Abstract
Background
Cervical cancer is a major public health problem in Latin America and the Caribbean (LA&C), showing some of the highest incidence and mortality rates worldwide. Information on HPV type distribution in high-grade cervical lesions (HSIL) and invasive cervical cancer (ICC) is crucial to predict the future impact of HPV16/18 vaccines and screening programmes, and to establish an appropriate post-vaccinal virologic surveillance. The aim was to assess the prevalence of HPV types in HSIL and ICC in studies in LA&C.
Methods and Findings
We performed a systematic review, following the MOOSE guidelines for systematic reviews of observational studies, and the PRISMA statement for reporting systematic reviews and meta-analyses. Inclusion criteria were at least ten cases of HSIL/ICC, and HPV-type elicitation. The search, without language restrictions, was performed in MEDLINE, Cochrane Library, EMBASE, LILACS from inception date to December 2009, proceedings, reference lists and consulting experts. A meta-analysis was performed using arc-sine transformations to stabilize the variance of simple proportions. Seventy-nine studies from 18 countries were identified, including 2446 cases of HSIL and 5540 of ICC. Overall, 46.5% of HSIL cases harbored HPV 16 and 8.9% HPV18; in ICC, 53.2% of cases harbored HPV 16 and13.2% HPV 18. The next five most common types, in decreasing frequency, were HPV 31, 58, 33, 45, and 52.
Study's limitations comprise the cross-sectional design of most included studies and their inherent risk of bias, the lack of representativeness, and variations in the HPV type-specific sensitivity of different PCR protocols.
Conclusions
This study is the broadest summary of HPV type distribution in HSIL and ICC in LA&C to date. These data are essential for local decision makers regarding HPV screening and vaccination policies. Continued HPV surveillance would be useful, to assess the potential for changing type-specific HPV prevalence in the post-vaccination era in Latin America.
Introduction
Human papillomavirus infection (HPV) is one of the most common sexually transmitted diseases worldwide [1]. Infection by certain types of HPV is recognized as a causal and necessary factor in the development of cervical cancer [2]. Cervical cancer represents the second-most common malignancy in women around the world and contributes to 9.8% of all female cancers [3]. Cervical cancer accounts for 10% of all female cancers, making it the second leading cause of cancer death in women. Worldwide, there were approximately 500,000 incident cases and 275,000 deaths due to cancer of the cervix in 2002. Latin America and the Caribbean accounted for 15% and 11%, respectively, of this burden [4]. The age-standardized cervical cancer incidence rate is 30.6 per 100,000 persons in Central America, and 28.6 per 100,000 persons in South America [5].
It is now recognized that virtually all cervical cancers (both the squamous and adenocarcinoma histological types) and their precursor lesions are causally related to cervical infections through at least 14 oncogenic HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68) [6], [7]. However, only a minority of pre-neoplastic lesions progress to cancer; the HPV type is a robust risk factor for differential progression [8]. Since cervical cancer affects relatively young women, it represents the single biggest cause of years of life lost (YLL) from cancer in the developing world, contributing more to this burden of disease measure than do tuberculosis, maternal conditions or acquired immunodeficiency syndrome (AIDS) [9]. In developed countries, Papanicolaou (PAP) smear test screening has decreased the incidence of cervical cancer by about 70% in recent decades; however, it still represents a major public health issue in LA&C because of the failure of prevention programs [4]. Previous meta-analyses have reported information about prevalence distribution of high-risk HPV types in HSIL or cervical cancer worldwide; however, this data is variable and incomplete for LA&C populations [10]–[14]. Regional data on type-distribution is essential for estimating the impact of vaccines on cervical cancer and for the development of screening programs. The aim of the present study is to assess exhaustively the HPV type distribution in HSIL and ICC in studies in LA&C region.
Materials and Methods
We performed a systematic review, following the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [15] for systematic reviews of observational studies, and the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews and meta-analyses [16], [17], which replaced the Quality Of Reporting Of Meta-analysis (QUOROM) statement [18].
Search methodology
A search, without language restrictions, was performed on the main international and regional literature databases MEDLINE; EMBASE; CINAHL; NHS R&D Health Technology Assessment Program; ClinicalTrials.gov; LILACS; Cab International Global Health; Pascal Biomed; generic and academic internet search and meta-search engines; and the specialized register of the Cochrane Gynecological Cancer Group from its inception date to December 2009. Databases containing regional proceedings or congress's annals, doctoral theses and experts were also consulted.
The Medline, LILACS, and EMBASE search strategy is available at the Appendix S1. An exhaustive strategy module was developed to localize studies from LA&C. According to pre-specified criteria, pairs of authors independently examined the title, abstract, and descriptors of the articles in order to identify potentially relevant studies for full review. The reference lists of the articles finally included were hand-searched for additional information. If data or data subsets of the same population were published in more than one article, only the publication with the largest sample size was selected, after consulting the principal investigator. Discrepancies were resolved by consensus or, finally, by a third author. The full texts of relevant articles retrieved were examined using a pre-designed form.
Types of studies and participants
Any descriptive epidemiological study with individual-level data was considered. Participant subjects were women from LA&C countries, in studies of cervical cancer/HSIL associated with HPV. The inclusion criteria were a) to inform at least ten cases of HSIL or ICC, b) confirmed by biopsy, and c) HPV-type elicitation. We excluded those papers that undoubtedly failed to meet the aforementioned inclusion criteria. Studies using both polymerase chain reaction (PCR)-amplified and non-amplified genotyping methods were included. There were no restrictions on PCR primers' utilization. HPV DNA tissue sources included fixed or fresh biopsies and/or exfoliated cells. Outcome measures included global and type-specific HPV prevalence. Two attempts of email contact with the author were made in order to recover missing data.
Methodological quality assessment
Two reviewers assessed the methodological quality of studies independently. Discrepancies were solved by consensus of the whole team. Observational studies or control arms of randomized controlled trials were assessed by a checklist of essential items stated in STROBE [19] (Strengthening the Reporting of Observational studies in Epidemiology) statement, two methodological papers [20], [21] and the general guidelines of MOOSE [15]. (See Appendix S2)
Pairs of reviewers independently abstracted the following key information: country where the samples were drawn, setting, population, sample size, study design, age, study year, distribution of cases by histological type, type of cervical specimen and PCR primers, type-specific and overall prevalence of HPV infection, reported duration of HPV infection, and quality score. Data on HPV-specific prevalence was extracted independently for squamous cell carcinoma (SCC) and for adeno- and adenosquamous carcinoma. Each study, or regional components of a study, was classified by the following criteria: 1) geographical region (Central America/Mexico/The Caribbean or South America) 2) income level as defined by the Gross Nation Income (GNI) World Bank Classification (lower-middle income, upper-middle income, high income), 3) tissue source (exfoliated cells, fixed biopsies, fresh biopsies, combined), and 4) genotyping method (Southern blot, Dot blot, FISH and In Situ Hybridization), PCR 1 (PCR MY09/11 or Consensus primers), PCR 2 (PCR SPF, GP5/6, E6, E7 and others) and PCR 3 (PCR MY and GP performed together).
Statistical analysis
HPV prevalence data was expressed as a percentage of all cases tested for HPV. Multiple infections were separated into constituent types, thus type-specific prevalence represents both single and multiple infections. For HPV type-specific prevalence, only studies testing for a particular HPV type contribute to the analysis for that type, and therefore sample size varied between the type-specific analyses. In order to perform a meta-analysis with prevalence data, we first transformed proportions into a quantity (the Freeman-Tukey variant of the arcsine square root transformed proportion) [22]. The pooled proportion was calculated as the back-transformation of the weighted mean of the transformed proportions, using inverse arcsine variance weights for the fixed effects model. The arcsine transformations were necessary to stabilize the variance of simple proportions.
One must consider that each HPV type proportion is a pooled estimate of only those studies reporting the particular HPV type. Hence, each proportion has its own denominator and must be considered regardless of the other types. Thus, cumulative point estimates do not sum to 100%. DerSimonian-Laird weights for the random effects model [23] were applied where heterogeneity between studies was found. The I2 statistic quantifies the heterogeneity between studies. This statistic describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance). [24] We used Statsdirect and STATA 8.0.
We hypothesized the following possible sources of heterogeneity: age, risk factors of HPV and/or HSIL/cervical cancer, country, geographical region, income level by the Gross National Income (GNI) World Bank Classification, type of cervical lesion, type of tissue source and type of genotyping method used. With the available data we could perform pre-designed subgroup analyses considering the country where the study was carried out, the geographical region, the income level of the country according the Gross National Income (GNI) World Bank Classification, the type of genotyping method and the tissue source. Additionally, we applied a meta-regression analysis in order to further study the possible sources of heterogeneity and to get the adjusted prevalence. Publication bias was unlikely as assessed by funnel plots although this type of bias is unlikely to occur in prevalence studies (data not shown). No ethical approval was required for this study.
Results
The present Systematic Review and Meta-analysis met the PRISMA statement requirements (See Checklist S1 and Diagram S1).
