Abstract
Measurement of individual organ tissue oxygen levels can provide information to help evaluate and optimize medical interventions in many areas including wound healing, resuscitation strategies, and cancer therapeutics. Echo planar 19F MRI has previously focused on tumor oxygen measurement at low oxygen levels (pO2) < 30 mmHg. It uses the linear relationship between spin-lattice relaxation rate (R1) of hexafluorobenzene (HFB) and pO2. The feasibility of this technique for a wider range of pO2 values and individual organ tissue pO2 measurement was investigated in a rat model. Spin-lattice relaxation times (T1=1/R1) of HFB were measured using 19F saturation recovery echo planar imaging (EPI). Initial in vitro studies validated the linear relationship between R1 and pO2 from 0 mmHg to 760 mmHg oxygen partial pressure at 25°C, 37°C, and 41°C at 7 Tesla for HFB. In vivo experiments measured rat tissue oxygen (ptO2) levels of brain, kidney, liver, gut, muscle and skin during inhalation of both 30% and 100% oxygen. All organ ptO2 values significantly increased with hyperoxia (p<0.001). This study demonstrates that 19F MRI of HFB offers a feasible tool to measure regional ptO2 in vivo, and that hyperoxia significantly increases ptO2 of multiple organs in a rat model.
Keywords: oximetry, tissue oxygen tension, fluorine MRI, hexafluorobenzene
Introduction
The primary goal in treating critically ill patients is to ensure adequate perfusion and oxygenation of vital organs. Tissue oxygenation is complex and depends upon the interaction of blood flow, arterial oxygen tension, hemoglobin dissociation conditions, oxygen carrying capacity, mass transfer resistances and local oxygen consumption. Tissue hypoxia has a high correlation with organ failure and poor prognosis in severe disease states(1,2). Frequently used surrogates for tissue oxygenation include blood pressure, heart rate, cardiac filling pressures, cardiac output, hemoglobin saturation, systemic blood gas measurement, and lactate levels. All are global measures and give no specific information about the status of individual organs. Abnormal values of these surrogate measures often signify a poor prognosis. However, using medical therapies to obtain normal values does not reliably predict a favorable result, and may lead to patient outcomes that are worse, better, or unchanged(3). Measurement of individual organ tissue oxygen levels could help physicians and investigators evaluate and optimize medical interventions in many areas including wound healing(4), resuscitation strategies(5), and cancer therapeutics(6) by providing defined endpoints to titrate therapies and evaluate interventions.
A variety of tissue oxygen measurement techniques exist, but all have limitations. Although polarographic electrodes are considered the gold standard of oximetry, they are invasive, consume oxygen, sample a limited tissue volume, and cannot display the heterogeneity of tissue oxygen levels(7). Although fiberoptic probes do not consume oxygen, they suffer from similar limitations regarding invasiveness and fragility(8). Methods based on hemoglobin (Hb) saturation, such as near infrared spectroscopy, measure an average of arterial, venous, and capillary blood in a large tissue volume. Electron paramagnetic resonance (EPR) gives precise tissue oxygen values and allows repeated measurements, but requires implantation of a paramagnetic material, is limited in its sampling area, and is hindered by a lack of available instrumentation(9). Positron emission tomography images tissue oxygen levels using intravenous reporter molecules that become trapped in the tissues, but the calibration range of this method is limited to hypoxic conditions(10). Blood oxygen level-dependent (BOLD) MRI is sensitive to intravascular Hb saturation but does not provide absolute pO2. Although the BOLD technique is positively correlated with tumor pO2, its intravascular based imaging does not reliably predict tissue pO2(11,12). In addition, it is heavily affected by changes in blood flow and vascular volume(13).
A quantitative oximetry method using fluorine-based MRI has been developed that allows regional dynamic changes to be measured from sequential images of tissue oxygen(14,15). The use of 19F MRI oximetry based on direct tissue injection of hexafluorobenzene (HFB) has primarily been directed at measurements of tumor oxygenation in animal models, and has consequently focused on pO2 quantitative imaging at low oxygen levels(16). We have chosen this method because it is minimally invasive, requiring only microliters of a fluorinated substance to be injected, it directly measures pO2 in the tissue, allows imaging of regional tissue oxygen distributions based on small voxels (typically, in-plane areas of 1.25 mm X 1.25 mm), and has been validated by direct comparison to multiple polarographic electrode measurements(7,15,16) and fluorescence quenching oximetry(17). Various perfluorocarbons (PFCs) have served as reporter molecules, each exhibiting a linear relationship between their corresponding spin lattice relaxation rate (R1) and pO2(18). The ability of a PFC to measure pO2 varies with its molecular properties and route of administration. PFCs injected intravenously tend to be largely sequestered by the reticuloendothelial system into the liver and spleen(19). This method of administration often results in levels of PFC accumulation in other tissues of interest that are too low to provide an adequate signal to noise ratio (SNR). Additionally, intravenous administration has the potential to produce even lower tissue concentrations of PFC in poorly vascularized regions. Even though the technique is not dependent on absolute reporter molecule concentration, these areas would have a SNR too low for accurate pO2 measurement, and could bias the technique to areas of higher perfusion and oxygenation. In contrast, the technique of direct tissue injection affords greater precision and sensitivity of pO2 measurements, as well as the ability to study potentially poorly perfused areas(20,21). The PFC hexafluorobenzene (HFB) has 6 magnetically equivalent fluorine atoms, resulting in a single resonance. This feature provides high sensitivity, an advantageous SNR, and dramatically simplifies imaging. HFB also has the advantage of a relatively low sensitivity to both temperature and pH variation, and is easily available through several commercial sources(15,22). HFB has minimal toxicity, with no mutagenicity, teratogenicity, or fetotoxicity reported, and an LD50 > 10g/kg when administered orally or intraperitoneally in rats(20). Thus HFB also has the potential for future use in humans.
Although the vast majority of oxygen content in blood is carried on hemoglobin, increasing inspired oxygen levels results in a significant increase in arterial plasma oxygen content (dissolved oxygen), and is associated with improved wound healing, decreased infection rates, and improved efficacy of cancer treatment(23,24). High levels of inspired oxygen (ranging up to 100%) are used daily in the operating rooms and critical care units to improve arterial oxygenation, although the effects on tissue oxygenation are not normally measured in the clinical setting. Even under normobaric conditions, high oxygen tension in the arterial blood has been reported to lead to hemodynamic changes including bradycardia, decreases in both pulmonary artery pressure and cardiac output, and increases in systemic vascular tone(25,26). Despite many attempts, animal studies have generally failed to identify specific organs or tissue areas of altered blood flow during hyperoxia. An increase in vascular resistance from hyperoxia has been observed in animal models, but blood flow to major organs appeared to be preserved(27–29). The specific mechanisms of both changes in vascular resistance and changes in tissue oxygen consumption remain unknown, leaving the regional effects of hyperoxia unclear, and highlighting the need for direct tissue oxygen measurement.
A better understanding of how hyperoxia affects individual organ oxygenation is needed to optimize the use of oxygen as a medical therapy. Therefore, one of the goals of this study was to examine the influence of increasing the dissolved arterial oxygen content on individual organ pO2 as measured by 19F MRI, while ensuring that temperature, arterial CO2, anesthetic depth, and intravascular volume status remained constant. For this study, we chose inspired oxygen levels that would ensure a nearly complete saturation of hemoglobin, yet provide markedly different arterial blood pO2. To examine the influence of hyperoxemia on individual organs, we measured brain, gut, kidney, liver, muscle, and skin oxygen tension during ventilation with the fraction of inspired oxygen (FiO2) set at either 0.3 or 1.0 in a euthermic, euvolemic, anesthetized rat model. An FiO2 of 0.3 rather than room air (0.21) was chosen because under general anesthesia there is an increase in pulmonary shunting which results in hypoxic arterial oxygen levels compared to awake subjects. In addition, an FiO2 of less than 0.3 is rarely used in either the intensive care unit (ICU) or operating room for patients that are intubated and anesthetized.
