Abstract
Background
Three-dose regimens for human papillomavirus (HPV) vaccines are expensive and difficult to complete, especially in settings where the need for cervical cancer prevention is greatest.
Methods
We evaluated the vaccine efficacy of fewer than three doses of the HPV16/18 vaccine Cervarix in our Costa Rica Vaccine Trial. Women were randomly assigned to receive three doses of the HPV16/18 vaccine or to a control vaccine and were followed for incident HPV16 or HPV18 infection that persisted in visits that were 10 or more months apart (median follow-up 4.2 years). After excluding women who had no follow-up or who were HPV16 and HPV18 DNA positive at enrollment, 5967 women received three vaccine doses (2957 HPV vaccine vs 3010 control vaccine), 802 received two doses (422 HPV vs 380 control), and 384 received one dose (196 HPV vs 188 control). Reasons for receiving fewer doses and other pre- and post-randomization characteristics were balanced within each dosage group between women receiving the HPV and control vaccines.
Results
Incident HPV16 or HPV18 infections that persisted for 1 year were unrelated to dosage of the control vaccine. Vaccine efficacy was 80.9% for three doses of the HPV vaccine (95% confidence interval [CI] = 71.1% to 87.7%; 25 and 133 events in the HPV and control arms, respectively), 84.1% for two doses (95% CI = 50.2% to 96.3%; 3 and 17 events), and 100% for one dose (95% CI = 66.5% to 100%; 0 and 10 events).
Conclusion
Four years after vaccination of women who appeared to be uninfected, this nonrandomized analysis suggests that two doses of the HPV16/18 vaccine, and maybe even one dose, are as protective as three doses.
CONTEXT AND CAVEATS
Prior knowledge
The HPV16/18 vaccine Cervarix is normally given in three doses. Previously, there were no efficacy data to establish whether fewer doses might protect women against cervical cancer.
Study design
Data were taken from the Costa Rica vaccine trial, in which many of the 7153 women missed one or more of three prescribed doses of a randomly assigned HPV16/18 vaccine or control (hepatitis A) vaccine mostly because of pregnancy and referral to colposcopy. Vaccine efficacy was evaluated in each dosage group by determination, via HPV DNA testing, of the number of newly detected HPV16 or HPV18 infections that persisted at least 1 year.
Contribution
The estimated vaccine efficacy against infection with HPV16 and HPV18 was similar whether the woman received one, two, or all three doses.
Implication
It appears that two doses, or even one dose, of the HPV16/18 vaccine, is highly efficacious in protecting against persistent HPV16/18 infections.
Limitations
It is still not known whether the three-dose regimen might provide longer duration of protection or more cross-protection against heterologous HPV types and whether the findings in this trial are applicable to populations in other geographical settings or to other HPV vaccines.
From the Editors
Cervical cancer is the third most common cancer in women worldwide, and it is the leading cause of cancer death among women in some areas (1). Approximately 85% of cervical cancers occur in developing countries without effective screening programs (1). Administering prophylactic human papillomavirus (HPV) vaccines to susceptible populations of young women could reduce a large fraction of the disease burden.
The standard three-dose regimen of either Cervarix, the bivalent HPV16/18 vaccine with AS04 adjuvant (GlaxoSmithKline Biologicals, Rixensart, Belgium), or Gardasil, the quadrivalent HPV6/11/16/18 vaccine (Merck and Co, Whitehouse Station, NJ) prevents HPV16 and HPV18 infections and related cervical precancers among unexposed women (2,3). Currently, the cost of these regimens and logistical difficulties associated with administering three doses over 6 months make it impractical to vaccinate preadolescent and young adult women in developing countries (4). Even in developed countries, vaccine programs often do not successfully administer all three doses; in the United States, a minority of vaccinees complete the full vaccine course (5); in countries that have school-based health programs, such as Australia (6) and the United Kingdom (7), vaccine uptake is higher. If vaccination with fewer than three doses were to retain the high efficacy of the standard regimen, the ability to vaccinate more women for the same cost could translate to a greater public health benefit in underserved areas.
Women in our clinical trial in Costa Rica were randomly assigned to receive three doses of either Cervarix or control vaccine, yet approximately 20% received fewer than three doses mostly because of pregnancy and referral to colposcopy. Here, we compare the efficacy of fewer than three doses of this HPV vaccine vs the standard regimen to prevent newly detected persistent HPV16 and HPV18 infections.
Methods
The 7153 women included in the present evaluation are among the participants in an ongoing randomized clinical trial of 7466 women (8,9). The primary aim of the trial is to evaluate the efficacy of a three-dose regimen of the Cervarix vaccine to prevent persistent type-specific infection with HPV16 or HPV18 and the subsequent development of HPV-associated precancerous lesions (8,10).
In June 2004 and December 2005, the study enrolled young women who resided in the regions of Guanacaste and Puntarenas, Costa Rica, and were identified via a census. To be eligible, women were required to be 18–25 years of age, in good general health, and neither pregnant nor breastfeeding. Women were excluded if they had a preexisting medical condition that would preclude vaccination, a history of hepatitis A infection or previous vaccination against hepatitis A, or if they were unwilling to use contraception during the vaccination period. The trial was approved by human subjects review committees of the US National Cancer Institute and Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud (INCIENSA) in Costa Rica. In the United States, it was registered as Clinical Trial number NCT00128661.
