Abstract
Background
Case–control studies have reported that exogenous estrogen use is associated with increased risk of skin cancer. The effects of menopausal hormone therapy on incidence of nonmelanoma skin cancer and melanoma were evaluated in post hoc analyses of the Women’s Health Initiative randomized placebo-controlled hormone therapy trials of combined estrogen plus progestin (E + P) and estrogen only (E-alone).
Methods
Postmenopausal women aged 50–79 years were randomly assigned to conjugated equine estrogen (0.625 mg/d) plus medroxyprogesterone acetate (2.5 mg/d) or placebo in the E + P trial if they had an intact uterus (N = 16 608) or to conjugated equine estrogen alone or placebo in the E-alone trial if they had a hysterectomy (N =10 739); the mean follow-up was 5.6 and 7.1 years, respectively. Incident nonmelanoma skin cancers (n = 980 [E + P trial]; n = 820 [E-alone trial]) and melanomas (n = 57 [E + P trial]; n =38 [E-alone trial]) were ascertained by self-report. Incident cases of cutaneous malignant melanoma were confirmed by physician review of medical records. Incidences of nonmelanoma skin cancer and melanoma were compared between the two randomization groups within each trial using hazard ratios (HRs), with corresponding 95% confidence intervals (CIs) and Wald statistic P values from Cox proportional hazards models. All statistical tests were two-sided.
Results
Rates of incident nonmelanoma skin cancer and melanoma were similar between the active hormone (combined analysis of E + P and E-alone) and placebo groups (nonmelanoma skin cancer: HR = 0.98, 95% CI = 0.89 to 1.07; melanoma: HR = 0.92, 95% CI = 0.61 to 1.37). Results were similar for the E + P and E-alone trials when analyzed individually.
Conclusions
Menopausal hormone therapy did not affect overall incidence of nonmelanoma skin cancer or melanoma. These findings do not support a role of menopausal estrogen, with or without progestin, in the development of skin cancer in postmenopausal women.
CONTEXT AND CAVEATS
Prior knowledge
Case–control studies have reported an association between the use of exogenous estrogen and an increased risk of skin cancer.
Study design
A post hoc analysis of the Women’s Health Initiative randomized placebo-controlled hormone therapy trials (combined estrogen plus progestin or estrogen only) to examine the effect of menopausal hormone therapy on the overall incidences of nonmelanoma skin cancer and melanoma.
Contribution
In these trials of 27 347 postmenopausal women, there was no association between hormone therapy use and skin cancer after approximately 6 years of follow-up.
Implication
Use of estrogen, with or without progestin, is not associated with the development of skin cancer in postmenopausal women.
Limitations
The analyses were not stratified by anatomical location or type of tumor. Information about major risk factors for skin cancer was not collected in these trials. Potential effects of hormone therapy on skin cancer incidence may take more than 6–7 years to become evident. The nonmelanoma skin cancer outcome was based on self-report.
From the Editors
Skin cancer is the most common malignancy in the United States, with more than 1 million new diagnoses each year (1). Over the past 40 years, the incidence of both melanoma and nonmelanoma skin cancer has increased dramatically (1,2). Although the etiologies of nonmelanoma skin cancer (particularly basal cell and squamous cell carcinomas) and melanoma are not completely understood, sun exposure plays a critical role and is a primary risk factor (3,4).
Preclinical and clinical findings suggest that estrogen may be involved in the development of skin cancer. Estrogen receptors are present on skin keratinocytes (5), and in clinical trials, oral contraceptives and hormone therapy have been shown to decrease acne (6) and skin aging (7), respectively. Two case–control studies found an association between oral contraceptive use and an increased risk of nonmelanoma skin cancer (8,9). It has also been suspected that estrogen influences the risk of melanoma; however, two reviews suggest that the evidence linking hormone therapy to melanoma is weak (10,11). Moreover, melanoma incidence and survival differ by sex as women have a better prognosis than men (12,13), and the estrogen receptor is expressed in benign nevi, dysplastic nevi, and cutaneous melanomas (14). Although epidemiological studies of menopausal hormone therapy and the risk of melanoma have produced mixed findings, several studies reported that hormone use was associated with increased risk of melanoma (15–17). To our knowledge, no large randomized controlled trials have investigated the effects of hormone therapy on risk of skin cancer. We analyzed the incidence of nonmelanoma skin cancer and of melanoma in the large multiethnic Women’s Health Initiative (WHI) clinical trials of estrogen plus progestin (E + P) vs placebo and estrogen alone (E-alone) vs placebo.
