 2006
| FSC | Crude OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Ever use in the last year | ||||
| No use in the last year | Reference | – | Reference | – |
| Use in the last year | 1.19 | (1.08–1.32) | 1.1 | (0.96–1.24) |
| Last use prior to index date (days) | ||||
| No use in the last year | Reference | – | Reference | – |
| Last use prior 12 days | 1.10 | (0.92–1.33) | 1.02 | (0.83–1.27) |
| Last use prior 13–25 days | 1.38 | (1.16–1.64) | 1.24 | (1.01–1.51) |
| Last use prior 26–52 days | 1.12 | (0.94–1.34) | 1.12 | (0.91–1.38) |
| Last use prior 53–365 days | 1.17 | (0.98–1.4) | 1.06 | (0.87–1.30) |
| Duration of use (days) | ||||
| No use in last year | Reference | – | Reference | – |
| 1–90 | 1.15 | (0.96–1.37) | 1.06 | (0.87–1.29) |
| 91–180 | 1.14 | (0.96–1.34) | 1.05 | (0.87–1.27) |
| 181–270 | 1.28 | (1.04–1.57) | 1.18 | (0.93–1.49) |
| ≥271 | 1.25 | (1.05–1.49) | 1.20 | (0.97–1.48) |
| Number of prescriptions | ||||
| No prescriptions in last year | Reference | – | Reference | – |
| 1–3 | 1.10 | (0.92–1.31) | 1.02 | (0.84–1.24) |
| 4–6 | 1.12 | (0.95–1.33) | 1.07 | (0.88–1.30) |
| 7–9 | 1.25 | (1.02–1.53) | 1.20 | (0.95–1.52) |
| ≥10 | 1.34 | (1.13–1.59) | 1.29 | (1.05–1.59) |
Notes: Population includes patients with COPD in the GPRD between 2003 and 2006. Controls matched to cases on gender, age within 2 years, general practice, and number of years in the cohort prior to the event of interest. Model adjusted for other inhaled corticosteroid use, asthma, long-acting beta agonists, short-acting bronchodilator, oral corticosteroid use, COPD hospitalizations, BMI, smoking status, statin use, depression, diabetes, rheumatoid arthritis, hypertension, oxygen use, nebulizer use, and count of antibiotic prescriptions.
Abbreviations: CI, confidence interval; FSC, fluticasone propionate/salmeterol fixed dose combination; GPRD, General Practice Research Database; OR, odds ratio.
There was no pattern of increased odds of cataracts associated with more recent use of FSC or other ICS. The only significant association with increased odds for cataracts was observed for the exposure category of FSC prescription written between 13 and 25 days prior to the index date relative to no FSC use in the last year (OR: 1.24; 95% CI: 1.01–1.51) (Table 2).
There was no pattern of increased odds for cataracts associated with duration of FSC or other ICS exposure. The odds for cataracts was increased for very long duration of exposure to other ICS (381+ days) compared to no other ICS use (OR: 1.22; 95% CI: 1.02–1.46) (Table 3). Similarly, there was no pattern of increased odds for cataracts associated with increasing number of prescriptions of either FSC or other ICS. A statistically significant association was observed for the highest frequency of prescription categories of either FSC (10+) (OR: 1.29; 95% CI: 1.05–1.59) or other ICS (9+) (OR: 1.22; 95% CI: 1.02–1.46) versus no FSC or no other ICS in the last year (Tables 2 and 3).
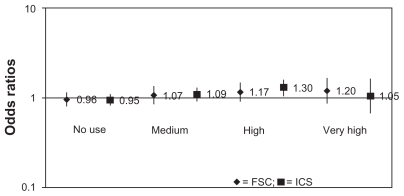
In the average daily dose analyses, no pattern of association between increasing levels of FSC or other ICS exposure and increased odds of cataracts was observed in the 12-month study period (Figure 1). Results were similar in the 3- and 6-month intervals prior to the index date (data not shown). Furthermore, the CIs for the FSC and other ICS estimates overlapped, suggesting no difference between the class-level and FSC-specific exposure odds estimates.
Figure 1.
Association between 1 year prior FSC or ICS prescriptions and odds of cataracts: average daily dose analysis.
Notes: Reference: low average daily dose.
Abreviations: FSC, fluticasone propionate/salmeterol fixed-close combination; ICS, inhaled corticosteroids.