At the enrollment visit and following informed consent, a risk factor interview was administered, a pelvic examination was performed on sexually experienced women, exfoliated cells were collected in PreservCyt liquid medium (Cytyc Corp, now Hologic, Marlborough, MA) for Thinprep (Cytyc Corp) cytological evaluation and HPV DNA testing, and blood was collected (for 90% of women, 30 mL; for the 10% of women in our immunogenicity subcohort, 70 mL). Then, women were randomly assigned in a double-blinded fashion to receive either Cervarix or a “control” hepatitis A vaccine (a modified preparation of Havrix; GlaxoSmithKline Biologicals) (0.5 mL per dose). Both vaccines had identical packaging and were intended to be administered at 0, 1, and 6 months. At the 6-month vaccination visit, sexually experienced women self-collected a cervicovaginal exfoliated cell specimen for HPV DNA testing. Women who were not vaccinated in the allowable time frames (ie, 21–120 days and 121–300 days after enrollment for doses two and three, respectively) did not receive the dose. Women who became pregnant during the vaccination phase or who were referred to colposcopy were deferred, so that they missed the dose if the vaccination window was missed. A Data Safety Monitoring Board reviewed safety data annually during the vaccination phase and as needed during the follow-up period (final review: November 10, 2010).
The protocol required all women to be seen each year during the 4 years of follow-up. At each annual study visit, clinicians collected exfoliated cells from sexually active women for cytological evaluation and HPV DNA testing. Women found to have low-grade squamous intraepithelial neoplasia (LSIL) or HPV-positive atypical squamous cells of undetermined significance (ASCUS) underwent the same procedures at 6-month intervals for safety until three consecutive normal cytological results, when they returned to yearly follow-up. Women with evidence of high-grade disease or persistent low-grade abnormalities were referred to colposcopy for evaluation and treatment if needed. After the 4-year visit, a modified algorithm for colposcopic referral, biopsy, and treatment was applied to assure safety of participants at the completion of the initial 4-year study period (8).
Cervical samples were shipped from the clinic to the laboratory in Costa Rica in controlled temperature coolers. Duplicate 0.5 mL aliquots were made for HPV DNA testing by polymerase chain reaction (PCR), and they were frozen in liquid nitrogen. These samples were stored frozen in the repository in Costa Rica and then shipped in frozen batches to the Netherlands for HPV DNA analysis.
PCR-based HPV DNA testing was performed using the SPF10 PCR primer system and a DNA enzyme immunoassay detection of amplimers (DEIA; DDL Diagnostic Laboratory, Voorburg, the Netherlands) (11). Briefly, 10 μL proteinase K–treated DNA was added to 40 μL of PCR mix. The SPF10 PCR primer set amplifies a small fragment of 65 bp from the L1 region of mucosal HPV genotypes. Amplification products were detected using the HPV SPF10 PCR DEIA system. DEIA-positive SPF10 amplimers were used to identify the HPV genotype by reverse hybridization with the HPV line probe assay (LiPA25), containing probes for 25 different HPV genotypes (HPV genotypes 6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68/73, 70, and 74; SPF10 HPV LiPA25 version 1 [Labo Biomedical Products, Rijswijk, the Netherlands, based on licensed Innogenetics technology]) (12). To ensure that HPV16 and HPV18 infections were not missed, all specimens that were positive for the presence of HPV DNA using the SPF10 DEIA assay but negative for presence of HPV16 or HPV18 by the LiPA25 assay were also tested with type-specific PCR primer sets used to selectively amplify a 92 bp HPV16 E7 fragment (TS16) and a 126 bp HPV18 L1 fragment (TS18). Amplimers from the type-specific PCRs were detected by DEIA, similar to the method for SPF10 amplimer detection (13).
Statistical Analysis
Results follow a statistical analysis plan that was prepared before the investigation of this question was initiated. Fieldwork is ongoing, and individual information remains blinded. Analyses were therefore conducted by an external group, Information Management Systems (Rockville, MD), under the direction of the investigators who remained masked to individual random assignments.
All women were included in the analysis except those who were both HPV16 and HPV18 DNA positive at enrollment or had no follow-up visits post-enrollment. Women in the HPV and control arms were grouped according to the number of doses they received. Reasons for not receiving the full-dosing regimen were compared by use of the χ2 test for categorical variables. Median follow-up time from enrollment was calculated in months by arm and dose and compared by use of the nonparametric Kruskal–Wallis test.
The primary endpoint for this analysis was a newly detected HPV16 or HPV18 infection that persisted for at least 10 months, which is an intermediate cancer endpoint that is associated with development of cervical intraepithelial neoplasia 3 (14). The definition of that endpoint (persistent infection) required two detections of infection by the same HPV type that occurred at least 10 months apart with no intervening negative tests. We required the first detection of the infection to be at the 6-month vaccine visit or later to avoid misclassification of infections prevalent at enrollment. In this woman-level analysis, each woman could only contribute once to the numerator (number of women who had an incident persistent HPV16 and/or HPV18 infection) and denominator (all women with at least one study visit post-enrollment and HPV 16 and/or HPV18 DNA negative at enrollment), even if multiple HPV types were detected. Furthermore, HPV infection status was assessed at both regular and colposcopy study visits.