Methods
Magnetic Resonance Pulse Sequence for Measuring the T1 of HFB
A saturation recovery echo planar imaging (EPI) sequence(15) was used to measure the T1 of directly injected HFB. A group of twenty 90° hard pulses with 50 ms spacing was applied to saturate the magnetization along the z-axis. Following a variable time delay (τ), a single-shot spin echo with blipped phase encoding EPI was used to collect images. The saturation recovery was favored, and chosen over the traditional inversion recovery since there is no need to wait more than a period of 5T1 between acquisitions for longitudinal relaxation recovery. This method is well suited for long T1 measurements, which were common in this study. The Alternated Relaxation Delays with Variable Acquisitions (ARDVARC) scheme (30) was modified and used to acquire 14 τ in the order: 90 s, 200 ms, 60 s, 400 ms, 40 s, 600 ms, 20 s, 800 ms, 16 s, 1 s, 8 s, 1.5 s, 4 s, and 2 s. This acquisition scheme is optimized to minimize any error from the clearance effects of HFB in vivo. A wide range of τ values was selected since the range of the expected T1 values under differing oxygen conditions is broad. Generally, the corresponding number of averages was set to one for all delays, but occasionally an average of two was needed when the signal-to-noise ratio (SNR) was low. The acquisition time per T1 measurement was approximately 5 minutes. The standard voxel size was 1.25 × 1.25 × 5 mm.
Calibration of HFB R1 and pO2 at 7 Tesla
Calibration techniques were based on previously described methods used at 4.7T magnetic field strength(15,30). Three different HFB samples were equilibrated at each of 5 different oxygen concentrations balanced with nitrogen. The concentrations examined were 0% O2 (pure N2), 5% O2, 10% O2, 21% O2, 50% O2, 100% O2. These oxygen concentrations were examined under atmospheric pressure (sea level) at 25°C, 37°C, and 41°C. Additionally, since 2% isoflurane was used to anesthetize animals, the entire calibration sample conditions were repeated with and without the presence of 2% isoflurane in order to determine whether the fluorine atoms in isoflurane affect the linear relationship between the R1 of HFB and pO2. The pO2 was calculated using standard partial pressure equations taking into account the barometric pressure, oxygen percentage, temperature, and presence of water vapor(31).
One ml of HFB was added to a gas-tight NMR tube (Wilmad Lab Glass, Buena, NJ, USA) together with 5 ml water. Since HFB is hydrophobic and immiscible with water, the denser HFB remained in a separate layer at the bottom of the tube. The NMR tube containing HFB and water was equilibrated under the desired temperature and atmospheric conditions within a sealed previously equilibrated chamber using a small catheter with the selected gas continuously bubbled into the sample. The desired temperature was maintained during imaging using a water bath containing D2O circulating in tubing surrounding the NMR tube. The T1 of HFB in the sample was then determined with 19F saturation recovery EPI. The experiment was repeated in triplicate for each oxygen and temperature condition of interest. Separate sets of experiments determined the time needed for oxygen equilibration in this experimental set-up and confirmed maintenance of a constant selected temperature during imaging.
Animal preparation
The University of California, San Francisco Institutional Animal Care and Use Committee (IACUC) approved this work and animal protocol prior to any experimentation. Twelve male Sprague-Dawley rats (250–350g, Charles River Labs, Wilmington, MA, USA) were anesthetized with isoflurane. General anesthesia was initially maintained with 2.0% isoflurane in 100% oxygen via nose cone during placement of invasive monitoring and HFB. The rat was shaved, and placed on an adjustable temperature water pad, which was used to keep the animal euthermic (37.5 °C ± 0.5°C). Temperature was measured by rectal probe. An MRI compatible pulse oximeter (Nonin Medical, Inc. Plymouth, MN) was placed on the animal’s right hind paw; heart rate and oxygen saturation were monitored for the duration of the experiment. The trachea was exposed and a tracheal ventilation cannula placed and secured. The rat was then mechanically ventilated throughout the experiment in order to control and maintain normal physiologic arterial CO2 levels and decrease atelectasis during the experiment. Initial ventilation settings of 70 breaths/min and tidal volume of 12 ml/kg were then adjusted based on end tidal carbon dioxide, which was monitored every 15 minutes throughout the remainder of the surgery and experimental procedure. These higher tidal volume settings were necessary due to the large capacitance in the longer length of tubing needed to position the ventilator outside the magnetic field of the scanner. Following the tracheotomy, the femoral artery was cannulated. A 1.5 cm incision was made along the crease formed by the abdomen and left thigh (contralateral to the side with the pulse oximeter). The femoral artery was then exposed. Approximately 1 cm of the vessel was dissected free under microscopic visualization and a saline-filled catheter inserted into the vessel and secured in place with suture. This arterial line was then connected to a blood pressure monitor (Spacelabs Healthcare, Issaquah, WA, USA) and used throughout the remainder of the preparation and data collection for blood pressure monitoring and blood gas sampling. After securing the arterial line, the tail vein was cannulated with a 24 gauge catheter and attached to a programmable syringe infusion pump (New Era Pump Systems, Inc., Wantagh, NY, USA); 10mL/kg normal saline was bolused at this time to replace losses during surgery, and then normal saline infused intravenously at a rate of 2.5mL/kg/hr for fluid maintenance. Total surgical time ranged from 60 to 120 minutes.
The animal was placed on its side, and a superficial skin nick (2mm) was made on the proximal hind thigh, contralateral to the arterial cannulation. Aliquots of HFB were injected into multiple tissues of each rat as follows: 50 µL HFB was incrementally injected under direct vision into the muscle with a 33 ga. needle. Percutaneous injection was used for placement of 50 µL HFB into the skin. Following a small scalp incision, a 1.5 mm diameter hole was drilled through the animal’s skull, 2mm anterior to the bregma and 4mm lateral from midline. 30 µL HFB was injected 1.5 mm below the dural layer over 10 minutes with the assistance of a stereotactic frame and microscope. A 1.5 to 2.0 cm incision was made just caudal to the right inferior costal margin, through the peritoneum to expose the kidney, liver, and gut. 50 µL HFB was injected into the liver tissue, kidney tissue, and gut wall under direct vision. Total time for HFB injection for all organs of interest was approximately 60 minutes.
