Abstract
Interferon-γ (IFN-γ) is a cytokine whose biological activity is conventionally associated with cytostatic/cytotoxic and antitumor mechanisms during cell-mediated adaptive immune response. It has been used clinically to treat a variety of malignancies, albeit with mixed results and side effects that can be severe. Despite ample evidence implicating a role for IFN-γ in tumor immune surveillance, there has been a steady flow of reports suggesting that it may also have pro-tumorigenic effects under certain circumstances. We propose that in fact IFN-γ treatment is a double-edged sword whose anti- and pro-tumorigenic activities are dependent on the cellular, microenvironmental, and/or molecular context. As such, inhibition of the IFN-γ/IFNγR pathway may prove to be a viable new therapeutic target for a subset of malignancies.
BACKGROUND
The canonical IFN-γ signaling pathway
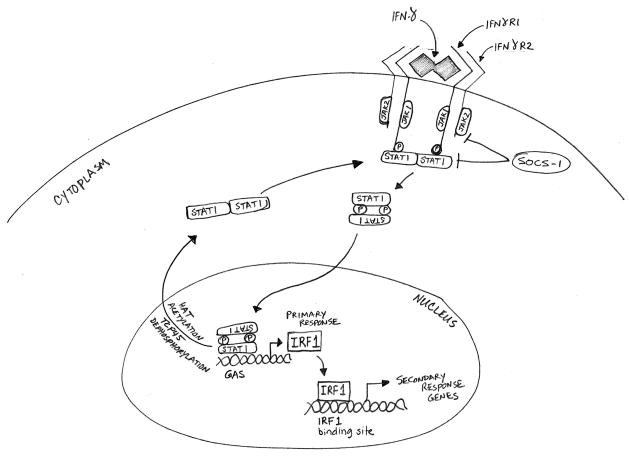
Interferons (IFNs) are a group of pleiotropic cytokines that play important roles in intercellular communication during innate and acquired immune responses and host defense against viral and bacterial infections, as well as tumor surveillance (1). IFNs are divided into two main categories in mammals – type I and type II – both of which differ substantially with respect to the relative potencies of their immunomodulatory and cell-surface molecular modification properties (2). The two major members of type I IFNs (IFN-α and IFN-β) are ubiquitously expressed and signal through the type I receptor. IFN-γ is the lone member of the type II IFN and is more restrictively expressed. It is structurally and functionally different from the type I IFNs and has its own receptor, consisting of IFNγR1 and IFNγR2 subunits (3–4). The biologically active form of IFN-γ is an antiparallel dimer that interacts with the extracellular domain of the receptor subunit IFNγR1 (3). Binding of the ligand engages the IFNγR2 subunit, which is responsible for the intracellular transmission of the signal. The intracellular carboxy termini of IFNγR1 and IFNγR2 carry the nonreceptor tyrosine kinases JAK1 and JAK2, respectively, which phosphorylate the receptor upon ligand binding (5–7). This phosphorylation creates binding sites for the signal transducer and activator of transcription (STAT) proteins, primarily STAT1 (4, 8–9). Phosphorylation leads to translocation of STAT1 homodimers into the nucleus, where they bind to GAS (gamma-activated sequence) sites on the promoters of downstream target genes. One of the major primary response genes transactivated by IFN-γ-activated JAK/STAT signaling is the transcription factor interferon response factor 1 (IRF1). IRF1 in turn activates a large number of secondary response genes (10). Figure 1 depicts a simplified canonical IFN-γ/JAK/STAT1 pathway. Details of interferon signaling pathways have been reviewed elsewhere (11).
Figure 1.
The canonical IFN-γ/JAK/STAT pathway. Binding of IFN-γ dimers to the extracellular domain of the IFNγR1 receptor subunit leads to engagement of the IFNγR2 subunit, which causes JAK1 and JAK2 to cross-phosphorylate each other and the receptor subunits. The parallel STAT1 homodimers are then recruited to the receptors, and their phosphorylation converts the homodimers into an antiparallel configuration. The reoriented STAT1 homodimers translocate to the nucleus, where they bind to gamma activated sequence (GAS) sites on the primary response genes including IRF1. IRF1 subsequently activates a large number of secondary response genes, which carry out a range of immunomodulatory functions. The SOCS proteins serve as the major negative regulators of the IFN-γ pathway by inhibiting the phosphorylation of JAKs and STAT1. Dephosphorylation and acetylation of STAT1 homodimers revert them to parallel configuration and causes their exit from the nucleus.