We evaluated 6-month persistence as a secondary endpoint because it is more distal to precancer; 6-month persistence was defined as two or more positive tests for a given HPV type that occurred at least 4 months apart with no intervening negative tests. We also assessed VE for incident 12-month persistent HPV31, HPV33, and HPV45 infections combined because these are the HPV types for which there is prior evidence of vaccine cross-protection (2,10); in this analysis, we excluded women with prevalent HPV31, HPV33, and HPV45 infections detected at enrollment. The endpoint for this analysis was defined as a new type-specific HPV31, HPV33, or HPV45 infection that was first detected at the 6-month vaccine visit or later, and again at a visit at least 10 months later, with no intervening negative tests for the HPV type in question.
For each dose group and arm, we defined the attack rate as the proportion of the number of events among the number of women over the 4 years of the study. Any difference in the attack rates among women who received one, two, or three doses of the control vaccine could reflect random variation or underlying differences in the risk of incident infection by the number of doses; the absence of a difference in the attack rates in the control vaccine arm (in which women are not protected from HPV infections unlike the HPV vaccine arm) would indicate that determinants of risk, like sexual behavior, are not biasing the comparison between number of doses received.
Within each dosage group, the complement of the ratios of the attack rates for the HPV and the control arms are the VE estimates. We used data from the control arm, instead of directly comparing by the number of doses within the HPV arm alone, because we were not certain whether the risk of a new persistent HPV16 or HPV18 infection might vary by number of doses received, even if no HPV vaccine had been administered. We calculated exact confidence intervals (15) for VE based on the binomial distribution of the number of events in the HPV arm among the total number of events in the HPV and control arms. The exact confidence limits for VE use numerators of the attack rates based on the product of the total number of events and the exact binomial limit (16).
The VE estimate for three doses is presented in this article to compare that for two doses and one dose. The three-dose VE estimate in the current analysis, however, differs from our previously presented estimate (90.9% for according to protocol efficacy) (10) because of slightly different analytical cohorts: the present analysis did not exclude based on disease, treatment, or vaccination windows, and it counted outcomes starting at the 6-month visit instead of the 12-month visit.
If we found statistically significant evidence (ie, a 95% CI excluding zero) that two or one dose(s) of vaccine conferred VE, we calculated the ratios of VEs for two vs three doses and for one vs three doses and their corresponding 95% confidence intervals using an unconditional bootstrap percentile method (17) with 1000 bootstrap datasets, where resampling was done with replacement. Because the cost of administering a series of vaccinations is approximately proportional to the number of doses given, we prespecified that a ratio of 2:3 for the VEs of HPV in women who received two and three doses would reflect equal efficacy per dose and per cost. Thus, we would claim that vaccination with fewer than three doses was successful if we rejected a one-sided test of the null hypothesis that the ratio of the two-dose and three-dose VEs equals two-thirds, equivalent to the lower bound of the 95% confidence interval for the estimate of the ratio being above two-thirds. For one dose, the corresponding criterion for claiming a positive result is that the lower bound of the 95% confidence interval for the estimate of the ratio is above one-third.
The 12 months of persistence required for the endpoint began with an incident infection at the 6-month visit, when the final vaccine dose was scheduled to be administered, or later. A sensitivity analysis that included only infections incident at the 12-month visit or later as endpoints addressed the possibility of bias from differential assessment of outcomes in women who missed vs received the 6-month vaccination.
The present analysis contains updated data until June 21, 2010, when the analytical database was frozen; using the available data at the initial January 1, 2010 data lock point, statistically significant vaccine efficacy (VE) was observed by dose (VE for three doses = 78%, 95% confidence interval [CI] = 67% to 86%; VE for two doses = 82%, 95% CI = 43% to 96%; VE for one dose = 100%, 95% CI = 57% to 100%).
Results
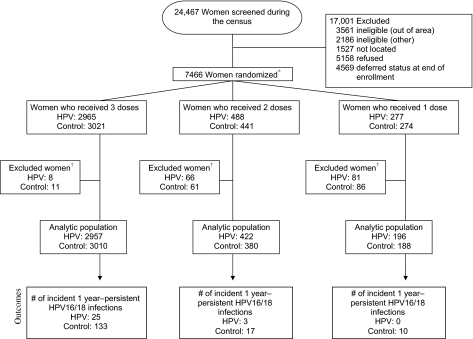
Of the 24 467 women who we screened, 7466 women were enrolled and were randomly assigned to the HPV16/18 vaccine Cervarix or to a control vaccine for hepatitis A (Figure 1). We excluded women who had no post-enrollment visits or who were HPV16 and HPV18 DNA positive at enrollment (155 in the HPV vaccine arm; 158 in the control vaccine arm); the analytic cohort comprised 5967 women who received three doses (of whom 2957 received the HPV vaccine and 3010 received the control vaccine), 802 women who received two doses (of whom 422 received the HPV and 380 received the control vaccine), and 384 women who received one dose (of whom, 196 received the HPV and 188 received the control vaccine) (P for differences in the number of women receiving the HPV vaccine vs the control vaccine by dose = 0.23). Median time of follow-up from date of first vaccine administration was 50 months (4.2 years) and was similar between arms within dose.
