Abstract
Background
The combination of chemotherapy with thoracic radiotherapy (TRT) compared with TRT alone has been shown to confer a survival advantage for good performance status patients with stage III non–small cell lung cancer. However, it is not known whether sequential or concurrent delivery of these therapies is the optimal combination strategy.
Methods
A total of 610 patients were randomly assigned to two concurrent regimens and one sequential chemotherapy and TRT regimen in a three-arm phase III trial. The sequential arm included cisplatin at 100 mg/m2 on days 1 and 29 and vinblastine at 5 mg/m2 per week for 5 weeks with 60 Gy TRT beginning on day 50. Arm 2 used the same chemotherapy regimen as arm 1 with 60 Gy TRT once daily beginning on day 1. Arm 3 used cisplatin at 50 mg/m2 on days 1, 8, 29, and 36 with oral etoposide at 50 mg twice daily for 10 weeks on days 1, 2, 5, and 6 with 69.6 Gy delivered as 1.2 Gy twice-daily fractions beginning on day 1. The primary endpoint was overall survival, and secondary endpoints included tumor response and time to tumor progression. Kaplan–Meier analyses were used to assess survival, and toxic effects were examined using the Wilcoxon rank sum test. All statistical tests were two-sided.
Results
Median survival times were 14.6, 17.0, and 15.6 months for arms 1–3, respectively. Five-year survival was statistically significantly higher for patients treated with the concurrent regimen with once-daily TRT compared with the sequential treatment (5-year survival: sequential, arm 1, 10% [20 patients], 95% confidence interval [CI] = 7% to 15%; concurrent, arm 2, 16% [31 patients], 95% CI = 11% to 22%, P = .046; concurrent, arm 3, 13% [22 patients], 95% CI = 9% to 18%). With a median follow-up time of 11 years, the rates of acute grade 3–5 nonhematologic toxic effects were higher with concurrent than sequential therapy, but late toxic effects were similar.
Conclusion
Concurrent delivery of cisplatin-based chemotherapy with TRT confers a long-term survival benefit compared with the sequential delivery of these therapies.
CONTEXTS AND CAVEATS
Prior knowledge
Patients with stage III non–small cell lung cancer have been shown to benefit from a combination of chemotherapy with thoracic radiotherapy. However, it is not known if sequential or concurrent delivery of these therapies is the more beneficial treatment strategy.
Study design
In a randomized phase III trial, 610 patients with inoperable stage II or III non–small cell lung cancer were randomly assigned to two different concurrent regimens (chemotherapy with once-daily or twice-daily radiotherapy) or to sequential treatment with these therapies.
Contribution
Five-year survival was statistically significantly higher for patients treated with the concurrent regimen with once-daily radiotherapy compared with the sequential treatment.
Implication
Long-term survival was better for patients in the concurrent delivery arms even though the rates of acute nonhematological toxic effects were higher than in the sequential arm.
Limitations
Patients with lower functional status or with other comorbid conditions were not included in the trial. Thus, the current findings may not be applicable to these patients.
From the Editors
Nearly 50 000 Americans are diagnosed each year with non–small cell lung cancer (NSCLC) that is confined to the thorax and not amenable to potentially curative resection because of invasion of adjacent structures and/or lymph node metastases. These patients have stage III disease according to both the 1992 and 2010 American Joint Commission on Cancer (AJCC) staging system (1), and the optimal management of this large and heterogeneous population remains controversial.
At least five randomized trials (2–6) conducted since the early 1980s have demonstrated a survival advantage for good performance status patients with stage III NSCLC when chemotherapy is combined with thoracic radiotherapy (TRT) compared with TRT alone (2–6). The median survival time (MST) of the combined modality arms in these trials ranged from 12 to 14 months compared with 9 to 12 months with TRT alone. Although each of these trials used a platinating compound as one of the chemotherapeutic agents, there was considerable variability among the combined modality arms with respect to chemotherapeutic agent selection, dosing, and schedule, dose and schedule of TRT, and the temporal relationship between chemotherapy and TRT. Two of these trials were conducted in North America by National Cancer Institute–sponsored cooperative groups, and both used the sequential chemoradiation regimen of two cycles of cisplatin and vinblastine followed by TRT (2,3). The survival benefit of this regimen compared with TRT alone and its relatively low toxicity helped establish sequential cisplatin-based chemoradiation as a standard approach to patients with unresected stage III NSCLC.
Since 1990, several phase II trials of concurrent chemoradiation regimens for such patients have resulted in promising MSTs of 16–20 months (7,8). In addition, there are at least two randomized trials comparing concurrent chemoradiation to TRT alone, which demonstrated a survival advantage favoring the concurrent therapy arms (5,6). Although the concurrent delivery of these therapies increased both the frequency of severe hematologic and nonhematologic toxicity seen with sequential chemoradiation, the survival results were considered sufficiently promising to the Radiation Therapy Oncology Group (RTOG) to conduct a phase III multi-institutional trial with two promising concurrent chemoradiation regimens and the sequential chemoradiation regimen tested in earlier cooperative group trials. This trial compared concurrent once-daily radiation against sequential therapy and then the better of those two against concurrent twice-daily radiation to determine whether the rate of overall survival is improved by concurrent and/or hyperfractionated radiotherapy administration.