Participants and Methods
Study Design
This study included postmenopausal women aged 50–79 years who were enrolled in the WHI hormone therapy trials (http://Clinicaltrials.gov identifier: NCT00000611) and recruited at 40 US clinical centers. In these studies, coronary heart disease and breast cancer were the primary monitored outcomes for benefit and harm, respectively. In an attempt to summarize important aspects of health benefits vs risks, a global index was defined as the earliest occurrence of coronary heart disease, invasive breast cancer, stroke, pulmonary embolism, endometrial cancer, colorectal cancer, hip fracture, or death (18). This global index was used to monitor the study for benefit or harm. The trials were not powered for skin cancer outcomes. Women were eligible for the WHI hormone therapy trials if they had no history of cancer, except nonmelanoma skin cancer, within the last 10 years and had a life expectancy of at least 3 years. Detailed descriptions of additional eligibility criteria and recruitment methods have been published (19).
The WHI hormone therapy trials enrolled 27 347 women between 1993 and 1998; those with an intact uterus were enrolled in the E + P trial (N = 16 608) and those with a previous hysterectomy were enrolled in the E-alone trial (N = 10 739). Women were randomly assigned by use of a permuted block algorithm in a double-blind fashion to daily administration of either 0.625 mg of conjugated equine estrogen with 2.5 mg of medroxyprogesterone acetate (Prempro; Wyeth-Ayerst, Philadelphia, PA) or placebo in the E + P trial or, in the E-alone trial, to either 0.625 mg conjugated equine estrogen (Premarin; Wyeth-Ayerst) or placebo. These doses are still commonly prescribed for systemic hormone therapy in postmenopausal women (20).
Demographic information, medical history, and other characteristics were obtained by questionnaire or physical measurement at study entry (baseline). The protocol was approved by each participating institution’s review board, and all women provided written informed consent.
Retention, Adherence, and Follow-up
Study participants were contacted by telephone 6 weeks after random assignment to assess symptoms and reinforce adherence. Follow-up for clinical events occurred by telephone every 6 months, and annual in-clinic visits were required. At each semiannual contact, a standardized self-administered questionnaire collected information on symptoms, safety concerns, and reports of changes in medical history, including any cancer diagnosis. Adherence to study interventions was assessed by weighing returned medication bottles at the annual visits and was predefined as taking 80% or more of study pills (active or placebo). Participants were followed for major outcomes, regardless of adherence to study interventions, until death or loss to follow-up. Over the course of the studies, 583 women (3.5%) in the E + P trial and 563 women (5.2%) in E-alone trial withdrew or were lost to follow-up (ie, had not provided outcomes information for >18 months).
The E + P trial was terminated at a mean follow-up of 5.6 years because the health risks, including increased incidence of coronary heart disease, stroke, pulmonary emboli, and breast cancer, exceeded the benefits of decreased incidence of colorectal cancer and hip fracture (18). The E-alone trial was terminated at 7.1 years of follow-up because of increased incidence of stroke and no suggestion of benefit regarding coronary heart disease (21). At the time each trial was terminated, 42% of E + P trial participants and 54% of E-alone trial participants had stopped taking study pills, whereas 6.2% of women assigned to active E + P trial pills and 10.7% of the placebo group had initiated hormone therapy through their own physicians, as had 5.7% of women assigned to active E-alone trial pills and 9.1% on placebo.
Determining Incident Cases of Skin Cancer
Women were provided questionnaires at 6-month intervals to report any hospitalizations and other outcomes, including nonmelanoma skin cancer and melanomas. Incident cases of cutaneous malignant melanoma were confirmed by central review of medical records, including pathology reports, and coded as invasive, in situ, or unknown (22). Incident cases of nonmelanoma skin cancer were not adjudicated.
Statistical Analysis
Descriptive characteristics and potential risk factors for skin cancer, including race, body mass index (BMI), smoking status, regional solar radiation (based on the amount of sunlight reaching the clinic site as measured by the US Weather Bureau), total outdoor walking energy expenditure (metabolic equivalent tasks per week), nonsteroidal anti-inflammatory drug use, and history of skin cancer, were evaluated by treatment group within each trial.
