Abstract
Introduction
The projection of vagina, uterine cervix, and nipple to the sensory cortex in humans has not been reported.
Aims
To map the sensory cortical fields of the clitoris, vagina, cervix and nipple, toward an elucidation of the neural systems underlying sexual response.
Methods
Using functional Magnetic Resonance Imaging (fMRI) we mapped sensory cortical responses to clitoral, vaginal, cervical, and nipple self-stimulation. For points of reference on the homunculus, we also mapped responses to the thumb and great toe (hallux) stimulation.
Main Outcome Measures
fMRI of brain regions activated by the various sensory stimuli.
Results
Clitoral, vaginal, and cervical self-stimulation activate differentiable sensory cortical regions, all clustered in the medial cortex (medial paracentral lobule). Nipple self-stimulation activated the genital sensory cortex (as well as the thoracic) region of the homuncular map.
Conclusion
The genital sensory cortex, identified in the classical Penfield homunculus based on electrical stimulation of the brain only in men, was confirmed for the first time in the literature by the present study in women, applying clitoral, vaginal, and cervical self-stimulation, and observing their regional brain responses using fMRI. Vaginal, clitoral, and cervical regions of activation were differentiable, consistent with innervation by different afferent nerves and different behavioral correlates. Activation of the genital sensory cortex by nipple self-stimulation was unexpected, but suggests a neurological basis for women’s reports of its erotogenic quality.
Introduction
The original map of the representation of the genitals in the sensory cortex in humans was generated by applying roving electrical stimulation to the brain in awake men, and asking the men from which part of their body the stimulation seemed to emanate. The men reported penile sensation when the interhemispheric region, i.e., the medial cortex (medial region of the paracentral lobule, Figure 1) was stimulated; they reported foot sensation when the electrical stimulation was applied immediately superior to the penile representation [1–3]. While Penfield and Rasmussen [3] did not report the effect of brain stimulation on genital sensation in women, they did report that their patient with spontaneous sensory seizures likely stimulated by a small glioma in the postcentral gyrus near the falx (i.e., the interhemispheric component of the dura mater) had labial, breast, and foot sensations during her seizures.
Figure 1.
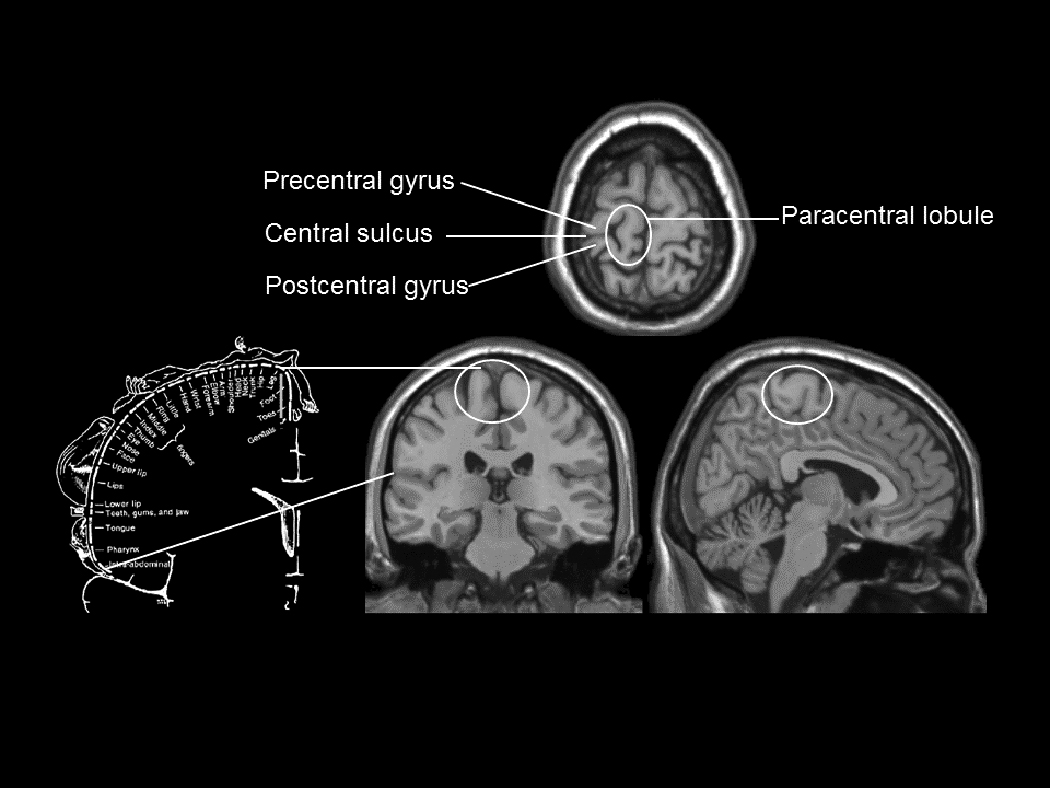
Three views of the paracentral lobule, showing its relation to adjacent cortical regions (adapted from [32]). The relation of the paracentral lobule to the sensory cortical homunculus of Penfield and Rasmussen [3] is shown by the lines connecting the corresponding regions.