Methods
Patient Eligibility
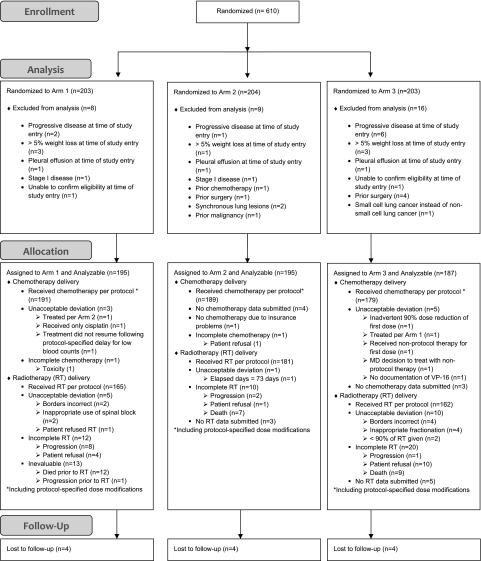
Patients with medically or surgically inoperable AJCC stage II, IIIA, or IIIB newly diagnosed histologically confirmed NSCLC (adenocarcinoma, squamous cell carcinoma, large cell carcinoma, or NSCLC—not otherwise specified) were eligible for the study. Patients were required to have a Karnofsky performance status (KPS) of 70 or greater, to have no more than 5% weight loss over 3 months before enrollment, to be 18 years of age or older, and to be without evidence of metastatic disease. Patients with pleural effusions with malignant cytology were ineligible as were those with pleural effusions visible on chest x-ray unless the effusion appeared only after a thoracotomy or another invasive procedure (Figure 1). Radiological assessment included chest x-ray, computerized axial tomography of the thorax and upper abdomen and brain, and technetium-99 bone scan. All studies, including a complete medical history and physical examination, required completion within 4 weeks of study enrollment. Patients were required to have a hemoglobin value of 8.0 g or higher, a granulocyte count of 2000/cc or higher, a platelet count of 100 000/cc or higher, a serum creatinine level 1.5 g or less, and serum levels of bilirubin and serum glutamic oxaloacetic transaminase less than 1.5 times the upper range of normal laboratory values, unless the abnormality was caused by documented benign disease. Pregnant women were ineligible, and patients with childbearing potential were required to practice appropriate contraception.
Figure 1.
CONSORT diagram for Radiation Therapy Oncology Group (RTOG) 9410 clinical trial. RT = radiotherapy.
Patients who underwent a complete tumor resection were ineligible, as were those having received prior chemotherapy or thoracic or neck radiotherapy. Patients were ineligible if they could be enrolled on an RTOG phase III trial for patients with confirmed N2 lymph node involvement evaluating the role of surgery for such patients (RTOG 9309). All patients signed an informed consent that was approved by their institutional review board before random assignment. Patients were randomly assigned to one of three treatment arms via phone call to RTOG headquarters and were stratified by KPS (70–80 vs 90–100) and clinical stage (II vs IIIA vs IIIB).
Treatment
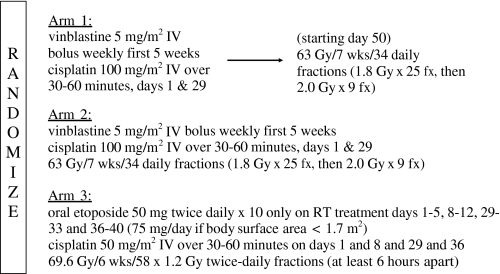
The chemotherapy regimen was identical in arms 1 and 2 and identical to that delivered in the investigational arm of the two American cooperative group phase III studies (2,3) that demonstrated a survival advantage for chemoradiation compared with RT alone (Figure 2) (2,3). Cisplatin chemotherapy was delivered intravenously at a dose of 100 mg/m2 over a 30- to 60-minute period on day 1 or 2, and vinblastine was delivered at a dose of 5 mg/m2 weekly for five consecutive weeks beginning on day 1. Arm 3 chemotherapy was based on the regimen piloted in an RTOG phase II trial in which the encouraging MST of 19.6 months was achieved (7). Cisplatin was delivered intravenously at 50 mg/m2 over 30–60 minutes on days 1, 8, 29, and 36, and oral etoposide was administered at a dose of 50 mg twice daily on days 1–5, 8–12, 29–33, and 36–40. The dosing of oral etoposide was reduced to 75 mg/d if the patient’s body surface area was less than 1.7 m2. In all three arms, chemotherapy dose modifications were based on the granulocyte and platelet counts. C. J. Langer reviewed chemotherapy records of all patients with RTOG data management staff. After completion of chemotherapy, all patient charts were reviewed and assessed for their adherence to protocol guidelines.