Regulatory features
IFN-γ/JAK/STAT signaling is regulated at several levels by positive and negative processes (Figure 1). The STAT1 homodimers exist in the cytoplasm in the inactive antiparallel configuration (12). Phosphorylation leads to a change into a parallel configuration, which exposes a nuclear localization signal leading to nuclear translocation and binding to the target GAS sequences (12–15). Intranuclear dephosphorylation by phosphatases such as TCP45 inactivates the STAT1 homodimer and causes its exodus from the nucleus into the cytoplasm (16). The activation of STAT1 is negatively regulated by lysine acetylation through histone acetyltransferases (e.g., CBP), but deacetylation by histone deacetylases (e.g., HDAC3) enhances activation (16–17). A major avenue of IFN-γ/JAK/STAT pathway regulation is the negative feedback inhibition by the suppressor of cytokine signaling (SOCS) molecules, which block the activity of JAKs (18–19). It has also been proposed that the transcriptional activity of STAT1 is enhanced by kinases such as MAPK, PKC and PI3K/AKT, which phosphorylate STAT1 in the transactivation domain (20). At the same time, these kinases themselves are activated through IFN-γ-induced STAT1-independent pathways. Although STAT1 is the primary transactivator immediately downstream of IFN-γ, there is evidence that under certain circumstances some STAT1-independent transactivators are also activated directly by IFN-γ-mediated signaling, including STAT3, STAT5, AP1 and NFκB (21–22).
TRANSLATIONAL ASPECTS
Diverse biological functions
Through the activation of a panoply of downstream effector molecules, IFN-γ signaling performs diverse biological functions, primarily related to host defense and immune regulation, including anti-viral and anti-bacterial defense, cell cycle, apoptosis, inflammation, and innate and acquired immunity (11). The most well-characterized function of IFN-γ is the upregulation of the major histocompatibility (MHC) Class I molecules to aid in the priming and presentation of antigens in the professional antigen presenting cells (23). IFN-γ regulates the differentiation and function of many types of immune cells. It is intimately involved in all aspects of Th1-mediated immune responses by regulating the differentiation, activation and homeostasis of T cells; it inhibits Th2 cell development, but promotes the development of regulatory T (Treg) cells (24). It also activates macrophages and induces production of chemokines, which recruit specific effector cells to the site of inflammation (25).
The profound immunomodulatory functions associated with IFN-γ quickly inspired clinical applications in a variety of disease conditions, including chronic granulomatous disease, fungal infections, autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease and lupus nephritis, as well as cancer (26). IFN-γ has long been associated with cytostatic/cytotoxic and anti-tumor functions (27). Fibrosarcoma cell lines refractory to IFN-γ signaling due to ectopic expression of dominant negative IFNγR1 were shown to grow better and resist rejection in syngeneic mice, suggesting that IFN-γ plays an important role in the detection and elimination of tumor cells (28). It was also suggested that IFN-γ takes part in tumor surveillance functions by enhancing tumor cell immunogenicity, as mice that were insensitive to IFN-γ (i.e., IFNγR−/− and Stat1−/− mice) exhibited enhanced methylcolanthrene-induced tumor growth (29). This was supported by the finding that approximately one-third of melanoma and lung adenocarcinoma cell lines had inactivating mutations in the IFN-γ pathway components (29), which raised the possibility that tumor insensitivity to IFN-γ may be a mechanism used by cancers to evade tumor surveillance. The effects of IFN-γ were shown to involve upregulation of MHC Class I genes, which increase tumor immunogenicity (30). This avenue of tumor surveillance was determined to involve recognition and elimination of tumor cells by cytotoxic T lymphocytes (CTLs) recruited to the tumor mass via IFN-γ-induced chemokine signaling (31–32).
Recombinant IFN-γ was shown to be involved in anti-proliferative (33–35), anti-angiogenic (36–38) and pro-apoptotic (39) effects against cancer cells. It was first clinically used to treat chronic myelogenous leukemia, alone and in combination with recombinant IFN-α, but failed to show any significant positive outcome (40–41). Since then, IFN-γ has been used in the clinical management of a variety of malignancies, including bladder carcinoma, colorectal cancer, ovarian cancer, and adult T cell leukemia; however, the results have been mixed (reviewed in (26) ).
The first demonstration of the anti-proliferative effects of IFN-γ in melanoma cells was reported by Fisher et al. (42). Subsequently, Brown et al. identified IFN-γ as one of the growth inhibitory factors present in conditioned media of activated T cells (27). Kortylewski et al. reported that IFN-γ had significant growth inhibitory activity on four different human melanoma cell lines, although the extent of growth inhibition was inconsistent (43). The growth inhibition was dependent on STAT1 activation. Curiously, however, although STAT1 was activated by a low concentration of IFN-γ, the growth inhibition was only evident at a much higher concentration, indicating the presence of complex and even divergent signals emanating from the IFN-γ/STAT1 axis. Further support of this notion comes from a study demonstrating that IFN-γ upregulates c-jun and c-myc in a Stat1-independent manner (44).