MRI Experiments
All experiments were performed on a Varian DirectDrive™ 7T horizontal bore magnet equipped with 21-cm inner diameter imaging gradients that provide ±200 mT/m (Varian, Inc., Palo Alto, CA, USA). A custom constructed 19F/1H dually tunable birdcage RF transmit/receive coil (Rapid Biomedical GmbH, Rimpar, Germany) with 7.2 cm inner diameter was used for proton and fluorine imaging. Each animal was randomly assigned to an initial FiO2 of 0.3 or 1.0. Following surgical preparation and HFB injection, the animal was allowed to equilibrate on the assigned FiO2 for 20 minutes, and an arterial blood gas (ABG; 0.25 ml) was sampled and analyzed using an i-STAT® hand held blood analyzer (Abbott Point of Care, Inc., East Windsor, NJ, USA). The rat was kept prone on a D2O circulating warming pad and covered with a plastic covered cotton drape throughout the experimentation to maintain euthermia. The animal and monitoring lines were secured onto a custom built Plexiglas bed and cradle inserted into the magnet such that the RF coil center was aligned to the isocenter of the imaging gradients. The bed was manually adjusted to position each different organ location at the center of the RF coil. The order of imaging each organ of interest was randomized. Conventional T1-weighted 2D spin echo 1H and 19F images with typical voxel size of 0.625×0.625×2 mm were acquired to identify the position of HFB signal for different organs of interest. Following the anatomical scans, the T1 of the implanted HFB was measured with the saturation recovery EPI. The field of view (FOV) of the saturation recovery EPI images was the same as the spin echo images for appropriate signal registration.
Ventilation, administration of isoflurane, and continuous monitoring of pulse and blood pressure were continued throughout the MRI scan, with all ferrous metal monitors and equipment located outside the 5-gauss field line. Rectal temperature was recorded prior to placement of the rat in the magnet and again following completion of the scan. Abdominal images were acquired with respiratory gating. The respiration signal was determined using a mechanical pressure transduction signal from the ventilator exhaust outlet that was transferred into a respiratory gating module (Small Animal Instruments, Inc, Stony Brook, NY, USA).
Following the organ scans at the initial FiO2, the inspired gas was changed to the alternate value. The animal was rescanned as described above after a 20-minute equilibration period. Following the scan, 0.25 ml of arterial blood was again taken for blood gas and hematocrit analysis. The total scan time for both FiO2 values was approximately 2.5 hrs.
After completion of imaging, the animal was removed from the magnet, a final blood gas drawn, and the animal euthanized.
Data Analysis
Raw data were imported into Matlab (MathWorks Inc., Natick, MA, USA) for image reconstruction and processing. The T1 was calculated voxel by voxel in the selected region of interest (ROI) with a three-parameter fit using the Levenberg-Marquardt algorithm(32). Error estimates of the derived T1 were calculated from the residuals and Jacobian matrix used in this fit method. The in vitro calibration data at each temperature were then fit to Eq. [1] as previously detailed by Zhao et al.(15):
| [1] |
Where R1 is the spin lattice relaxation rate (s−1), psO2 is the oxygen pressure in the sample (mmHg), A and B are constants at a given temperature and magnetic field. R1 was derived from the reciprocal of the mean T1 averaged over selected ROIs in three individual measurements.
Prior research has demonstrated a linear dependence of R1 on temperature over a small range(33). Assuming a linear dependence of A and B with temperature T, then A= C+D*T and B= E+ F*T, and Eq. [1] is transferred into a temperature-dependent model described by Eq. [2]:
| [2] |
where C, D, E, F are constants, T is the temperature in the unit of Celsius. These results were used to examine the effects of temperature at a field strength of 7T.
The in vivo tissue oxygen pressure (ptO2) was calculated for each voxel in the selected ROI using the linear calibration equation derived at 37°C shown in Eq. [3] in the Results section. To improve data quality, similar to previous analysis methods(15), any voxels with calculated T1 error greater than 40% (determined from the curve fit to derive T1) were omitted from statistical analysis. In addition, any extreme outliers were also removed by combining voxels from all animals for a given condition, and removing voxels with ptO2 outside the mean ptO2 ± 2.5SD. Application of these two criteria resulted in removal of 169 voxels and 21 voxels respectively from an original total of 1190.
Statistical Analysis
Baseline physiologic rat data were compared between an FiO2 of 0.3 and 1.0 using a paired t-test. A linear mixed effects model was used for statistical data analysis of ptO2 as the primary study outcome, with condition (FiO2 of 0.3 or 1.0) and organ as the two primary fixed effects. The entire data set was analyzed using a two-way repeated measures analysis of variance (ANOVA). Because we were specifically interested in different effects by organ, we tested all pair-wise differences in the regression-adjusted ("least squares") estimates of organ differences, using the post hoc Tukey-Kramer test to adjust for multiple comparisons. In addition, separate one-way ANOVA’s were completed across organs for each inhaled oxygen condition in order to alleviate the reduced power of the numerous multiple comparisons of the two-way ANOVA. All calculations were done using SAS/STAT software (Version 9.1.3 of the SAS System for Windows Copyright ® 2002–2003 and JMP 7.01, SAS Institute Inc., Cary, NC, USA). Under this study design, with a planned sample size of 12 and the anticipated effect size based on previously published values of rat organ ptO2(9,34,35), our a priori power analysis determined that we should have > 85% power to detect a statistically significant difference with an alpha level set at 0.05 under the null hypothesis of no organ site difference.
Results
Calibration Results
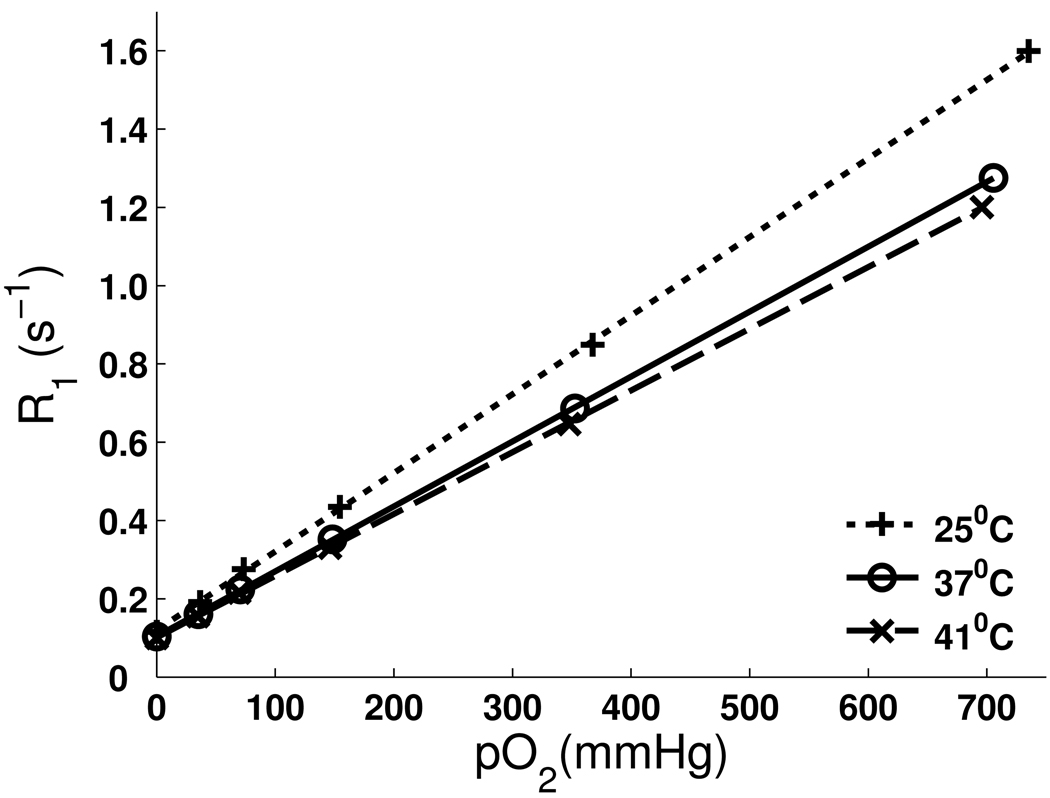
No significant differences were found in sample calibration results (Table 1) with and without 2% isoflurane for a given temperature. Therefore only results of samples with the addition of 2% isoflurane are shown in Figure 1 to simulate the in vivo environment. The T1 of HFB ranged from 9.8 s at 0% oxygen to 0.77 s at 100% oxygen at 37°C. The R1 of HFB was linearly dependent on pO2 from 0% oxygen to 100% oxygen at 25°C, 37°C and 41°C, shown in Figure 2. At 37°C, A = 0.1041 ± 0.0014 s−1, B = 0.001659 ± 0.000004 (s mmHg)−1 were derived with linear fit (R2 = 0.99998) according to Eq. [1]. Thereby the calibration equation at 37°C can be written as follows:
| [3] |
As shown in Figure 1, the HFB R1 temperature dependence increased as pO2 increased. Fitting the R1 of HFB at different pO2’s and temperatures to the temperature-dependent model Eq. [2] gave C = 0.1500 ± 0.0040 s−1, D = −(1.21 ± 0.12) × 10−3 (s °C)−1, E = (2.69 ± 0.06) × 10−3 (s mmHg)−1, and F = −(2.74 ± 0.18) × 10−5 (s mmHg °C)−1.