Figure 2.
Flow diagram of treatment arms. Patients were stratified by Karnofsky performance status and stage before being randomly assigned to one of three treatment arms, a control arm of sequential chemotherapy followed by standard radiation and two experimental arms in which chemotherapy was delivered concurrently with radiation. fx = fraction; IV = intravenous; RT = Radiotherapy.
The TRT guidelines for arms 1 and 2 were identical except that arm 1 TRT started on day 50 and arm 2 TRT began on day 1. In both arms, the initial TRT target volume consisted of the primary pulmonary tumor, the regional draining lymph nodes, and any intrathoracic or supraclavicular lymph nodes measuring greater than 2.5 cm. TRT was delivered to this volume at a daily dose of 1.80 Gy to a total dose of 45.00 Gy over 5 weeks. The sixth and seventh weeks of TRT were delivered to a smaller target volume encompassing the primary tumor and lymph nodes known to be involved with disease and any lymph node measuring greater than 2.0 cm. This treatment was delivered in a technique avoiding the spinal cord at a daily dose of 2.00 Gy for nine fractions to 18.00 Gy. The total TRT dose in arms 1 and 2 to the tumor was 63.00 Gy, and the total dose to the spinal cord was restricted to 48.00 Gy or less. In arm 3, TRT was delivered twice daily in 20 Gy fractions to a total dose of 69.60 Gy separated with an interfraction time interval of 6–8 hours. Target volume definitions were identical to those in arms 1 and 2, and the total dose was 50.40 Gy for the initial volume and 19.20 Gy for the secondary target volume. Spinal cord dose was also restricted to 48.00 Gy or less. W. J. Curran, R. Komaki, and RTOG dosimetry staff reviewed dosimetry, treatment plans, and simulation and verification films of all treatment fields for all evaluable patients. Similarly to chemotherapy, all patients’ radiation treatment delivery was reviewed for adherence to the protocol treatment guidelines.
Statistical Analysis
The primary objective of this study was to evaluate overall survival. The survival outcome from two prior RTOG phase II trials (7,8) of concurrent chemoradiation and the survival results of sequential chemoradiation in the two prior phase III trials (2,3) determined the patient sample size. RTOG 90-15 (8) used a concurrent chemoradiation regimen identical to arm 2 except for the RT fractionation, and the MST of patients meeting eligibility criteria of this study was 17.5 months. RTOG 91-06 (7) used the same concurrent chemoradiation regimen as arm 3, and the MST of these patients was 19.7 months. In both prior North American phase III randomized trials testing the sequential chemoradiation regimen used as arm 1 in this trial, the MST was 13.8 months (2,3). The sequential regimen was considered the standard arm for this study (RTOG 9410). The Makuch and Simon (9) sample size formula, which assumes constant proportionality of hazards, was used to calculate the sample size. A total of 597 evaluable patients (199 per arm) accrued over 4.2 years and followed for a minimum of 2 years were needed to ensure an 80% (20% type II error rate) probability of detecting a 43% relative improvement in MST between the worst and best regimen while rejecting the null hypothesis at the 95% level (a 5% type I error rate) (10). This sample size was also sufficient to detect an absolute 10% difference in acute grade 3 or worse esophageal toxicity. Secondary endpoints included tumor response and time to tumor progression. Patients were stratified by stage (II vs IIIA vs IIIB) and KPS (90–100 vs 70–80) and were randomly assigned to a given treatment arm, according to Zelen permuted block randomization method (11). A failure for the primary endpoint of overall survival was death due to any cause, and the corresponding event time was measured from date of random assignment to the date of death or last follow-up if the patient was still alive. Response to treatment was evaluated using the World Health Organization classification of response. A response was considered either a clinical complete response or a clinical partial response. A failure for tumor progression was considered the first instance of progressive disease; patients who were alive without progression were censored, and those who died without progression were considered a competing risk. The corresponding failure time was measured from the date of random assignment until the first instance of progression or the date of last follow-up/death if there was no progression.
Three interim analyses of the primary study endpoint of survival were performed at 50%, 67%, and 75% of the total planned accrual. The results of the blinded interim analysis were reported to the RTOG Data Monitoring Committee. The log-rank test was used to examine differences in Kaplan–Meier curves between arms. The forward selection method of Chen and Simon (12), which is analogous to the Makuch and Simon method (9) for determining sample size, was used for the primary analysis. This method compared arm 1 with arm 2 at the P = .055 level and then compared the superior arm of this test to arm 3 at the P = .069 level to select the best arm overall. For example, if arm 1 was dropped at the interim analysis, then arm 2 would be compared with arm 3 at the P = .040 level. Hazard ratios (HRs) with 95% confidence intervals (CIs) were also calculated. A multivariable analysis testing for effects of pretreatment characteristics was done using the Cox proportional hazards model. Proportionality was confirmed using a Kolmogorov-type supremum test. Differences in time to progression were examined using Gray test. Differences in toxicity distributions were examined using the Wilcoxon rank sum test. All reported P values are two-sided.