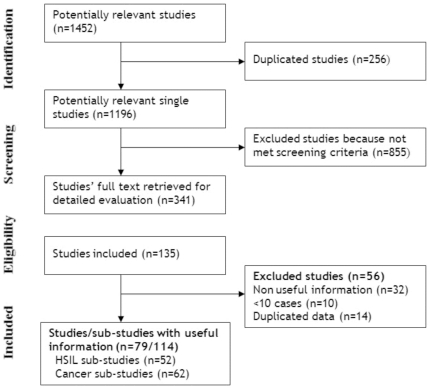
Overall, 1452 citations were retrieved from the search strategy. After the assessment ( Figure 1 ), 79 studies from 18 countries, totaling 7986 women, met the inclusion criteria [25]–[100]. Study characteristics are presented in Appendix S3. Nine countries were from Central America/Mexico/The Caribbean (31.8% of the women) and nine countries from South America (68.2%). One country, was a high-income nation (0.3% of women), six countries were middle-income (72.3%), and eleven countries were low-income (27.4%).
Figure 1. Study flow diagram.
We considered 114 sub-studies for the analysis, including seven country-level sub-studies from Bosch 1995 [29] and discriminated sub-studies by cervical lesion (52 sub-studies evaluated patients with HSIL and 62 evaluated patients with cervical cancer). Thirteen studies had a moderate risk of bias [29], [41], [44], [68], [70], [73], [82], [92], [101]–[105] and the rest carried a high risk of bias. HPV DNA was retrieved from fixed biopsies in 34.2%, from exfoliated cells in 34.2%, from fresh biopsies in 19.7%, and from exfoliated cells and fresh biopsies in 11.8% of the studies. Most of the authors used PCR MY09/11 or non-specified consensus primers (n = 30), while the rest used membrane or in-situ hybridization (n = 9), PCR GP5/6 or SPF or others (i.e. E6 and E7) (n = 30), or PCR using MY and GP together (n = 8) (Appendix S3).
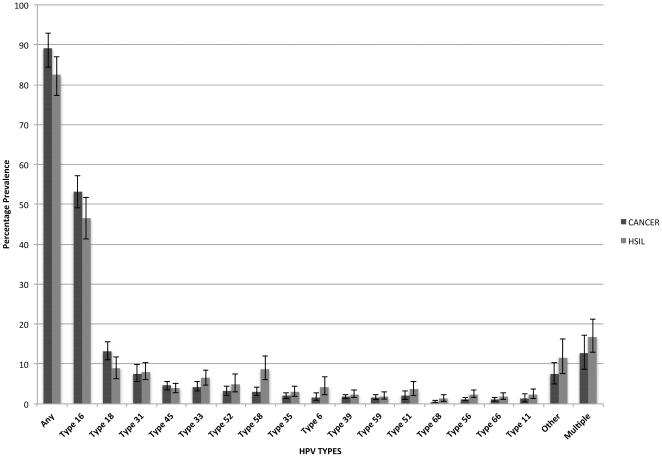
HSIL/ICC cases came mainly from Brazil (23.7%), Argentina (19.0%), and Mexico (17.9%). The HSIL and ICC prevalence, and ICC∶HSIL prevalence ratio by type are presented in Table 1 . HPV16 and HPV18, were the first- and second-most common types, respectively for both HSIL and ICC. HPV18, 45 and 16 had the highest ICC∶HSIL prevalence ratio (1.48, 1.18, and 1.14 respectively). Conversely, HPV11, 56, 6, 68 and 58, were each 2 to 3-fold more prevalent in HSIL than in ICC.
Table 1. HSIL and CANCER prevalence by HPV type.
| HPV TYPE | HSIL | CANCER | CANCER∶HSIL | ||
| N° of patients | Prevalence % | N° of patients | Prevalence % | Prevalence | |
| (N° of Studies) | (95% CI) | (N° of Studies) | (95% CI) | ratio | |
| Global | 2446 (52) | 5540 (62) | |||
| Any | 1749 (36) | 82.5 (77.3–87.1) | 3435 (43) | 89.0 (84.3–92.9) | 1.08 |
| Type 6 | 1415 (29) | 4.2 (2.2–6.7) | 2274 (32) | 1.7 (0.9–2.8) | 0.4 |
| Type 11 | 1414 (29) | 2.4 (1.3–3.8) | 2274 (32) | 1.3 (0.5–2.5) | 0.54 |
| Type 16 | 2327 (49) | 46.5 (41.3–51.7) | 5463 (60) | 53.2 (49.1–57.2) | 1.14 |
| Type 18 | 2194 (45) | 8.9 (6.3–11.8) | 4962 (56) | 13.2 (11.0–15.6) | 1.48 |
| Type 31 | 1785 (36) | 8.0 (6.0–10.4) | 3903 (45) | 7.5 (5.5–9.8) | 0.94 |
| Type 33 | 1722 (35) | 6.5 (4.7–8.5) | 3821 (42) | 4.3 (3.2–5.5) | 0.66 |
| Type 35 | 1228 (24) | 3.0 (1.9–4.4) | 2332 (31) | 2.0 (1.3–2.7) | 0.67 |
| Type 39 | 885 (20) | 2.4 (1.5–3.5) | 1977 (27) | 1.8 (1.3–2.4) | 0.75 |
| Type 45 | 1077 (24) | 3.9 (2.8–5.2) | 3389 (37) | 4.6 (3.5–5.7) | 1.18 |
| Type 51 | 1013 (21) | 3.7 (2.1–5.7) | 2131 (30) | 2.1 (1.1–3.3) | 0.57 |
| Type 52 | 1152 (25) | 4.9 (2.9–7.4) | 2544 (34) | 3.2 (2.1–4.4) | 0.65 |
| Type 56 | 892 (19) | 2.4 (1.5–3.4) | 2155 (28) | 1.2 (0.8–1.7) | 0.5 |
| Type 58 | 1197 (26) | 8.7 (6.0–11.9) | 2564 (34) | 3.0 (2.1–4.1) | 0.34 |
| Type 59 | 954 (21) | 1.9 (1.2–2.9) | 2199 (30) | 1.6 (1.1–2.2) | 0.84 |
| Type 66 | 926 (20) | 1.8 (1.1–2.8) | 2095 (28) | 1.1 (0.7–1.6) | 0.61 |
| Type 68 | 619 (14) | 1.3 (0.6–2.3) | 1864 (23) | 0.5 (0.3–0.9) | 0.38 |
| Other* | 1479 (32) | 11.6 (7.6–16.2) | 3177 (34) | 7.5 (5.0–10.4) | 0.65 |
| Multiple | 1431 (29) | 16.8 (12.9–21.2) | 2090 (27) | 12.6 (8.7–17.2) | 0.75 |
The comparison of HPV type-specific prevalence cancer and HSIL cases is illustrated by Figure 2 .
Figure 2. HPV type-specific prevalence in Cancer and HSIL, with 95% CIs.
High grade intraepithelial lesions (HSIL)
In the 52 sub-studies included in the HSIL systematic review, 16 were performed in Mexico or Central America and 36 in South America. Overall, a total of 2446 patients' samples were analyzed with a median of 47.5 specimens in each sub-study (range 6 to 130). Most data came from cross-sectional studies (n = 39) while seven came from case-control studies, four from cohort studies/prospective follow up, one from a nested case-control study, one from a before-after study and one from a randomized controlled trial. Mean age of women was 40.4±7.6 years old.