Glaucoma
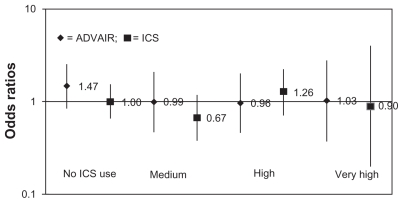
There was only one statistically significant unadjusted OR observed between FSC and other ICS use (Tables 4 and 5) with glaucoma in this population of COPD patients (last use in prior 39–100 days crude OR: 1.82; CI: 1.19–2.76). However, no adjusted ORs had 95% CIs, which excluded the null value and therefore provided no evidence of an increased risk for glaucoma associated with FSC or other ICS use. Moreover, no association was observed between average daily dose of FSC or ICS and incident glaucoma in the 12-month analysis (Figure 2) or 3- and 6-month analyses (not shown).
Table 4.
Crude and adjusted OR and 95% CI of the association between FSC use and incident glaucoma between 2003 and 2006
| FSC | Crude OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Ever use in the last year | ||||
| No use | Reference | – | Reference | – |
| Use | 0.84 | (0.62–1.15) | 0.68 | (0.45–1.01) |
| Last use prior to index date (days) | ||||
| No use | Reference | – | Reference | – |
| 12 days | 1.02 | (0.61–1.71) | 1.00 | (0.53–1.89) |
| 13–25 days | 0.92 | (0.52–1.62) | 0.67 | (0.34–1.30) |
| 26–52 days | 0.54 | (0.3–0.97) | 0.55 | (0.28–1.09) |
| 53–365 days | 0.98 | (0.58–1.65) | 0.81 | (0.44–1.52) |
| Duration of use (days) | ||||
| No use | Reference | – | Reference | – |
| 1–90 | 1.14 | (0.66–1.97) | 1.02 | (0.54–1.90) |
| 91–180 | 0.62 | (0.36–1.08) | 0.50 | (0.26–0.96) |
| 181–270 | 0.68 | (0.36–1.28) | 0.52 | (0.25–1.08) |
| ≥271 | 0.99 | (0.61–1.59) | 0.75 | (0.42–1.34) |
| Number of prescriptions | ||||
| No prescriptions in last year | Reference | – | Reference | – |
| 1–3 | 1.14 | (0.66–1.97) | 1.01 | (0.54–1.89) |
| 4–6 | 0.63 | (0.36–1.10) | 0.49 | (0.26–0.93) |
| 7–9 | 0.70 | (0.38–1.30) | 0.50 | (0.24–1.03) |
| ≥10 | 0.96 | (0.59–1.56) | 0.72 | (0.39–1.30) |
Notes: Population includes patients with COPD in the GPRD between 2003 and 2006. Controls matched to cases on gender, age within 2 years, general practice, and number of years in the cohort prior to the event of interest. Model adjusted for other inhaled corticosteroids, asthma, long-acting beta agonists, short-acting bronchodilators, oral corticosteroids, BMI, smoking status, statin use, depression, diabetes, oxygen, nebulizer, and count of antibiotic prescriptions.
Abbreviations: BMI, body mass index; CI, confidence interval; FSC, fluticasone propionate/salmeterol fixed-dose combination; GPRD, General Practice Research Database OR, odds ratio.
Table 5.
Crude and adjusted OR and 95% CI of the association between ICS use and incident glaucoma between 2003 and 2006
| ICS | Crude OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Ever use in the last year | ||||
| No use | Reference | – | Reference | – |
| Use | 0.98 | (0.72–1.34) | 0.94 | (0.64–1.38) |
| Last use prior to index date (days) | ||||
| No use | Reference | – | Reference | – |
| 18 days | 1.18 | (0.77–1.81) | 1.07 | (0.62–1.82) |
| 19–38 days | 0.85 | (0.53–1.35) | 0.74 | (0.41–1.34) |
| 39–100 days | 1.82 | (1.19–2.76) | 1.82 | (1.08–3.05) |
| 101–365 days | 0.63 | (0.38–1.04) | 0.55 | (0.31–0.99) |
| Duration of use (days) | ||||
| No use | Reference | – | Reference | – |
| 1–120 | 0.94 | (0.6–1.47) | 0.83 | (0.49–1.43) |
| 121–230 | 0.99 | (0.61–1.6) | 0.78 | (0.43–1.4) |
| 231–380 | 1.10 | (0.71–1.68) | 0.92 | (0.53–1.58) |
| ≥381 | 1.27 | (0.83–1.94) | 1.15 | (0.68–1.95) |
| Number of prescriptions | ||||
| None | Reference | – | Reference | – |
| 1–3 | 0.91 | (0.59–1.39) | 0.85 | (0.51–1.41) |
| 4–5 | 1.29 | (0.81–2.07) | 1.17 | (0.67–2.04) |
| 6–8 | 1.12 | (0.72–1.75) | 0.86 | (0.49–1.52) |
| ≥9 | 1.07 | (0.69–1.66) | 0.86 | (0.49–1.49) |
Notes: Population includes patients with COPD in the GPRD between 2003 and 2006. Controls matched to cases on gender, age within 2 years, general practice, and number of years in the cohort prior to the event of interest. Models Adjusted for FSC, asthma, long-acting beta agonists, short-acting bronchodilators, oral corticosteroids, BMI, smoking status, statin use, depression, diabetes, oxygen use, nebulizer use, and count of antibiotic prescriptions.