Results
Between July 1994 and July 1998, a total of 610 patients from 153 institutions located in the United States and Canada were enrolled on this trial (203, 204, and 203 for arms 1, 2, and 3, respectively). The average monthly accrual rate was 11.4 patients per month, and the three blinded interim analyses performed during the accrual period of this trial did not result in any recommended changes by the RTOG Data Monitoring Committee. The median age of enrolled patients was 61 years (Table 1), and there were no statistically significant differences among the three arms with respect to age, performance status, sex, histological subtype, or clinical stage. Thirty-three patients were deemed ineligible for the trial (8, 9, and 16 for arms 1, 2, and 3, respectively). A total of 577 patients were eligible for analysis, constituting 95% of enrolled patients, and were analyzed based on the treatment arm to which they were assigned. All data received at RTOG headquarters as of November 4, 2009, were included in the analysis. The median follow-up time for the 28 patients not reported dead was 11 years (range 0.8–14.0 years). Two patients were lost to follow-up at 10 and 12 months.
Table 1.
Demographics of enrolled patients*
| Patient characteristic | Arm 1 (n = 195) | Arm 2 (n = 195) | Arm 3 (n = 187) | Total (n = 577) |
| Age, No. (%) | ||||
| <60,y | 82 (42) | 90 (46) | 72 (39) | 244 (42) |
| ≥60,y | 113 (58) | 105 (54) | 115 (62) | 333 (58) |
| Median | 63 | 60 | 63 | 62 |
| Range | 33–79 | 33–79 | 35–80 | 33–80 |
| Sex, No. (%) | ||||
| Men | 122 (63) | 125 (64) | 124 (66) | 371 (64) |
| Women | 73 (37) | 70 (36) | 63 (34) | 206 (36) |
| KPS, No. (%) | ||||
| 70–80 | 45 (23) | 47 (24) | 45 (24) | 137 (24) |
| 90–100 | 150 (77) | 148 (76) | 142 (76) | 440 (76) |
| Histology, No. (%) | ||||
| Squamous | 75 (38) | 75 (38) | 70 (37) | 220 (38) |
| Adenocarcinoma | 53 (27) | 73 (37) | 52 (28) | 178 (31) |
| Large Cell | 29 (15) | 27 (14) | 23 (12) | 79 (14) |
| Combined | 2 (1) | 2 (1) | 8 (4) | 12 (2) |
| Carcinoma NOS | 34 (17) | 18 (9) | 30 (16) | 82 (14) |
| Other | 2 (1) | 0 (0) | 4 (2) | 6 (1) |
| AJCC stage, No. (%) | ||||
| II | 4 (2) | 3 (2) | 4 (2) | 11 (2) |
| IIIA | 81 (42) | 84 (43) | 75 (40) | 240 (42) |
| IIIB | 110 (56) | 108 (55) | 108 (58) | 326 (57) |
| Race, No. (%) | ||||
| White | 167 (86) | 171 (88) | 161 (86) | 499 (86) |
| Hispanic | 3 (2) | 2 (1) | 2 (1) | 7 (1) |
| Black or African American | 20 (10) | 19 (10) | 17 (9) | 56 (10) |
| Asian | 3 (2) | 2 (1) | 5 (3) | 10 (2) |
| Native American | 0 (0) | 1 (<1) | 1 (<1) | 2 (<1) |
| Other | 2 (1) | 0 (0) | 1 (<1) | 3 (1) |
Arm 1 was the sequential arm. Arm 2 used the same chemotherapy regimen as arm 1 with 60 Gy thoracic radiotherapy beginning on day 1. Arm 3 used cisplatin at 50 mg/m2 on days 1, 8, 29, and 36 with oral etoposide at 50 mg twice daily for 10 weeks on days 1, 2, 5, and 6 with 69.6 Gy delivered as 1.2-Gy twice-daily fractions beginning on day 1. AJCC = American Joint Commission on Cancer staging; KPS = Karnofsky performance status; NOS = not otherwise specified.
Compliance with treatment guidelines was excellent. TRT was given in compliance with guidelines or with minor deviations in 83% of patients in arm 1, 92% in arm 2, and 81% in arm 3. Major unacceptable variations were observed in only 3% of patients. Treatment interruptions in the delivery of the TRT of more than 1 week were seen in 9% of patients. Chemotherapy as per protocol or with minor deviations was received by 97% of patients.