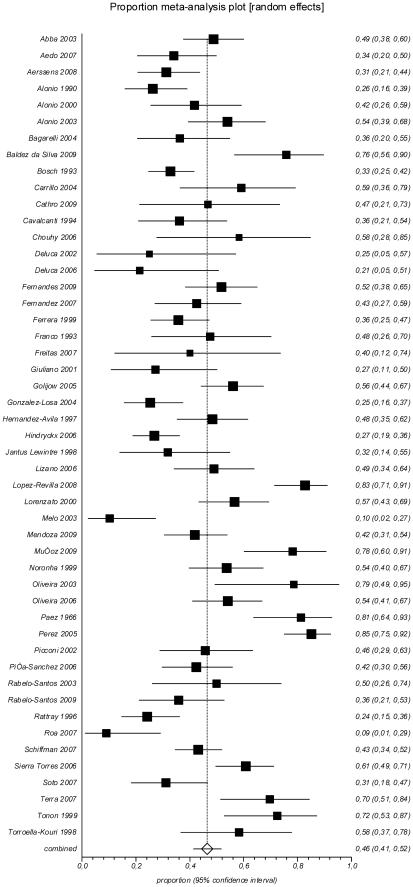
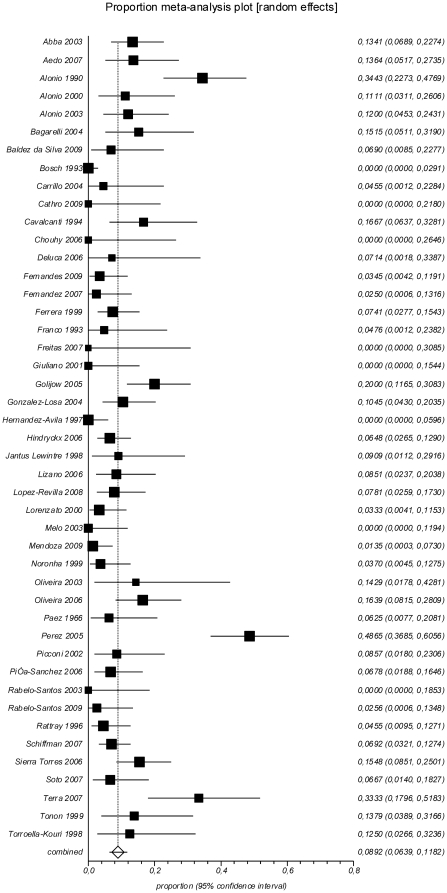
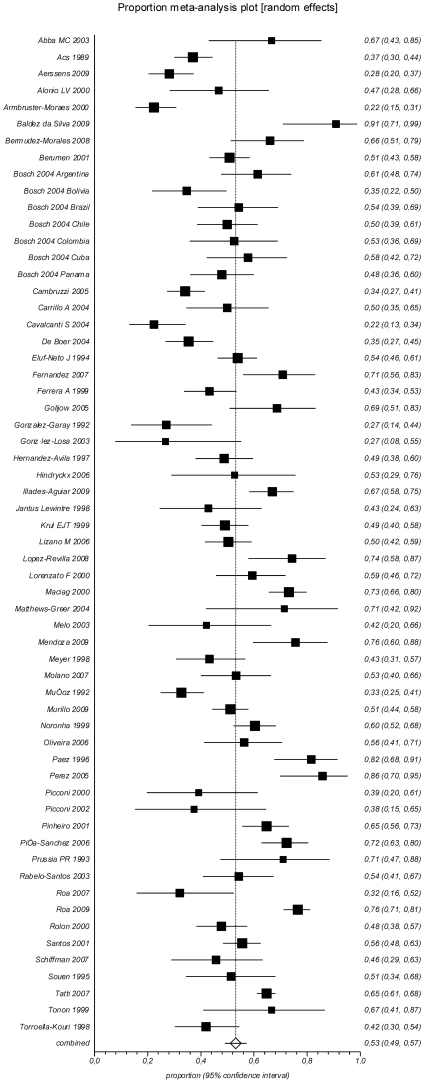
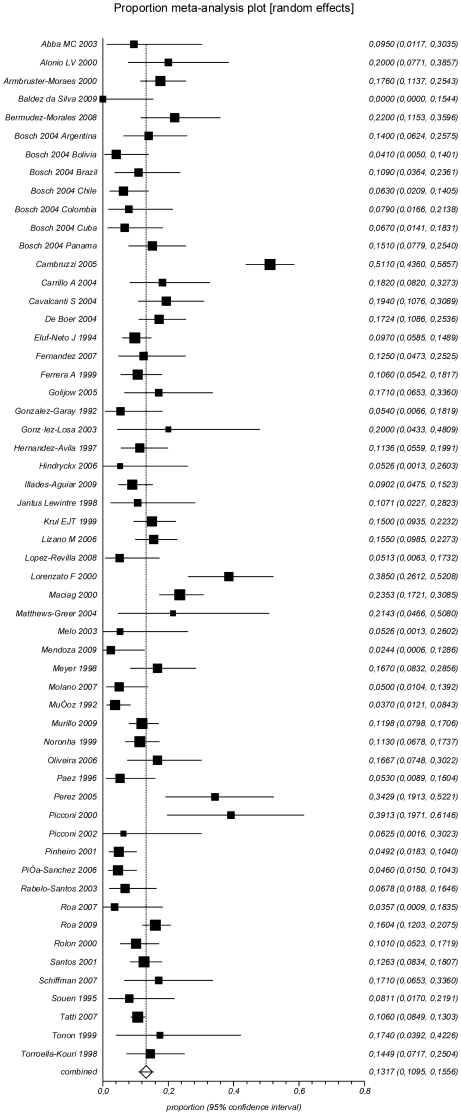
Any HPV in HSIL was found in a pooled proportion of 82.5% (95% CI 77.3–87.1%; I2 = 86.4%) of samples, while prevalence of HPV16 was 46.5% (95% CI 41.3–51.7%; I2 = 84.6%) and prevalence of HPV18 was 8.9% (95% CI 6.3–11.8%; I2 = 80.0%) ( Table 1 , Figure 3 , 4 ). Multiple HPV infections were seen in 16.8% (95% CI 12.8–21.4; I2 = 77.0%) of the analyzed samples.
Figure 3. Prevalence of HPV16 in HSIL.
Figure 4. Prevalence of HPV18 in HSIL.
Table 2 presents the HPV16/18 prevalence in ICC and HSIL by country, region, and Gross National Income (GNI) from the World Bank's classification. In Argentina (12 studies) the pooled prevalence of HPV16 in HSIL samples was 48.5% (95% CI 36.7–60.3%; I2 = 85.8%). In Brazil (13 studies) the pooled prevalence of HPV16 in HSIL samples was 52.7% (95% CI 45.6–59.6%; I2 56.8%). In Mexico (9 studies), the pooled prevalence of HPV16 in HSIL samples was 48.5% (95% CI 35.5–61.6%; I2 86.1%).
Table 2. HPV16/18 prevalence in ICC and HSIL: subgroup analysis by country, region, and GNI World Bank classification.
| Subgroups | HSIL | CERVICAL CANCER | ||||||
| HPV TYPE: 16 | HPV TYPE: 18 | HPV TYPE: 16 | HPV TYPE: 18 | |||||
| N patients(studies) | Prevalence(95% CI) | N patients(studies) | Prevalence(95% CI) | N patients(studies) | Prevalence(95% CI) | N patients(studies) | Prevalence(95% CI) | |
| GLOBAL | 2327 (49) | 46.5 (41.3–51.7) | 2194 (45) | 8.9 (6.3–11.8) | 5463 (60) | 53.2 (49.1–57.2) | 4962 (56) | 13.2 (11–15.6) |
| By country | ||||||||
| Argentina | 502 (12) | 48.5 (36.7–60.3) | 490 (11) | 16.9 (9.8–25.4) | 1013 (10) | 59.5 (51.3–67.5) | 1013 (10) | 17.6 (12–24.1) |
| Barbados | - | - | - | - | 21 (1) | 71.4 (47.8–88.7) | - | - |
| Belize | 15 (1) | 46.7 (21.3–73.4) | 15 (1) | 0 (0–0) | - | - | - | - |
| Bolivia | - | - | - | - | 49 (1) | 34.7 (21.7–49.6) | 49 (1) | 4.1 (0.5–14) |
| Brazil | 466 (13) | 52.7 (45.6–59.6) | 466 (13) | 9 (5–14.1) | 1269 (13) | 53.2 (42.9–63.3) | 1269 (13) | 15.8 (8.9–24.2) |
| Chile | 95 (3) | 18.5 (5.8–36.3) | 73 (2) | 5.9 (0.2–26.2) | 420 (4) | 51.8 (29.7–73.5) | 420 (4) | 9.5 (4.2–16.7) |
| Colombia | 241 (3) | 56.7 (31.2–80.4) | 209 (2) | 4.9 (1.7–29.5) | 450 (4) | 46.7 (35.9–57.7) | 450 (4) | 7.5 (3.7–12.6) |
| Costa Rica | 130 (1) | 43.1 (34.4–52) | 130 (1) | 7.4 (2.8–15.4) | 35 (1) | 45.7 (28.8–63.4) | 35 (1) | 17.1 (6.6–33.6) |
| Cuba | 45 (1) | 31.1 (18.2–46.6) | 45 (1) | 6.3 (0.8–20.8) | 45 (1) | 57.8 (42.2–72.3) | 45 (1) | 6.7 (1.4–18.3) |
| Ecuador | 32 (1) | 81.3 (63.6–92.8) | 32 (1) | 4.5 (0.9–12.7) | 47 (1) | 80.9 (66.7–90.9) | 47 (1) | 4.3 (0.5–14.5) |
| Honduras | 81 (1) | 35.8 (25.4–47.2) | 81 (1) | 6.9 (3.2–12.7) | 104 (1) | 43.3 (33.6–53.3) | 104 (1) | 10.6 (5.4–18.1) |
| Jamaica | 66 (1) | 24.2 (14.5–36.4) | 66 (1) | 6.7 (1.4–18.3) | - | - | - | - |
| Mexico | 405 (9) | 48.5 (35.5–61.6) | 405 (9) | 6 (3.1–9.7) | 1021 (14) | 54.9 (47.6–61.9) | 840 (13) | 12.8 (9.7–16.2) |
| Nicaragua | 175 (2) | 28.8 (22.4–35.7) | 108 (1) | 6.7 (1.4–18.3) | 136 (2) | 38.1 (17–61.9) | 19 (1) | 5.3 (0.1–26) |
| Panama | - | - | - | - | 255 (2) | 41.6 (31.3–52.2) | 73 (1) | 15.1 (7.8–25.4) |
| Paraguay | 74 (1) | 41.9 (30.5–53.9) | 74 (1) | 1.4 (0–7.3) | 154 (2) | 61.3 (33.9–85.2) | 154 (2) | 7.2 (1.8–15.7) |
| Peru | - | - | - | - | 198 (1) | 55.6 (48.3–62.6) | 198 (1) | 12.6 (8.3–18.1) |
| Suriname | - | - | - | - | 246 (2) | 42.2 (29.4–55.7) | 246 (2) | 16.3 (12–21.2) |
| By geographic region | ||||||||
| Central America and Mexico | 917 (16) | 41.7 (33.8–49.8) | 850 (15) | 6.3 (4.6–8.3) | 1617 (22) | 51.7 (45.6–57.8) | 1116 (18) | 12.5 (10.1–15.1) |
| South America | 1410 (33) | 48.9 (42.2–55.5) | 1344 (30) | 10.5 (6.6–15.1) | 3846 (38) | 54.0 (48.6–59.2) | 3846 (38) | 13.3 (10.4–16.5) |
| By GNI World Bank classification | ||||||||
| Lower middle income | 714 (10) | 43.6 (32.8–54.8) | 615 (8) | 5.5 (2.3–10.1) | 1429 (15) | 49.4 (42.6–56.2) | 1312 (14) | 9.5 (7.2–12) |
| Upper middle income | 1613 (39) | 47.3 (41.5–53.2) | 1579 (37) | 9.8 (6.8–13.2) | 4013 (44) | 54.1 (49.2–58.9) | 3650 (42) | 14.8 (11.9–18) |
| High income | - | - | - | - | 21 (1) | 71.4 (47.8–88.7) | - | - |
We found a pooled prevalence of HPV18 in HSIL of 16.9% (95% CI 9.8–25.4%; I2 81.2%) in Argentina, 9.0% (95% CI 5.0–14.1%; I2 = 66.0%) in Brazil, and 6% (95% CI 3.1–9.7%; I2 = 50.6%) in Mexico. HPV prevalence according to subgroups of geographic region and by GNI World Bank Classification are shown in Table 2 . The subgroup analyses by primers used and by tissue source are shown in Table 3 .