Figure 1.
CONSORT diagram of women in the Costa Rica Vaccine Trial. The primary aim of the trial was to evaluate the efficacy of a three-dose regimen of the Cervarix vaccine to prevent persistent type-specific infection with HPV16 or HPV18 and the subsequent development of HPV-associated precancerous lesions. Although 7466 women were randomized to receive three doses of either Cervarix or control vaccine, approximately 20% received fewer than three doses of Cervarix or control vaccine mostly because of pregnancy and referral to colposcopy. Thus, we were able to investigate the protection afforded by two and one dose(s) of the HPV vaccine because the cost and logistical difficulties of the standard three-dose vaccine regimen compromises implementation. Asterisk indicates that four women received discordant vaccines (one woman was enrolled twice and received three doses of each vaccine and three women received two doses of one vaccine and one dose of the other vaccine). For the purpose of this analysis, the control dosing was ignored and they were categorized based on the number of HPV vaccines they received. Dagger indicates that women who were both HPV16 and HPV18 DNA positive at enrollment were excluded, as were women with no follow-up visits post-enrollment.
Participants’ reasons for not receiving all doses were similar in both arms conditional on the number of doses actually received (P > .05 for all comparisons; Table 1). The most common reasons for not receiving all three doses were involuntary, including pregnancy and colposcopy referral; it was less common for participants to refuse the vaccine.
Table 1.
Reasons for missing doses among women who received two doses or one dose of vaccine*
| Reason for missed dose | Missing second or third dose, among women who received two doses |
Missing second dose, among women who received one dose† |
Missing third dose, among women who received one dose† |
|||
| HPV, No. (%) | Control, No. (%) | HPV, No. (%) | Control, No. (%) | HPV, No. (%) | Control, No. (%) | |
| Pregnancy | 155 (31.8) | 145 (32.9) | 35 (12.6) | 35 (12.8) | 50 (18.1) | 57 (20.8) |
| Colposcopy referral | 13 (2.7) | 12 (2.7) | 57 (20.6) | 45 (16.4) | 57 (20.6) | 42 (15.3) |
| Medical condition | 79 (16.2) | 66 (15.0) | 49 (17.7) | 60 (21.9) | 43 (15.5) | 57 (20.8) |
| Vaccine refusal by participant | 63 (12.9) | 58 (13.2) | 42 (15.2) | 38 (13.9) | 87 (31.4) | 84 (30.7) |
| Missed study visit | 98 (20.1) | 96 (21.8) | 58 (20.9) | 55 (20.1) | 20 (7.2) | 23 (8.4) |
| Other‡ | 80 (16.4) | 64 (14.5) | 36 (13.0) | 41 (15.0) | 20 (7.2) | 11 (4.0) |
This table includes all vaccinated women to prevent unblinding that could happen with cells that had small sample size (ie, fewer than five women). HPV = human papillomavirus.
For women who received only one dose, it was possible to have different reasons for missing each of the two subsequent doses.
The three most common “other” reasons included: the woman could not get time off from work to come into the clinic for a vaccination, personal reasons, or the woman was not using an acceptable form of birth control.
For all dosage groups, women who received the HPV vaccine vs the control vaccine were comparable with respect to age at entry and total number of visits (Table 2). Among those who received two doses or one dose of the vaccine, HPV16 and HPV18 DNA and serologic status at enrollment were comparable by arm within each dose group. Among women who received three doses, we observed no differences in serologic status at enrollment; however, women in the control group were marginally more likely to have been HPV16 and/or HPV18 DNA positive at enrollment than women who received the HPV vaccine (8.9% vs 7.5%, P = .05), as noted previously (8,9). In the control arm, the attack rates of incident HPV16 or HPV18 infections that persisted for 1 year were similar among women who received three doses (4.4%), two doses (4.5%), or one dose (5.3%) indicating that they were at similar risk for acquiring HPV infections regardless of the number of doses received (Table 3).
Table 2.