The dark side of IFN-γ
At about the same time that IFN-γ was being touted as a promising anti-tumor agent, the opposite was being reported as well. Taniguchi et al. showed that IFN-γ was a much more potent enhancer of lung colonization of intravenously inoculated B16 melanoma cells than either IFN-α or IFN-β (45). Low-dose IFN-γ treatment of B16 cells enhanced resistance to NK cells and was accompanied by upregulated expression of MHC Class I molecules H-2Kb and H-2Db. Human lymphocytes expressing low levels of IFNGR2 showed anti-apoptotic and proliferative responses to IFN-γ, while those expressing high IFNGR2 levels demonstrated a pro-apoptotic phenotype (46). Intratumoral expression of IFN-γ was shown to be associated with expression of MHC Class II molecules and a more aggressive phenotype in human melanomas (47). Garbe et al. reported that treatment of human melanoma cells in culture induced characteristics of a biologically aggressive phenotype (48). Gorbacheva et al. showed that IFN-γ accelerated the proliferation of NIH-3T3 cells by upregulating guanylate-binding protein 2 (GBP2) (49). Autocrine IFN-γ signaling was shown to enhance experimental metastatic ability of IFN-γ gene-transfected TS/A mammary adenocarcinoma cells, and was attributed to increased resistance to NK cells (50).
Despite these early indications, IFN-γ was taken into clinical trials for melanoma. Early small-scale clinical trials were largely inconclusive (51–54). However, due to moderate success of recombinant IFN-α in melanoma clinical trials, IFN-γ was further tested in relatively larger studies. Schiller et al. reported a phase II/III clinical trial of IFN-γ for good prognosis melanoma patients (55). This study failed to detect any efficacious effects of IFN-γ, as the response rate was only 5%, with significant side effects. Importantly, suppression of helper T cells was observed (55). Yet another melanoma trial for adjuvant application of IFN-γ had to be prematurely terminated as the IFN-γ-treated patients fared worse than the untreated population (56–57).
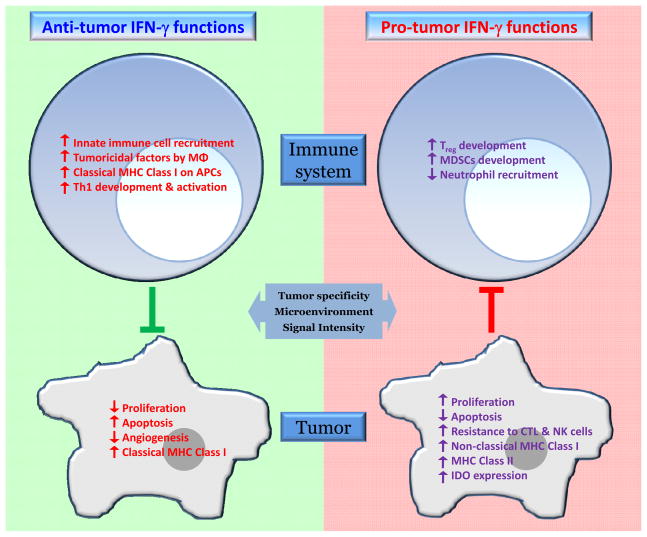
These failed attempts to treat melanoma with recombinant IFN-γ, combined with the occasional but conspicuous reports of its pro-growth activities, raises the possibility that IFN-γ has, in fact, two faces; it can have cytostatic/cytotoxic as well as cytoproliferative effects depending on the context (Figure 2). Such a scenario is not a new concept. Transforming growth factor (TGF)-β and tumor necrosis factor (TNF) are well-known examples of secreted factors that display this kind of dual contrasting behavior (58–59).
Figure 2.
The two faces of IFN-γ. IFN-γ exhibits both anti-tumor and pro-tumor activities. Under both scenarios, IFN-γ influences the tumor cells directly as well as the development, recruitment and/or activation of immune response cells. The anti-tumor effects result in direct inhibition of tumor cell growth, and recognition and elimination of the tumor cells by the immune response cells. On the other hand, the pro-tumor functions of IFN-γ involve proliferative and anti-apoptotic signals, as well as escape of the tumor cells from recognition and cytolysis by CTLs and NK cells. Which face is ultimately displayed may depend on the contexts of tumor specificity, microenvironmental factors, and signaling intensity.
Possible mechanisms underlying pro-tumorigenic IFN-γ
For the last three decades IFN-γ has established a reputation for being an immunological guardian against neoplastic disease. Schreiber and colleagues have implicated IFN-γ as a central player in their “immunoediting” model of the war between the immune defense systems of the host (tumor surveillance) and the oncogenic machinery of the tumor bent on escape (60–61). Their model suggests that while most oncogenic cells are recognized and eliminated by the immune system, some evolve strategies to survive and live in a dormant state where equilibrium with the immune system is achieved. Further accumulation of capabilities (mutations) may push the tumor to the stage of complete evasion of the immune system, leading to overt disease.