In post hoc analyses, incidences of nonmelanoma skin cancer and melanoma were compared between the two randomization groups within each trial by using hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) and Wald statistic P values from Cox proportional hazards models. We confirmed the proportionality assumption by running a proportional hazards model that modeled each outcome as a function of the interaction between the hormone therapy effect and the log survival time. We also verified the proportional assumption graphically. Modeling analyses used time-to-event methods according to the intention-to-treat principle. Kaplan–Meier estimates were used to describe event rates over time. Potential differential effects across subgroups of risk factors for both nonmelanoma skin cancer and melanoma were tested by running proportional hazards models with the E + P or E-alone intervention, the categorical subgroup of interest, and their interaction. Hazard ratios for active hormone therapy treatment vs placebo within each subgroup are presented along with the P value for interaction. Sensitivity analyses were performed by restricting the analysis to participants who were adherent to the study interventions (ie, those who took ≥80% of study pills) during the trial. All P values are two-sided, and P values less than or equal to .05 were considered to be statistically significant.
For nonmelanoma skin cancer and melanoma, six predefined subgroup analyses were performed for each of the two trials as well as for combined hormone therapy trial results (ie, active E + P and E-alone groups vs combined placebo groups) to assess possible statistical interactions between active hormone therapy use and the following variables: 1) age (50–59, 60–69, or 70–79 years), 2) BMI (<25 or ≥25 kg/m2), 3) regional solar radiation (≤375 or >375 langleys) based on the amount of sunlight reaching the clinic site as measured by the US Weather Bureau, 4) history of nonmelanoma skin cancer (yes, no), 5) smoking status (never, past, or current), and 6) ever use of nonsteroidal anti-inflammatory drug at study entry (baseline; yes or no). Cut points for age, BMI, and regional solar radiation were as previously defined in the WHI clinical trials. All statistical analyses were conducted using SAS (version 9.2; SAS Institute Inc, Cary, NC). All statistical tests were two-sided.
Results
Demographic Characteristics of Participants
Baseline demographic characteristics, medical history, health behaviors, and measures of sun exposure (regional solar radiation and outdoor walking) were generally balanced between randomization groups for each trial (Table 1). A minor difference in the history of cancer was seen between randomization groups in the E-alone trial (4.5% E-alone vs 5.7% placebo), and total outdoor walking energy expenditure differed by less than 2% between randomization groups in both trials.
Table 1.
Baseline characteristics of participants*
| Characteristic | E + P trial |
E-alone trial |
||
| Active HT (N = 8506) | Placebo (N = 8102) | Active HT (N = 5310) | Placebo (N = 5429) | |
| No. (%) | No. (%) | No. (%) | No. (%) | |
| Age, y | ||||
| 50–59 | 2837 (33.4) | 2683 (33.1) | 1639 (30.9) | 1674 (30.8) |
| 60–69 | 3854 (45.3) | 3655 (45.1) | 2386 (44.9) | 2465 (45.4) |
| 70–79 | 1815 (21.3) | 1764 (21.8) | 1285 (24.2) | 1290 (23.8) |
| Race/ethnicity | ||||
| White | 7141 (84.0) | 6805 (84.0) | 4009 (75.5) | 4075 (75.1) |
| Black | 548 (6.4) | 574 (7.1) | 781 (14.7) | 835 (15.4) |
| Hispanic | 471 (5.5) | 415 (5.1) | 319 (6.0) | 332 (6.1) |
| American Indian | 25 (0.3) | 30 (0.4) | 41 (0.8) | 34 (0.6) |
| Asian or Pacific Islander | 194 (2.3) | 169 (2.1) | 86 (1.6) | 78 (1.4) |