Table 3. HPV16/18 prevalence in ICC and HSIL: subgroup analysis by genotyping method and tissue source.
| Subgroups | HSIL | CERVICAL CANCER | ||||||
| HPV TYPE: 16 | HPV TYPE: 18 | HPV TYPE: 16 | HPV TYPE: 18 | |||||
| N patients(studies) | Prevalence(95% CI) | N patients(studies) | Prevalence(95% CI) | N patients(studies) | Prevalence(95% CI) | N patients(studies) | Prevalence(95% CI) | |
| By Genotyping Method | ||||||||
| Hybridization techniques* | 494 (8) | 37.1 (31.6–42.7) | 427 (7) | 8.2 (1.6–19.3) | 998 (15) | 47.7 (39.1–56.4) | 816 (14) | 12.0 (9–15.4) |
| PCR 1** | 948 (23) | 48.2 (39.7–56.7) | 882 (20) | 7.6 (6–9.4) | 1355 (18) | 58.5 (51.2–65.7) | 1174 (17) | 11.3 (7.5–15.7) |
| PCR 2† | 560 (12) | 42.9 (33.5–52.7) | 560 (12) | 7.5 (4.2–11.6) | 2618 (19) | 49.9 (42.8–56.9) | 2480 (17) | 14.9 (10.2–20.3) |
| PCR 3‡ | 292 (5) | 57.7 (39.7–74.6) | 294 (5) | 16.6 (4.7–33.7) | 420 (6) | 62.4 (51.9–72.4) | 420 (6) | 16.9 (11.7–22.9) |
| By tissue source | ||||||||
| Exfoliated cells | 1330 (26) | 44.7 (38.4–51.1) | 1251 (24) | 6.5 (4.3–9.2) | 914 (16) | 58.4 (52.3–64.4) | 914 (16) | 12.2 (8.4–16.5) |
| Fixed biopsies | 805 (13) | 43.4 (31.4–55.7) | 586 (12) | 13.2 (6.3–22.3) | 2352 (30) | 52.4 (46.2–58.6) | 2149 (28) | 14.6 (10.9–18.8) |
| Fresh biopsies | 266 (7) | 50.5 (36.1–64.7) | 266 (7) | 9.1 (4.5–15.2) | 1592 (9) | 50.7 (42.3–59) | 1411 (8) | 8.8 (6.3–11.8) |
| Combined | 32 (1) | 78.1 (60–90.7) | - | - | 605 (5) | 46.5 (25.6–68) | 488 (4) | 16.3 (10.2–23.3) |
*Southern blot, Dot blot, FISH and In Situ Hybridization.
**Polymerase Chain Reaction MY09/11 or Consensus primers.
Polymerase Chain Reaction SPF, GP5/6, E6, E7 and others.
Polymerase Chain Reaction MY and GP performed together.
Cervical cancer
In the 62 sub-studies included in the ICC systematic review, a total of 5540 patients' samples were analyzed with a median of 56 specimens in each study (range 14 to 750). Most data came from cross-sectional studies (n = 52) while 10 came from case-control studies, and one nested case-control study. Mean age of women was 41.1±7.0 years old.
Any HPV in cervical cancer was found in a pooled proportion of 89.0% (CI 84.3–92.9%; I2 = 94.0%) of the samples, while the prevalence of HPV16 was 53.2% (CI 49.1–57.2%; I2 = 88.5%) and the prevalence of HPV18 was 13.2% (CI 11.0–15.6%; I2 = 81.1%) ( Table 1 , Figure 5 , 6 ). Multiple HPV infections were seen in 12.6% (CI 8.7–17.2%; I2 = 87.8%) of the samples.
Figure 5. Prevalence of HPV16 in ICC.
Figure 6. Prevalence of HPV18 in ICC.
Table 2 shows the prevalence of HPV16/18 in ICC and HSIL by country, region, and GNI World Bank Classification. In Argentina (10 studies), the pooled prevalence of HPV16 in cancer samples was 59.5% (95% CI 51.3–67.5%; I2 = 68.2%), in Brazil (13 studies) 53.2% (95% CI 42.9–63.3%; I2 = 92.5%), and in Mexico (14 studies) 54.9% (95% CI 47.6–61.9%; I2 = 80.3%).
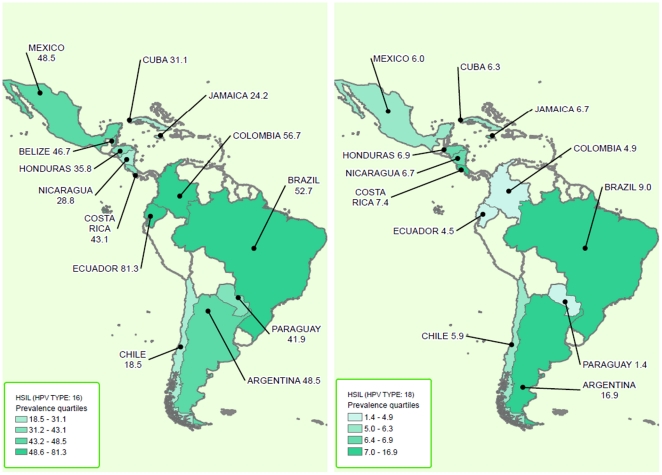
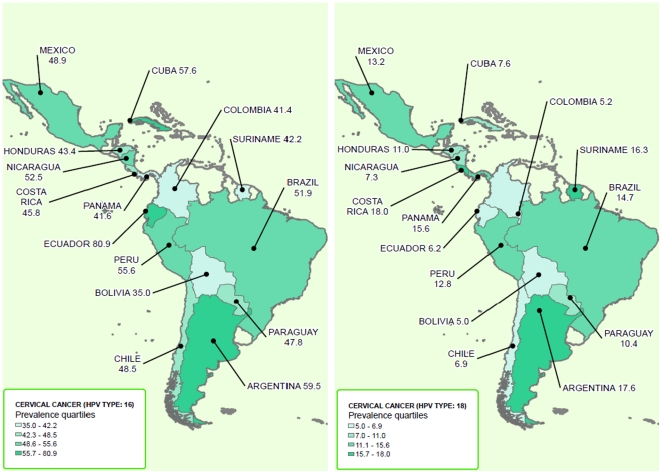
When we analyzed the prevalence of HPV18 in ICC samples, we found a pooled prevalence of 17.6% (95% CI 12–24.1%; I2 = 65.7%) in Argentina, 15.8% (95% CI 8.9–24.2%; I2 = 92.8%) in Brazil, and 12.8% (95% CI 9.7–16.2%; I2 = 47.6%) in Mexico. HPV prevalence by the geographic region and by GNI World Bank Classification are shown in Table 2 . The analyses by primers used and by tissue source are shown in Table 3 . The distributions of HPV 16/18 in HSIL and ICC in LA&C according to quartiles of prevalence are shown in maps in Figures 7 and 8 .
Figure 7. Distribution HPV 16/18 in HSIL in LA&C.
Figure 8. Distribution HPV 16/18 in ICC in LA&C.
We also applied a meta-regression analysis adjusting by GNI World Bank Classification, Geographic region, genotyping method and HPV tissue source to obtain adjusted estimates. There were no statistically significant differences for HPV16 in cancer and in HSIL. For HPV18, the statistically significant difference were seen for HSIL when the tissue source was fixed biopsies (compared to exfoliated cells) and when MY and GP performed together were used compared to Hybridization techniques and for cancer when the tissue source were Polymerase Chain Reaction SPF, GP5/6, E6, E7 and others compared to Hybridization techniques. However the adjusted prevalence, by the means of each variable and considering the SE of the meta-analysis, remained stable: HPV16 in HSIL women 45.7% (CI 95% 42.9–48.5%) HPV18 in HSIL 8.7% (7.2–10.3%); HPV16 in ICC 55.3% (52.5–58.1%); and HPV18 in ICC 13.4 (11.5–15.3).
Funnel plots showed no evidence of publication bias (data not shown).