There were statistically significantly higher rates of acute esophagitis in both concurrent arms than in the sequential arm, with grade 3 or worse rates of 4%, 22%, and 45% for arms 1, 2, and 3, respectively (Table 2). A comparison of severe acute esophagitis by the Wilcoxon test demonstrated significant differences between arms 1 and 2 (P < .001) and arms 3 and 2 (P < .001). Of interest was the lack of difference in late esophagitis rates among these arms, with rates of 1%–4% among the three arms. Grade 3 or worse acute pulmonary toxicity was higher for the sequential arm than either concurrent arm, and although no difference in late toxicity was observed, the grade 3–5 late pulmonary toxicities ranged between 13% and 17% for the three arms. The rates of grade 3 or worse granulocyte level depressions were highest in arms 1 and 2, which included vinblastine (arm 1, 77%; arm 2, 81%; and arm 3, 53%). A total of 14 patients (2%) experienced acute fatal toxicity; 13 were attributable to neutropenic sepsis. Of the eight late fatal toxicities, seven were pulmonary in nature, and one was infectious. No differences between the arms were noted in the rates of these grade 5 toxicities.
Table 2.
Chemotherapy and acute and late toxicities*
| Toxicity | Grade |
||||||||
| 3 | 4 | 5 | 3 | 4 | 5 | 3 | 4 | 5 | |
| Chemotherapy and acute radiotherapy toxicities, No. (%) | Arm 1 (n = 195) | Arm 2 (n = 193) | Arm 3 (n = 187) | ||||||
| Granulocytopenia | 37 (19) | 111 (57) | 2 (1) | 40 (21) | 114 (59) | 3 (2) | 51 (27) | 46 (25) | 3 (2) |
| Leukopenia | 68 (35) | 42 (22) | 1 (<1) | 64 (33) | 94 (49) | 4 (2) | 76 (40) | 51 (27) | 1 (<1) |
| Thrombocytopenia | 4 (2) | 5 (3) | 0 | 11 (6) | 6 (3) | 1 (<1) | 14 (7) | 16 (9) | 0 |
| Worst hematological | 40 (21) | 114 (58) | 2 (1) | 46 (24) | 117 (61) | 4 (2) | 77 (41) | 53 (28) | 3 (2) |
| Pulmonary | 13 (7) | 2 (1) | 2 (1) | 6 (3) | 1 (<1) | 0 | 3 (2) | 1 (<1) | 0 |
| Esophagus | 7 (4) | 0 | 0 | 40 (21) | 3 (2) | 0 | 78 (42) | 6 (3) | 0 |
| Cardiac | 2 (1) | 1 (<1) | 0 | 0 | 0 | 1 (<1) | 4 (2) | 3 (2) | 0 |
| Mucositis | 2 (1) | 0 | 0 | 21 (11) | 0 | 0 | 39 (21) | 3 (2) | 0 |
| Nausea/Vomiting | 22 (11) | 7 (4) | 0 | 36 (19) | 7 (4) | 1 (<1) | 31 (17) | 14 (7) | 0 |
| Anemia | 13 (7) | 1 (<1) | 0 | 20 (10) | 3 (2) | 0 | 31 (17) | 4 (2) | 0 |
| Other nonhematological | 21 (11) | 7 (4) | 5 (3) | 33 (17) | 9 (5) | 1 (<1) | 42 (22) | 8 (4) | 3 (2) |
| Worst nonhematological | 47 (24) | 14 (7) | 7 (4) | 82 (42) | 20 (10) | 1 (<1) | 90 (48) | 31 (17) | 3 (2) |
| Worst overall toxicity | 47 (24) | 114 (57) | 7 (4) | 50 (26) | 121 (63) | 4 (2) | 79 (42) | 75 (40) | 3 (2) |
| Late radiotherapy toxicities, No. (%) | Arm 1 (n = 176) | Arm 2 (n = 184) | Arm 3 (n = 171) | ||||||
| Granulocytopenia | 1 (1) | 0 | 0 | 0 | 1 (1) | 0 | 1 (1) | 0 | 0 |
| Leukopenia | 0 | 1 (1) | 0 | 1 (1) | 1 (1) | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 0 | 1 (1) | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 |
| Worst hematological | 1 (1) | 2 (1) | 0 | 1 (1) | 1 (1) | 0 | 2 (1) | 0 | 0 |
| Pulmonary | 20 (11) | 4 (2) | 2 (1) | 20 (11) | 0 | 3 (2) | 24 (14) | 3 (2) | 2 (1) |
| Esophagus | 1 (1) | 0 | 0 | 5 (3) | 1 (1) | 0 | 6 (4) | 0 | 0 |
| Cardiac | 1 (1) | 0 | 0 | 3 (2) | 1 (1) | 0 | 7 (4) | 1 (1) | 0 |
| Nausea/Vomiting | 1 (1) | 0 | 0 | 1 (1) | 0 | 0 | 0 | 0 | 0 |
| Anemia | 1 (1) | 0 | 0 | 1 (1) | 0 | 0 | 2 (1) | 1 (1) | 0 |
| Other nonhematological | 1 (1) | 1 (1) | 0 | 8 (4) | 2 (1) | 0 | 4 (2) | 0 | 1 (1) |
| Worst nonhematological | 24 (14) | 5 (3) | 2 (1) | 29 (16) | 4 (2) | 3 (2) | 31 (18) | 4 (2) | 3 (2) |
| Worst overall toxicity | 25 (14) | 7 (4) | 2 (1) | 30 (16) | 5 (3) | 3 (2) | 32 (19) | 4 (2) | 3 (2) |
Arm 1 was the sequential arm. Arm 2 used the same chemotherapy regimen as arm 1 with 60 Gy thoracic radiotherapy beginning on day 1. Arm 3 used cisplatin at 50 mg/m2 on days 1, 8, 29, and 36 with oral etoposide at 50 mg twice daily for 10 weeks on days 1, 2, 5, and 6 with 69.6 Gy delivered as 1.2-Gy twice-daily fractions beginning on day 1.
