Abstract
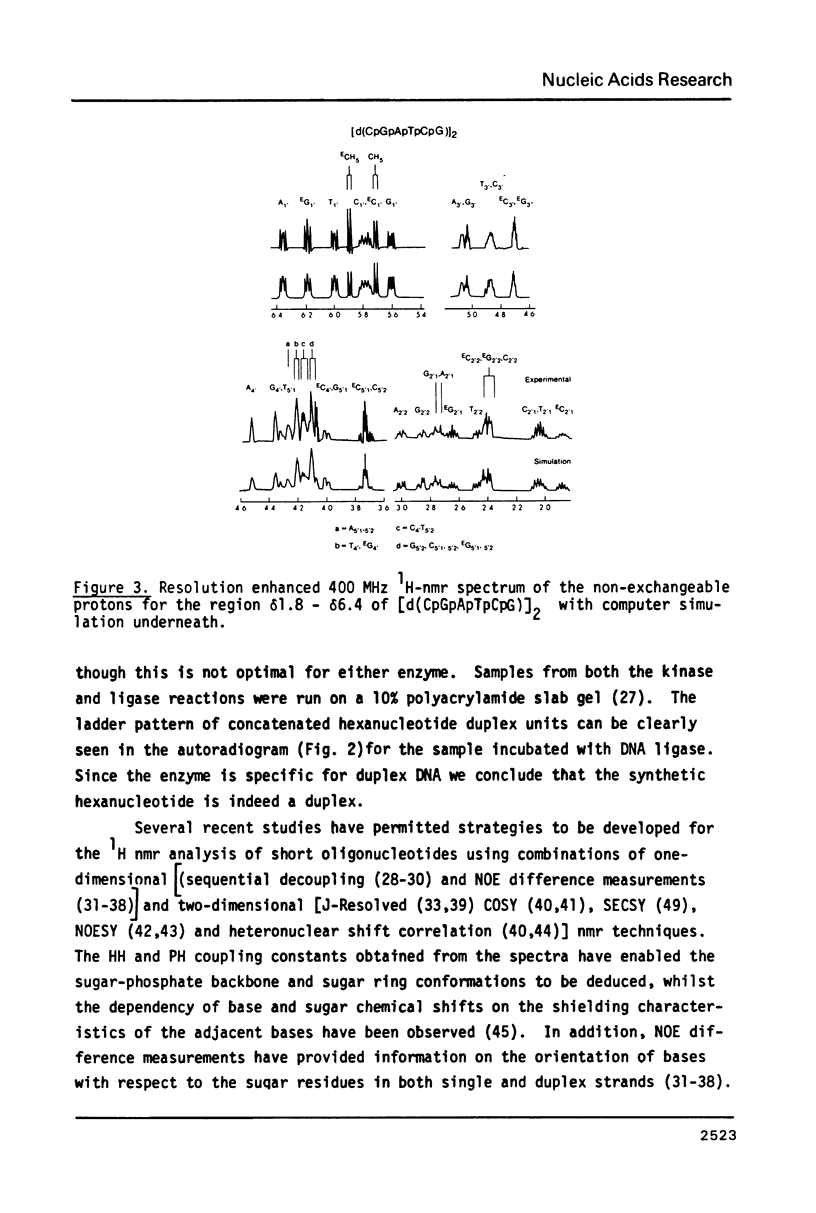
The two deoxyribonucleotides [d(CpGpApTpCpG)]2 and [d(CpGpCpG)]2 were synthesized by the phosphotriester method. Their duplex form under the conditions of the 1H-nmr experiments was proven by end 32P labeling with T4 polynucleotide kinase followed by butt end joining employing the absolute specificity of T4 ligase for double stranded DNA and analysis using gel electrophoresis and autoradiography. Complete nmr assignment of the 1H chemical shifts and coupling constants was achieved. The assignments were secured using sequential decoupling, NOE difference measurements, and two-dimensional COSY and SECSY experiments. Spectrum simulation confirmed the experimental values of chemical shifts and coupling constants. The techniques for the assignment outlined together with 31P and 2-D heteronuclear shift correlation permit an approach to a systematic analysis of more complex single-strand and duplex oligodeoxyribonucleotides.