Discussion
Data on the geographic distribution of HPV type in HSIL and ICC are crucial for estimating the impact of HPV vaccines on cervical cancer and cervical screening programs. [106], [107] Epidemiological studies employing a variety of HPV typing protocols have been aggregated in some meta-analyses. However, the number of samples from LA&C considered in these studies was relatively low.
This review brings representative estimations of HPV type distribution from the LA&C region. Since multiple HPV genotyping techniques have been included, varying sensitivities of the techniques considered might impact the HPV type-specific prevalence reported [108]. Currently, identification of specific HPV types in biological specimens is preferentially done by PCR-based methods due to its higher sensitivity; in this study, however, hybridization techniques without PCR amplification (membrane and in situ hybridization) were also included in order to incorporate the largest number of HSIL and ICC cases, and to increase the representativeness of the data. Nevertheless, only 6% of studies -the oldest ones- used non-PCR-based techniques.
In 2003, Smith et al. [14] updated a meta-analysis of over 10,000 cases published [10], [11]. It retrieved 1,427 cancer cases and 833 HSIL cases from 13 countries in the LA&C region; the prevalence of HPV 16/18 in cervical cancer for South/Central America was 65%. Muñoz et al., in 2004, included 1,084 cervical cancer cases from Central/South America and found an HPV16/18 prevalence of 69%. [13]. Later, Li et al have published a worldwide meta-analysis of HPV type-specific including a total of 30,848 cervical cancers. It included 3,010 cancer cases from 15 countries of LA&C; in this region for 1990–2010, HPV16 and HPV18 were the first and second most common types, respectively (54% and 15% respectively); being the third to eighth most common types HPVs 31, 45, 33, 58, 52 and 35. [12]. The present systematic tripled the number HSIL cases included in the previous reports of Clifford et al. [10], [11] and Smith et al. [14]. Overall, 55% of HSIL cases harbored HPV 16/18, confirming that HPV type distribution in HSIL does not entirely match that of ICC. HPV types 16, 18 and 45 are less common in HSIL than in ICC, whereas other HPV types are more frequent (particularly, HPV58, the third-most prevalent type in HSIL). These differences emphasize the importance of HPV type in the risk of progression to cancer, even from HSIL. The proportions of HSIL cases attributable to both HPV16 and HPV 18 in this study were higher than those in previous meta-analyses [11],[14], which estimated 48% for the region. Our prevalence HPV 16/18 rate is similar to Europe (57.6%) and North America (55.1%), according to the study published by Smith et al. [14]
Data on ICC has greatly enriched previous reports; we increased the number of Latin American cases included from 3,010 considered by the last published meta-analysis [14] to 5,542 in our study. Regarding ICC cases, 53.2% harbored HPV 16 and 13.2% HPV18, confirming that they are the first- and second-most prevalent types, respectively, which agrees with data previously obtained on other continents and worldwide. The next five-most common types, (HPV 31, 58, 33, 45, and 52) added 22.6% of cases. The proportions of cases attributable to HPV16/18 in this study were similar to previous meta-analyses [10], [11], [14], which estimated nearly 65% for the region. Our findings corroborate that in LA&C the HPV16/18 prevalence of ICC is similar to that of Asia (66.9%) and lower than that of Africa (70%), Europe (73.8%) and North America (76.4%), according Smith et al. [14]
Some intra-regional variations of the most common HPV types have been observed, although these apparent differences may happen simply by random fluctuation and/or a lack of sample representativeness of certain countries. For ICC, Mexico, Central America and the Caribbean showed a slightly lower HPV16/18 prevalence than South America (64.2% vs. 67.3% respectively). Particularly, Argentina shows the highest prevalence rate for HPV16/18 in both HSIL (65.4%) and ICC (77.1%). It is interesting to point out that the 12 Argentine studies incorporated samples from women of different provinces of the country, including aboriginal communities (Quechua [37] and Guarani [39] populations), revealing similar HPV16/18 prevalence data.
In 11.6% of HSIL and 7.5% of ICC, HPV detection resulted positive, but the viral type could not be identified (“other type”); these cases most likely represent the failed detection of known types (almost certainly different than HPV 16 and 18) rather than infections of yet-undiscovered types.
In this review, multiple-type HPV infections were detected in 16.8% of HSIL and 12.6% of ICC, although the frequency of multiple infections depends largely on the number of HPV types tested for within a given study. The attribution of ICC etiology to HPV types is increasingly complicated by the rising prevalence of multiple co-existing types. It was suggested that infections with multiple HPV types seem to act synergistically in cervical carcinogenesis [109], and it was also associated with poor response and with reduced survival in cervical cancer patients. [110]. However, other study indicates that despite the presence of many viruses infecting the same anatomical site, only one genotype would be responsible for the disease [111].
HPV18 and 16 had the highest ICC∶HSIL prevalence ratio in our studies, as found in Smith et al. meta-analysis [14]. Conversely, HPV11, 56, 6, 68 and 58, were each 2 to 3-fold more prevalent in HSIL than in ICC. These lowest ratios were observed for many different types and lower than reported [14].
As more data is accumulated, it is supportive to observe that HPV16/18 accounts for two-thirds of ICC in LA&C. The proportion of ICC cases potentially averted by the current approved vaccines may be even higher than the aforementioned one if cross-protection against non-vaccine high-risk HPV types (like HPV31 and 45) is found to be clinically effective in reducing the incidence of ICC and HSIL caused by these genotypes. The information given by this work would be also useful in LA&C for the evaluation of polyvalent vaccines (currently in development) for the prevention of ICC associated to more than eight or nine high-risk HPV types.
Limitations of our meta-analysis include the cross-sectional design of the included studies and their inherent risk of bias, lack of representativeness, the HPV type-specific prevalence variation and HPV type-specific sensitivity of different PCR protocols [112]. There is evidence of considerable heterogeneity between studies. Heterogeneity could not be ruled out even by the pre-designed subgroup analysis: by country, region, and GNI World Bank classification. However inconsistencies might be explained by variations in the population and methods utilized. To address this issue we chose the random effect model meta-analysis to combine data in order to obtain conservative (wider) confidence intervals, which may result more informative than central estimates. In addition 61% of the patients included in the meta-analysis came from only three countries (Argentina, Brazil and Mexico) and one should be cautious when extrapolating our summary results to the entire region. Further, many studies did not type for a broad range of HPV types, and cyto-histological diagnoses across studies were not standardized. The poor infrastructure of research in molecular biology in many countries highlights the need to consider strategic alliances and promoting regional research consortia on the topic of HPV. In this way, according to the World Health Organization HPV Laboratory Network (WHO HPV LabNet) guidelines, the establishment of a Regional HPV LabNet would be extremely useful [113]. This is initiative would support the laboratory standardization and quality assurance of HPV typing methods to promote international comparability of results, promoting an appropriate vaccine introduction and virological surveillance in the vaccine era.
Although information on the histological type of ICC was collected, its discrimination was not always clear and the data came mostly from SCC. For this reason we presented only global data of ICC.
This study is the broadest summary of HPV type distribution in HSIL and ICC in LA&C to date, and it has included the majority of American countries which have the highest cervical cancer burdens in the region and worldwide. The presented information may be of importance for local decision makers to consider the cervical cancer prevention as a whole, taking into account the relevance of vaccination and updating screening strategies using type-specific high-risk HPV-DNA-based tests. This work comes available at the time some Latin American and Caribbean countries are evaluating the HPV vaccine introduction in their National Vaccination Schedules, in the frame of the Pan American Health Organization purchase using revolving fund, which makes vaccines affordable. Continued surveillance of HPV types in HSIL and ICC as HPV vaccines are introduced would be useful, to assess the potential for changing type-specific HPV prevalence in the post-vaccination era in Latin America.
Supporting Information
Search Strategy.
(DOC)
Methodological Quality Assessment.
(DOC)
Study characteristics and HPV-specific prevalence by study and country.
(DOC)
PRISMA checklist for reporting systematic reviews and meta-analyses.
(DOC)
PRISMA study flow diagram for reporting systematic reviews and meta-analyse.
(DOC)
Acknowledgments
We deeply thank Dr. Eduardo Franco, Dr. Nubia Muñoz, Dr. Jorge A. Gómez, Dr. Mark Schiffman and other collaborators for their advice and support. We are also indebted to Dr. Virginia Alonio for her critical review of the manuscript, Jonathan Willner for language editing and librarian Daniel Comandé for his efforts.
Footnotes
Competing Interests: The authors have read the journal's policy and have the following conflicts: The study was supported by an independent grant from GlaxoSmithKline and the Institute for Clinical Effectiveness and Health Policy - IECS. The authors have declared that no other competing interests exist. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: The study was supported by an independent grant from GlaxoSmithKline and the Institute for Clinical Effectiveness and Health Policy (IECS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3–8. doi: 10.1016/s0002-9343(97)00177-0. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J BF, Pisani P, Parkin DM. GLOBOCAN 2002: cancer incidence, mortality and prevalence worldwide. Lyon 2004 [Google Scholar]
- 4.Lewis M. Análisis de la situación del Cáncer Cervicouterino en América Latina y el Caribe. OPS Journal Washington DC 2004 [Google Scholar]
- 5.Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 6.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, et al. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 7.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 8.Lorincz AT, Reid R, Jenson AB, Greenberg MD, Lancaster W, et al. Human papillomavirus infection of the cervix: relative risk associations of 15 common anogenital types. Obstet Gynecol. 1992;79:328–337. doi: 10.1097/00006250-199203000-00002. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organisation. 2006. Available: http://wwwwhoint/healthinfo/statistics/en/Indexhtml. Accessed March 10th, 2011.