Several lines of evidence place IFN-γ at the elimination stage of the immunoediting paradigm (62). However, there is now emerging evidence that IFN-γ may also be involved at the equilibrium and/or evasion stages, roles that may be more pro-tumorigenic. If so, under what conditions and by which mechanisms might IFN-γ behave as a “bad guy”? One key may lie in the homeostatic functions of IFN-γ. While the well-known primary function of IFN-γ is to enhance the inflammatory response, it also plays a crucial role in limiting the destruction of tissues in the aftermath of inflammation. The IFN-γ-induced inflammatory cascade summons a variety of immune-related cell types such as macrophages, NK cells and CTLs that play a central role in tissue repair and remodeling at the site of inflammation. We propose that the actions of IFN-γ can help protect normal cells from the collateral damage associated with tissue remodeling and repair; however, these same mechanisms may allow cells harboring oncogenic mutations to evade destruction, and exist in a state of equilibrium until they become more fully transformed. This concept agrees with the model of tumor immune privilege put forth by Mellor and Munn, in which localized inflammation may lead to an immunosuppressive and tolerogenic tumor microenvironment (63).
Suppression of CTL- and NK cell-mediated immune responses is central to tumor immune escape, and a number of studies have indicated that IFN-γ may be intimately involved in these immunosuppressive mechanisms. It has been shown that IFN-γ upregulates the development of Treg and suppresses CTLs by inducing the expression of indoleamine 2,3-dioxygenase (IDO) in melanoma cells (64–66). IFN-γ attenuates infiltration of neutrophils and myeloid cells into the tissue microenvironments (67–68). It activates constitutive expression of CIITA in melanoma leading to upregulation of MHC Class II antigens, which are associated with malignant progression and resistance to Fas-L+ T-cell-mediated apoptosis (69–71). Two separate studies have shown that incubation of IGR39D, FO-1, and MELA melanoma cell lines with IFN-γ decreases NK cell-mediated cytolysis, with or without activation of MHC Class I antigens (72-73). Using the CT26 colon carcinoma tumor model, Beatty et al. showed that IFN-γ enhanced the expression of MHC Class I molecules, which led to reduced tumor recognition and CTL-mediated lysis (74). Morel et al. reported that melanoma cell lines treated with IFN-γ lost Melan-A and gp100 tumor antigen processing, enabling the tumor cells to evade CTL recognition (75).
The presence of monocytic and granulocytic myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment, both of which are dependent on IFN-γ, cause suppression of T cell response (76). Non-classical MHC Class I molecules (e.g., HLA-G and HLA-E) are IFN-γ-regulated genes that are implicated in resistance to CTL and NK cell responses, and in immune escape in a variety of cancers (77–78). Recently, Cho et al. attributed the clinical failure of melanoma peptide vaccines to IFN-γ-driven expression of non-classical MHC Class I molecules, which enable melanoma cells to evade CTL-mediated cytolysis (79).
We ourselves have provided evidence that a substantial proportion of human melanomas harbor IFN-γ-producing macrophages, consistent with the proposed role for infiltrating macrophages in the IFN-γ-driven pro-tumorigenic microenvironment created in UVB-irradiated skin (80-81). Although strong data implicate lymphocytic infiltration in primary melanoma as a favorable prognostic marker (82–83), little is known about the prognostic significance of macrophage infiltration. The prospect of validating IFN-γ+ macrophages as a new and simple cellular marker of poor prognosis deserves further investigation. It is also noteworthy that the presence of IFN-γ in serum has already been implicated as an independent prognostic indicator for disease recurrence in melanoma patients (84).
In conclusion, recent advances have provided evidence for the existence of a dark side of IFN-γ. IFN-γ appears capable of driving novel cellular and molecular inflammatory mechanisms that may underlie tumor initiation, immunoevasion, survival and/or outgrowth. Which side wins the tussle between the anti- and pro-tumorigenic functions of IFN-γ seems to be dependent on the contexts of tumor specificity, microenvironmental factors, and signaling intensity (Figure 2). Despite the frequent, albeit typically ineffective, use of high dose type I interferons as conventional chemotherapy (85), we believe there is now a case to be made for exploring a paradigm-shifting strategy in which IFN-γ/IFNγR and/or downstream pathway members become viable therapeutic targets for at least a subset of melanomas, and perhaps other cancers as well.
Acknowledgments
We thank Howard Young for many helpful comments on the manuscript. We have attempted to be as comprehensive as space limitations would allow. We apologize to those authors whose work we did not cite, even though their work may have been relevant to the topic discussed in this article. This work was supported in part by the Intramural Research Program of the NIH, NCI, Center for Cancer Research.