| Unknown | 127 (1.5) | 109 (1.3) | 74 (1.4) | 75 (1.4) |
| Education level | ||||
| Less than high school diploma or GED | 2090 (25.7) | 2148 (26.5) | 1769 (33.3) | 1707 (31.4) |
| Some school after high school diploma | 3357 (39.5) | 3060 (37.8) | 2271 (42.8) | 2351 (43.3) |
| College degree or higher | 2915 (34.3) | 2839 (35.0) | 1217 (22.9) | 1327 (24.4) |
| Body mass index, kg/m2 | ||||
| <25 | 2579 (30.3) | 2479 (30.6) | 1110 (20.9) | 1096 (20.2) |
| 25 to <30 | 2992 (35.2) | 2835 (35.0) | 1798 (33.9) | 1915 (35.3) |
| ≥30 | 2899 (34.1) | 2737 (33.8) | 2375 (44.7) | 2385 (43.9) |
| Smoking status | ||||
| Never | 4178 (49.1) | 3999 (49.4) | 2723 (51.3) | 2705 (49.8) |
| Past | 3362 (39.5) | 3157 (39.0) | 1986 (37.4) | 2090 (38.5) |
| Current | 880 (10.3) | 838 (10.3) | 542 (10.2) | 571 (10.5) |
| NSAID use (ever) | ||||
| Yes | 2853 (33.5) | 2767 (34.2) | 1881 (35.4) | 1945 (35.8) |
| No | 5653 (66.5) | 5335 (65.8) | 3429 (64.6) | 3484 (64.2) |
| Total vitamin D intake, IU† | ||||
| <200 | 3320 (39.0) | 3164 (39.1) | 2379 (44.8) | 2350 (43.3) |
| 200 to <400 | 1608 (18.9) | 1547 (19.1) | 885 (16.7) | 937 (17.3) |
| 400 to <600 | 1875 (22.00) | 1775 (21.9) | 1083 (20.4) | 1161 (21.4) |
| ≥600 | 1414 (16.6) | 1347 (16.6) | 731 (13.8) | 727 (13.4) |
| Regional solar radiation, langleys‡ | ||||
| 300–325 | 2517 (29.6) | 2370 (29.3) | 1458 (27.5) | 1501 (27.6) |
| 350 | 2000 (23.5) | 1927 (23.8) | 1118 (21.1) | 1129 (20.8) |
| 375–380 | 965 (11.3) | 905 (11.2) | 640 (12.1) | 662 (12.2) |
| 400–430 | 1334 (15.7) | 1258 (15.5) | 966 (18.2) | 991 (18.3) |
| 475–500 | 1690 (19.9) | 1641 (20.3) | 1123 (21.1) | 1142 (21.0) |
| Total outdoor walking energy expenditure, METs/wk | ||||
| 0 | 2705 (31.8) | 2670 (33.0) | 1951 (36.7) | 1897 (34.9) |
| ≤3.5 | 1585 (18.6) | 1573 (19.4) | 1078 (20.3) | 1064 (19.6) |
| 3.6–7.0 | 1515 (17.8) | 1476 (18.2) | 901 (17.0) | 913 (16.8) |
| >7.0 | 1866 (21.9) | 1877 (23.2) | 950 (17.9) | 1031 (19.0) |
| History of cancer§ | ||||
| Yes | 167 (2.0) | 158 (2.0) | 241 (4.5) | 308 (5.7) |
| No | 8339 (98.0) | 7944 (98.0) | 5069 (95.5) | 5121 (94.3) |
| History of melanoma skin cancer | ||||
| Yes | 11 (0.1) | 10 (0.1) | 6 (0.1) | 10 (0.2) |
| No | 8495 (99.9) | 8092 (99.9) | 5304 (99.9) | 5419 (99.8) |
| History of nonmelanoma skin cancer | ||||
| Yes | 491 (5.8) | 456 (5.6) | 257 (4.8) | 290 (5.3) |
| No | 8015 (94.2) | 7646 (94.4) | 5053 (95.2) | 5137 (94.7) |
| Hormone therapy use | ||||
| Never used | 6277 (73.8) | 6022 (74.3) | 2769 (52.1) | 2769 (51.0) |
| Past user | 1671 (19.6) | 1587 (19.6) | 1871 (35.2) | 1947 (35.9) |
| Current user | 554 (6.5) | 490 (6.0) | 669 (12.6) | 709 (13.1) |
| Dietary modification intervention assignment | ||||
| Not randomly assigned | 6077 (71.4) | 5873 (72.5) | 3656 (68.9) | 3691 (68.0) |
| Intervention | 972 (11.4) | 925 (11.4) | 615 (11.6) | 670 (12.3) |
| Comparison | 1457 (17.1) | 1304 (16.1) | 1039 (19.6) | 1068 (19.7) |
| Calcium vitamin D intervention assignment | ||||
| Not randomly assigned | 3463 (40.7) | 3232 (39.9) | 2236 (42.1) | 2327 (42.9) |
| Active | 2508 (29.5) | 2475 (30.5) | 1531 (28.8) | 1540 (28.4) |
| Placebo | 2535 (29.8) | 2395 (29.6) | 1543 (29.1) | 1562 (28.8) |
Percentages may not total 100% because of missing data. E-alone = estrogen alone; E + P = estrogen plus progestin; GED = general equivalency diploma; HT = hormone therapy; NSAID = nonsteroidal anti-inflammatory drug; MET = metabolic equivalent tasks.
From diet and supplements.
Based on the mean annual amount of sunlight reaching the clinic site as measured by the US Weather Bureau; 1 langley = 1 g-cal/cm2.
History of cancer (cancers diagnosed more than 10 years before enrollment) is defined as any cancer except nonmelanoma skin cancer.