- 10.Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer. 2003;89:101–105. doi: 10.1038/sj.bjc.6601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88:63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2010 doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- 13.Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278–285. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 14.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 20.Fowkes FG, Fulton PM. Critical appraisal of published research: introductory guidelines. BMJ. 1991;302:1136–1140. doi: 10.1136/bmj.302.6785.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36:666–676. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 22.Stuart AOJ. Kendall's Advanced Theory of Statistics (6th edition) London: Edward Arnold; 1994. [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abba MC, Gomez MA, Golijow CD. [Human papillomavirus genotype distribution in cervical infections among woman in La Plata, Argentina]. Rev Argent Microbiol. 2003;35:74–79. [PubMed] [Google Scholar]
- 26.Alonio LV, Dalbert D, Mural J, Bartt O, Bazan G, et al. Different papillomaviruses in uterine cervical lesions: Detection and location by ‘in situ’ hydridization with biotinylated probes. Cervix & the Lower Female Genital Tract. 1990;8:339–348. [Google Scholar]
- 27.Alonio LV, Dalbert D, Picconi MA, Cervantes Vazquez G, Garcia Carranca A, et al. [Ha-ras and p53 gene mutations scanned by PCR-SSCP in premalignant and malignant lesions of the uterine cervix associated with human papillomavirus]. Medicina (B Aires) 2000;60:895–901. [PubMed] [Google Scholar]
- 28.Alonio LV, Picconi MA, Dalbert D, Mural J, Bartt O, et al. Ha-ras oncogene mutation associated to progression of papillomavirus induced lesions of uterine cervix. J Clin Virol. 2003;27:263–269. doi: 10.1016/s1386-6532(02)00181-6. [DOI] [PubMed] [Google Scholar]
- 29.Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, et al. Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. Journal of the National Cancer Institute. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 30.Chouhy D, Gil LB, Nocito AL, Wojdyla D, Ornella L, et al. Development and evaluation of a colorimetric PCR system for the detection and typing of human papillomaviruses. Int J Mol Med. 2006;18:995–1003. [PubMed] [Google Scholar]
- 31.Deluca GD, Lucero RH, Martin de Civetta MT, Vicente L, de Gorodner OL, et al. Genotipificación del Virus Papiloma Humano (HPV) por PCR-RFLP en alteraciones cervicales 2002 [Google Scholar]
- 32.Deluca GD, Marin HM, Schelover E, Chamorro EM, Vicente L, et al. Chlamydia trachomatis and papillomavirus infection in women with cytohistological abnormalities in uterine cervix. [Spanish]. Medicina. 2006;66:303–306. [PubMed] [Google Scholar]
- 33.Golijow CD, Abba MC, Mouron SA, Laguens RM, Dulout FN, et al. Chlamydia trachomatis and Human papillomavirus infections in cervical disease in Argentine women. Gynecol Oncol. 2005;96:181–186. doi: 10.1016/j.ygyno.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 34.Jantus Lewintre EMdCM. Cancer de cuello uterino en Corrientes (Argentina): Tipificación de virus del papiloma humano (HPV) en lesiones cervicales por PCR-Hibridación. Nuevas tendencias en Oncología. 1998;7:134–139. [Google Scholar]
- 35.Perez LO, Barbisan G, Abba MC, Laguens RM, Dulout FN, et al. Herpes simplex virus and human papillomavirus infection in cervical disease in Argentine women. Int J Gynecol Pathol. 2006;25:42–47. doi: 10.1097/01.pgp.0000177996.30427.2b. [DOI] [PubMed] [Google Scholar]
- 36.Picconi MA, Alonio LV, Garcia Carranca A, Lizano M, Cervantes Vazquez G, et al. [Molecular variants of human papillomavirus (HPV) types 16 and 18 in adenocarcinomas of the cervix]. Medicina (B Aires) 2000;60:889–894. [PubMed] [Google Scholar]
- 37.Picconi MA, Gronda J, Alonio LV, Villa LL, Sichero L, et al. Virus papiloma humano en mujeres quechuas jujeñas con alta frecuencia de cancer de cuello uterino: tipos virales y variantes de HPV 16. Medicina (BAires) 2002;62:209–220. [PubMed] [Google Scholar]
- 38.Tatti S, Fleider L, Tinnirello M, Chabelski C, Vighi S, et al. AVANCES EN LA PREVENCION DEL CANCER CERVICAL: “del PAPANICOLAOU a la VACUNA PROFILACTICA para el PAPILOMAVIRUS HUMANO (HPV)”. 2007. Buenos Aires.
- 39.Tonon SA, Picconi MA, Zinovich JB, Liotta DJ, Bos PD, et al. Human papillomavirus cervical infection and associated risk factors in a region of Argentina with a high incidence of cervical carcinoma. Infect Dis Obstet Gynecol. 1999;7:237–243. doi: 10.1002/(SICI)1098-0997(1999)7:5<237::AID-IDOG6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prussia PR, Schegget J, Smits HL. Detection of oncogenic HPV DNA by a consensus polymerase chain reaction method in genital carcinomas in twenty women in Barbados. West Indian med j. 1993;42:144–146. [PubMed] [Google Scholar]
- 41.Cathro HP, Loya T, Dominguez F, Howe SL, Howell R, et al. Human papillomavirus profile of women in Belize City, Belize: correlation with cervical cytopathologic findings. Hum Pathol. 2009;40:942–949. doi: 10.1016/j.humpath.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 42.Armbruster-Moraes E, Ioshimoto LM, Leao E, Zugaib M. Prevalence of ‘high risk’ human papillomavirus in the lower genital tract of Brazilian gravidas. Int J Gynaecol Obstet. 2000;69:223–227. doi: 10.1016/s0020-7292(00)00191-0. [DOI] [PubMed] [Google Scholar]
- 43.Bagarelli LB, Oliani AH. Tipagem e estado físico de papilomavírus humano por hibridização in situ em lesões intra-epiteliais do colo uterino. Rev bras ginecol obstet. 2004;26:59–64. [Google Scholar]
- 44.Cambruzzi E, Zettler CG, Alexandre CO. Expression of Ki-67 and squamous intraepithelial lesions are related with HPV in endocervical adenocarcinoma. Pathol Oncol Res. 2005;11:114–120. doi: 10.1007/BF02893378. [DOI] [PubMed] [Google Scholar]
- 45.Cavalcanti SM, Frugulhetti IC, Passos MR, Fonseca ME, Oliveira LH. Prevalence of human papillomavirus DNA in female cervical lesions from Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 1994;89:575–580. doi: 10.1590/s0074-02761994000400013. [DOI] [PubMed] [Google Scholar]
- 46.Cavalcanti SM, Deus FC, Zardo LG, Frugulhetti IC, Oliveira LH. Human papillomavirus infection and cervical cancer in Brazil: a retrospective study. Mem Inst Oswaldo Cruz. 1996;91:433–440. doi: 10.1590/s0074-02761996000400009. [DOI] [PubMed] [Google Scholar]
- 47.Eluf-Neto J, Booth M, Munoz N, Bosch FX, Meijer CJ, et al. Human papillomavirus and invasive cervical cancer in Brazil. Br J Cancer. 1994;69:114–119. doi: 10.1038/bjc.1994.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandes JV, de Vasconcellos Meissner R, de Carvalho MGF, de Medeiros Fernandes TAA, de Azevedo PRM, et al. Prevalence of HPV infection by cervical cytologic status in Brazil. International Journal of Gynecology and Obstetrics. 2009;105:21–24. doi: 10.1016/j.ijgo.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Franco E, Villa L, Rohan T, Ferenczy A, Petzl-Erler M, et al. Design and methods of the Ludwig-McGill longitudinal study of the natural history of human papillomavirus infection and cervical neoplasia in Brazil. Ludwig-McGill Study Group. Rev Panam Salud Publica. 1999;6:223–233. doi: 10.1590/s1020-49891999000900001. [DOI] [PubMed] [Google Scholar]
- 50.Freitas TP, Carmo BBd, Paula FDF, Rodrigues LF, Fernandes AP, et al. Molecular detection of HPV 16 and 18 in cervical samples of patients from Belo Horizonte, Minas Gerais, Brazil. Rev Inst Med Trop SAo Paulo. 2007;49:297–301. doi: 10.1590/s0036-46652007000500005. [DOI] [PubMed] [Google Scholar]
- 51.Lorenzato F, Ho L, Terry G, Singer A, Santos LC, et al. The use of human papillomavirus typing in detection of cervical neoplasia in Recife (Brazil). International Journal of Gynecological Cancer. 2000;10:143–150. doi: 10.1046/j.1525-1438.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 52.Maciag PC, Schlecht NF, Souza PS, Franco EL, Villa LL, et al. Major histocompatibility complex class II polymorphisms and risk of cervical cancer and human papillomavirus infection in Brazilian women. Cancer Epidemiol Biomarkers Prev. 2000;9:1183–1191. [PubMed] [Google Scholar]