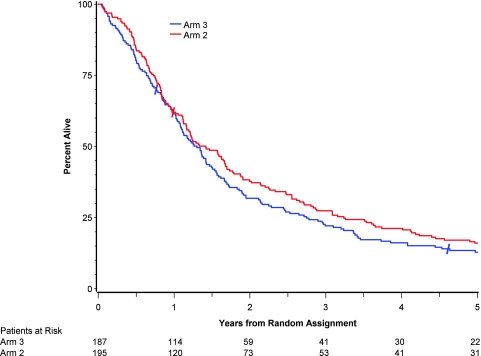
MSTs were 14.6 months (95% CI = 12.1 to 17.0 months) in arm 1, 17.0 months (95% CI = 14.0 to 20.2 months) in arm 2, and 15.6 months (95% CI = 13 to 18 months) in arm 3. Overall 5-year survival rates were 10% (20 patients, 95% CI = 7% to 15%) for arm 1, 16% (31 patients, 95% CI = 11% to 22%) for arm 2, and 13% (22 patients, 95% CI = 9% to 18%) for arm 3. Overall survival was not statistically significantly longer in arm 2 compared with arm 1 (HR of death = 0.81, 95% CI = 0.66 to 0.996, P = .46, log-rank test, Figure 3) Overall survival was statistically significantly longer in arm 2 compared with arm 3 (HR of death = 0.93, 95% CI = 0.75 to 1.14, P = .046, log-rank test, Figure 4). Multivariable analysis using treatment, age, KPS, sex, histology, stage, and race/ethnicity showed that, after controlling for treatment, only age, KPS, and stage were statistically significantly associated with survival (P = .001, .007, and .02, respectively).
Figure 3.
Five-year survival results for patients assigned to receive standard radiation with concurrent chemotherapy compared with patients assigned to receive sequential chemotherapy and radiotherapy. Hazard ratio for death = 0.812, 95% confidence interval = 0.663 to 0.996, P = .046, two-sided log-rank test. Total dead at any time: Arm 1 = 189 and Arm 2 = 185. Slash marks indicate censored observations.
Figure 4.
Five-year survival results for patients assigned to receive standard radiation with concurrent chemotherapy (arm 2) compared with patients assigned to receive hyperfractionated radiation with concurrent chemotherapy (arm 3). Hazard ratio for death = 0.925, 95% confidence interval = 0.752 to 1.138, P = .46, two-sided log-rank test. Total dead at any time: Arm 2 = 185 and Arm 3 = 175. Slash marks indicate a censored observation.
Response rate was highest in arm 2 at 70% (95% CI = 63% to 76%). This included a clinical complete response (cCR) rate of 42% (95% CI = 35% to 49%). Arm 1 had a 61% response rate (95% CI = 54% to 68%) and a cCR of 30% (95% CI = 24% to 37%). Arm 3 had a 65% response rate (95% CI = 58% to 71%) and a cCR of 33% (95% CI = 27% to 40%). The response rate in arm 2 was statistically significantly higher (P < .05) compared with arm 1, but arms 2 and 3 were not statistically significantly different in response rate. Local tumor failure rate (progression of tumor within the full dose radiation region) was highest in arm 1 (39%, 95% CI = 32 to 46 at 2 years) and lower in arm 2 (30%, 95% CI = 23 to 36) and arm 3 (29%, 95% CI = 22 to 35). The differences in local tumor failure rates between arms 2 and 1 and between arms 1 and 3 were not statistically significant (P = .09 and .053, respectively).
Patterns of disease progression were defined for all patients as occurring “infield,” distant, or both (Table 3). There was no difference in the pattern of occurrence of first sites of disease failure between arms 1 and 2. There were, however, fewer patients in arm 3 with disease progression within the TRT target volume as compared with arm 1 (P = .03). The time to infield disease progression was also compared between arms 1 and 2 and between arms 1 and 3. No difference was observed between arms 1 and 2; however, infield disease progression rates were reduced in arm 3 compared with arm 1 (P = .01).
Table 3.