References
- 1.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004 Dec;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 2.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010 Sep 27;207(10):2053–63. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ealick SE, Cook WJ, Vijay-Kumar S, Carson M, Nagabhushan TL, Trotta PP, et al. Three-dimensional structure of recombinant human interferon-gamma. Science. 1991 May 3;252(5006):698–702. doi: 10.1126/science.1902591. [DOI] [PubMed] [Google Scholar]
- 4.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005 May 5;(5):375–86. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 5.Haan C, Kreis S, Margue C, Behrmann I. Jaks and cytokine receptors--an intimate relationship. Biochem Pharmacol. 2006 Nov 30;72(11):1538–46. doi: 10.1016/j.bcp.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998 May 1;93(3):373–83. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 7.Yeh TC, Pellegrini S. The Janus kinase family of protein tyrosine kinases and their role in signaling. Cell Mol Life Sci. 1999 Sep 55;(12):1523–34. doi: 10.1007/s000180050392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varinou L, Ramsauer K, Karaghiosoff M, Kolbe T, Pfeffer K, Muller M, et al. Phosphorylation of the Stat1 transactivation domain is required for full-fledged IFN-gamma-dependent innate immunity. Immunity. 2003 Dec 19;(6):793–802. doi: 10.1016/s1074-7613(03)00322-4. [DOI] [PubMed] [Google Scholar]
- 9.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995 Jul 28;82(2):241–50. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 10.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 11.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004 Feb;75(2):163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 12.Zhong M, Henriksen MA, Takeuchi K, Schaefer O, Liu B, ten Hoeve J, et al. Implications of an antiparallel dimeric structure of nonphosphorylated STAT1 for the activation-inactivation cycle. Proc Natl Acad Sci U S A. 2005 Mar 15;102(11):3966–71. doi: 10.1073/pnas.0501063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao X, Ren Z, Parker GN, Sondermann H, Pastorello MA, Wang W, et al. Structural bases of unphosphorylated STAT1 association and receptor binding. Mol Cell. 2005 Mar 18;17(6):761–71. doi: 10.1016/j.molcel.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Mertens C, Zhong M, Krishnaraj R, Zou W, Chen X, Darnell JE., Jr Dephosphorylation of phosphotyrosine on STAT1 dimers requires extensive spatial reorientation of the monomers facilitated by the N-terminal domain. Genes Dev. 2006 Dec 15;20(24):3372–81. doi: 10.1101/gad.1485406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wenta N, Strauss H, Meyer S, Vinkemeier U. Tyrosine phosphorylation regulates the partitioning of STAT1 between different dimer conformations. Proc Natl Acad Sci U S A. 2008 Jul 8;105(27):9238–43. doi: 10.1073/pnas.0802130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer OH, Knauer SK, Greiner G, Jandt E, Reichardt S, Guhrs KH, et al. A phosphorylation-acetylation switch regulates STAT1 signaling. Genes Dev. 2009 Jan 15;23(2):223–35. doi: 10.1101/gad.479209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer OH, Baus D, Knauer SK, Stein S, Jager E, Stauber RH, et al. Acetylation of Stat1 modulates NF-kappaB activity. Genes Dev. 2006 Feb 15;20(4):473–85. doi: 10.1101/gad.364306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, et al. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999 Sep 3;98(5):597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 19.Marine JC, Topham DJ, McKay C, Wang D, Parganas E, Stravopodis D, et al. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell. 1999 Sep 3;98(5):609–16. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 20.Choudhury GG. A linear signal transduction pathway involving phosphatidylinositol 3-kinase, protein kinase Cepsilon, and MAPK in mesangial cells regulates interferon-gamma-induced STAT1alpha transcriptional activation. J Biol Chem. 2004 Jun 25;279(26):27399–409. doi: 10.1074/jbc.M403530200. [DOI] [PubMed] [Google Scholar]
- 21.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009 Oct 16;31(4):539–50. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramana CV, Gil MP, Schreiber RD, Stark GR. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 2002 Feb;23(2):96–101. doi: 10.1016/s1471-4906(01)02118-4. [DOI] [PubMed] [Google Scholar]