Subsequent studies used electrical stimulation of the dorsal nerve of the penis to measure the distribution of evoked potentials in the cortex. Those studies confirmed the earlier homuncular map, for each reported that the evoked potentials were focused in the medial cortex, (in the medial region of the paracentral lobule), i.e., in the genital region as represented in the Penfield map [4–6]. Similar findings were reported by Allison et al [7] in response to electrical stimulation of the clitoris as well as the penis.
However, more recent studies using PET or fMRI, and another using penile evoked potentials, reported that a more dorsolateral portion of the paracentral lobule, rather than its medial region, was activated when direct penile stimulation was applied by the experimenter (using a toothbrush and recording fMRI [8]), when penile stimulation was applied by the subject’s partner (using manual stimulation and recording PET [9]), or in response to electrical stimulation of the penis [10]. While the basis for this discrepancy in the penile map is still not reconciled, it is possible that sensory activation of the more dorsolateral region of the paracentral lobule may result from inadvertent and incidental stimulation of the groin, on the basis that Penfield’s [3] map and even Kell’s [8] “revised” map both show the transition zone between upper thigh and trunk (i.e., “groin”) to be located on the dorsolateral region of the paracentral lobule.
A parallel discrepancy in the genital map of women has now become evident. Two recent studies, using fMRI with electrical stimulation of the clitoris [11] or using PET and mechanical stimulation of the clitoris by the subject’s partner [12], reported that clitoral stimulation activated the dorsolateral, rather than the medial, region of the paracentral lobule. . In those studies, the clitoral stimulation was applied by the experimenter or the subject’s partner.
As seen in the present findings, using fMRI, in which the women applied clitoral, vaginal or uterine cervical self-stimulation, there is clear evidence of activation of the medial region of the paracentral lobule, in the sensory genital region of the homuncular map of Penfield and Rasmussen [3]. In addition, there is an occasional secondary activation in the dorsolateral paracentral lobule, indicative of groin stimulation.
Rationale for the present research
The map of the genital sensory cortical representation is based almost exclusively on responses to penile and clitoral stimulation, both of whose afferent innervation is provided by the pudendal nerve. However, additional nerves convey sensation from the vagina and cervix, i.e., the pelvic, hypogastric and vagus nerves [13; for review: 14]. To our knowledge, the projection of vagina and uterine cervix to the sensory cortex in humans has not been reported previously. To address this gap, in the present study using fMRI, we mapped the regions of the sensory cortex that are activated by clitoral, vaginal, and cervical self-stimulation. For points of reference on the homunculus, we also mapped responses to the thumb and great toe (hallux) stimulation and nipple self-stimulation. Portions of these findings have been reported in abstract form [15].
Methods
Research Participants
Eleven healthy right-handed women, ages 23–56, recruited by word of mouth, were prescreened with the SCL-90 questionnaire to rule out any psychological contraindications to study participation. Each participant tested negative for pregnancy prior to scanning. All participants gave informed consent as required by the research protocol approved by the New Jersey Medical School – University of Medicine and Dentistry of New Jersey IRB. Participants were compensated $100 for participating in the study; the duration of each scan session was 1–2 hours.
Experimental Paradigm
A “boxcar” experimental design was employed with each 5-minute trial consisting of 30 sec of rest, then 30 sec of stimulation, repeated 5 times in succession. Control trials consisted of an experimenter rhythmically tapping a participant's thumb or toe in separate trials to establish reference points on the sensory cortex. Experimental mapping trials consisted of participants self-stimulating, by hand or personal device, using “comfortable” intensity, the clitoris, anterior wall of the vagina, the cervix, or the nipple, in separate, randomized-sequence trials. Clitoral self-stimulation was applied using rhythmical tapping with the right hand. Vaginal self-stimulation (of the anterior wall) was applied using the participant's own stimulator (typically a 15mm-diameter S-shaped acrylic rounded-top cylinder). Cervical self-stimulation was applied using a similar-diameter, glass or acrylic straight rounded-tip cylinder brought to the study by each participant. Nipple self-stimulation was applied using the right hand to tap the left nipple rhythmically. All trials started with a 30-sec rest period. The participants were instructed by an experimenter via headphones as to when to start and stop self-stimulation. The participants were in continuous audio contact with the experimenters for the duration of the experimental paradigm.