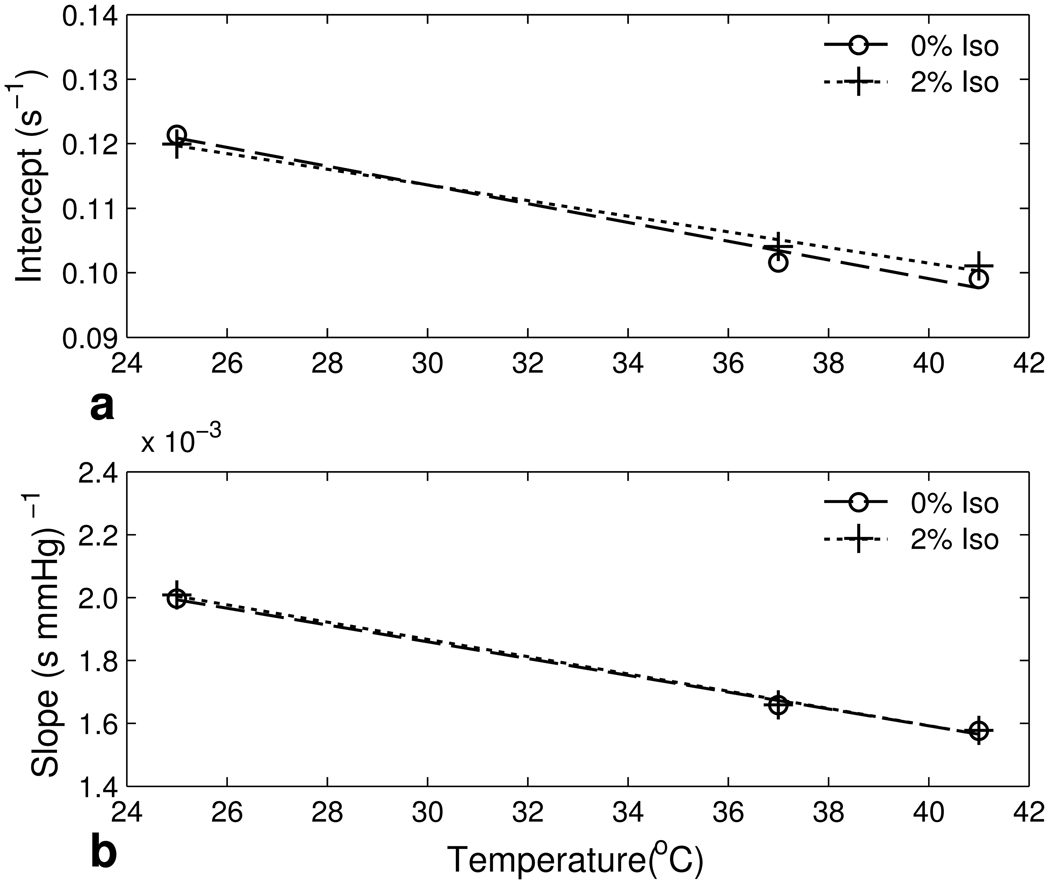
Table 1.
The intercept and slope obtained from calibration data at 0% or 2% isoflurane at three temperatures
| Isoflurane (%) | Temperature(°C) | Intercept (s−1) | Slope 10−3 (s mmHg)−1 |
|---|---|---|---|
| 0 | 25 | 0.121 ± 0.004 | 1.997 ± 0.013 |
| 0 | 37 | 0.102 ± 0.003 | 1.658 ± 0.008 |
| 0 | 41 | 0.099 ± 0.003 | 1.576 ± 0.010 |
| 2 | 25 | 0.120 ± 0.004 | 2.008 ± 0.012 |
| 2 | 37 | 0.104 ± 0.001 | 1.659 ± 0.004 |
| 2 | 41 | 0.101 ± 0.002 | 1.578 ± 0.006 |
Data are mean ± SD from three individual samples using the linear fit from Eq. [1]. No significant effect on the intercept or slope from the presence of isoflurane.
Fig. 1.
Linear fits of R1 of HFB (with 2% Isoflurane) vs. pO2 from 0% to 100% at 25°C, 37°C and 41°C. Each sample point represents the mean of 3 individual samples (SD < 0.0153 s−1 at all points displayed).
Fig. 2.
The dependence of intercept and slope on temperature as depicted from Table 1. Data from calibration with and without the presence of isoflurane are displayed. a: Linear fits of intercept vs. temperature according to Eq. [2]. b: Linear fits of slope vs. temperature according to Eq. [2].
Figure 2 displays the calibration curve temperature dependence of both the intercept (2a) and slope (2b) with and without the presence of isoflurane.
In vivo Results
The mean rat physiologic data from the in vivo experimentation are provided in Table 2. Mean arterial pO2 was 98 ± 12 mmHg at an FiO2 of 0.3 and 400 ± 85 mmHg at an FiO2 of 1.0, reflecting the specific significant increase of dissolved oxygen in plasma due to the increase of FiO2 (p<.01). No significant differences in the remaining measured physiologic data were noted. Mean arterial CO2 was 38 ± 5 mmHg, mean Hb was 12.8 ± 0.9 g/dL. Both parameters remained in the physiological range throughout the in vivo experiments. Mean weight of 12 rats was 312 ± 39 grams. Core temperature of animals remained euthermic ranging from 37.0°C to 38.0°C during all experiments.
Table 2.
Physiological values measured during in vivo experimentation at FiO2 of 0.3 and 1.0
| Physiological Variables | FiO2 = 0.3 | FiO2 = 1.0 |
|---|---|---|
| Arterial pO2 (mmHg) * | 98 ± 12 | 400 ± 85 |
| Arterial pCO2 (mmHg) | 36 ± 5 | 40 ± 4 |
| Hb (g/dL) | 12.8 ± 0.8 | 12.9 ± 0.9 |
| Mean arterial pressure(mmHg) | 90 ± 6 | 86 ± 9 |
| Temperature (°C) | 37.3 ± 0.3 | 37.3 ± 0.6 |
| Heart rate (beats/minute) | 352 ± 38 | 353 ± 23 |
Data are mean ± SD. A significant difference (*) was noted in the arterial pO2 (p<.001) between the conditions. No significant difference (p<.05) was noted between conditions for the remaining physiologic variables.