- 53.Noronha V, Mello W, Villa L, Brito A, Macêdo R, et al. Papilomavírus humano associado a lesöes de cérvice uterina. Rev Soc Bras Med Trop. 1999;32:235–240. [PubMed] [Google Scholar]
- 54.Oliveira LdHdS, Rodrigues EdVM, Lopes APTAdS, Fernandez AdP, Cavalcanti SMB. HPV 16 detection in cervical lesions, physical state of viral DNA and changes in p53 gene. SAo Paulo med j. 2003;121:67–71. doi: 10.1590/S1516-31802003000200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oliveira LHS, Rosa MLG, Pereira CRN, Vasconcelos GALBM, Silva RA, et al. Human papillomavirus status and cervical abnormalities in women from public and private health care in Rio de Janeiro State, Brazil. Rev Inst Med Trop São Paulo. 2006;48:279–285. doi: 10.1590/s0036-46652006000500008. [DOI] [PubMed] [Google Scholar]
- 56.Pinheiro NA, Villa LL. Low frequency of p53 mutations in cervical carcinomas among Brazilian women. Braz j med biol res = Rev bras pesqui m'd biol. 2001;34:727–733. doi: 10.1590/s0100-879x2001000600005. [DOI] [PubMed] [Google Scholar]
- 57.Rabelo-Santos SH, Zeferino L, Villa LL, Sobrinho JP, Amaral RG, et al. Human papillomavirus prevalence among women with cervical intraepithelial neoplasia III and invasive cervical cancer from Goiania, Brazil. Mem Inst Oswaldo Cruz. 2003;98:181–184. doi: 10.1590/s0074-02762003000200003. [DOI] [PubMed] [Google Scholar]
- 58.Souen J, Ramos LO, Motta E, Eluf Neto J. Prevalência de híbridos do HPV entre portadoras de carcinoma do colo do útero. Rev bras ginecol obstet. 1995;17:509–512. [Google Scholar]
- 59.Terra AP, Murta EF, Maluf PJ, Caballero OL, Brait M, et al. Aberrant promoter methylation can be useful as a marker of recurrent disease in patients with cervical intraepithelial neoplasia grade III. Tumori. 2007;93:572–579. doi: 10.1177/030089160709300610. [DOI] [PubMed] [Google Scholar]
- 60.Aedo SA, Melo AA, García P, Guzmán PG, Capurro IV, et al. Detección y tipificación de virus papiloma humano en lesiones preneoplásicas del cuello uterino mediante PCR-RFLP. Rev méd Chile. 2007;135:167–173. doi: 10.4067/s0034-98872007000200004. [DOI] [PubMed] [Google Scholar]
- 61.Melo A, Montenegro S, Hooper T, Capurro I, Roa JC, et al. [Human papilloma virus (HPV) typing in preneoplastic and neoplastic lesions of the uterine cervix in the IX region-Chile]. Rev Med Chil. 2003;131:1382–1390. [PubMed] [Google Scholar]
- 62.Roa JCS, Martínez RS, Montenegro S, Roa IE, Capurro IV, et al. Inestabilidad microsatelital en lesiones preneoplásicas y neoplásicas del cuello uterino: Correlación con el genotipo del virus papiloma humano. Rev méd Chile. 2007;135:37–44. doi: 10.4067/s0034-98872007000100006. [DOI] [PubMed] [Google Scholar]
- 63.Roa JC, Garcia P, Gomez J, Fernandez W, Gaete F, et al. HPV genotyping from invasive cervical cancer in Chile. Int J Gynaecol Obstet. 2009;105:150–153. doi: 10.1016/j.ijgo.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 64.Bosch FX, Munoz N, de Sanjose S, Navarro C, Moreo P, et al. Human papillomavirus and cervical intraepithelial neoplasia grade III/carcinoma in situ: a case-control study in Spain and Colombia. Cancer Epidemiol Biomarkers Prev. 1993;2:415–422. [PubMed] [Google Scholar]
- 65.Molano M, Moreno Acosta P, Bravo MM. Types and variants of human papillomavirus in patients with cervical cancer submitted to radiotherapy. Biosalud. 2007:45–57. [Google Scholar]
- 66.Munoz N, Bosch FX, de Sanjose S, Tafur L, Izarzugaza I, et al. The causal link between human papillomavirus and invasive cervical cancer: a population-based case-control study in Colombia and Spain. Int J Cancer. 1992;52:743–749. doi: 10.1002/ijc.2910520513. [DOI] [PubMed] [Google Scholar]
- 67.Muñoz N, Hernandez-Suarez G, Mendez F, Molano M, Posso H, et al. Persistence of HPV infection and risk of high-grade cervical intraepithelial neoplasia in a cohort of Colombian women. Br J Cancer. 2009;100:1184–1190. doi: 10.1038/sj.bjc.6604972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murillo R, Molano M, Martinez G, Mejia JC, Gamboa O. HPV prevalence in Colombian women with cervical cancer: implications for vaccination in a developing country. Infect Dis Obstet Gynecol. 2009;2009:653598. doi: 10.1155/2009/653598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sierra-Torres CH, Arboleda-Moreno YY, Orejuela-Aristizabal L. Exposure to wood smoke, HPV infection, and genetic susceptibility for cervical neoplasia among women in Colombia. Environ Mol Mutagen. 2006;47:553–561. doi: 10.1002/em.20228. [DOI] [PubMed] [Google Scholar]
- 70.Schiffman M, Herrero R, Desalle R, Hildesheim A, Wacholder S, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337:76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Soto Y, Mune M, Morales E, Goicolea A, Mora J, et al. Human papillomavirus infections in Cuban women with cervical intraepithelial neoplasia. Sex Transm Dis. 2007;34:974–976. doi: 10.1097/olq.0b013e31812e6b89. [DOI] [PubMed] [Google Scholar]
- 72.Paez C, Konno R, Yaegashi N, Matsunaga G, Araujo I, et al. Prevalence of HPV DNA in cervical lesions in patients from Ecuador and Japan. Tohoku J Exp Med. 1996;180:261–272. doi: 10.1620/tjem.180.261. [DOI] [PubMed] [Google Scholar]
- 73.Ferrera A, Velema JP, Figueroa M, Bulnes R, Toro LA, et al. Human papillomavirus infection, cervical dysplasia and invasive cervical cancer in Honduras: a case-control study. Int J Cancer. 1999;82:799–803. doi: 10.1002/(sici)1097-0215(19990909)82:6<799::aid-ijc5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 74.Rattray C, Strickler HD, Escoffery C, Cranston B, Brown C, et al. Type-specific prevalence of human papillomavirus DNA among Jamaican colposcopy patients. J Infect Dis. 1996;173:718–721. doi: 10.1093/infdis/173.3.718. [DOI] [PubMed] [Google Scholar]
- 75.Bermudez-Morales VH, Gutierrez LX, Alcocer-Gonzalez JM, Burguete A, Madrid-Marina V. Correlation between IL-10 gene expression and HPV infection in cervical cancer: a mechanism for immune response escape. Cancer Invest. 2008;26:1037–1043. doi: 10.1080/07357900802112693. [DOI] [PubMed] [Google Scholar]
- 76.Berumen J, Ordonez RM, Lazcano E, Salmeron J, Galvan SC, et al. Asian-American variants of human papillomavirus 16 and risk for cervical cancer: a case-control study. J Natl Cancer Inst. 2001;93:1325–1330. doi: 10.1093/jnci/93.17.1325. [DOI] [PubMed] [Google Scholar]
- 77.Carrillo A, Mohar A, Meneses A, Frias-Mendivil M, Solorza G, et al. Usefulness of combining universal oligonucleotides in detecting human papillomavirus in cervical cancer and premalignant lesions. [Spanish]. Salud Publica de Mexico. 2004;46:7–15. doi: 10.1590/s0036-36342004000100002. [DOI] [PubMed] [Google Scholar]
- 78.Giuliano AR, Papenfuss M, Abrahamsen M, Denman C, de Zapien JG, et al. Human papillomavirus infection at the United States-Mexico border: implications for cervical cancer prevention and control. Cancer Epidemiol Biomarkers Prev. 2001;10:1129–1136. [PubMed] [Google Scholar]
- 79.Fernandez-Tilapa G, Illades-Aguiar B, Martinez-Carrillo DN, del Carmen Alarcon-Romero L, Vences-Velazquez A, et al. Prevalence of human papillomavirus types among Mexican women with intraepithelial lesions and cervical cancer: Detection with MY09/MY011 and GP5+/GP6+ primer systems. American Journal of Infectious Diseases. 2007;3:62–67. [Google Scholar]
- 80.Gonzalez-Garay ML, Barrera-Saldana HA, Aviles LB, Alvarez-Salas LM, Gariglio P. Prevalence in two mexican cities of human papillomavirus DNA sequences in cervical cancer. Rev Invest Clin. 1992;44:491–499. [PubMed] [Google Scholar]
- 81.Gonzalez-Losa MDR, Rosado-Lopez I, Valdez-Gonzalez N, Puerto-Solis M. High prevalence of human papillomavirus type 58 in Mexican colposcopy patients. Journal of Clinical Virology. 2004;29:202–205. doi: 10.1016/S1386-6532(03)00138-0. [DOI] [PubMed] [Google Scholar]
- 82.Hernandez-Avila M, Lazcano-Ponce EC, Berumen-Campos J, Cruz-Valdez A, Alonso de Ruiz PP, et al. Human papilloma virus 16–18 infection and cervical cancer in Mexico: a case-control study. Arch Med Res. 1997;28:265–271. [PubMed] [Google Scholar]