Nonmelanoma Skin Cancer
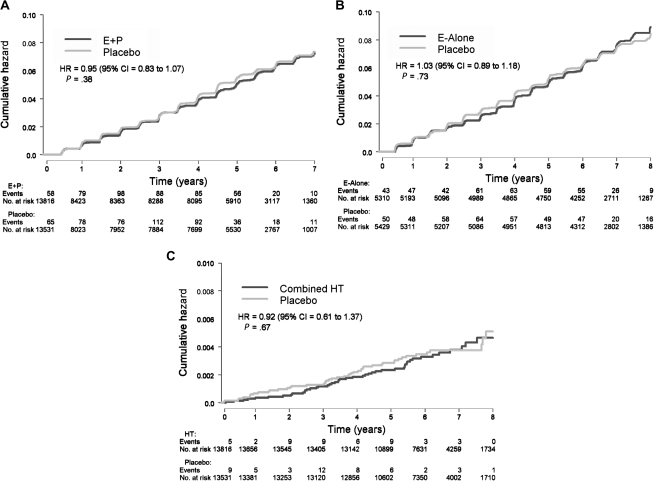
Postmenopausal women were followed for a mean of 5.6 years in the E + P trial and 7.1 years in the E-alone trial. There was no effect of combined E + P or E-alone on the number of incident cases of self-reported nonmelanoma skin cancer (E + P vs placebo: 494 vs 486 cases, HR = 0.95, 95% CI = 0.83 to 1.07, P = .38; E-alone vs placebo: 406 vs 414 cases, HR = 1.03, 95% CI = 0.89 to 1.18, P = .73) (Figure 1, A and B). Because of the null findings in the each of the two trials, we combined the data from the E + P and E-alone trials to enhance the statistical power. Hormone therapy use had no effect on the incidence of nonmelanoma skin cancer in the combined analysis (HR = 0.98, 95% CI = 0.89 to 1.07, P = .65) (Table 2) nor did hormone therapy use affect the incidence of nonmelanoma skin cancer within any of the six predetermined subgroups (ie, age, BMI, regional solar radiation, history of nonmelanoma skin cancer, smoking status, or nonsteroidal anti-inflammatory drug use) Supplementary Tables 1 and 2, available online).
Figure 1.
Kaplan–Meier estimates of cumulative hazards for nonmelanoma skin cancer and melanoma events. A) Nonmelanoma skin cancer events in the E + P trial (N = 980). B) Nonmelanoma skin cancer events in the E-alone trial (N = 820). C) Melanoma events in hormone therapy users from the E + P and E-alone trials combined (N = 95). Cox proportional hazards, Wald statistic P value. CI = confidence interval; E-alone = estrogen alone; E + P = estrogen plus progestin; HR = hazard ratio; HT = combined hormone therapy; NMSC = nonmelanoma skin cancer.
Table 2.
Nonmelanoma skin cancer and melanoma events by trial and overall*
| Number of cases (annualized %) |
||||
| Event | E + P (N = 8506) | Placebo (N = 8102) | HR (95% CI) | P† |
| NMSC | 494 (1.05) | 486 (1.11) | 0.95 (0.83 to 1.07) | .38 |
| Melanoma | 29 (0.06) | 28 (0.06) | 0.96 (0.57 to 1.61) | .86 |
| E-alone (N = 5310) | Placebo (N = 5429) | |||
| NMSC | 406 (1.12) | 414 (1.11) | 1.03 (0.89 to 1.18) | .73 |
| Melanoma | 17 (0.05) | 21 (0.05) | 0.85 (0.45 to 1.61) | .61 |
| Any HT (N = 13 816) | Placebo (N = 13 531) | |||
| NMSC | 900 (1.08) | 900 (1.11) | 0.98 (0.89 to 1.07) | .65 |
| Melanoma | 46 (0.05) | 49 (0.06) | 0.92 (0.61 to 1.37) | .67 |
CI = confidence interval; E-alone = estrogen alone; E+P = estrogen plus progestin; HT = hormone therapy; HR = hazard ratio; ; NMSC = nonmelanoma skin cancer.
Two-sided (from Cox proportional hazards model).