fMRI Acquisition
Data were collected using a 3T Siemens Allegra (head only) system using gradient-echo echo-planar sequence (EPIBOLD) with the following acquisition parameters: 2000/40 (TR/TE); 64X64 matrix, 22 cm field of view, 5-mm thick contiguous sections, and 90 degree flip angle. For each 5-min sensory paradigm, 150 image sets of 32 slices per TR were obtained using a standard quadrature “bird cage” head coil. The participant's head was stabilized with an individually-fitted thermoplastic frame that was affixed to the head coil to limit motion. Images were reconstructed from Siemens proprietary software (Advanced Neuropackage) and transferred to a remote workstation for processing and analysis.
Anatomical images
T1 weighted (TR/TE = 450/14, FOV=24 cm, Matrix=256x256, Slice thickness = 5 mm skip 0, 32 slices) high-resolution anatomical images were acquired in the transaxial plane in identical slice locations during each imaging session. This data set was used for image underlay with functional data to identify the anatomical landmarks.
Data analysis
Statistical parametric mapping (SPM-8) was utilized. In SPM-8, the blood-oxygenation-level-dependent (BOLD) signal intensity of each voxel during the stimulus conditions was compared statistically with its activity during the prestimulus condition (baseline condition). The images from each trial were pre-processed for realignment, normalized to MNI space, motion-corrected, and smoothed using an 8x8x10 kernel. The high pass filter was set to a default value of 128 sec to remove the slow signal drifts.
In order to obtain the overall presentation for each paradigm, group maps were generated with second level analysis using a random effects model. A canonical hemodynamic function was selected for the basis function to estimate the hemodynamics. To calculate the model parameters, the Restricted Maximum Likelihood (ReML) algorithm was used. In addition, an autoregressive model (AR) to correlate the time series was used with ReML to account for the aliased biorhythms and unmodeled neuronal activity.