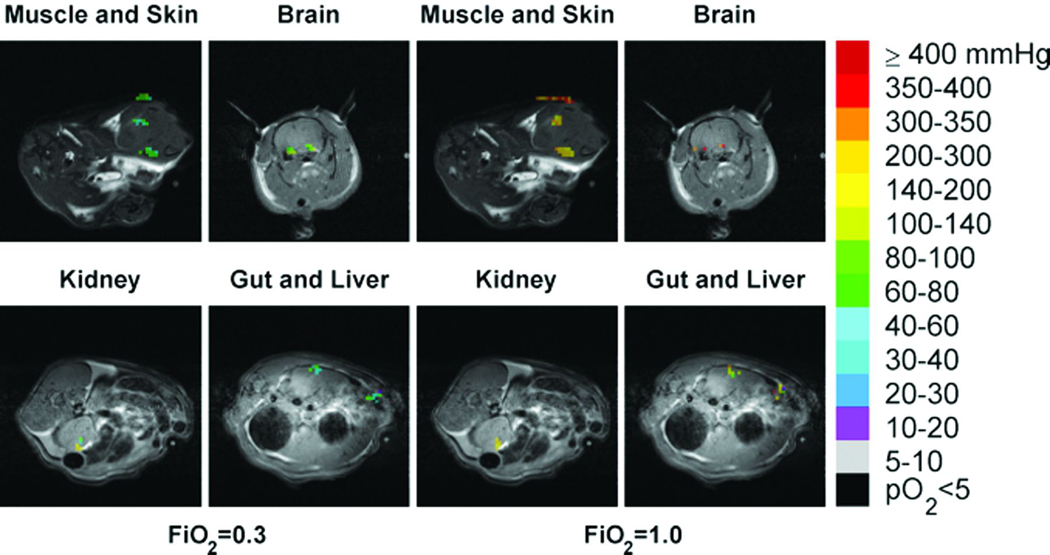
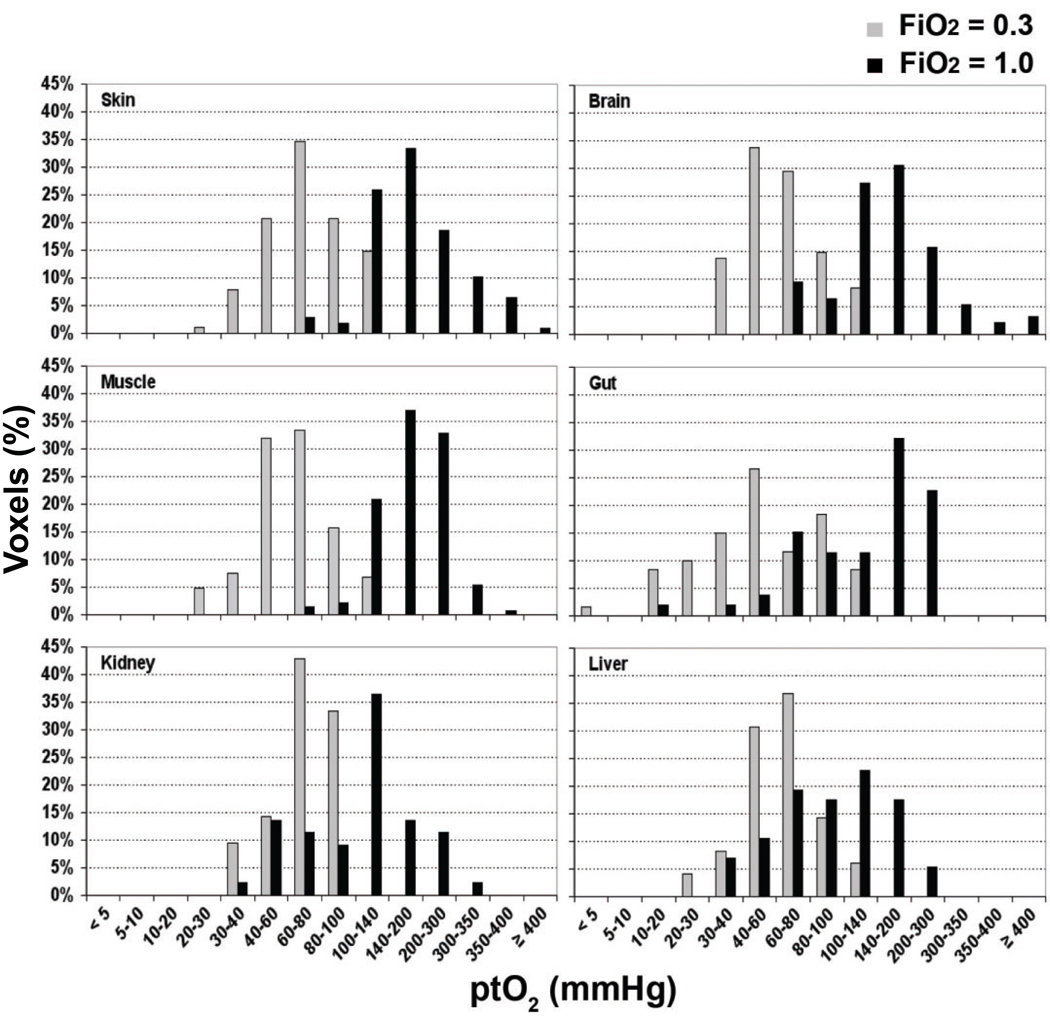
The distributions of ptO2 in all six organs of interest at FiO2 conditions of 0.3 and 1.0 are shown in Figure 3. These 19F MRI based images are from a single representative animal. The shift towards yellow and orange colors displayed in the ptO2 mapping on the right side represents higher values ptO2 in all scanned organs at FiO2 of 1.0 compared to 0.3. Separate organ histograms of the ptO2 values recorded from all animals are displayed in Figure 4. Tissue oxygenation is heterogeneous in individual organs and is noted to vary significantly between organs at the higher level of inspired oxygen (FiO2 = 1.0).
Fig. 3.
Tissue oxygen distributions of six organs at FiO2 of 0.3 (left) and 1.0 (right) shown in color superimposed on proton anatomical images shown in black and white. PO2 reference ranges corresponding to different colors are shown on the side bar color key.
Fig. 4.
Separate organ histograms showing the distribution of individual voxel ptO2 values recorded from all 12 animals are displayed at both an FiO2 of 0.3 (gray) and 1.0 (black).