Full text
PDF


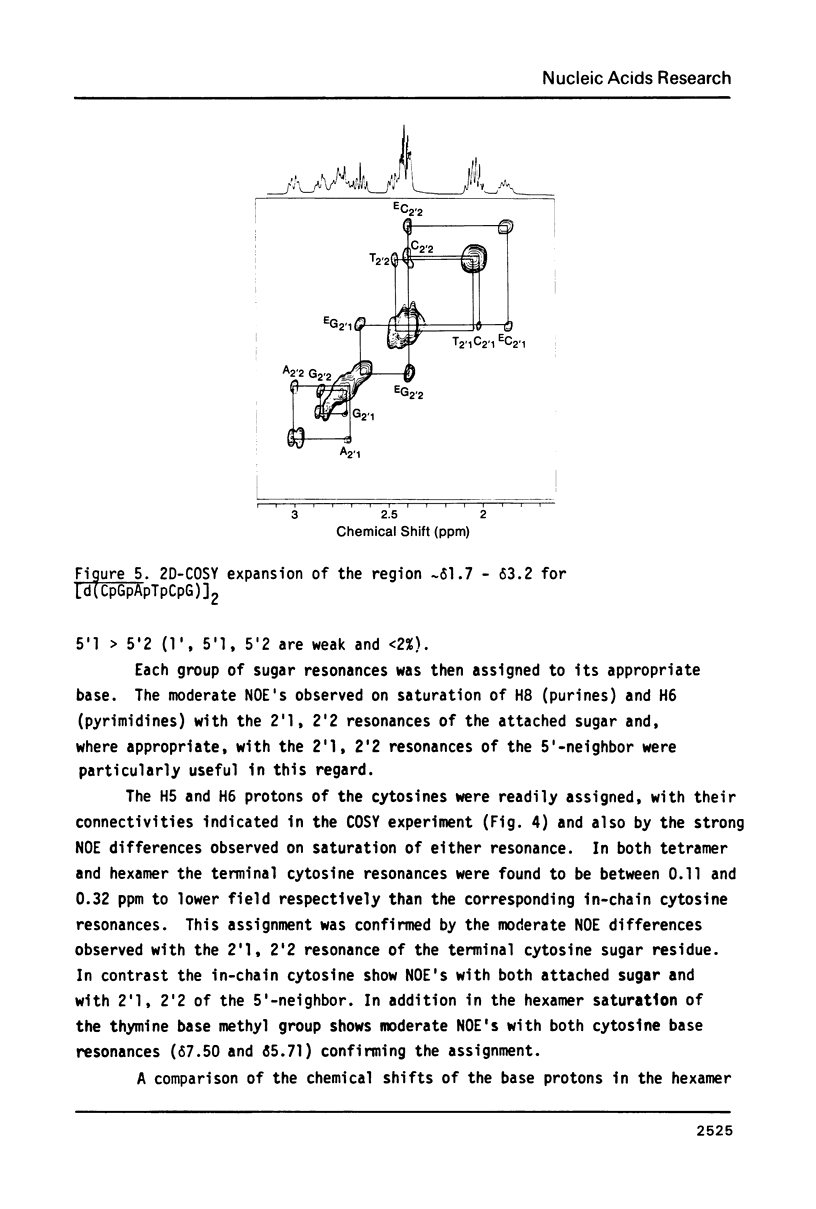
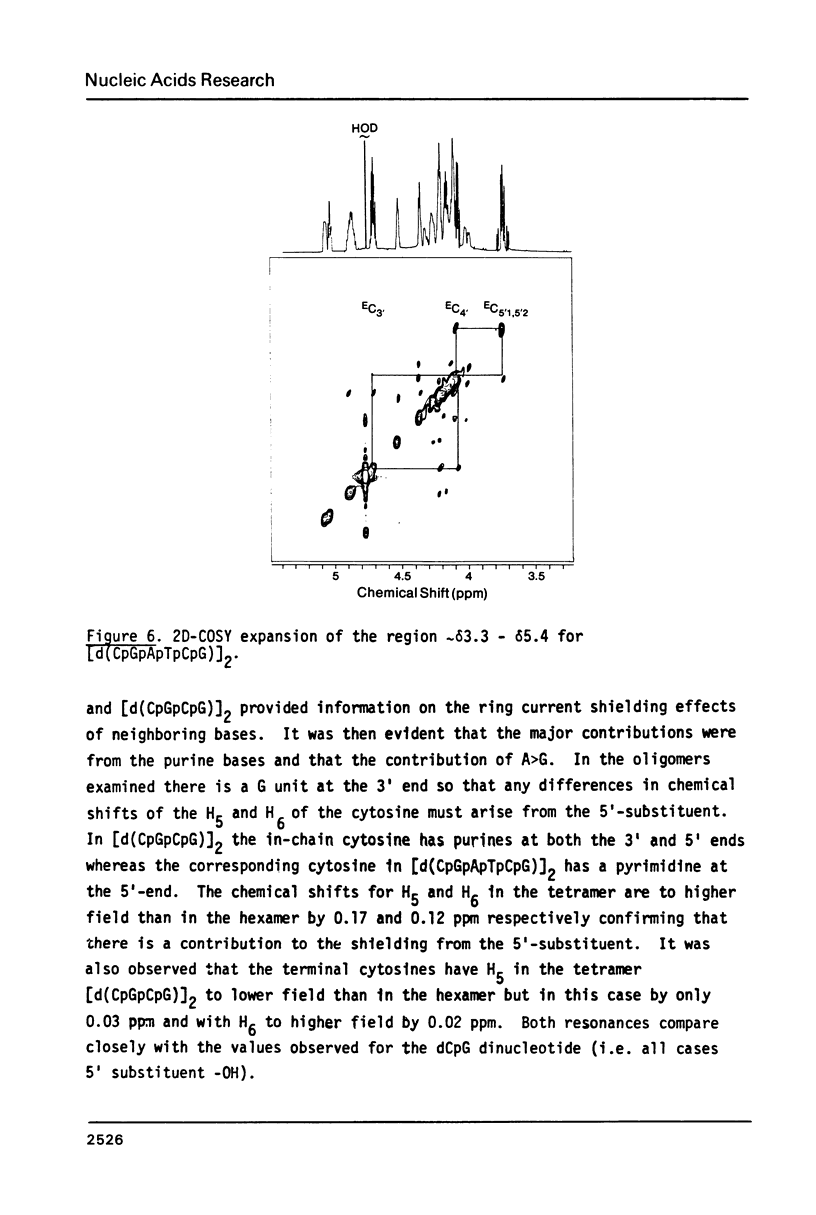
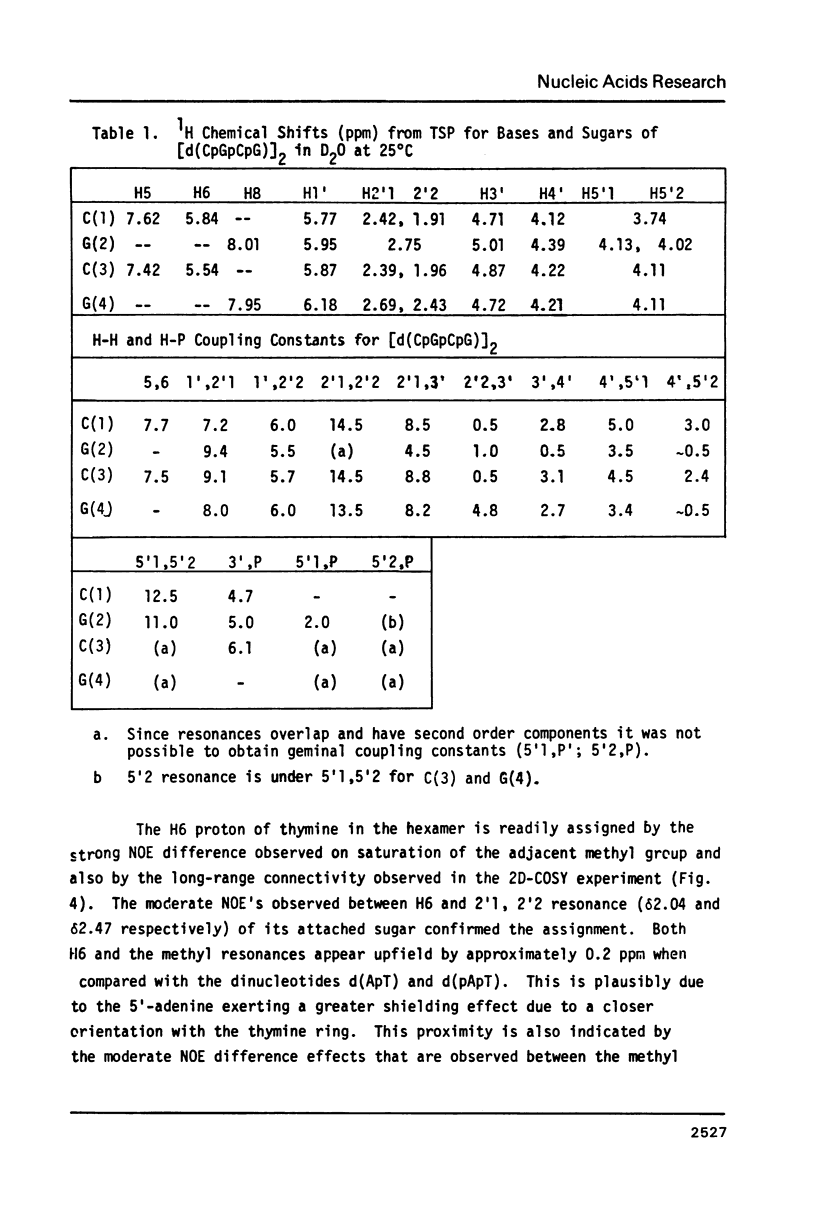
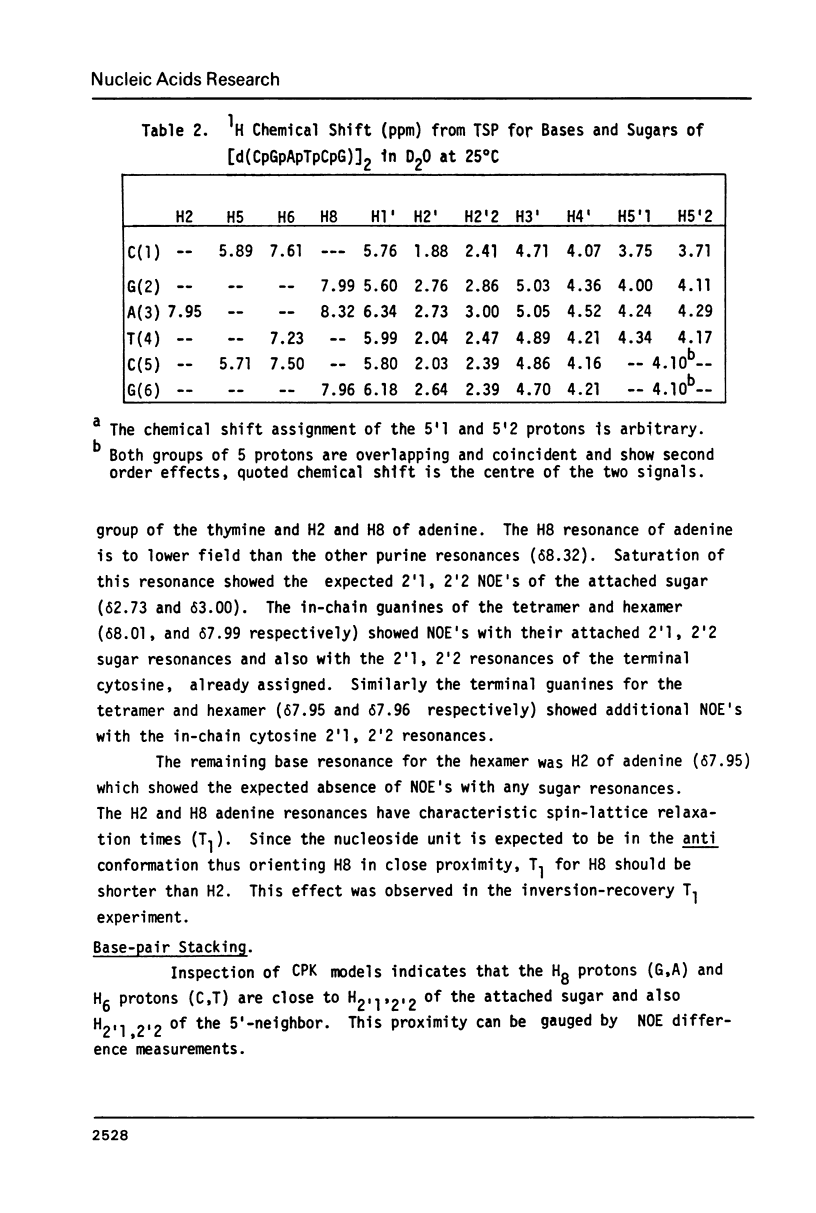



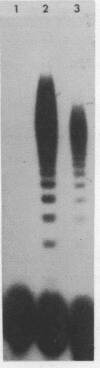
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borer P. N., Kan L. S., Ts'o P. O. Conformation and interaction of short nucleic acid double-stranded helices. I. Proton magnetic resonance studies on the nonexchangeable protons of ribosyl ApApGpCpUpU. Biochemistry. 1975 Nov 4;14(22):4847–4863. doi: 10.1021/bi00693a012. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya J. B., Reese C. B. Chemical synthesis of tridecanucleoside dodecaphosphate sequence of SV40 DNA. Nucleic Acids Res. 1980 May 10;8(9):2039–2053. doi: 10.1093/nar/8.9.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D. M., Kan L. S., Leutzinger E. E., Jayaraman K., Miller P. S., Ts'o P. O. Conformational study of two short pentadeoxyribonucleotides, d-CpCpApApG and d-CpTpTpGpG, and their fragments by proton nuclear magnetic resonance. Biochemistry. 1982 Feb 16;21(4):621–630. doi: 10.1021/bi00533a004. [DOI] [PubMed] [Google Scholar]
- Cheng D. M., Sarma R. H. Intimate details of the conformational characteristics of deoxyribodinucleoside monophosphates in aqueous solution. J Am Chem Soc. 1977 Oct 26;99(22):7333–7348. doi: 10.1021/ja00464a038. [DOI] [PubMed] [Google Scholar]