MRIcro [32] was used for visualization of group maps on a standardized anatomical template. The numbers in the calibration bars in Figures 2 and 3c are the range of Z scores that correspond to the “hot metal” representation of the fMRI activity levels in the adjacent brain images. Thus, the closer the color of the brain activity is to “white hot”, the more highly significant is that activity. Z scores: 1.96 = p< 0.05; 2.3 = p<0.01.
Figure 2.
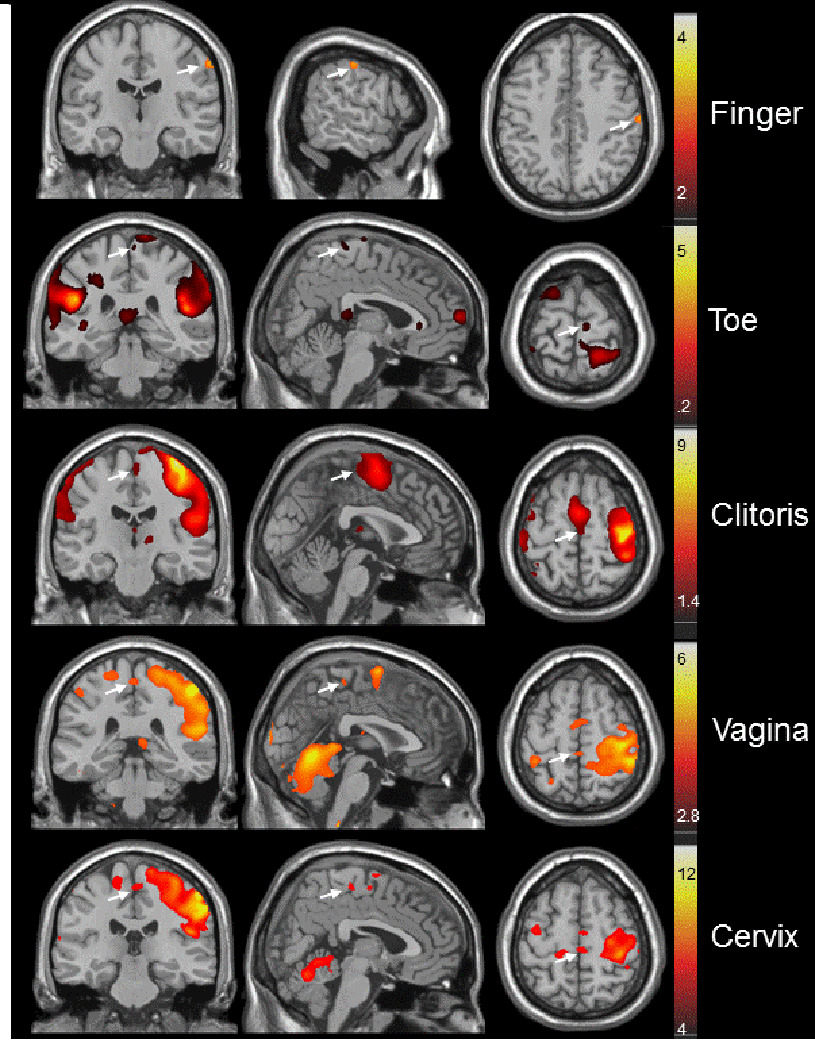
Three-axis (columns: coronal, sagittal and transaxial) views of the group-based responses to experimenter-applied (finger and toe) or participant self-applied (clitoris, vagina, or cervix) stimulation in relation to the homuncular map (adapted) generated by Penfield and Rasmussen [3]. The arrows indicate the sensory cortical regions activated by the various stimulated body regions. Finger stimulation activated the postcentral (sensory) gyrus. Hallux (large toe) stimulation activated the medial paracentral lobule. Clitoral, vaginal, cervical, and nipple self-stimulation also activated the medial paracentral lobule. Note that the perineal (groin) region just lateral to the midline in the paracentral lobule was also activated by clitoral, vaginal, and cervical self-stimulation. There was marked hand-related activation in the postcentral gyrus, and continuation of activation into the supplementary motor area immediately rostral to the sensory cortical responses, in the self-stimulation conditions. The secondary sensory cortex (SII; at the base of the homunculus) was activated under all the stimulus conditions (not evident in these images in the thumb stimulation condition). The “hot-metal” calibrations show the range of Z-scores for the intensity of the fMRI responses.
Figure 3.
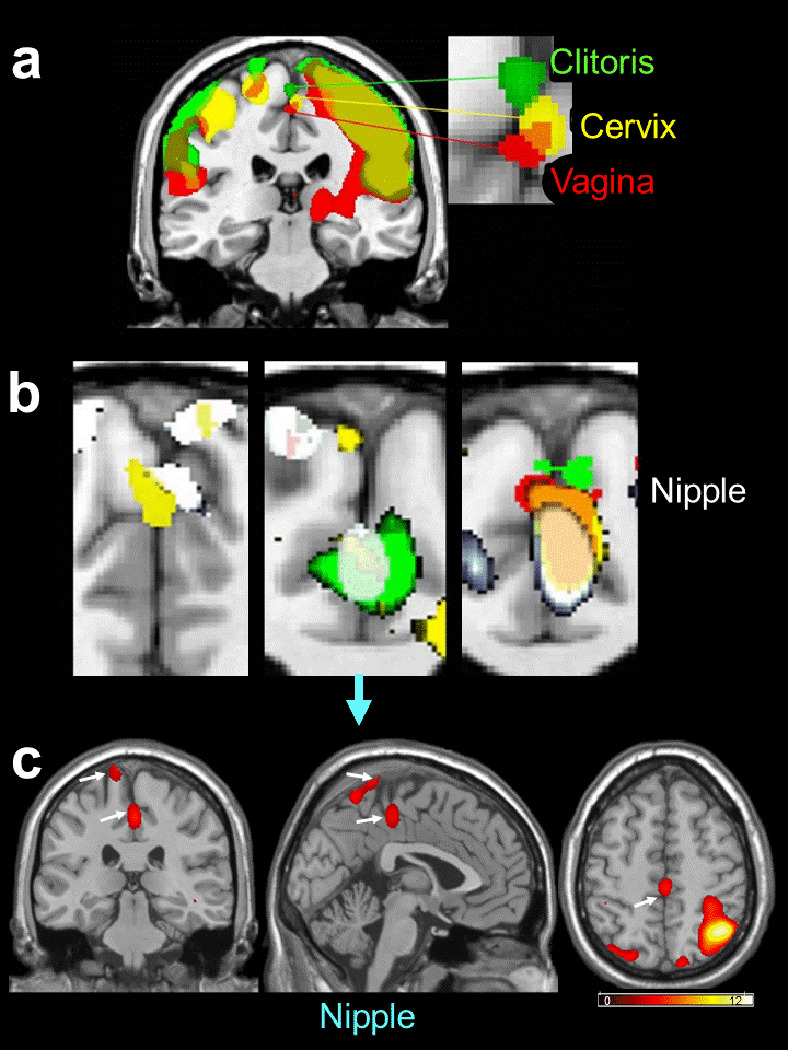
a: Group-based composite view of the clitoral, vaginal and cervical activation sites, all in the medial paracentral lobule, but regionally differentiated. We interpret this as due to the differential sensory innervation of these genital structures, i.e., clitoris: pudendal nerve, vagina: pelvic nerve, and cervix: hypogastric and vagus nerves (e.g., [14]).