Participant characteristics by number of vaccine doses received and vaccine arm*
| Characteristic | One dose† |
Two doses† |
Three doses† |
|||
| HPV, No. (%) | Control, No. (%) | HPV, No. (%) | Control, No. (%) | HPV, No. (%) | Control, No. (%) | |
| Age at entry, y‡ | ||||||
| 18–19 | 53 (27.0) | 52 (27.7) | 140 (33.2) | 143 (37.6) | 933 (31.6) | 982 (32.6) |
| 20–21 | 56 (28.6) | 49 (26.1) | 117 (27.7) | 85 (22.4) | 739 (25.0) | 725 (24.1) |
| 22–23 | 42 (21.4) | 40 (21.3) | 90 (21.3) | 80 (21.1) | 661 (22.4) | 704 (23.4) |
| 24–25 | 45 (23.0) | 47 (25.0) | 75 (17.8) | 72 (18.9) | 624 (21.1) | 599 (19.9) |
| No. of clinic visits attended§ | ||||||
| 1–2 | 13 (6.6) | 17 (9.0) | 36 (8.5) | 37 (9.7) | 74 (2.5) | 68 (2.3) |
| 3–5 | 96 (49.0) | 99 (52.7) | 275 (65.2) | 224 (58.9) | 1912 (64.7) | 1867 (62.0) |
| 6–8 | 67 (34.2) | 62 (33.0) | 97 (23.0) | 106 (27.9) | 813 (27.5) | 896 (29.8) |
| ≥9 | 20 (10.2) | 10 (5.3) | 14 (3.3) | 13 (3.4) | 158 (5.3) | 179 (5.9) |
| Median (IQR) | 5 (4 to 7) | 5 (4 to 7) | 5 (4 to 6) | 5 (4 to 6) | 5 (5 to 6) | 5 (5 to 6) |
| HPV16/18 DNA status at enrollment‖ | ||||||
| Negative¶ | 245 (88.4) | 241 (88.0) | 438 (89.8) | 403 (91.4) | 2742 (92.5) | 2751 (91.1) |
| Positive# | 32 (11.6) | 33 (12.0) | 50 (10.2) | 38 (8.6) | 223 (7.5)** | 270 (8.9) |
| HPV16/18 serostatus at enrollment‖ | ||||||
| Negative | 173 (62.5) | 165 (60.2) | 294 (60.2) | 269 (61.0) | 1899 (64.0) | 1899 (62.9) |
| Positive# | 104 (37.5) | 109 (39.8) | 194 (39.8) | 172 (39.0) | 1066 (36.0) | 1122 (37.1) |
IQR = interquartile range; HPV = human papillomavirus.
The women who received discordant vaccines were categorized according to the number of HPV vaccine doses they received.
Two women enrolled at age 17 years were included in the 18–19 year age group; one woman enrolled at age 26 and one woman enrolled at age 27 were included in the 24–25 year age group.
Includes clinic visits for vaccination, annual screening, colposcopy, and treatment (when needed).
Data on HPV16 and HPV18 status at enrollment include all vaccinated women to prevent unblinding that could happen with cells that had small sample size (ie, fewer than five women).
Included in the negative category are virgins (who did not provide a cervical specimen for HPV testing) and three women who were missing enrollment HPV polymerase chain reaction results
Indicates positive for either or both HPV16 and HPV18 at enrollment.
Table 3.
Estimated vaccine efficacy against 12-month incident persistent infection for women who received one, two, and three doses of a HPV vaccine compared with a control vaccine
| Doses, No. | Arm | Women, No. | Events, No. | Proportion of women with incident, 12-month persistent HPV16 or HPV18 infections, % (95% CI)* | HPV vaccine efficacy, % (95% CI)* | Efficacy relative to three-dose regimen, % (95% CI)* |
| 3 (standard regimen)† | Control | 3010 | 133 | 4.4% (3.7% to 5.2%) | 80.9% (71.1% to 87.7%) | Referent |
| HPV | 2957 | 25 | 0.85% (0.56% to 1.2%) | |||
| 2‡ | Control | 380 | 17 | 4.5% (2.7% to 6.9%) | 84.1% (50.2% to 96.3%) | 104% (69.3% to 129%) |
| HPV | 422 | 3 | 0.71% (0.18% to 1.9%) | |||
| 1 | Control | 188 | 10 | 5.3% (2.7% to 9.3%) | 100% (66.5% to 100%) | 124%§ |
| HPV | 196 | 0 | 0.0% (0.0% to 1.5%) |
Human papillomavirus = HPV; 95% CI = 95% confidence interval.
The distribution of the time at diagnosis of the case patients in the HPV and control arms was qualitatively assessed to determine whether the protection afforded by two doses may be short lived compared with that of three doses. Twenty (80.0%) of 25 breakthrough 1-year persistent HPV infections in the vaccine arm were first detected in the first year of follow-up (suggesting missed prevalent infections at enrollment) compared with 40 (30.1%) of 133 infections detected in the control arm. Sixteen (64.0%) of 25 breakthrough infections occurred among women who were HPV16 seropositive at enrollment.
One of the three breakthrough infections was detected in each of the first 3 years of the study compared with 0%, 64.7%, 23.5%, and 11.8% of the 17 infections in years 1, 2, 3, and 4 of the study, respectively. One (33.3%) of the three breakthrough infections occurred in a woman who was HPV16 seropositive at enrollment.
No bootstrap confidence interval could be estimated due to the presence of zero events in the HPV arm after one dose of vaccine.
VE for three doses against newly detected HPV16 or HPV18 that persisted at least 1 year was 80.9% (95% CI = 71.1% to 87.7%; 25 and 133 events in the HPV and control arms, respectively), for two doses was 84.1% (95% CI = 50.2% to 96.3%; three and 17), and for one dose was 100% (95% CI = 66.5% to 100%; zero and 10); no statistically significant trend for increasing VE with fewer doses was observed (Ptrend = .21) (Table 3). VE results were similar for the 6-month persistent HPV16 and/or HPV18 infection endpoint (Supplementary Table 1, available online). Although we did see cross-protection against incident 1-year persistent infection with HPV31, HPV33, and HPV45 combined in women who received the standard three-dose regimen (41.3% [95% CI = 18.9% to 57.9%]; 57 and 99 events in the HPV and control arms, respectively) (8), there was no evidence of such cross-protection in women who received two doses: −25.9% (95% CI = −334% to 61.1%; seven and five). The small number of total events (n = 7) limited our ability to evaluate cross-protection among women who received only one dose.