- 23.Seliger B, Ruiz-Cabello F, Garrido F. IFN inducibility of major histocompatibility antigens in tumors. Adv Cancer Res. 2008;101:249–76. doi: 10.1016/S0065-230X(08)00407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O'Shea JJ, et al. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol. 2003 May;23(3):147–61. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 25.Hu X, Chakravarty SD, Ivashkiv LB. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol Rev. 2008 Dec;226:41–56. doi: 10.1111/j.1600-065X.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller CH, Maher SG, Young HA. Clinical Use of Interferon-gamma. Ann N Y Acad Sci. 2009 Dec;1182:69–79. doi: 10.1111/j.1749-6632.2009.05069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown TJ, Lioubin MN, Marquardt H. Purification and characterization of cytostatic lymphokines produced by activated human T lymphocytes. Synergistic antiproliferative activity of transforming growth factor beta 1, interferon-gamma, and oncostatin M for human melanoma cells. J Immunol. 1987 Nov 1;139(9):2977–83. [PubMed] [Google Scholar]
- 28.Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994 Sep;1(6):447–56. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998 Jun 23;95(13):7556–61. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber JS, Rosenberg SA. Modulation of murine tumor major histocompatibility antigens by cytokines in vivo and in vitro. Cancer Res. 1988 Oct 15;48(20):5818–24. [PubMed] [Google Scholar]
- 31.Kunz M, Toksoy A, Goebeler M, Engelhardt E, Brocker E, Gillitzer R. Strong expression of the lymphoattractant C-X-C chemokine Mig is associated with heavy infiltration of T cells in human malignant melanoma. J Pathol. 1999 Dec;189(4):552–8. doi: 10.1002/(SICI)1096-9896(199912)189:4<552::AID-PATH469>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 32.Mukai S, Kjaergaard J, Shu S, Plautz GE. Infiltration of tumors by systemically transferred tumor-reactive T lymphocytes is required for antitumor efficacy. Cancer Res. 1999 Oct 15;59(20):5245–9. [PubMed] [Google Scholar]
- 33.Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science. 1996 May 3;272(5262):719–22. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- 34.Hobeika AC, Etienne W, Torres BA, Johnson HM, Subramaniam PS. IFN-gamma induction of p21(WAF1) is required for cell cycle inhibition and suppression of apoptosis. J Interferon Cytokine Res. 1999 Dec;19(12):1351–61. doi: 10.1089/107999099312812. [DOI] [PubMed] [Google Scholar]
- 35.Platanias LC, Uddin S, Bruno E, Korkmaz M, Ahmad S, Alsayed Y, et al. CrkL and CrkII participate in the generation of the growth inhibitory effects of interferons on primary hematopoietic progenitors. Exp Hematol. 1999 Aug;27(8):1315–21. doi: 10.1016/s0301-472x(99)00060-0. [DOI] [PubMed] [Google Scholar]
- 36.Beatty G, Paterson Y. IFN-gamma-dependent inhibition of tumor angiogenesis by tumor-infiltrating CD4+ T cells requires tumor responsiveness to IFN-gamma. J Immunol. 2001 Feb 15;166(4):2276–82. doi: 10.4049/jimmunol.166.4.2276. [DOI] [PubMed] [Google Scholar]
- 37.Coughlin CM, Salhany KE, Gee MS, LaTemple DC, Kotenko S, Ma X, et al. Tumor cell responses to IFNgamma affect tumorigenicity and response to IL-12 therapy and antiangiogenesis. Immunity. 1998 Jul;9(1):25–34. doi: 10.1016/s1074-7613(00)80585-3. [DOI] [PubMed] [Google Scholar]
- 38.Ruegg C, Yilmaz A, Bieler G, Bamat J, Chaubert P, Lejeune FJ. Evidence for the involvement of endothelial cell integrin alphaVbeta3 in the disruption of the tumor vasculature induced by TNF and IFN-gamma. Nat Med. 1998 Apr;4(4):408–14. doi: 10.1038/nm0498-408. [DOI] [PubMed] [Google Scholar]
- 39.Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, et al. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003 Jun;8(3):237–49. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- 40.Kloke O, Wandl U, Opalka B, Moritz T, Nagel-Hiemke M, Franz T, et al. A prospective randomized comparison of single-agent interferon (IFN)-alpha with the combination of IFN-alpha and low-dose IFN-gamma in chronic myelogenous leukaemia. Eur J Haematol. 1992 Feb;48(2):93–8. doi: 10.1111/j.1600-0609.1992.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 41.Kurzrock R, Talpaz M, Kantarjian H, Walters R, Saks S, Trujillo JM, et al. Therapy of chronic myelogenous leukemia with recombinant interferon-gamma. Blood. 1987 Oct;70(4):943–7. [PubMed] [Google Scholar]
- 42.Fisher PB, Miranda AF, Babiss LE. Measurement of the effect of interferons on cellular differentiation in murine and human melanoma cells. Methods Enzymol. 1986;119:611–8. doi: 10.1016/0076-6879(86)19082-3. [DOI] [PubMed] [Google Scholar]
- 43.Kortylewski M, Komyod W, Kauffmann ME, Bosserhoff A, Heinrich PC, Behrmann I. Interferon-gamma-mediated growth regulation of melanoma cells: involvement of STAT1-dependent and STAT1-independent signals. J Invest Dermatol. 2004 Feb;122(2):414–22. doi: 10.1046/j.0022-202X.2004.22237.x. [DOI] [PubMed] [Google Scholar]