Melanoma
There was no effect of combined E + P or E-alone on the number of incident cases of melanoma after a mean follow-up of 5.6 and 7.1 years in the respective trials (E + P vs placebo: 29 vs 28 cases, HR = 0.96, 95% CI = 0.57 to 1.61, P = .86; E-alone vs placebo: 17 vs 21 cases, HR = 0.85, 95% CI = 0.45 to 1.61, P = .61) (Table 2). Because there was no evidence that the results differed between trials, we combined the melanoma data for the E + P and E-alone trials to assess active hormone therapy use vs placebo (Table 2, Figure 1, C). Hormone therapy use had no effect on the incidence of melanoma in the combined analysis (HR = 0.92, 95% CI = 0.61 to 1.37) nor did hormone therapy use affect the incidence of melanoma within any of the six predetermined subgroups (Supplementary Table 3, available online). In a sensitivity analysis that was restricted to participants who were adherent to the study interventions (ie, those who took 80% or more of study pills) during the trial, there was no effect of hormone therapy use on the incidence of melanoma or nonmelanoma skin cancer (data not shown). Of the 95 incident cases of melanoma, 50 were invasive and 45 were in situ. We found no statistically significant differences in subtypes of melanomas (invasive vs in situ) between women randomly assigned to E-alone vs placebo, E + P vs placebo, or any hormone therapy vs placebo.
Discussion
To our knowledge, these are the first data on hormone therapy use and the risk of skin cancer from a randomized double-blind trial. In the WHI hormone therapy trials, neither E + P nor E-alone affected the incidence of nonmelanoma skin cancer or melanoma in postmenopausal women. The findings with regard to melanoma incidence are consistent with the preponderance of data from observational studies [as reviewed in (10,11)]. Of 14 observational studies that examined the association between hormone therapy and melanoma (15–17,23–33), only three (15–17) reported that hormone therapy use was statistically significantly associated with a higher incidence of melanoma. Findings from these three reports may have been confounded because of the lack of detailed information on sun exposure and constitutional risk factors such as skin color, and even among the 14 studies, the results were inconsistent (15). Because melanoma is associated with sunburn in childhood or teenage years (34), hormone exposure in early life may have a stronger effect on skin cancer development than exposures after menopause.
Although endogenous estrogens have been reported to modulate the growth of basal and squamous cell carcinomas in mouse models of skin cancer (35), we are unaware of any previous clinical studies that have examined the association between hormone therapy and nonmelanoma skin cancer.
The strengths of this study include the randomization and double-blind design of the two trials, the large ethnically diverse study population, the length of follow-up in the trials, the large number of nonmelanoma skin cancer cases, and the central adjudication of the melanoma cases. This study has several limitations. First, the small number of melanoma cases precluded analyses stratified by anatomical location (eg, sun-exposed vs non–sun-exposed body sites) or type of tumor (invasive vs in situ). Second, there is a possibility of chance findings due to the nature of post hoc analyses. Third, the low rate of adherence to study medication, albeit higher than that observed in clinical practice (20), could have potentially attenuated the hormone effect estimates; however, a sensitivity analysis that was restricted to women who were adherent to treatment did not show an effect of hormone therapy on the risk of skin cancer. Fourth, major risk factors for skin cancer (ie, individual sun exposure, history of sunburns, number of nevi, family history of skin cancer, and skin type) were not collected in these WHI trials. However, the 40 WHI clinical centers were geographically diverse, which may serve as a proxy of sun exposure in our analyses (36). In addition, the lack of major differences in characteristics between randomization groups (Table 1) and skin cancer risk factors are likely to be balanced between the groups. Fifth, because follow-up time was limited to the period of the intervention, it is possible that any potential effects of hormone therapy on skin cancer incidence will take more than 6–7 years to become evident. Sixth, the nonmelanoma skin cancer outcome was based on self-report. However, studies assessing the validity of self-reports of skin cancer have found that they are highly correlated with pathology review (>90% correlation) (37,38). Finally, because information on the type of nonmelanoma skin cancer was not collected, we could not determine if there were differential effects of hormone therapy on the risks of basal cell vs squamous cell carcinoma.
In these trials of 27 347 postmenopausal women, we found no association between hormone therapy use and skin cancer. Although this cohort was followed for approximately 6 years, we cannot rule out a delayed effect of hormone therapy on risk of skin cancer. Given that the WHI continues to monitor most of the participants for additional outcomes even though the intervention has ended (39), in a future study, we plan to examine the effect of hormone therapy with 11 years of follow-up. In conclusion, the use of estrogen therapy, alone or in combination with a progestin, did not influence the incidence of nonmelanoma skin cancer or of melanoma in postmenopausal women.
Funding
This work was supported by the Women’s Health Initiative Program, which is funded by the National Heart, Lung, and Blood Institute at the National Institutes of Health (contract number N01-WH-32108); the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health (1K23AR056736-01 to J.T.); and the National Center for Research Resources at the National Institutes of Health (KL2 RR024130 to J.T.).