b: Nipple self-stimulation activated not only the thoracic region, but also unexpectedly, the genital region of the medial paracentral lobule. Shown are superimposed responses to nipple and genital self-stimulation in three participants. Note the congruence between activation produced by stimulation of nipple and cervix (left panel), nipple, cervix and clitoris (center panel), and nipple, vagina, cervix and clitoris (right panel). Not unexpectedly, cervical self-stimulation activated the groin region (center panel). However, it is surprising that vaginal self-stimulation activated the thoracic nipple region (center panel), and nipple self-stimulation activated the groin sensory region (left panel). Color coding: nipple (white), cervix (yellow), clitoris (green), vagina (red, or when congruent with nipple - pink).
c: Three-axis view of the response to the nipple self-stimulation in the case of the center image of Figure 3(b) (downward pointing blue arrow).
Results
Figure 2 shows the group maps of cortical responses to self-stimulation of clitoris, vagina, or cervix, or investigator-applied stimulation of left thumb or left hallux. The columns, (left to right: coronal, sagittal and transaxial views) show the maps of group data based upon Ns between 9 and 11. In these views, the convention is that the subjects’ right side is on the left side of the image, as if their feet are closer to the observer than their head. For reference, in Figure 1, the homuncular map of Penfield and Rasmussen [3] is shown with lines indicating the relation between the boundaries of the postcentral gyrus laterally and the paracentral lobule medially on the schematic map and the brain anatomical template. As labeled in Figure 1, the paracentral lobule is the medial continuation of the more lateral postcentral (sensory) gyrus. In the Penfield and Rasmussen [3] homunculus, the face, hands and arms are represented in the postcentral gyrus, whereas the groin, legs, feet and genitals are located in the paracentral lobule. In Figure 2, the arrows indicate sensory cortical brain regions activated by the specific stimuli. Note that the response to stimulation of the thumb, in the postcentral gyrus, corresponded closely to the homuncular map. The group response to stimulation of the toe (weaker than that to the thumb) was in the medial region of the paracentral lobule, corresponding precisely to the homuncular map.
The group responses to clitoral, vaginal, and cervical self-stimulation were all located in the medial paracentral lobule, with the precise localizations being distinct from each other. Note that the groin region of the paracentral lobule (i.e., its dorsolateral region) was also activated in all but the thumb stimulation conditions. Note also the region of activation of the ventrolateral (sensory) thalamus by the clitoral self-stimulation condition. We have intentionally avoided “modeling out” the participants’ hand movement involved in the self-stimulation. Note the absence of hand movement in the case of experimenter-applied thumb stimulation. Two unexpected observations were that a) although the participants were all using just their right hand to apply the self-stimulation, both the contralateral and ipsilateral hand areas were activated, a highly reliable effect, and b) in the case of investigator-applied toe stimulation, the participants’ hand areas were also activated. These findings are addressed in the Discussion.
An unexpected finding was that nipple self-stimulation, which we had selected as a reference point on the homunculus, also activated the medial paracentral lobule, in the region activated by genital self-stimulation (Figure 3b and c).
A composite coronal view of the clitoral, vaginal and cervical activation sites is shown in Figure 3a. The sites are all in the medial paracentral lobule, but regionally differentiated. This clustered, but differential, localization pattern is likely due to the differential sensory innervation of these genital structures, i.e., clitoris: pudendal nerve, vagina: pelvic nerve, and cervix: pelvic, hypogastric and vagus nerves (for review: [14]). It is likely that the overlap between the sites activated by vaginal and cervical self-stimulation (using a passive dildo) is due at least in part to the stretching stimulation of the vagina that inevitably accompanied the cervical self-stimulation.
The sagittal views in Figure 2 show that in each of the self-stimulation conditions, activation was evident also in the supplementary motor area, which is immediately rostral (anterior) to the paracentral lobule.