- 44.Ramana CV, Gil MP, Han Y, Ransohoff RM, Schreiber RD, Stark GR. Stat1-independent regulation of gene expression in response to IFN-gamma. Proc Natl Acad Sci U S A. 2001 Jun 5;98(12):6674–9. doi: 10.1073/pnas.111164198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taniguchi K, Petersson M, Hoglund P, Kiessling R, Klein G, Karre K. Interferon gamma induces lung colonization by intravenously inoculated B16 melanoma cells in parallel with enhanced expression of class I major histocompatibility complex antigens. Proc Natl Acad Sci U S A. 1987 May;84(10):3405–9. doi: 10.1073/pnas.84.10.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernabei P, Coccia EM, Rigamonti L, Bosticardo M, Forni G, Pestka S, et al. Interferon-gamma receptor 2 expression as the deciding factor in human T, B, and myeloid cell proliferation or death. J Leukoc Biol. 2001 Dec;70(6):950–60. [PubMed] [Google Scholar]
- 47.Brocker EB, Zwadlo G, Holzmann B, Macher E, Sorg C. Inflammatory cell infiltrates in human melanoma at different stages of tumor progression. Int J Cancer. 1988 Apr 15;41(4):562–7. doi: 10.1002/ijc.2910410415. [DOI] [PubMed] [Google Scholar]
- 48.Garbe C, Krasagakis K, Zouboulis CC, Schroder K, Kruger S, Stadler R, et al. Antitumor activities of interferon alpha, beta, and gamma and their combinations on human melanoma cells in vitro: changes of proliferation, melanin synthesis, and immunophenotype. J Invest Dermatol. 1990 Dec;95(6 Suppl):231S–7S. doi: 10.1111/1523-1747.ep12875837. [DOI] [PubMed] [Google Scholar]
- 49.Gorbacheva VY, Lindner D, Sen GC, Vestal DJ. The interferon (IFN)-induced GTPase, mGBP-2. Role in IFN-gamma-induced murine fibroblast proliferation. J Biol Chem. 2002 Feb 22;277(8):6080–7. doi: 10.1074/jbc.M110542200. [DOI] [PubMed] [Google Scholar]
- 50.Lollini PL, Bosco MC, Cavallo F, De Giovanni C, Giovarelli M, Landuzzi L, et al. Inhibition of tumor growth and enhancement of metastasis after transfection of the gamma-interferon gene. Int J Cancer. 1993 Sep 9;55(2):320–9. doi: 10.1002/ijc.2910550224. [DOI] [PubMed] [Google Scholar]
- 51.Creagan ET, Ahmann DL, Long HJ, Frytak S, Sherwin SA, Chang MN. Phase II study of recombinant interferon-gamma in patients with disseminated malignant melanoma. Cancer Treat Rep. 1987 Sep;71(9):843–4. [PubMed] [Google Scholar]
- 52.Ernstoff MS, Trautman T, Davis CA, Reich SD, Witman P, Balser J, et al. A randomized phase I/II study of continuous versus intermittent intravenous interferon gamma in patients with metastatic melanoma. J Clin Oncol. 1987 Nov;5(11):1804–10. doi: 10.1200/JCO.1987.5.11.1804. [DOI] [PubMed] [Google Scholar]
- 53.Kopp WC, Smith JW, 2nd, Ewel CH, Alvord WG, Main C, Guyre PM, et al. Immunomodulatory effects of interferon-gamma in patients with metastatic malignant melanoma. J Immunother Emphasis Tumor Immunol. 1993 Apr;13(3):181–90. doi: 10.1097/00002371-199304000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Kowalzick L, Weyer U, Lange P, Breitbart EW. Systemic therapy of advanced metastatic malignant melanoma with a combination of fibroblast interferon-beta and recombinant interferon-gamma. Dermatologica. 1990;181(4):298–303. doi: 10.1159/000247830. [DOI] [PubMed] [Google Scholar]
- 55.Schiller JH, Pugh M, Kirkwood JM, Karp D, Larson M, Borden E. Eastern cooperative group trial of interferon gamma in metastatic melanoma: an innovative study design. Clin Cancer Res. 1996 Jan;2(1):29–36. [PubMed] [Google Scholar]
- 56.Meyskens FL, Jr, Kopecky K, Samson M, Hersh E, Macdonald J, Jaffe H, et al. Recombinant human interferon gamma: adverse effects in high-risk stage I and II cutaneous malignant melanoma. J Natl Cancer Inst. 1990 Jun 20;82(12):1071. doi: 10.1093/jnci/82.12.1071-a. [DOI] [PubMed] [Google Scholar]
- 57.Meyskens FL, Jr, Kopecky KJ, Taylor CW, Noyes RD, Tuthill RJ, Hersh EM, et al. Randomized trial of adjuvant human interferon gamma versus observation in high-risk cutaneous melanoma: a Southwest Oncology Group study. J Natl Cancer Inst. 1995 Nov 15;87(22):1710–3. doi: 10.1093/jnci/87.22.1710. [DOI] [PubMed] [Google Scholar]
- 58.Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A. 2003 Jul 22;100(15):8621–3. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wajant H. The role of TNF in cancer. Results Probl Cell Differ. 2009;49:1–15. doi: 10.1007/400_2008_26. [DOI] [PubMed] [Google Scholar]
- 60.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 61.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011 Mar 25;331(6024):1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 62.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006 Nov;6(11):836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 63.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008 Jan;8(1):74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 64.Brody JR, Costantino CL, Berger AC, Sato T, Lisanti MP, Yeo CJ, et al. Expression of indoleamine 2,3-dioxygenase in metastatic malignant melanoma recruits regulatory T cells to avoid immune detection and affects survival. Cell Cycle. 2009 Jun 15;8(12):1930–4. doi: 10.4161/cc.8.12.8745. [DOI] [PubMed] [Google Scholar]