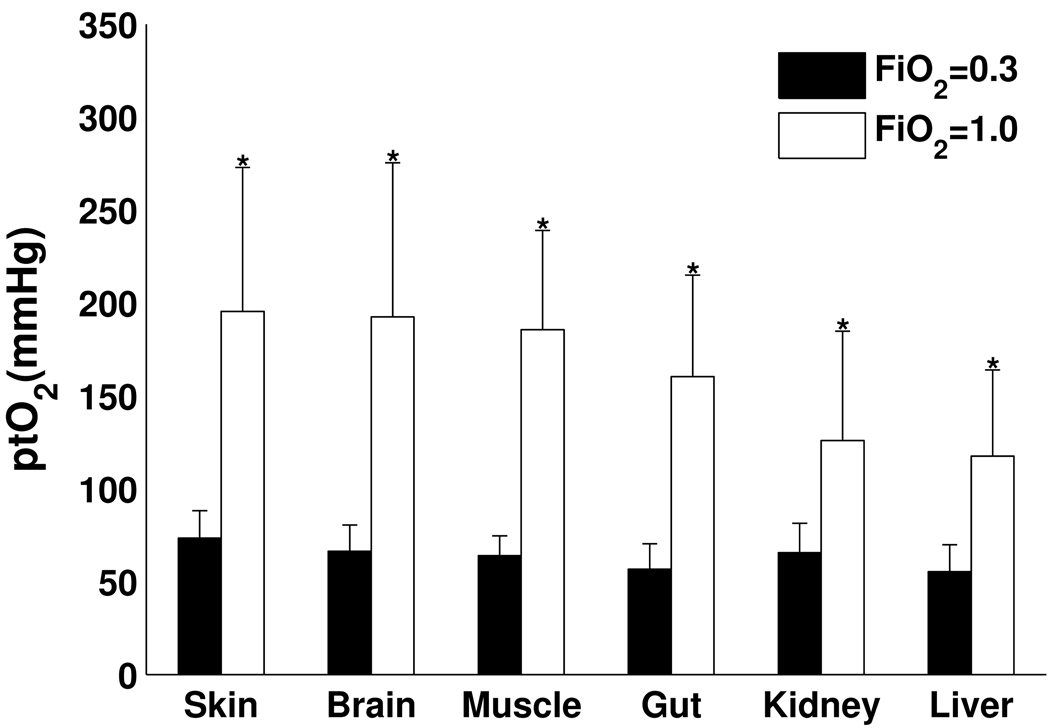
Mean values of ptO2 were calculated over all voxels in the selected ROI for each organ in each individual animal. The mean organ ptO2 from all animals under each inspired oxygen condition are displayed graphically in Figure 5. The mean organ ptO2 values at an FiO2 of 0.3 ranged from 57 ±13 mmHg to 75 ± 15 mmHg. The mean organ ptO2 values at an FiO2 of 1.0 ranged from 127 ± 44 mmHg to 197 ± 86 mmHg. Our final mixed model (two-way ANOVA) showed both "condition" (p-value <.001) and "organ" (p-value <.001) to be significant predictors of ptO2 level. For each organ studied, the ptO2 measured with an FiO2 = 1.0 was significantly greater than that measured while the animal was ventilated with an FiO2 = 0.3 (p < .05). This result demonstrates a significant positive increase in organ ptO2 values at the higher inspired oxygen condition. Comparison of organs showed that the subgroup ptO2 values of skin and muscle were significantly greater than brain and gut which in turn were significantly greater than kidney and liver ptO2 values (p<.05). A second analysis across organs using a one-way ANOVA at each FiO2 condition found a significant organ ptO2 difference noted with an FiO2 = 1.0 (similar to the two-way ANOVA), but no significant difference between organ ptO2 values at an FiO2 =0.3.
Fig. 5.
Bar plots of mean ptO2 over all voxels in brain, kidney, liver, gut, muscle and skin among 12 studied animals at FiO2 of 0.3 and 1.0. Significant differences (*) are noted for all organs as the ptO2 at an FiO2 of 1.0 is significantly increased compared to values at an FiO2 of 0.3 (p<0.05).
DISCUSSION
We demonstrated the feasibility of quantitative tissue oxygen mapping in a rat model with 19F MRI measurement of directly injected HFB in multiple organs under two inspired oxygen conditions. Calibration results showed that the spin-lattice relaxation rate R1 of HFB is linearly dependent on oxygen pressure pO2 at a fixed temperature in the physiological range 25–41°C from 0% to 100% oxygen. Calibration Eq. [3] is similar to the calibration equation previously derived from 0% to 21% oxygen at 7T(22). The R1 of HFB is highly sensitive to pO2, but minimally sensitive to temperature. Since the rat core temperature was carefully maintained between 37°C and 38°C throughout in vivo experimentation, the error of ptO2 measurement was minimized (6 mmHg/°C at pO2 300 mmHg). According to calibration results, 2% isoflurane used for anesthesia in vivo has no significant effects on the calibration equation, and consequently inhaled perfluorocarbon anesthetics can be used without worry of affecting the results of a 19F MRI study using HFB. Prior studies demonstrated that the linear dependence of R1 of PFCs on pO2 is also essentially unresponsive to pH, CO2, charged paramagnetic ions, mixing with blood, or emulsification(15,36). This allows 19F MRI to remain a viable option for measuring ptO2 under a variety of physiologic conditions.
The mean values of ptO2 using 19F MRI in this study are consistently somewhat higher than literature values previously reported for corresponding measurements of brain, kidney, and muscle in rats measured by EPR(9,34,35). The use of EPR ptO2 measurement shows a similar significant rise in ptO2 with increasing inspired oxygen concentration in measurement of both rat and mouse specific organs(9,34,37). The 19F MRI oximetry values are also somewhat higher than previous rat organ measurements of ptO2 obtained using polarographic electrodes(38–42). In a previous study by Jordan et al. using both 19F MRI oximetry and fluorescence quenching fiber-optic probe oximetry to quantify the in vivo response of mouse tumor pO2 with inhalation of carbogen, the tumor pO2 determined with the 19F MRI technique was typically higher(17). The exact reason for the higher absolute values with organ ptO2 measurement using 19F MRI compared to EPR, polarographic electrode, and fluorescence quenching measurements remains uncertain, but may be due to differences in tissue disruption, use of a mean of multiple measurement voxels (with 19F MRI) vs. a single value averaging a larger area of contacting tissue, length of time between placement of reporter material and measurement, anesthetic technique, and control of specific variables known to change tissue perfusion such as temperature, volume status, and spontaneous vs. controlled ventilation. We observed that HFB signal was often imaged near the ventricular floor of the brain instead of cortex layer at the top in Fig. 3. We suspect that the HFB entered the cerebral spinal fluid (CSF) in either ventricles or subdural space in many studies despite a careful slow injection aided by a stereotactic syringe holder and microscope. The parenchymal injection is difficult to reliably reproduce as this layer in the rat brain is approximately 1 – 2 mm thick and HFB will enter the CSF if the needle tip is either to shallow or deep, frequently traveling to the ventricle and sinking to the floor due to its hydrophobic nature and greater density than water. The failure of HFB injection in the parenchyma may overestimate brain tissue oxygen levels since pO2 in CSF is often higher than in brain tissue itself(43).
The marked variability and different absolute increases in the organ ptO2 noted in our study using 19F MRI, show the diversities of oxygenation in various organs and the potential benefit of the regional versus global measurements this technique allows. The increased amount of oxygen bound to hemoglobin due to increasing the FiO2 from 0.3 to 1.0 is small since both levels of corresponding arterial pO2 (98 and 400 mmHg respectively) represent conditions in which hemoglobin is nearly fully saturated. However the mean arterial pO2 at an FiO2 of 1.0 was four fold higher than values corresponding to an FiO2 of 0.3, which reflects the large increase of oxygen dissolved in plasma. Therefore, we conclude that the increases in ptO2 quantified in all studied organs were primarily caused by the increased dissolved oxygen level in plasma. Although the increase in arterial blood oxygen content due to the increased FiO2 is small when compared to the total oxygen content, the corresponding increase of arterial pO2 tension significantly increases the driving force of oxygen diffusion from the capillary bed into tissue, and thus increases the absolute organ ptO2. We believe the 19F MRI technique does not reflect an unintended direct measure of the arterial pO2, as all organ tissue values are significantly less than the arterial oxygen levels, and any HFB in direct contact with the vascular system would be rapidly removed from the tissue, taken to the lungs and exhaled.