Figure 3b and c presents evidence that nipple self-stimulation activated not only, as expected, the thoracic (rib) region (as situated between the abdomen and the neck on the Penfield & Rasmussen homuncular map), but also, unexpectedly, the genital sensory cortex, i.e., the genital (medial) region of the paracentral lobule. Shown (Figure 3b) are superimposed responses to nipple and genital self-stimulation in three participants. Left panel: Note the overlap between activation produced by stimulation of nipple and cervix. Center panel: overlap between activation produced by stimulation of nipple, cervix and clitoris. Not unexpectedly, cervical self-stimulation activated the groin region of the dorsolateral paracentral lobule, probably as a consequence of unavoidable mechanical stimulation of the groin in the course of self-stimulating the cervix. Right panel: overlap among activation produced by stimulation of nipple, vagina, cervix and clitoris. Two unexpected observations were that nipple self-stimulation also activated the groin sensory region (dorsolateral paracentral lobule; Left panel) and, conversely, vaginal self-stimulation activated the thoracic nipple region (Center panel). Figure 3c is a three-axis view of the response to nipple self-stimulation in the brain shown in the center panel of Figure 3b.
Discussion
Clitoral, vaginal, and cervical self-stimulation differentially activated regions of the sensory cortex, but all were clustered in the medial paracentral lobule.
Because the perineal (groin) region is also stimulated incidentally during the clitoral, vaginal, and cervical self-stimulation, its corresponding sensory cortical region -- i. e, the dorsal convexity of the paracentral lobule, immediately lateral to the midline -- was also activated.
The present findings may help to resolve a discrepancy in the literature that claims that the location of the genital sensory cortical representation is on the dorsolateral paracentral lobule, rather than the medial paracentral lobule [8–12]. That is, based on the present findings, the discrepancy in the literature may be due to responses to indirect stimulation of the perineal (groin) region rather than to adequate stimulation of the genitals per se.
It is likely that the clitoris is indirectly stimulated by self-stimulation of the cervix or vagina. Under the conditions of the present study, it is not possible to discern whether the overlap among regions of the sensory cortex activated in response to self-stimulation of each of these three genital regions is due to true overlap of the brain regions that would be activated by “pure” stimulation of each of these three genital regions separately, or whether the overlap is due to incidental stimulation of one genital region (e.g., vagina) during self-stimulation of a different genital region (e.g., cervix). What is clear, however, is that to some extent, the sensory cortical regions activated by each of these three genital regions are to some extent separable and distinct. Unexpectedly, nipple/breast self-stimulation activated not only the (expected) thoracic sensory homuncular region, but also the region of the paracentral lobule that overlaps with the region activated by clitoral, vaginal, or cervical self-stimulation. This finding is consistent with many women's reports that nipple/breast stimulation is erotogenic and can elicit orgasms ([16–18] and personal communication).
The present finding of convergence between nipple and genital input in the genital sensory cortex is supported by an intriguing observation by Penfield and Rasmussen ([3], p.26): “One patient, Case E.D., a woman of 27 years who had a small glioma in the right postcentral gyrus next to the falx [i.e., the dura mater in the midline, separating the two cerebral hemispheres], experienced spontaneous sensory seizures that involved the left labium and left breast. At times…[the sensation] began in the left labium, spread to the left breast and continued to tingle in the labium and nipple. On one occasion this sensory aura was followed by twitching of the left foot…there was nothing in the sensation that resembled sexual excitement. But the description does suggest that the labium and nipple have a neighboring localization in the contralateral sensorimotor area near the motor representation for foot.”
The ability of nipple stimulation to activate genital sensory cortex could have an indirect basis. Thus, nipple/breast self-stimulation-induced oxytocin secretion could stimulate uterine contractions that in turn generate afferent activity that projects to the paracentral lobule. However, it is also possible that nipple/breast and genital sensory activity converge directly not only on oxytocinergic neurons of the hypothalamic paraventricular nucleus [19], but also on paracentral lobule neurons of the genital sensory cortex.
The cerebellum activation observed in the present study during vaginal and cervical self-stimulation is a common observation during genital stimulation, especially during orgasm [12, 13]. It is likely that it is involved in controlling muscle tension during genital stimulation [14]. Two other brain regions that were seen to be activated in the present study are the supplementary motor area and SII (Secondary somatosensory cortex). Other brain regions activated more variably were thalamus, frontal and parietal cortices.
Regarding the observation of bilateral activation of the hand representation in sensory cortex in response to unilateral hand-applied self-stimulation that was noted in the Results section for clitoral and vaginal self-stimulation, it is likely that the sensory stimulation emanating from that single hand, by utilizing the corpus callosum, generates contra- as well as ipsi-lateral activation of the hand representation in sensory cortex. This observation is supported by substantial evidence in the literature of bilateral sensory cortical response to unilateral hand stimulation [20, 21]. A more curious observation was the activation of the hand representation in sensory cortex during investigator-applied toe stimulation. One speculation to account for this observation is that subtle muscle-induced contractions of the hand in response to toe stimulation (a compensatory response preparatory to breaking the fall in the “stumble” response: [22]) activates the hand representation area in the sensory cortex, although no obvious hand movement was observed. Another possibility is that the response is among the class of atypical forms of referred sensation (e.g., [23]).