Abbreviations: BMI, body mass index; CI, confidence interval; FSC, fluticasene propionate/salmeterol fixed-close combination; GPRD, General Practice Research Database OR, odds ratio.
Figure 2.
Association between 1 year prior drug exposure and odds of glaucoma: average daily dose analysis.
Notes: Reference: low average daily dose.
Abbreviation: ICS, inhaled corticosteroids.
Discussion
The results of our analysis in a UK population-based COPD patient cohort were not consistent with an association between exposure to FSC or other ICS and increased risk of ocular events (cataracts or glaucoma).
Several observational studies have shown an association between ICS use and increased risk of either cataracts or glaucoma, although the applicability of these studies to risks for COPD patients is limited. The previous studies were not specific to COPD patients who tend to be exposed to both ICS for routine management of their symptoms and OCS for managing acute exacerbations.7,12,14,15 Further, the sample sizes within strata of high average daily dose of ICS exposure or longer duration of ICS exposure were relatively small, leading to imprecise estimates with wide CIs.
Mitchell and colleagues reported an association between ICS use and glaucoma among patients with a family history of glaucoma or elevated intraocular pressure.6 However, these data were from a cross-sectional design, which does not allow for clear assessment of the temporality of the association, for example, that the exposure clearly preceded the event of interest. A dose–response relationship was reported, yet the 95% CIs for the estimates were wide; the OR for the highest dose ranged from 1.0 to 38.6. Further, Chylack and colleagues reported results from a randomized clinical trial of asthma patients comparing either 640 μg daily of ciclesonide to beclomethasone dipropionate. The mean change from baseline in LOCIII scores was small and did not differ from a normal aging population over 1 year.23 The results from this randomized clinical study are consistent with our findings.
The many strengths of our GPRD study were methodological, including the nesting of the case-control study within a population-based cohort of patients with diagnosed COPD. The cohort included over 53,000 patients allowing for increased precision in the effect estimates. The sensitivity and specificity of both COPD diagnosis and outcomes coding were good in previous validation studies in the GPRD despite our inability to use a standardized method for ascertainment of glaucoma cases.22,24 Matching controls on age and sex are critical considering that they are the two strongest risk factors for the outcomes of interest. Matching on general practice serves as a proxy for both physician prescribing patterns and socioeconomic status of neighborhood. Multiple indicators of COPD severity were included in multivariate models in order to reduce the potential confounding by severity. Finally, the effect of FSC was modeled in a number of ways to characterize exposure and explore the biologic plausibility of any observed increase in risk.
There were several limitations in this study. The first is the potential for confounding by severity/indication. If disease severity is associated with both the likelihood of receiving the medication and the probability of the outcome, then good markers of disease severity are needed to adjust for this potential confounding. We did not have access to clinical parameters that could further characterize COPD severity. Information about other parameters linked to outcome risk, such as physical activity, functional status (performance of activities of daily living), community involvement, and assistance with ambulation, are also absent from electronic medical records and could not be adjusted for in multivariate models. However, we included the number of GP visits and hospitalizations as proxies for exacerbations, as well as number of antibiotic and OCS prescriptions. We also included comorbid conditions and medications in the multivariate logistic regression that were associated with exposure, as well as the outcomes of interest, to reduce confounding bias in the adjusted risk estimates.
There are limitations in measuring the exposure to OCS use, which is an important marker of disease severity and a critical risk factor for the outcomes of interest. OCSs administered in the emergency room or inpatient hospital stays are not always captured in the medical record. In addition, we were limited to prescriptions recorded in the database during the study period. Lifetime exposure to OCS was not available in the database, but we assume that disease severity measured via the covariates included in the model (comorbid conditions, health care utilization, and medication use) in the period prior to index date has reasonably adjusted for COPD severity. However, there is the possibility of residual confounding after the adjustment for other medication use; this potential confounding by severity would result in a bias away from the null.