Because there was statistically significant evidence that two doses or even one dose of the vaccine had VE against HPV16 and HPV18 (ie, for all VEs, the lower bound of the 95% CI was substantially greater than 0%), we computed the ratios of the VEs for fewer than three doses vs full regimen. The ratio of 2:3 dose VEs was 104% (95% CI = 69.3% to 129%), consistent with two doses being more than two-thirds as effective as three doses (Table 3). The 1:3 ratio of VEs was 124%, but no bootstrap confidence interval could be estimated because of the absence of events in the HPV arm.
In the sensitivity analysis that investigated the potential bias from differential assessment of outcomes by dose, VEs excluding the 6-month study visit were comparable: 82.4% (95% CI = 72.7% to 89.0%) for three doses, 84.1% (95% CI = 50.2% to 96.3%) for two doses, and 100% (95% CI = 66.5% to 100%) for one dose.
Discussion
We investigated the protection afforded by two and one dose(s) of the HPV vaccine because the cost and logistical difficulties of the standard three-dose vaccine regimen compromises implementation of this life-saving measure in resource-poor settings. Nested within our phase III randomized clinical trial in Costa Rica, we provide the first clinical evidence that two doses of the bivalent HPV vaccine are highly efficacious in the prevention of incident HPV16 and HPV18 infections that persist for at least 1 year (VE = 84.1% [95% CI = 50.2% to 96.3%]), and that even a single dose may be highly efficacious (VE = 100% [95% CI = 66.5% to 100%]).
Although this analysis was not randomized, the attack rates of new infections were essentially equal among women who received one, two, and three doses of the control vaccine. Equal attack rates in the control arm suggested that risks of infection were the same regardless of number of doses received. Furthermore, pregnancy, the most common reason that women received a reduced number of doses, was unrelated to vaccine assignment (18) and therefore unlikely to bias the VE estimates. These observations provide support for the robustness of our analysis.
Further evaluation of the efficacy of one dose is particularly important for several reasons. The protection afforded by a single dose of vaccine was unexpected because other subunit vaccines typically require boosting following the prime dose to confer long-term protection, although perhaps many of our sexually active participants had been previously exposed to HPV16 or HPV18. Also, the number of events (N = 10 for ≥12-month persistent infection; N = 15 for ≥6-month persistent infection) was small, and follow-up time was limited.
Available evidence from immunologic studies supports our efficacy findings. Girls who received two and three doses of Gardasil had similar antibody titers several years post-vaccination (19). Women in the control arm of our trial with relatively high antibody titers after natural infection were partially protected against subsequent incident HPV16 and HPV18 infection (20). However, the exact relationship between these antibody markers and future risk of disease is not clear.
Important questions remain unanswered. The full three-dose regimen may confer greater cross-protection (2,10) against heterologous HPV types, as suggested by the limited evidence here. Additionally, data from a trial of the bivalent HPV16/18 vaccine in Costa Rica may not apply to other vaccine formulations (such as the quadrivalent HPV vaccine with alum adjuvant) or to other populations who may have more comorbidities than our study population (eg, endemic parasitic infections that could, in turn, produce malnutrition and possibly a less robust immune response). Finally, the duration of protection for fewer than three doses must be quantified. Future studies, such as the cluster-randomized trial that is being conducted in India by the World Health Organization, are the next critical step in addressing these important research questions.
Our clinical efficacy data provide suggestive evidence that an HPV vaccine program that could vaccinate 50% more women with a two-dose regimen could potentially reduce cervical cancer incidence more than a standard three-dose program that uses the same number of total doses but in fewer women. Surveillance of the ongoing programs with extended intervals (vaccination at 0, 6, and 60 months), such as those currently implemented in Quebec and Mexico, can monitor the short-term effectiveness of administering fewer than three doses. If randomized studies and cost-effectiveness analyses confirm the net benefits of administering fewer doses, and the duration of protection is sufficient, then the need for fewer doses may make primary prevention of cervical cancer a reality, especially for women in areas where most cervical cancers occur.
Funding
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the US National Cancer Institute (NCI). The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women’s Health.
Supplementary Material
Footnotes
This study was conducted with the support from the Ministry of Health of Costa Rica. Vaccine was provided for our trial by GlaxoSmithKline Biologicals (GSK), under a Clinical Trials Agreement with the NCI. GSK also provided support for aspects of the trial associated with regulatory submission needs of the company under FDA BB-IND 7920. SchillerJ. T. Schiller and D. R. Lowy report that they are named inventors on US government–owned HPV vaccine patents that are licensed to GSK and Merck and for which the NCI receives licensing fees. J. T. Schiller and D. R. Lowy are entitled to limited royalties as specified by federal law. No other financial disclosures were reported.