The present findings provide evidence that, rather than vaginal stimulation being just an indirect means of stimulating the clitoris [17, 24], vaginal and cervical stimulation per se activate specific sensory cortical regions that are distinct from the clitoral sensory projection. These differential routes of entry into the brain are undoubtedly of significance in activating the diverse and differential consequences of clitoral, vaginal or cervical stimulation; they include differential physiological effects, e.g., on prolactin secretion [25], analgesia [26], and blood pressure reactivity to stress [27], and differential behavioral effects, e.g., on orgasm [28], sexual satisfaction [29], and intimate relationship quality [30, 31].
While the present study mapped the primary sensory field of genital input to the sensory cortex, it would be of interest in future studies to extend this analysis to brain fields beyond the sensory cortex that are activated when genital stimulation is perceived as ‘erotic’ versus when it is perceived as ‘just pressure’.
Acknowledgement
We are especially grateful to Dr. Robert Savoy for his generous provision of the head stabilization instrument. We thank Ms. Gladys Martinez for her insights and help, and gratefully acknowledge Ms. Sebina Versi, Ms. Sophie Greller and Ms. Grace Maher for their excellent technical assistance. Support: NIH 2 R 25 GM060826, the Rutgers University Research Fund, and The Carnegie Trust for the Universities of Scotland.
Footnotes
Conflict of Interest:
None
References
- 1.Foerster O. Handbuch der Neurologie. Berlin: Springer; 1936. Sensible corticale Felder. [Google Scholar]
- 2.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 3.Penfield W, Rasmussen T. The Cerebral Cortex of Man. New York: Macmillan; 1950. [Google Scholar]
- 4.Makela JP, Illman M, Jousmaki V, Numminen J, Lehecka M, Salenius S, Forss N, Hari R. Dorsal penile nerve stimulation elicits left-hemisphere dominant activation in the second somatosensory cortex. Hum Brain Map. 2003;18:90–99. doi: 10.1002/hbm.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakagawa H, Namima T, Aizawa M, Uchi K, Kaiho Y, Yoshikawa S, Orikasa S, Nakasato N. Somatosensory evoked magnetic fields elicited by dorsal penile, posterior tibial, and median nerve stimulation. Electroenceph Clin Neurophysiol. 1998;108:57–61. doi: 10.1016/s0168-5597(97)00093-2. [DOI] [PubMed] [Google Scholar]
- 6.Narici L, Modena I, Opsomer RJ, Pizzella V, Romani GL, Traversa R, Rossini PM. Neuromagnetic somatosensory homunculus: a non-invasive approach in humans. Neurosci Lett. 1991;2:51–54. doi: 10.1016/0304-3940(91)90647-c. 121. [DOI] [PubMed] [Google Scholar]
- 7.Allison T, McCarthy G, Luby M, Puce A, Spencer D. Localization of functional regions of human mesial cortex by somatosensory evoked potential recording and by cortical stimulation. Electroenceph Clin Neurophysiol. 1996;100:126–140. doi: 10.1016/0013-4694(95)00226-x. [DOI] [PubMed] [Google Scholar]
- 8.Kell CA, Kriegstein K, Rosller A, Kleinschmidt A, Lauf H. The sensory cortical representation of the human penis: revisiting somatotopy in the male homunculus. J Neurosci. 2005;25:5984–5987. doi: 10.1523/JNEUROSCI.0712-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgiadis J, Holstege G. Human brain activation during sexual stimulation of the penis. J Comp Neurol. 2005;493:33–38. doi: 10.1002/cne.20735. [DOI] [PubMed] [Google Scholar]
- 10.Bradley WE, Farrell DF, Ojemann G. Human cerebrocortical potentials evoked by stimulation of the dorsal nerve of the penis. Somatosens Motor Res. 1998;15:118–127. doi: 10.1080/08990229870844. [DOI] [PubMed] [Google Scholar]
- 11.Michels L, Mehnert U, Boy S, Schurch B, Kollias S. The somatosensory representation of the human clitoris: an fMRI study. Neuroimage. 2010;49:177–184. doi: 10.1016/j.neuroimage.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Georgiadis J, Kortekaas R, Kulpers R, Nieuwenburg A, Pruim H, Reinders S, Holstege G. Regional cerebral blood flow changes associated with clitorally induced orgasm in healthy women. Eur J Neurosci. 2006;24:3305–3316. doi: 10.1111/j.1460-9568.2006.05206.x. [DOI] [PubMed] [Google Scholar]