- 83.Illades-Aguiar B, Cortes-Malagon EM, Antonio-Vejar V, Zamudio-Lopez N, Alarcon-Romero LdC, et al. Cervical carcinoma in Southern Mexico: Human papillomavirus and cofactors. Cancer Detect Prev. 2009;32:300–307. doi: 10.1016/j.cdp.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 84.Lazcano-Ponce E, Perez G, Cruz-Valdez A, Zamilpa L, Aranda-Flores C, et al. Impact of a Quadrivalent HPV6/11/16/18 Vaccine in Mexican Women: Public Health Implications for the Region. Arch Med Res. 2009;40:514–524. doi: 10.1016/j.arcmed.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 85.Lizano M, De la Cruz-Hernandez E, Carrillo-Garcia A, Garcia-Carranca A, Ponce de Leon-Rosales S, et al. Distribution of HPV16 and 18 intratypic variants in normal cytology, intraepithelial lesions, and cervical cancer in a Mexican population. Gynecologic Oncology. 2006;102:230–235. doi: 10.1016/j.ygyno.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 86.Lopez-Revilla R, Martinez-Contreras LA, Sanchez-Garza M. Prevalence of high-risk human papillomavirus types in Mexican women with cervical intraepithelial neoplasia and invasive carcinoma. Infectious Agents and Cancer. 2008;3 doi: 10.1186/1750-9378-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matthews-Greer J, Dominguez-Malagon H, Herrera GA, Unger J, Chanona-Vilchis J, et al. Human Papillomavirus Typing of Rare Cervical Carcinomas. Archives of Pathology & Laboratory Medicine. 2004;128:553–556. doi: 10.5858/2004-128-553-HPTORC. [DOI] [PubMed] [Google Scholar]
- 88.Meyer T, Arndt R, Christophers E, Beckmann ER, Schroder S, et al. Association of rare human papillomavirus types with genital premalignant and malignant lesions. J Infect Dis. 1998;178:252–255. doi: 10.1086/517447. [DOI] [PubMed] [Google Scholar]
- 89.Pinña-Sanchez P, Hernandez-Hernandez DM, Lopez-Romero R, Vazquez-Ortiz G, Perez-Plasencia C, et al. Human papillomavirus-specific viral types are common in Mexican women affected by cervical lesions. International Journal of Gynecological Cancer. 2006;16:1041–1047. doi: 10.1111/j.1525-1438.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 90.Torroella-Kouri M, Morsberger S, Carrillo A, Mohar A, Meneses A, et al. HPV prevalence among Mexican women with neoplastic and normal cervixes. Gynecol Oncol. 1998;70:115–120. doi: 10.1006/gyno.1998.5055. [DOI] [PubMed] [Google Scholar]
- 91.Aerssens A, Claeys P, Garcia A, Sturtewagen Y, Velasquez R, et al. Natural history and clearance of HPV after treatment of precancerous cervical lesions. Histopathology. 2008;52:381–386. doi: 10.1111/j.1365-2559.2007.02956.x. [DOI] [PubMed] [Google Scholar]
- 92.Aerssens A, Claeys P, Beerens E, Garcia A, Weyers S, et al. Prediction of recurrent disease by cytology and HPV testing after treatment of cervical intraepithelial neoplasia. Cytopathology. 2009;20:27–35. doi: 10.1111/j.1365-2303.2008.00567.x. [DOI] [PubMed] [Google Scholar]
- 93.Hindryckx P, Garcia A, Claeys P, Gonzalez C, Velasquez R, et al. Prevalence of high risk human papillomavirus types among Nicaraguan women with histological proved pre-neoplastic and neoplastic lesions of the cervix. Sex Transm Infect. 2006;82:334–336. doi: 10.1136/sti.2006.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Acs J, Hildesheim A, Reeves WC, Brenes M, Brinton L, et al. Regional distribution of human papillomavirus DNA and other risk factors for invasive cervical cancer in Panama. Cancer Res. 1989;49:5725–5729. [PubMed] [Google Scholar]
- 95.Mendoza L, Arbiza J, Paez M, Kasamatsu E, Castro A, et al. Molecular epidemiology of human papillomavirus infection in Paraguayan women, according to the severity of cervical lesion. Journal of Medical Virology. 2010 (In press) doi: 10.1002/jmv.22112. [DOI] [PubMed] [Google Scholar]
- 96.Rolon PA, Smith JS, Munoz N, Klug SJ, Herrero R, et al. Human papillomavirus infection and invasive cervical cancer in Paraguay. Int J Cancer. 2000;85:486–491. [PubMed] [Google Scholar]
- 97.Lorincz AT, Lancaster WD, Temple GF. Cloning and characterization of the DNA of a new human papillomavirus from a woman with dysplasia of the uterine cervix. Journal of Virology. 1986;58:225–229. doi: 10.1128/jvi.58.1.225-229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Santos C, Munoz N, Klug S, Almonte M, Guerrero I, et al. HPV types and cofactors causing cervical cancer in Peru. Br J Cancer. 2001;85:966–971. doi: 10.1054/bjoc.2001.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Boer MA, Peters LA, Aziz MF, Siregar B, Cornain S, et al. Human papillomavirus type 16 E6, E7, and L1 variants in cervical cancer in Indonesia, Suriname, and The Netherlands. Gynecol Oncol. 2004;94:488–494. doi: 10.1016/j.ygyno.2004.05.037. [DOI] [PubMed] [Google Scholar]
- 100.Krul EJT, Van De Vijver MJ, Schuuring E, Van Kanten RW, Peters AAW, et al. Human papillomavirus in malignant cervical lesions in Surinam a high- risk country, compared to the Netherlands, a low-risk country. International Journal of Gynecological Cancer. 1999;9:206–211. doi: 10.1046/j.1525-1438.1999.99020.x. [DOI] [PubMed] [Google Scholar]
- 101.Cathro HP, Loya T, Dominguez F, Howe SL, Howell R, et al. Human papillomavirus profile of women in Belize City, Belize: correlation with cervical cytopathologic findings. Human Pathology. 2009;40:942–949. doi: 10.1016/j.humpath.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 102.Freitas TP, Carmo BBd, Paula FDF, Rodrigues LF, Fernandes AP, et al. Molecular detection of HPV 16 and 18 in cervical samples of patients from Belo Horizonte, Minas Gerais, Brazil. Rev Inst Med Trop São Paulo. 2007;49:297–301. doi: 10.1590/s0036-46652007000500005. [DOI] [PubMed] [Google Scholar]
- 103.Lazcano-Ponce E, Perez G, Cruz-Valdez A, Zamilpa L, Aranda-Flores C, et al. Impact of a Quadrivalent HPV6/11/16/18 Vaccine in Mexican Women: Public Health Implications for the Region. Archives of Medical Research. 2009;40:514–524. doi: 10.1016/j.arcmed.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 104.Muñoz N, Hernandez-Suarez G, Mendez F, Molano M, Posso H, et al. Persistence of HPV infection and risk of high-grade cervical intraepithelial neoplasia in a cohort of Colombian women. British Journal of Cancer. 2009;100:1184–1190. doi: 10.1038/sj.bjc.6604972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rabelo-Santos SH, Derchain SF, Villa LL, Costa MC, Sarian LO, et al. Human papillomavirus-specific genotypes in cervical lesions of women referred for smears with atypical glandular cells or adenocarcinoma in situ. Int J Gynecol Pathol. 2009;28:272–278. doi: 10.1097/PGP.0b013e318190ed27. [DOI] [PubMed] [Google Scholar]
- 106.Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589–1597. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 107.Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 108.Chan PK, Cheung TH, Tam AO, Lo KW, Yim SF, et al. Biases in human papillomavirus genotype prevalence assessment associated with commonly used consensus primers. Int J Cancer. 2006;118:243–245. doi: 10.1002/ijc.21299. [DOI] [PubMed] [Google Scholar]
- 109.Trottier H, Mahmud S, Costa MC, Sobrinho JP, Duarte-Franco E, et al. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2006;15:1274–1280. doi: 10.1158/1055-9965.EPI-06-0129. [DOI] [PubMed] [Google Scholar]
- 110.Bachtiary B, Obermair A, Dreier B, Birner P, Breitenecker G, et al. Impact of multiple HPV infection on response to treatment and survival in patients receiving radical radiotherapy for cervical cancer. Int J Cancer. 2002:237–243. doi: 10.1002/ijc.10708. [DOI] [PubMed] [Google Scholar]
- 111.Quint W, Molijn A, Colau A, et al. One HPV Virus, one lesion as determined by LCM/PCR technology. 2009. May 8–14, 2009; Malmö, Sweden. pp. Abstract O-06.04, page 06.03.
- 112.Iftner T, Villa LL. Chapter 12: Human papillomavirus technologies. J Natl Cancer Inst Monogr. 2003:80–88. doi: 10.1093/oxfordjournals.jncimonographs.a003487. [DOI] [PubMed] [Google Scholar]
- 113.WHO World Health Organization HPV Laboratory Network (WHO HPV LabNet) guidelines. Geneve.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search Strategy.
(DOC)
Methodological Quality Assessment.
(DOC)
Study characteristics and HPV-specific prevalence by study and country.
(DOC)
PRISMA checklist for reporting systematic reviews and meta-analyses.
(DOC)
PRISMA study flow diagram for reporting systematic reviews and meta-analyse.
(DOC)