Patterns of failure*
| Component of first failure | No. (%) |
||
| Arm 1 (n = 195) | Arm 2 (n = 195) | Arm 3 (n = 187) | |
| Primary tumor | 65 (33) | 56 (29) | 47 (25) |
| Thoracic lymph nodes (infield) | 34 (17) | 24 (12) | 18 (10) |
| Thoracic lymph nodes (out of field) | 4 (2) | 8 (4) | 3 (2) |
| Brain metastases | 24 (12) | 28 (14) | 24 (13) |
| Other metastases | 65 (33) | 64 (33) | 60 (32) |
| Infield only | 59 (30) | 49 (25) | 38 (20) |
| Out of field only | 67 (34) | 73 (37) | 69 (37) |
| Both infield and out of field | 22 (11) | 20 (10) | 16 (9) |
Arm 1 was the sequential arm. Arm 2 used the same chemotherapy regimen as arm 1 with 60 Gy thoracic radiotherapy beginning on day 1. Arm 3 used cisplatin at 50 mg/m2 on days 1, 8, 29, and 36 with oral etoposide at 50 mg twice daily for 10 weeks on days 1, 2, 5, and 6 with 69.6 Gy delivered as 1.2-Gy twice-daily fractions beginning on day 1
Discussion
The long-term results of this large phase III trial confirm the hypothesis generated by the promising results from one of two prior phase II multi-institutional trials that the concurrent delivery of cisplatin-based chemotherapy with TRT improves survival compared with the sequential delivery of such therapies (7). The acute toxicity, particularly esophagitis, is statistically significantly worse with concurrent therapy, but long-term toxicity and treatment-related mortality are equivalent.
The management of patients with locally advanced unresected NSCLC has undergone considerable change since 1990 when 60 Gy TRT alone was the standard of care. TRT alone resulted in MST of 10–12 months and 3-year survival rates of 10%–15% among patients with a good performance status and low levels of disease-related weight loss (2,3,13). The past two decades have witnessed the maturation of at least five major randomized trials demonstrating that the addition of platinum-based chemotherapy before or during TRT statistically significantly improved survival in this patient cohort to MST of 13–15 months and 3-year survival rates of 15%–20% (2–6). The magnitude of benefit from the addition of chemotherapy to TRT was similar among these trials, and the optimal sequencing of chemotherapy and TRT for these patients remained uncertain.
In 1994, the RTOG initiated this trial after observing encouraging phase II results with two concurrent chemoradiation regimens. The MST of 17.5 months and 19.6 months seen among patients with good performance status and low weight loss in these phase II trials appeared to compare favorably with the 13.8-month MST observed with sequential chemoradiation in two large phase III trials (7,8). These phase II regimens were therefore used as the basis for the investigational arms (2,3) in this trial.
The statistically significant long-term survival benefit observed in arm 2 compared with arm 1 supports the hypothesis that the concurrent delivery of cisplatin-based chemoradiation provides an improved outcome in comparison to the sequential delivery of the same therapies. The promising phase II results observed in a prior RTOG trial of delivering concurrent cisplatin-based chemoradiotherapy have been confirmed in this phase III randomized trial. The only therapeutic variable between arms 1 and 2 was the timing of TRT, and no other factors such as the selection of chemotherapeutic agents or the dose and schedule of any therapy have confounded this result. It is likely that the radiosensitizing antitumor effect of the chemotherapy in arm 2 contributed to the improvement in patient survival seen in this treatment arm. The promising phase II results for the arm 3 regimen have not been supported in this trial. Although the reasons for this result are not clear, it is possible that the higher rates of acute nonhematologic toxicity observed in this arm may have diminished the benefit of this aggressive concurrent regimen.
Of note is another report (14) of a phase III comparison of concurrent vs sequential cisplatin-based chemoradiation for patients with stage III NSCLC, in which a survival advantage was also noted. In a 314-patient trial conducted by the West Japan Lung Cancer Oncology Group, MST was 17 vs 13 months and 5-year survival was 16% vs 9% (P = .039), with concurrent chemoradiation compared with sequential treatment (14). The remarkable similarity in the magnitude of difference between that result and the present report lends further support to the importance of optimizing the temporal relationship between these therapies. In addition, several other smaller European randomized trials have provided support for the use of concurrent chemoradiation as compared with sequential therapy (15–18).
This study also had some limitations. It is estimated that less than 30% of all stage III NSCLC patients would meet entry criteria for this study. The majority of patients with stage III NSCLC have a functional status that is too poor and have suffered too much disease-related weight loss, as well as other comorbid conditions that would disqualify them from enrollment in this trial. It is likely that the higher rates of severe esophagitis observed with concurrent therapy would be less well tolerated by patients with lower functional status.
Future approaches to patients with locally advanced NSCLC should continue to capitalize on the clinically relevant integration between these therapies. Since the conduct of this trial, a number of innovative changes have occurred in radiotherapeutic planning and delivery, which may improve the therapeutic ratio of concurrent therapy (19). In addition, the integration of new anticancer agents such as those targeting the epidermal growth factor receptor with concurrent chemoradiation is under active clinical investigation. This study does support the hypothesis that concurrent therapy should be the standard nonoperative regimen for eligible patients. Promising phase II reports of newer chemotherapeutic regimens, including taxane-based regimens, delivered concurrently with TRT continue to be published and will require additional testing to define the optimal regimen (20,21).