- 65.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008 Apr;222:206–21. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 66.Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008 Jun 26;27(28):3889–900. doi: 10.1038/onc.2008.35. [DOI] [PubMed] [Google Scholar]
- 67.Irmler IM, Gajda M, Brauer R. Exacerbation of antigen-induced arthritis in IFN-gamma-deficient mice as a result of unrestricted IL-17 response. J Immunol. 2007 Nov 1;179(9):6228–36. doi: 10.4049/jimmunol.179.9.6228. [DOI] [PubMed] [Google Scholar]
- 68.Manoury-Schwartz B, Chiocchia G, Bessis N, Abehsira-Amar O, Batteux F, Muller S, et al. High susceptibility to collagen-induced arthritis in mice lacking IFN-gamma receptors. J Immunol. 1997 Jun 1;158(11):5501–6. [PubMed] [Google Scholar]
- 69.Herlyn M, Guerry D, Koprowski H. Recombinant gamma-interferon induces changes in expression and shedding of antigens associated with normal human melanocytes, nevus cells, and primary and metastatic melanoma cells. J Immunol. 1985 Jun;134(6):4226–30. [PubMed] [Google Scholar]
- 70.Tsujisaki M, Igarashi M, Sakaguchi K, Eisinger M, Herlyn M, Ferrone S. Immunochemical and functional analysis of HLA class II antigens induced by recombinant immune interferon on normal epidermal melanocytes. J Immunol. 1987 Feb 15;138(4):1310–6. [PubMed] [Google Scholar]
- 71.Hemon P, Jean-Louis F, Ramgolam K, Brignone C, Viguier M, Bachelez H, et al. MHC Class II Engagement by Its Ligand LAG-3 (CD223) Contributes to Melanoma Resistance to Apoptosis. J Immunol. 2011 May 1;186(9):5173–83. doi: 10.4049/jimmunol.1002050. [DOI] [PubMed] [Google Scholar]
- 72.Maio M, Altomonte M, Tatake R, Zeff RA, Ferrone S. Reduction in susceptibility to natural killer cell-mediated lysis of human FO-1 melanoma cells after induction of HLA class I antigen expression by transfection with B2m gene. J Clin Invest. 1991 Jul;88(1):282–9. doi: 10.1172/JCI115289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pena J, Alonso C, Solana R, Serrano R, Carracedo J, Ramirez R. Natural killer susceptibility is independent of HLA class I antigen expression on cell lines obtained from human solid tumors. Eur J Immunol. 1990 Nov;20(11):2445–8. doi: 10.1002/eji.1830201113. [DOI] [PubMed] [Google Scholar]
- 74.Beatty GL, Paterson Y. IFN-gamma can promote tumor evasion of the immune system in vivo by down-regulating cellular levels of an endogenous tumor antigen. J Immunol. 2000 Nov 15;165(10):5502–8. doi: 10.4049/jimmunol.165.10.5502. [DOI] [PubMed] [Google Scholar]
- 75.Morel S, Levy F, Burlet-Schiltz O, Brasseur F, Probst-Kepper M, Peitrequin AL, et al. Processing of some antigens by the standard proteasome but not by the immunoproteasome results in poor presentation by dendritic cells. Immunity. 2000 Jan;12(1):107–17. doi: 10.1016/s1074-7613(00)80163-6. [DOI] [PubMed] [Google Scholar]
- 76.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009 Apr 15;182(8):4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wischhusen J, Waschbisch A, Wiendl H. Immune-refractory cancers and their little helpers--an extended role for immunetolerogenic MHC molecules HLA-G and HLA-E? Semin Cancer Biol. 2007 Dec;17(6):459–68. doi: 10.1016/j.semcancer.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 78.Gobin SJ, van den Elsen PJ. Transcriptional regulation of the MHC class Ib genes HLA-E, HLA-F, and HLA-G. Hum Immunol. 2000 Nov;61(11):1102–7. doi: 10.1016/s0198-8859(00)00198-1. [DOI] [PubMed] [Google Scholar]
- 79.Cho HI, Lee YR, Celis E. Interferon gamma limits the effectiveness of melanoma peptide vaccines. Blood. 2011 Jan 6;117(1):135–44. doi: 10.1182/blood-2010-08-298117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zaidi MR, Davis S, Noonan FP, Graff-Cherry C, Hawley TS, Walker RL, et al. Interferon-gamma links ultraviolet radiation to melanomagenesis in mice. Nature. 2011 Jan 27;469(7331):548–53. doi: 10.1038/nature09666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009 Aug 4;16(2):91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oble DA, Loewe R, Yu P, Mihm MC., Jr Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human melanoma. Cancer Immun. 2009;9:3. [PMC free article] [PubMed] [Google Scholar]
- 83.Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. 2007 Mar 1;25(7):869–75. doi: 10.1200/JCO.2006.08.9755. [DOI] [PubMed] [Google Scholar]
- 84.Porter GA, Abdalla J, Lu M, Smith S, Montgomery D, Grimm E, et al. Significance of plasma cytokine levels in melanoma patients with histologically negative sentinel lymph nodes. Ann Surg Oncol. 2001 Mar;8(2):116–22. doi: 10.1007/s10434-001-0116-3. [DOI] [PubMed] [Google Scholar]
- 85.Ascierto PA, Kirkwood JM. Adjuvant therapy of melanoma with interferon: lessons of the past decade. J Transl Med. 2008;6:62. doi: 10.1186/1479-5876-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]