Exposures longer than 12 months could not be addressed in this study, since creating categories with longer exposure yielded small sample sizes with very few events, such that the precision of the estimates was no longer informative. Thus, patients who used ICS medications and then discontinued for 13 months or longer prior to the ocular event could have been misclassified as a nonuser. We would expect the effect of this potential misclassification to be nondifferential with respect to case status. Thus, our estimate may have been biased toward no association. Our study evaluated a shorter exposure history than that of Garbe and colleagues who assessed exposure to ICS medications at 1–2 years and more than 2 years prior to the ocular event. However, Gonzalez et al assessed ICS use in the same exposure time window and reported a positive association between ICS use and ocular events, suggesting that study of 12 months of exposure could detect an effect should one exist.7 Finally, potential misclassification of glaucoma cases was also a limitation of our data source, which relied upon the electronic medical health record. We were unable to use a standardized prospectively collected data approach to evaluate lenticular opacities, yet our results were consistent with results obtained by Chylack et al who used LOCIII scores to classify glaucoma.
Another methodological concern with control selection among diseases with insidious onset was raised by Garbe and colleagues in 1998, where they indicate that serious selection bias may occur if the controls do not have the same opportunity for diagnosis as the cases.25 Given that there is a similar opportunity in the study population to be diagnosed and that matching by GP site accounts for the possible differences in GP referral practice to a specialist, we feel that the bias concern is greatly reduced. Additionally, we excluded cases from our analysis that did not have matched controls for the major confounders measured in the database (age, gender, GP, and length of time in the cohort), which resulted in fewer cases younger than 50 years in the final analysis. Consequently, the final results are less generalizable to a younger population of patients with COPD with the trade-off of improved adjustment for confounding in the matched sets of cases and controls.
We did not observe an association between prescriptions of FSC or other ICS in the previous year and increases in odds of incident cataracts or glaucoma in these nested case- control analyses of a large population-based COPD cohort of 53,191 patients from the United Kingdom. However, given the presence of significant risk factors for cataracts and glaucoma in COPD patients, primary care physicians should be further sensitized to screening for the presence of increased ocular pressure and cataracts prior to initiating therapy and continued monitoring. These data should be considered together with results from other observational studies and those from randomized clinical studies of longer duration in order to obtain a clearer understanding of the long-term benefit to risk profile of ICS-containing products in the treatment of COPD, including the risk of cataracts and glaucoma.
Acknowledgments
Funding for this study was provided by GlaxoSmithKline (WEUSRTP1127). All authors are employees of GlaxoSmithKline and meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. The authors acknowledge the following individuals for their critical review during the development of this manuscript: Rachael DiSantostefano and Michael Irizarry.
Footnotes
Disclosure
Dr Miller, Dr Davis, and Mr Sampson were full-time employees of GSK during the study design, analysis, and manuscript development phases. Ms Watkins worked part-time for GSK during the manuscript development.
References
- 1.Anderson HR, Gupta R, Strachan DP, Limb ES. 50 years of asthma: UK trends from 1955 to 2004. Thorax. 2007;62(1):85–90. doi: 10.1136/thx.2006.066407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stafford RS, Ma J, Finkelstein SN, Haver K, Cockburn I. National trends in asthma visits and asthma pharmacotherapy, 1978–2002. J Allergy Clin Immunol. 2003;111(4):729–735. doi: 10.1067/mai.2003.177. [DOI] [PubMed] [Google Scholar]
- 3.Rabe KF. Guidelines for chronic obstructive pulmonary disease treatment and issues of implementation. Proc Am Thorac Soc. 2006;3(7):641–644. doi: 10.1513/pats.200604-099SS. [DOI] [PubMed] [Google Scholar]
- 4.Skuta GL, Morgan RK. Corticosteroid-induced glaucoma. In: Ritch R, Shields MD, Krupin T, editors. The Glaucomas. St. Louis, MO: Mosby; 1996. pp. 1177–1188. [Google Scholar]