- Doornbos J., Wreesmann C. T., Van Boom J. H., Altona C. Conformational analysis of the single-stranded ribonucleic acid A-A-C-C. A one-dimensional and two-dimensional proton NMR study at 500 MHz. Eur J Biochem. 1983 Apr 5;131(3):571–579. doi: 10.1111/j.1432-1033.1983.tb07301.x. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B., Borer P. N., Kan L. S., Ts'o P. O. Ring-current effects in the Nmr of nucleic acids: a graphical approach. Biopolymers. 1976 Nov;15(11):2277–2286. doi: 10.1002/bip.1976.360151114. [DOI] [PubMed] [Google Scholar]
- Lankhorst P. P., Erkelens C., Haasnoot C. A., Altona C. Carbon-13 NMR in conformational analysis of nucleic acid fragments. Heteronuclear chemical shift correlation spectroscopy of RNA constituents. Nucleic Acids Res. 1983 Oct 25;11(20):7215–7230. doi: 10.1093/nar/11.20.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankhorst P. P., Wille G., van Boom J. H., Altona C., Haasnoot C. A. Conformational analysis of a ribopentanucleoside tetraphosphate in aqueous solution. A two-dimensional NMR study at 500 MHz. Nucleic Acids Res. 1983 May 11;11(9):2839–2856. doi: 10.1093/nar/11.9.