Supplementary Material
Footnotes
Both the Women’s Health Initiative (WHI) Investigators and the National Institutes of Health sponsors contributed to study design and execution. Statistical analyses and data management were conducted at the WHI Clinical Coordinating Center at the Fred Hutchinson Cancer Research Center (Seattle, WA).
The authors have no relevant financial disclosures or conflicts of interest to disclose. The study sponsors had no role in the study design, collection of the data, interpretation of the results, preparation of the article, or the decision to submit the article for publication.
References
- 1.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 2.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129(7):1666–1674. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41(1):45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Rosso S, Zanetti R, Martinez C, et al. The multicentre south European study ’Helios’. II: different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. Br J Cancer. 1996;73(11):1447–1454. doi: 10.1038/bjc.1996.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verdier-Sevrain S, Yaar M, Cantatore J, Traish A, Gilchrest BA. Estradiol induces proliferation of keratinocytes via receptor-mediated mechanisms. FASEB J. 2004;18(11):1252–1254. doi: 10.1096/fj.03-1088fje. [DOI] [PubMed] [Google Scholar]
- 6.Plewig G, Cunliffe WJ, Binder N, Höschen K. Efficacy of an oral contraceptive containing EE 0.03 mg and CMA 2 mg (Belara) in moderate acne resolution: a randomized, double-blind, placebo-controlled phase III trial. Contraception. 2009;80(1):25–33. doi: 10.1016/j.contraception.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Phillips T, Symons J, Menon S. Does hormone therapy improve age-related skin changes in postmenopausal women? A randomized, double-blind, double-dummy, placebo-controlled multicenter study assessing the effects of norethindrone acetate and ethinyl estradiol in the improvement of mild to moderate age-related skin changes in postmenopausal women. J Am Acad Dermatol. 2008;59(3):397–404. doi: 10.1016/j.jaad.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Applebaum KM, Nelson HH, Zens MS, Stukel TA, Spencer SK, Karagas MR. Oral contraceptives: a risk factor for squamous cell carcinoma? J Invest Dermatol. 2009;129(12):2760–2765. doi: 10.1038/jid.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asgari MM, Efird JT, Warton EM, Friedman GD. Potential risk factors for cutaneous squamous cell carcinoma include oral contraceptives: results of a nested case-control study. Int J Environ Res Public Health. 2010;7(2):427–442. doi: 10.3390/ijerph7020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Driscoll MS. Do hormones influence melanoma? Facts and controversies. Clin Dermatol. 2010;28(3):287–292. doi: 10.1016/j.clindermatol.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Mor Z, Caspi E. Cutaneous complications of hormonal replacement therapy. Clin Dermatol. 1997;15(1):147–154. doi: 10.1016/s0738-081x(96)00117-4. [DOI] [PubMed] [Google Scholar]
- 12.de Vries E, Nijsten TEC, Visser O, et al. Superior survival of females among 10,538 Dutch melanoma patients is independent of Breslow thickness, histologic type and tumor site. Ann Oncol. 2008;19(3):583–589. doi: 10.1093/annonc/mdm498. [DOI] [PubMed] [Google Scholar]
- 13.Karagas MR, Stukel TA. A pooled analysis of 10 case-control studies of melanoma and oral contraceptive use. Br J Cancer. 2002;86(7):1085–1092. doi: 10.1038/sj.bjc.6600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt AN, Nanney LB, Boyd AS, King LE, Ellis DL. Oestrogen receptor-beta expression in melanocytic lesions. Exp Dermatol. 2006;15(12):971–980. doi: 10.1111/j.1600-0625.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- 15.Holly EA, Cress RD, Ahn DK. Cutaneous melanoma in women: ovulatory life, menopause, and use of exogenous estrogens. Cancer Epidemiol Biomarkers Prev. 1994;3(8):661–668. [PubMed] [Google Scholar]
- 16.Adami H-O, Persson I, Hoover R, Schairer C, Bergkvist L. Risk of cancer in women receiving hormone replacement therapy. Int J Cancer. 1989;44(5):833–839. doi: 10.1002/ijc.2910440515. [DOI] [PubMed] [Google Scholar]
- 17.Koomen ER, Joosse A, Herings RMC, Casparie MK, Guchelaar HJ, Nijsten T. Estrogens, oral contraceptives and hormonal replacement therapy increase the incidence of cutaneous melanoma: a population-based case-control study. Ann Oncol. 2009;20(2):358–364. doi: 10.1093/annonc/mdn589. [DOI] [PubMed] [Google Scholar]