Similar absolute increases of ptO2 were found regardless of the order of inspired oxygen levels. This indicates that the tissue damage from the HFB injection was likely minimal. However, during the injection of HFB, transient reductions of blood pressure were observed occasionally. HFB has been used in the past as an inhaled anesthetic(44). These brief hypotensive episodes were most likely caused from a small portion of the HFB entering the intravascular compartment during the injection and exerting an anesthetic effect and its associated brief period of vasodilation or decreased myocardial contractility associated with most anesthetics. In order to prevent the transient acute blood pressure drop, we slowed the injection rate in each organ and reduced the isoflurane concentration briefly during the HFB injection period. The observed retention period of HFB was different in each organ. We believe that the residence time and clearance of HFB is most likely dependent on the individual organ perfusion. Half lives of HFB in less perfused organs such as muscle and skin are reported to be approximately 10 hours(18). HFB signal decayed faster in the more vascularized organs, eg. liver and kidney. The alternated relaxation delays were used to minimize the error in T1 measurement that might otherwise result from the clearance effect of HFB. Two signal averages were used to compensate for the signal loss when a low SNR of HFB was a concern for MRI scans at later post-injection times.
In summary, this minimally invasive 19F MRI oximetry technique using HFB as a reporter probe provides a sensitive quantitative method for measuring regional organ tissue oxygen tension and dynamic changes. These measures represent the balance between oxygen delivery and metabolic use, providing an endpoint to help guide future medical research and optimize a variety of therapeutic interventions, resuscitation strategies, and disease processes.
Acknowledgements
Funding for this project was from the following sources:
Foundation for Anesthesia Education and Research
SW-SAIRP grant U24 CA126608
NIH grant R01 CA139043-01A1
REFERENCES
- 1.Donati A, Loggi S, Preiser JC, Orsetti G, Munch C, Gabbanelli V, Pelaia P, Pietropaoli P. Goal-directed intraoperative therapy reduces morbidity and length of hospital stay in high-risk surgical patients. Chest. 2007;132(6):1817–1824. doi: 10.1378/chest.07-0621. [DOI] [PubMed] [Google Scholar]
- 2.Lima A, van Bommel J, Jansen TC, Ince C, Bakker J. Low tissue oxygen saturation at the end of early goal-directed therapy is associated with worse outcome in critically ill patients. Crit Care. 2009;13 Suppl 5:S13. doi: 10.1186/cc8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA. 1993;270(22):2699–2707. [PubMed] [Google Scholar]
- 4.Fife CE, Smart DR, Sheffield PJ, Hopf HW, Hawkins G, Clarke D. Transcutaneous oximetry in clinical practice: consensus statements from an expert panel based on evidence. Undersea Hyperb Med. 2009;36(1):43–53. [PubMed] [Google Scholar]
- 5.Wan JJ, Cohen MJ, Rosenthal G, Haitsma IK, Morabito DJ, Derugin N, Knudson MM, Manley GT. Refining resuscitation strategies using tissue oxygen and perfusion monitoring in critical organ beds. J Trauma. 2009;66(2):353–357. doi: 10.1097/TA.0b013e318195e222. [DOI] [PubMed] [Google Scholar]
- 6.Bache M, Kappler M, Said HM, Staab A, Vordermark D. Detection and specific targeting of hypoxic regions within solid tumors: current preclinical and clinical strategies. Curr Med Chem. 2008;15(4):322–338. doi: 10.2174/092986708783497391. [DOI] [PubMed] [Google Scholar]
- 7.Mason RP, Hunjan S, Constantinescu A, Song Y, Zhao D, Hahn EW, Antich PP, Peschke P. Tumor oximetry: comparison of 19F MR EPI and electrodes. Adv Exp Med Biol. 2003;530:19–27. doi: 10.1007/978-1-4615-0075-9_3. [DOI] [PubMed] [Google Scholar]
- 8.Lubbers DW. Oxygen electrodes and optodes and their application in vivo. Adv Exp Med Biol. 1996;388:13–34. doi: 10.1007/978-1-4613-0333-6_2. [DOI] [PubMed] [Google Scholar]
- 9.Khan N, Williams BB, Hou H, Li H, Swartz HM. Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: current status and future potential for experimental and clinical studies. Antioxid Redox Signal. 2007;9(8):1169–1182. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman JD, Schneider RF, Urbain JL, Hanks GE. Single-photon emission computed tomography and positron-emission tomography assays for tissue oxygenation. Semin Radiat Oncol. 2001;11(1):47–57. doi: 10.1053/srao.2001.18103. [DOI] [PubMed] [Google Scholar]
- 11.Baudelet C, Gallez B. How does blood oxygen level-dependent (BOLD) contrast correlate with oxygen partial pressure (pO2) inside tumors? Magn Reson Med. 2002;48(6):980–986. doi: 10.1002/mrm.10318. [DOI] [PubMed] [Google Scholar]
- 12.Zhao D, Jiang L, Hahn EW, Mason RP. Comparison of 1H blood oxygen level-dependent (BOLD) and 19F MRI to investigate tumor oxygenation. Magn Reson Med. 2009;62(2):357–364. doi: 10.1002/mrm.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howe FA, Robinson SP, McIntyre DJ, Stubbs M, Griffiths JR. Issues in flow and oxygenation dependent contrast (FLOOD) imaging of tumours. NMR Biomed. 2001;14(7–8):497–506. doi: 10.1002/nbm.716. [DOI] [PubMed] [Google Scholar]
- 14.Zhao D, Constantinescu A, Jiang L, Hahn EW, Mason RP. Prognostic radiology: quantitative assessment of tumor oxygen dynamics by MRI. Am J Clin Oncol. 2001;24(5):462–466. doi: 10.1097/00000421-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Zhao D, Jiang L, Mason RP. Measuring changes in tumor oxygenation. Methods Enzymol. 2004;386:378–418. doi: 10.1016/S0076-6879(04)86018-X. [DOI] [PubMed] [Google Scholar]
- 16.Mason RP, Ran S, Thorpe PE. Quantitative assessment of tumor oxygen dynamics: molecular imaging for prognostic radiology. J Cell Biochem Suppl. 2002;39:45–53. doi: 10.1002/jcb.10404. [DOI] [PubMed] [Google Scholar]
- 17.Jordan BF, Cron GO, Gallez B. Rapid monitoring of oxygenation by 19F magnetic resonance imaging: Simultaneous comparison with fluorescence quenching. Magn Reson Med. 2009;61(3):634–638. doi: 10.1002/mrm.21594. [DOI] [PubMed] [Google Scholar]
- 18.Kodibagkar VD, Wang X, Mason RP. Physical principles of quantitative nuclear magnetic resonance oximetry. Front Biosci. 2008;13:1371–1384. doi: 10.2741/2768. [DOI] [PubMed] [Google Scholar]