Funding
This study was supported by grants CA21661 (Radiation Therapy Oncology Group [RTOG] Operational Grant), CA37422 (RTOG Statistical Grant), and CA32115 (RTOG CCOP Research Base) from the National Cancer Institute.
Footnotes
The funders did not have any involvement in the design of the study; the collection, analysis, and interpretation of the data; the writing of the article; or the decision to submit the article for publication.
References
- 1.Beahrs OH, Henson DE, Hutter RVP, et al. American Joint Committee on Cancer Manual for Staging of Cancer. Philadelphia, PA: JB Lippincott Co; 1992. [Google Scholar]
- 2.Dillman RO, Seagren SL, Herndon J, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med. 1990;323:940–945. doi: 10.1056/NEJM199010043231403. [DOI] [PubMed] [Google Scholar]
- 3.Sause W, Scott C, Taylor S, et al. Radiation Therapy Oncology Group (RTOG) 88-08 and Eastern Cooperative Oncology Group (ECOG) 4588: preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. J Natl Cancer Inst. 1995;87:198–205. doi: 10.1093/jnci/87.3.198. [DOI] [PubMed] [Google Scholar]
- 4.Le Chevalier T, Arriagada R, Quoix E, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst. 1991;83:417–423. doi: 10.1093/jnci/83.6.417. [DOI] [PubMed] [Google Scholar]
- 5.Schaake-Koning C, Van Den Bogert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy in inoperable non-small cell lung cancer. N Engl J Med. 1992;326:524–530. doi: 10.1056/NEJM199202203260805. [DOI] [PubMed] [Google Scholar]
- 6.Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Hyperfractionated radiation therapy with or without concurrent low-dose daily carboplatin/etoposide for stage III non-small-cell lung cancer: a randomized study. J Clin Oncol. 1996;14:1065–1070. doi: 10.1200/JCO.1996.14.4.1065. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Scott C, Komaki R, et al. Concurrent chemoradiation therapy with oral etoposide and cisplatin for locally advanced inoperable non-small-cell lung cancer: radiation therapy oncology group protocol 91-06. J Clin Oncol. 1996;14:1055–1064. doi: 10.1200/JCO.1996.14.4.1055. [DOI] [PubMed] [Google Scholar]
- 8.Byhardt R, Scott C, Ettinger D, et al. Concurrent hyperfractionated irradiation and chemotherapy for unresectable non-small cell lung cancer. Results of Radiation Therapy Oncology Group 90-15. Cancer. 1995;75:2337–2344. doi: 10.1002/1097-0142(19950501)75:9<2337::aid-cncr2820750924>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Makuch RW, Simon RM. Sample size requirements for comparing time-to-failure among k treatment groups. J Chronic Dis. 1982;35:861–897. doi: 10.1016/0021-9681(82)90051-0. [DOI] [PubMed] [Google Scholar]
- 10.Fleming TR, Harrington DP, O’Brien PC. Designs for group sequential tests. Control Clin Trials. 1984;5:348–361. doi: 10.1016/s0197-2456(84)80014-8. [DOI] [PubMed] [Google Scholar]
- 11.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1994;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 12.Chen TT, Simon R. Two multi-step selection procedures with possible selection of two treatments of equal prior preference. Commun Stat Theory Methods. 1994;23:781–801. [Google Scholar]
- 13.Perez C, Stanley K, Grundy G, et al. Impact of irradiation technique and tumor extent in tumor control and survival of patients with unresectable non-oat cell carcinoma of the lung: report by the Radiation Therapy Oncology Group. Cancer. 1982;50:1091–1099. doi: 10.1002/1097-0142(19820915)50:6<1091::aid-cncr2820500612>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 15.Ulutin HC, Güden M, Oysul K, Sürenkök S, Pak Y. Split-course radiotherapy with or without concurrent or sequential chemotherapy in non-small cell lung cancer. Radiat Med. 2000;18:93–96. [PubMed] [Google Scholar]
- 16.Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non–small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique–Groupe Francais de Pneumo-Cancérologie NPC 95-01 Study. J Clin Oncol. 2005;23:5910–5917. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 17.Zatloukal P, Petruzelka L, Zemanova M, et al. Concurrent versus sequential radiochemotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer. A randomized study. Lung Cancer. 2004;46:87–98. doi: 10.1016/j.lungcan.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Belderbos J, Uitterhoeve L, van Zandwijk N, et al. EORTC LCG and RT Group. Randomised trial of sequential versus concurrent chemo-radiotherapy in patients with inoperable non-small cell lung cancer (EORTC 08972-22973) Eur J Cancer. 2007;43:114–121. doi: 10.1016/j.ejca.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Bradley J, Graham M, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I–II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:318–328. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 20.Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23:5883–5891. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 21.Vokes EE, Herndon JE, Crawford J, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. J Clin Oncol. 2002;20:4191–4198. doi: 10.1200/JCO.2002.03.054. [DOI] [PubMed] [Google Scholar]