- 18.Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 19.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative Clinical Trial and Observational Study. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 20.Tsai S, Stefanick M, Stafford R. Trends in menopausal hormone therapy use of US office-based physicians, 2000-2009. Menopause. 2011;18(4):385–392. doi: 10.1097/gme.0b013e3181f43404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 22.Curb JD, McTiernan A, Heckbert SR. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13(S9):S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 23.Beral V, Ramcharan S, Faris R. Malignant melanoma and oral contraceptive use among women in California. Br J Cancer. 1977;36(6):804–809. doi: 10.1038/bjc.1977.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beral V, Evans S, Shaw H, Milton G. Oral contraceptive use and malignant melanoma in Australia. Br J Cancer. 1984;50(5):681–685. doi: 10.1038/bjc.1984.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holly EA, Weiss NS, Liff JM. Cutaneous melanoma in relation to exogenous hormones and reproductive factors. J Natl Cancer Inst. 1983;70(5):827–831. [PubMed] [Google Scholar]
- 26.Holman CD, Armstrong BK, Heenan PJ. Cutaneous malignant melanoma in women: exogenous sex hormones and reproductive factors. Br J Cancer. 1984;50(5):673–680. doi: 10.1038/bjc.1984.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallagher RP, Elwood JM, Hill GB, Coldman AJ, Threlfall WJ, Spinelli JJ. Reproductive factors, oral contraceptives and risk of malignant melanoma: Western Canada Melanoma Study. Br J Cancer. 1985;52(6):901–907. doi: 10.1038/bjc.1985.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osterlind A, Tucker MA, Stone BJ, Jensen OM. The Danish case-control study of cutaneous malignant melanoma. III. Hormonal and reproductive factors in women. Int J Cancer. 1988;42(6):821–824. doi: 10.1002/ijc.2910420603. [DOI] [PubMed] [Google Scholar]
- 29.Smith MA, Fine JA, Barnhill RL, Berwick M. Hormonal and reproductive influences and risk of melanoma in women. Int J Epidemiol. 1998;27(5):751–757. doi: 10.1093/ije/27.5.751. [DOI] [PubMed] [Google Scholar]
- 30.Lea CS, Holly EA, Hartge P, et al. Reproductive risk factors for cutaneous melanoma in women: a case-control study. Am J Epidemiol. 2007;165(5):505–513. doi: 10.1093/aje/kwk040. [DOI] [PubMed] [Google Scholar]
- 31.Naldi L, Altieri A, Imberti G, Giordano L, Gallus S, Lavecchia C. Cutaneous malignant melanoma in women. phenotypic characteristics, sun exposure, and hormonal factors: a case–control study from Italy. Ann Epidemiol. 2005;15(7):545–550. doi: 10.1016/j.annepidem.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Persson I, Yuen J, Bergkvist L, Schairer C. Cancer incidence and mortality in women receiving estrogen and estrogen-progestin replacement therapy—long-term follow-up of a Swedish cohort. Int J Cancer. 1996;67(3):327–332. doi: 10.1002/(SICI)1097-0215(19960729)67:3<327::AID-IJC4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 33.Lederman J, Lew R, Koh H, Sober AJ. Influence of estrogen administration on tumor characteristics and survival in women with cutaneous melanoma. J Natl Cancer Inst. 1985;74(5):981–985. [PubMed] [Google Scholar]
- 34.Rosso S, Zanetti R, Pippione M, Sancho-Garnier H. Parallel risk assessment of melanoma and basal cell carcinoma: skin characteristics and sun exposure. Melanoma Res. 1998;8(6):573–583. doi: 10.1097/00008390-199812000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Mancuso M, Gallo D, Leonardi S, et al. Modulation of basal and squamous cell carcinoma by endogenous estrogen in mouse models of skin cancer. Carcinogenesis. 2009;30(2):340–347. doi: 10.1093/carcin/bgn243. [DOI] [PubMed] [Google Scholar]
- 36.Oliveria SA. Sun exposure and risk of melanoma. Arch Dis Child. 2005;91(2):131–138. doi: 10.1136/adc.2005.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ming ME, Levy RM, Hoffstad OJ, Filip J, Gimotty PA, Margolis DJ. Validity of patient self-reported history of skin cancer. Arch Dermatol. 2004;140(6):730–735. doi: 10.1001/archderm.140.6.730. [DOI] [PubMed] [Google Scholar]
- 38.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 39.LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy. JAMA. 2011;305(13):1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.