- 19.Mason RP, Antich PP, Babcock EE, Gerberich JL, Nunnally RL. Perfluorocarbon imaging in vivo: a 19F MRI study in tumor-bearing mice. Magn Reson Imaging. 1989;7(5):475–485. doi: 10.1016/0730-725x(89)90402-5. [DOI] [PubMed] [Google Scholar]
- 20.Hunjan S, Mason RP, Constantinescu A, Peschke P, Hahn EW, Antich PP. Regional tumor oximetry: 19F NMR spectroscopy of hexafluorobenzene. Int J Radiat Oncol Biol Phys. 1998;41(1):161–171. doi: 10.1016/s0360-3016(98)00020-0. [DOI] [PubMed] [Google Scholar]
- 21.Robinson SP, Griffiths JR. Current issues in the utility of 19F nuclear magnetic resonance methodologies for the assessment of tumour hypoxia. Philos Trans R Soc Lond B Biol Sci. 2004;359(1446):987–996. doi: 10.1098/rstb.2003.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mason RP, Rodbumrung W, Antich PP. Hexafluorobenzene: a sensitive 19F NMR indicator of tumor oxygenation. NMR Biomed. 1996;9(3):125–134. doi: 10.1002/(SICI)1099-1492(199605)9:3<125::AID-NBM405>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 23.Evans SM, Koch CJ. Prognostic significance of tumor oxygenation in humans. Cancer Lett. 2003;195(1):1–16. doi: 10.1016/s0304-3835(03)00012-0. [DOI] [PubMed] [Google Scholar]
- 24.Hopf HW, Rollins MD. Wounds: an overview of the role of oxygen. Antioxid Redox Signal. 2007;9(8):1183–1192. doi: 10.1089/ars.2007.1641. [DOI] [PubMed] [Google Scholar]
- 25.Kenmure AC, Murdoch WR, Hutton I, Cameron AJ. Hemodynamic effects of oxygen at 1 and 2 Ata pressure in healthy subjects. J Appl Physiol. 1972;32(2):223–226. doi: 10.1152/jappl.1972.32.2.223. [DOI] [PubMed] [Google Scholar]
- 26.Waring WS, Thomson AJ, Adwani SH, Rosseel AJ, Potter JF, Webb DJ, Maxwell SR. Cardiovascular effects of acute oxygen administration in healthy adults. J Cardiovasc Pharmacol. 2003;42(2):245–250. doi: 10.1097/00005344-200308000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Berry JM, Doursout MF, Butler BD. Effects of hyperbaric hyperoxia on cardiac and regional hemodynamics in conscious dogs. Aviat Space Environ Med. 1998;69(8):761–765. [PubMed] [Google Scholar]
- 28.Busing CM, von Gerstenbergk L, Dressler P, Rumm D, Wentz K. Experimental studies on microcirculation under normobaric hyperoxia using the microspheres method. Exp Pathol. 1981;19(3):146–153. doi: 10.1016/s0232-1513(81)80057-6. [DOI] [PubMed] [Google Scholar]
- 29.Matalon S, Nesarajah MS, Farhi LE. Pulmonary and circulatory changes in conscious sheep exposed to 100% O2 at 1 ATA. J Appl Physiol. 1982;53(1):110–116. doi: 10.1152/jappl.1982.53.1.110. [DOI] [PubMed] [Google Scholar]
- 30.Hunjan S, Zhao D, Constantinescu A, Hahn EW, Antich PP, Mason RP. Tumor oximetry: demonstration of an enhanced dynamic mapping procedure using fluorine-19 echo planar magnetic resonance imaging in the Dunning prostate R3327-AT1 rat tumor. Int J Radiat Oncol Biol Phys. 2001;49(4):1097–1108. doi: 10.1016/s0360-3016(00)01460-7. [DOI] [PubMed] [Google Scholar]
- 31.Perry RH, Green DW. Perry's chemical engineers' handbook. New York: McGraw-Hill; 2008. 1 v. (various pagings) p. [Google Scholar]
- 32.Barker BR, Mason RP, Bansal N, Peshock RM. Oxygen tension mapping with F-19 echo-planar MR imaging of sequestered perfluorocarbon. J Magn Reson Imaging. 1994;4(4):595–602. doi: 10.1002/jmri.1880040414. [DOI] [PubMed] [Google Scholar]
- 33.Mason RP, Shukla H, Antich PP. In vivo oxygen tension and temperature: simultaneous determination using 19F NMR spectroscopy of perfluorocarbon. Magn Reson Med. 1993;29(3):296–302. doi: 10.1002/mrm.1910290304. [DOI] [PubMed] [Google Scholar]
- 34.Hou H, Grinberg OY, Taie S, Leichtweis S, Miyake M, Grinberg S, Xie H, Csete M, Swartz HM. Electron paramagnetic resonance assessment of brain tissue oxygen tension in anesthetized rats. Anesth Analg. 2003;96(5):1467–1472. doi: 10.1213/01.ANE.0000055648.41152.63. table of contents. [DOI] [PubMed] [Google Scholar]
- 35.Liu KJ, Gast P, Moussavi M, Norby SW, Vahidi N, Walczak T, Wu M, Swartz HM. Lithium phthalocyanine: a probe for electron paramagnetic resonance oximetry in viable biological systems. Proc Natl Acad Sci U S A. 1993;90(12):5438–5442. doi: 10.1073/pnas.90.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas SR, Pratt RG, Millard RW, Samaratunga RC, Shiferaw Y, Clark LC, Jr, Hoffmann RE. Evaluation of the influence of the aqueous phase bioconstituent environment on the F-19 T1 of perfluorocarbon blood substitute emulsions. J Magn Reson Imaging. 1994;4(4):631–635. doi: 10.1002/jmri.1880040421. [DOI] [PubMed] [Google Scholar]
- 37.Glockner JF, Chan HC, Swartz HM. In vivo oximetry using a nitroxide-liposome system. Magn Reson Med. 1991;20(1):123–133. doi: 10.1002/mrm.1910200113. [DOI] [PubMed] [Google Scholar]
- 38.O'Hara JA, Khan N, Hou H, Wilmo CM, Demidenko E, Dunn JF, Swartz HM. Comparison of EPR oximetry and Eppendorf polarographic electrode assessments of rat brain PtO2. Physiol Meas. 2004;25(6):1413–1423. doi: 10.1088/0967-3334/25/6/007. [DOI] [PubMed] [Google Scholar]
- 39.Rink R, Kessler M. Signs of hypoxia in the small intestine of the rat during hemorrhagic shock. Adv Exp Med Biol. 1973;37A:469–475. doi: 10.1007/978-1-4684-3288-6_59. [DOI] [PubMed] [Google Scholar]
- 40.Shaw AD, Li Z, Thomas Z, Stevens CW. Assessment of tissue oxygen tension: comparison of dynamic fluorescence quenching and polarographic electrode technique. Crit Care. 2002;6(1):76–80. doi: 10.1186/cc1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brezis M, Heyman SN, Epstein FH. Determinants of intrarenal oxygenation. II. Hemodynamic effects. Am J Physiol. 1994;267(6 Pt 2):F1063–F1068. doi: 10.1152/ajprenal.1994.267.6.F1063. [DOI] [PubMed] [Google Scholar]
- 42.Sato N, Kamada T, Kawano S, Hayashi N, Kishida Y, Meren H, Yoshihara H, Abe H. Effect of acute and chronic ethanol consumption on hepatic tissue oxygen tension in rats. Pharmacol Biochem Behav. 1983;18 Suppl 1:443–447. doi: 10.1016/0091-3057(83)90215-0. [DOI] [PubMed] [Google Scholar]
- 43.Maas AI, Fleckenstein W, de Jong DA, van Santbrink H. Monitoring cerebral oxygenation: experimental studies and preliminary clinical results of continuous monitoring of cerebrospinal fluid and brain tissue oxygen tension. Acta Neurochir Suppl (Wien) 1993;59:50–57. doi: 10.1007/978-3-7091-9302-0_9. [DOI] [PubMed] [Google Scholar]
- 44.Hall LW, Jackson SR. Hexafluorobenzene. Further studies as an anaesthetic agent. Anaesthesia. 1973;28(2):155–159. doi: 10.1111/j.1365-2044.1973.tb00307.x. [DOI] [PubMed] [Google Scholar]