The NCI and Costa Rica investigators are responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the article. The NCI and Costa Rica investigators make final editorial decisions on this and subsequent publications; GSK has the right to review and comment.
Cervarix is a registered trademark of the GlaxoSmithKline group of companies.
ARK, SW, ACR, AH, RH, CP, DS, and MS designed the analysis. JS conducted all statistical programming under direction of ARK and SW. PW, SW, ACR, AH, RH, CP, DS, MS, MES, and SJ were responsible for data collection. WQ was responsible for all HPV-related test results. ARK, SW, ACR, AH, RH, CP, DS, and MS analyzed the data. AKR, SW, ACR, AH, RH, CP, DS, MS, DRL, and JTS interpreted the data. ARK and SW wrote the article. ARK, SW, ACR, AH, RH, DS, MS, DRL, JTS, MES, and JS critically reviewed all material for important intellectual content. ARK and SW are the guarantors of all material contained herein.
The names and affiliations of investigators in the Costa Rica Vaccine Trial (CVT) group are given below.
Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica: Mario Alfaro (Cytopathologist); Manuel Barrantes (Field Supervisor); M. Concepción Bratti (co-Investigator); Fernando Cárdenas (General Field Supervisor); Bernal Cortés (Specimen and Repository Manager); Albert Espinoza (Head, Coding and Data Entry); Yenory Estrada (Pharmacist); Paula González (co-Investigator); Diego Guillén (Pathologist); Rolando Herrero (co-Principal Investigator); Silvia E. Jiménez (Trial Coordinator); Jorge Morales (Colposcopist); Luis Villegas (Colposcopist); Lidia Ana Morera (Head Study Nurse); Elmer Pérez (Field Supervisor); Carolina Porras (co-Investigator); Ana Cecilia Rodríguez (co-Investigator); Libia Rivas (Clinical coordinator).
University of Costa Rica, San José, Costa Rica: Enrique Freer (Director, HPV Diagnostics Laboratory); José Bonilla (Head, HPV Immunology Laboratory); Alfonso García-Piñeres (Immunologist); Sandra Silva (Head Microbiologist, HPV Diagnostics Laboratory); Ivannia Atmella (Microbiologist, Immunology Laboratory); Margarita Ramírez (Microbiologist, Immunology Laboratory).
US National Cancer Institute, Bethesda, MD: Allan Hildesheim (co-Principal Investigator & NCI co-Project Officer); Aimée R. Kreimer (Investigator); Douglas R. Lowy (HPV Virologist); Nora Macklin (Trial Coordinator); Mark Schiffman (Medical Monitor & NCI co-Project Officer); John T. Schiller (HPV Virologist); Mark Sherman (QC Pathologist); Diane Solomon (Medical Monitor & QC Pathologist); Sholom Wacholder (Statistician).
SAIC, NCI-Frederick, Frederick, MD: Ligia Pinto (Head, HPV Immunology Laboratory); Troy Kemp (Scientist, HPV Immunology Laboratory).
Women’s and Infants’ Hospital, Providence, RI: Claire Eklund (QC Cytology); Martha Hutchinson (QC Cytology).
Georgetown University, Washington, DC: Mary Sidawy (Histopathologist).
DDL Diagnostic Laboratory, the Netherlands: Wim Quint (Virologist, HPV DNA Testing); Leen-Jan van Doorn (HPV DNA Testing).
We would like to extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. We also acknowledge the tremendous effort and dedication of the staff in Costa Rica involved in this project, including Bernardo Blanco and his team (census); Ricardo Cerdas and Ana Hernández (blood processing); José Miguel González, Osman López, Johnny Matamoros, Manuel Sánchez, Rafael Thompson, and Jorge Umaña (field activity coordinators); Su Yen Araya, Hazel Barquero, Hayleen Campos, Muriel Grijalba, Ana Cristina Monge, Ana Peraza, Diana Robles, María Fernanda Sáenz, Dorita Vargas, and Jessica Vindas (clinic coordinators); Paola Alvarez, Dinia Angulo, Ana Live Arias, Betzaida Barrantes, Marianela Bonilla, Mary José Calvo, Loretto Carvajal, Jessenia Chinchilla, Blanca Cruz, Marianela Herrera, Andrea Interiano, Fabiola Jiménez, Erick Lagos, Viviana Loría, Andrea Messeguer, Rebeca Ocampo, Silvia Padilla, Angie Ramírez, Libia Rivas, Daniela Romero, Byron Romero, Jessenia Ruiz, Daniela Ruiz, Genie Saborío, Sofía Ssoto, Malena Salas, Adriana Torrez, Natalia Ugalde, Ana Cristina Ugalde, Adriana Vallejos, Yesenia Vázquez, Maricela Villegas (clinicians); Marta Alvarado, Ana Cristina Arroyo, Gloriana Barrientos, Diana Díaz, Marlen Jara, Maureen Matarrita, María Ester Molina, Elida Ordóñez, Gina Sánchez, and Zihara Villegas (nurses); Arianne Castrillo and Vivian López (education and outreach effort coordinators); Karla Coronado (appointment coordinator); Ricardo Alfaro (quality control coordinator); Charles Sánchez and Livia Romero (document center coordinators); Cristian Montero (quality assurance, regulatory); and Carlos Avila and Eric Alpízar (IT coordinators). Special recognition is also extended to Sofía Elizondo, Executive Director of Fundación INCIENSA and her staff for their administrative support. In the United States, we would like to extend our appreciation to the team from Information Management Services (IMS) responsible for the development and maintenance of the data system used in the trial and who serve as the data management center for