- 5.Becker B, Mills DW. Corticosteroids and intraocular pressure. Arch Ophthalmol. 1963;70(4):500–507. doi: 10.1001/archopht.1963.00960050502012. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell P, Cumming RG, Mackey DA. Inhaled corticosteroids, family history, and risk of glaucoma. Ophthalmology. 1999;106(12):2301–2306. doi: 10.1016/S0161-6420(99)90530-4. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez AV, Li G, Suissa S, Ernst P. Risk of glaucoma in elderly patients treated with inhaled corticosteroids for chronic airflow obstruction. Pulm Pharmacol Ther. 2010;23(2):65–70. doi: 10.1016/j.pupt.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Simons FE, Persaud MP, Gillespie CA, Cheang M, Shuckett EP. Absence of posterior subcapsular cataracts in young patients treated with inhaled glucocorticoids. Lancet. 1993;342(8874):776–778. doi: 10.1016/0140-6736(93)91541-s. [DOI] [PubMed] [Google Scholar]
- 9.Toogood JH, Markov AE, Baskerville J, Dyson C. Association of ocular cataracts with inhaled and oral steroid therapy during long-term treatment of asthma. J Allergy Clin Immunol. 1993;91(2):571–579. doi: 10.1016/0091-6749(93)90263-f. [DOI] [PubMed] [Google Scholar]
- 10.Abuekteish F, Kirkpatrick JN, Russell G. Posterior subcapsular cataract and inhaled corticosteroid therapy. Thorax. 1995;50(6):674–676. doi: 10.1136/thx.50.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sevel D, Weinberg EG, van Niekerk CH. Lenticular complications of long-term steroid therapy in children with asthma and eczema. J Allergy Clin Immunol. 1977;60(4):215–217. doi: 10.1016/0091-6749(77)90132-4. [DOI] [PubMed] [Google Scholar]
- 12.Jick SS, Vasilakis-Scaramozza C, Maier WC. The risk of cataract among users of inhaled steroids. Epidemiology. 2001;12(2):229–234. doi: 10.1097/00001648-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Garbe E, Suissa S, LeLorier J. Association of inhaled corticosteroid use with cataract extraction in elderly patients. JAMA. 1998;280(6):539–543. doi: 10.1001/jama.280.6.539. [DOI] [PubMed] [Google Scholar]
- 14.Ernst P, Baltzan M, Deschênes J, Suissa S. Low-dose inhaled and nasal corticosteroid use and the risk of cataracts. Eur Respir J. 2006;27(6):1168–1174. doi: 10.1183/09031936.06.00043005. [DOI] [PubMed] [Google Scholar]
- 15.Smeeth L, Boulis M, Hubbard R, Fletcher AE. A population based case- control study of cataract and inhaled corticosteroids. Br J Ophthalmol. 2003;87(10):1247–1251. doi: 10.1136/bjo.87.10.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weatherall M, Clay J, James K, Perrin K, Shirtcliffe P, Beasley R. Dose- response relationship of inhaled corticosteroids and cataracts: a systematic review and meta-analysis. Respirology. 2009;14(7):983–990. doi: 10.1111/j.1440-1843.2009.01589.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang JJ, Rochtchina E, Tan AG, Cumming RG, Leeder SR, Mitchell P. Use of inhaled and oral corticosteroids and the long-term risk of cataract. Ophthalmology. 2009;116(4):652–657. doi: 10.1016/j.ophtha.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Lawson DH, Sherman V, Hollowell J. The general practice research database. Scientific and ethical advisory group. QJM. 1998;91(6):445–452. doi: 10.1093/qjmed/91.6.445. [DOI] [PubMed] [Google Scholar]
- 19.Nazareth I, King M, Haines A, Rangel L, Myers S. Accuracy of diagnosis of psychosis on general practice computer system. BMJ. 1993;307(6895):32–34. doi: 10.1136/bmj.307.6895.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansell A, Hollowell J, Nichols T, McNiece R, Strachan D. Use of the General Practice Research Database (GPRD) for respiratory epidemiology: a comparison with the 4th Morbidity Survey in General Practice (MSGP4) Thorax. 1999;54(5):413–419. doi: 10.1136/thx.54.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soriano JB, Maier WC, Egger P, et al. Recent trends in physician diagnosed COPD in women and men in the UK. Thorax. 2000;55(9):789–794. doi: 10.1136/thorax.55.9.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soriano JB, Maier WC, Visick G, Pride NB. Validation of general practitioner-diagnosed COPD in the UK General Practice Research Database. Eur J Epidemiol. 2001;17(12):1075–1080. doi: 10.1023/a:1021235123382. [DOI] [PubMed] [Google Scholar]
- 23.Chylack LT, Jr, Gross GN, Pedinoff A. A randomized, controlled trial to investigate the effect of ciclesonide and beclomethasone dipropionate on eye lens opacity. J Asthma. 2008;45(10):893–902. doi: 10.1080/02770900802353636. [DOI] [PubMed] [Google Scholar]
- 24.Van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29(6):517–522. doi: 10.1016/s8756-3282(01)00614-7. [DOI] [PubMed] [Google Scholar]
- 25.Garbe E, Boivin JF, LeLorier J, Suissa S. Selection of controls in database case-control studies: glucocorticoids and the risk of glaucoma. J Clin Epidemiol. 1998;51(2):129–135. doi: 10.1016/s0895-4356(97)00263-1. [DOI] [PubMed] [Google Scholar]