- 13.Komisaruk BR, Whipple B, Crawford A, Grimes S, Liu W-C, Kalnin A, Mosier K. Brain activation during vaginocervical self-stimulation and orgasm in women with complete spinal cord injury: fMRI evidence of mediation by the Vagus nerves. Brain Res. 2004;1024:77–88. doi: 10.1016/j.brainres.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 14.Komisaruk BR, Beyer C, Whipple B. The Science of Orgasm. Baltimore: The Johns Hopkins University Press; 2006. p. 358. [Google Scholar]
- 15.Komisaruk BR, Wise N, Frangos E, Liu W. Women’s clitoris, vagina and cervix mapped on the sensory cortex, using fMRI. Soc Neurosci. 2009 doi: 10.1111/j.1743-6109.2011.02388.x. 562.18, October. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinsey A, Pomeroy W, Martin C, Gebhard P. Sexual Behavior in the Human Female. Philadelphia: WB Saunders; 1953. [Google Scholar]
- 17.Masters W, Johnson V. Human Sexual Inadequacy. Boston: Little, Brown; 1970. [Google Scholar]
- 18.Paget L. The Big O. New York: Broadway Books; 2001. [Google Scholar]
- 19.Netter FH. Anatomy and Physiology. Summit, NJ: Ciba Pharmaceutical; 1986. The Ciba Collection of Medical Illustrations. Nervous System. Part I. [Google Scholar]
- 20.Blatow M, Nennig E, Durst A, Sartor K, Stippich C. FMRI reflects functional connectivity of human somatosensory cortex. Neuroimage. 2007;37:927–936. doi: 10.1016/j.neuroimage.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland MT. The hand and the ipsilateral primary somatosensory cortex. J Neurosci. 2006;26:8217–8218. doi: 10.1523/JNEUROSCI.2698-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haridas C, Zehr EP. Coordinated interlimb compensatory responses to electrical stimulation of cutaneous nerves in the hand and foot during walking. J Neurophysiol. 2003;90:2850–2861. doi: 10.1152/jn.00531.2003. [DOI] [PubMed] [Google Scholar]
- 23.Richter C. Mysterious form of referred sensation in man. Proc Natl Acad Sci USA. 1977;74:4702–4705. doi: 10.1073/pnas.74.10.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connell HE, Eizenberg N, Rahman M, Cleeve J. The anatomy of the distal vagina; towards unity. J Sex Med. 2008;5:1883–1891. doi: 10.1111/j.1743-6109.2008.00875.x. [DOI] [PubMed] [Google Scholar]
- 25.Brody S, Kruger THC. The post-orgasmic prolactin increase following intercourse is greater than following masturbation and suggests greater satiety. Biol Psychol. 2006;71:312–315. doi: 10.1016/j.biopsycho.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Whipple B, Komisaruk BR. Analgesia produced in women by genital self-stimulation. J Sex Research. 1988;24:130–140. doi: 10.1080/00224498809551403. [DOI] [PubMed] [Google Scholar]
- 27.Brody S. Blood pressure reactivity to stress is better for people who recently had penile-vaginal intercourse than for people who had other or no sexual activity. Biol Psychol. 2006;71:214–222. doi: 10.1016/j.biopsycho.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Cutler WB, Zacker M, McCoy N, Genoves-Stone E, Friedman E. Sexual response in women. Obstet Gynecol. 2000;95(4,suppl 1):S19. [Google Scholar]
- 29.Tao P, Brody S. Sexual behavior predictors of satisfaction in a Chinese sample. J Sex Med. 2011;8:455–460. doi: 10.1111/j.1743-6109.2010.02129.x. [DOI] [PubMed] [Google Scholar]
- 30.Costa RM, Brody S. Women’s relationship quality is associated with specifically penile-vaginal intercourse orgasm and frequency. J Sex Marital Ther. 2007;33:319–327. doi: 10.1080/00926230701385548. [DOI] [PubMed] [Google Scholar]
- 31.Brody S. The relative health benefits of different sexual activities. J Sex Med. 2010;7:1336–1361. doi: 10.1111/j.1743-6109.2009.01677.x. [DOI] [PubMed] [Google Scholar]
- 32.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. See also http://www.cabiatl.com/mricro/mricro/index.html. [DOI] [PubMed] [Google Scholar]