this effort. We would like to specifically acknowledge the invaluable contributions made by Jean Cyr, Julie Buckland, Laurie Rich, Brian Befano, and Dennis Buckman. We acknowledge the contributions made by individuals at Westat, Inc, who provided project development and/or monitoring support, including Kerry Grace Morrisey, Kirk Midkiff, Susan Truitt, Sonia Stoszek, Maribel Gomez, and Isabel Trejos. We acknowledge the assistance provided by Carla Chorley, Troy Moore, Kathi Shea, and Heather Siefers in the establishment of a specimen and vaccine repository for our trial and in their continued assistance with the handling and shipment of specimens. We would like to acknowledge Gary Dubin, Anne Schuind, Frank Struyf, Kelechi Lawrence, Darrick Fu, and Bruce Innis from GSK Biologicals for their contribution to discussions regarding trial conduct and Francis Dessy and Catherine Bougelet for HPV16/18 antibody testing. We would like to thank members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants in our trial (Steve Self [Chair], Adriana Benavides, Luis Diego Calzada, Ruth Karron, Ritu Nayar, and Nancy Roach) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (Joanna Cain, Chair, Diane Davey, David DeMets, Francisco Fuster, Ann Gershon, Elizabeth Holly, Silvia Lara, Henriette Raventós, Wasima Rida, Luis Rosero-Bixby, Kristen Suthers, Sarah Thomas, and Raphael Viscidi). We thank Nora Macklin for her support in preparing the article for submission.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 3.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 4.Goldie SJ, O’Shea M, Diaz M, Kim SY. Benefits, cost requirements and cost-effectiveness of the HPV16,18 vaccine for cervical cancer prevention in developing countries: policy implications. Reprod Health Matters. 2008;16(32):86–96. doi: 10.1016/S0968-8080(08)32409-4. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. National, state, and local area vaccination coverage among adolescents aged 13–17 years—United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(36):997–1001. [PubMed] [Google Scholar]
- 6.Immunise Australia Program. Human Papillomavirus (HPV) http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/immunise-hpv. Accessed July 12, 2011. [Google Scholar]
- 7. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_123826.pdf. Accessed July 12, 2011. [Google Scholar]
- 8.Herrero R, Hildesheim A, Rodriguez AC, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26(37):4795–4808. doi: 10.1016/j.vaccine.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hildesheim A, Herrero R, Wacholder S, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298(7):743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 10.Herrero R, Wacholder S, Rodríguez AC, et al. Prevention of persistent HPV infection by a HPV 16–18 vaccine: a community based randomized clinical trial in Guanacaste, Costa Rica. Oral presentation #459, presented at the 25th International Papillomavirus Conference; July 3-8, 2010. [Google Scholar]
- 11.Kleter B, van Doorn LJ, ter Schegget J, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am.J Pathol. 1998;153(6):1731–1739. doi: 10.1016/S0002-9440(10)65688-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleter B, van Doorn LJ, Schrauwen L, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37(8):2508–2517. doi: 10.1128/jcm.37.8.2508-2517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Doorn LJ, Molijn A, Kleter B, Quint W, Colau B. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol. 2006;44(9):3292–3298. doi: 10.1128/JCM.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koshiol J, Lindsay L, Pimenta JM, et al. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am J Epidemiol. 2008;168(2):123–137. doi: 10.1093/aje/kwn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agresti A. Categorical Data Analysis. 2nd ed. Hoboken, NJ: John Wiley and Sons, Inc; 2002. [Google Scholar]
- 16.Rothman KJ, Boice JD. Epidemiologic Analysis with a Programmable Calculator. New Edition. Boston, MA: Epidemiology Resources, Inc; 1982. [Google Scholar]
- 17.Efron B. The Jackknife, the Bootstrap and Other Resampling Plans. Philadelphia, PA: Society for Industrial and Applied Mathematics; 1982. [Google Scholar]
- 18.Wacholder S, Chen BE, Wilcox A, et al. Risk of misscarriage with bivalent vaccine against human papillomavirus (HPV) types 16 and 18: pooled analysis of two randomised controlled trials. BMJ. 2010;340:c712. doi: 10.1136/bmj.c712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobson S, Dawar M, Kollman T, et al. A two dose HPV vaccine schedule in girls: immunogenecity at 24 months. Poster #691, presented at the 25th International Papillomavirus Conference. July 3-8, 2010. [Google Scholar]
- 20.Safaeian M, Porras C, Schiffman M, et al. Costa Rican Vaccine Trial Group. Epidemiological study of anti-HPV 16/18 seropositivity and subsequent risk of HPV 16 and -18 infections. J Natl Cancer Inst. 2010;102(21):1653–1662. doi: 10.1093/jnci/djq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.