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mellema J. R., Haasnoot C. A., van der Marel G. A., Wille G., van Boeckel C. A., van Boom J. H., Altona C. Proton NMR studies on the covalently linked RNA-DNA hybrid r(GCG)d(TATACGC). Assignment of proton resonances by application of the nuclear Overhauser effect. Nucleic Acids Res. 1983 Aug 25;11(16):5717–5738. doi: 10.1093/nar/11.16.5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. R., Lee J. S., Pulleyblank D. E., Murray N. L., Evans D. H. Review: ethidium fluorescence assays. Part 1. Physicochemical studies. Nucleic Acids Res. 1979 Oct 10;7(3):547–569. doi: 10.1093/nar/7.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama K., Wüthrich K. Systematic application of two-dimensional 1H nuclear-magnetic-resonance techniques for studies of proteins. 1. Combined use of spin-echo-correlated spectroscopy and J-resolved spectroscopy for the identification of complete spin systems of non-labile protons in amino-acid residues. Eur J Biochem. 1981 Feb;114(2):365–374. doi: 10.1111/j.1432-1033.1981.tb05156.x. [DOI] [PubMed] [Google Scholar]
- Nosikov V. V., Sain B. Protection of particular endonuclease R. Hind III cleavage sites by distamycin A, propyl-distamycin and netropsin. Nucleic Acids Res. 1977 Jul;4(7):2263–2273. doi: 10.1093/nar/4.7.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Bhatt R. Sequence dependence of base-pair stacking in right-handed DNA in solution: proton nuclear Overhauser effect NMR measurements. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3908–3912. doi: 10.1073/pnas.80.13.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Nordheim A., Rich A. Right-handed and left-handed DNA: studies of B- and Z-DNA by using proton nuclear Overhauser effect and P NMR. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1413–1417. doi: 10.1073/pnas.79.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Pardi A., Itakura K. DNA conformation, dynamics, and interactions in solution. Science. 1982 May 7;216(4546):581–590. doi: 10.1126/science.6280281. [DOI] [PubMed] [Google Scholar]
- Petersheim M., Turner D. H. Nuclear overhauser studies of CCGGAp, ACCGGp, and ACCGGUp. Biochemistry. 1983 Jan 18;22(2):264–268. doi: 10.1021/bi00271a005. [DOI] [PubMed] [Google Scholar]
- Reid D. G., Salisbury S. A., Bellard S., Shakked Z., Williams D. H. Proton nuclear Overhauser effect study of the structure of a deoxyoligonucleotide duplex in aqueous solution. Biochemistry. 1983 Apr 12;22(8):2019–2025. doi: 10.1021/bi00277a044. [DOI] [PubMed] [Google Scholar]
- Reid D. G., Salisbury S. A., Brown T., Williams D. H., Vasseur J. J., Rayner B., Imbach J. L. Use of inter-proton nuclear Overhauser effects to assign the nuclear magnetic resonance spectra of oligodeoxynucleotide and hybrid duplexes in aqueous solution. Eur J Biochem. 1983 Sep 15;135(2):307–314. doi: 10.1111/j.1432-1033.1983.tb07654.x. [DOI] [PubMed] [Google Scholar]
- Sanderson M. R., Mellema J. R., van der Marel G. A., Wille G., van Boom J. H., Altona C. Assignment of non-exchangeable base proton and H1' resonances of a deoxyoctanucleoside heptaphosphate d(G-G-C*-C*-G-G-C-C) by using the nuclear Overhauser effect. Nucleic Acids Res. 1983 May 25;11(10):3333–3346. doi: 10.1093/nar/11.10.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgaramella V., Van de Sande J. H., Khorana H. G. Studies on polynucleotides, C. A novel joining reaction catalyzed by the T4-polynucleotide ligase. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1468–1475. doi: 10.1073/pnas.67.3.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawinski J., Hozumi T., Narang S. A., Bahl C. P., Wu R. Arylsulfonyltetrazoles, new coupling reagents and further improvements in the triester method for the synthesis of deoxyribooligonucleotides. Nucleic Acids Res. 1977 Feb;4(2):353–371. doi: 10.1093/nar/4.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Davidson N. An electron microscopic method for the mapping of proteins attached to nucleic acids. Nucleic Acids Res. 1978 Aug;5(8):2825–2846. doi: 10.1093/nar/5.8.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]