Abstract
Background
Drug-associated cues can elicit stress-like responses in addicted individuals, indicating thatcue- and stress-induceddrug relapse may share some neural mechanisms.It is unknown whetherα2 adrenergic receptor agonists, which are known toattenuate stress-induced reinstatement of drug-seeking in rats,also reduce cue-induced reinstatement.
Methods
Rats were tested for reinstatement of drug-seekingfollowing cocaine self-administration and extinction. We first evaluated the effects ofclonidine, an agonist at α2 and imidazoline-1 (I1) receptors, on relapse to cocaine-seeking. To explore possible mechanisms of clonidine’s effects, we then tested more specific α2 or I1 agonists, post-synaptic adrenergic receptor (α1 and β) antagonists, andcorticotropin-releasing factor receptor-1 (CRF R1) antagonists.
Results
We found that clonidine,andthe more selective α2 agonists UK-14,304 and guanfacine, decreased cue-induced reinstatement of cocaine-seeking.The specific I1 receptor agonist moxonidine reduced cue-induced as well as cocaine-induced reinstatement.Clonidine or moxonidine effects on cue-induced reinstatementwere reversed by the selective α2 receptor antagonist RS-79948, indicatinga role for α2 receptors.Prazosin and propranolol, antagonists at the α1and β receptor, respectively, reducedcue-induced reinstatement only when administered in combination. Finally, the CRF R1 antagonist CP-154,526reduced cue-induced reinstatement, as previouslyobservedfor stress-induced reinstatement, indicating possible overlap between stress and cue mechanisms.
Conclusions
These results indicate that α2 and I1 receptor agonists are novel therapeutic options for prevention of cue-induced cocaine relapse. Given that α2 receptor stimulation is associated with sedation in humans, the I1agonist moxonidineseems to have substantial potential for treating addictive disorders.
Keywords: cocaine, self-administration, relapse, norepinephrine, imidazoline, corticotropin-releasing factor
Introduction
Prevention of relapseisa primary goal of addiction recovery. Understanding the neural mechanisms involved in relapse facilitates rationale development of new therapeutics to treat addictive disorders. Animal modelsof relapse revealeda role for the central noradrenergic (NA)and corticotropin-releasing factor (CRF) systemsinstress-induced relapse (reviewed in 1, 2-3). Administration of α2adrenergic agonistsor CRF receptor-1 (R1) antagonists attenuatedstress-induced reinstatement of extinguished drug-seeking for cocaine, heroin, ethanol, and nicotine in rats(4-11). Further, lesioningthe ventral noradrenergic fiber bundle blocked stress-induced reinstatement of heroin-seeking (6). However, it is unknown whether adrenergicsignaling alsoplays a role in relapse triggered by drug-associated cues, and to what degree the neural mechanisms of cue- and stress-induced reinstatement may overlap.
Human studies indicate that cues and stress may share common neural mechanisms for provoking drug craving. Cocaine-dependent individuals exhibited increased drug craving, anxiety, and activation of the hypothalamic-pituitary-adrenal (HPA) axis in response to both drug-related stimuli and stress-related imagery(12-13). Similar HPA axis activationwas seen in rats following cue- or stress-induced reinstatement of cocaine-seeking (14-15). Additionally, stress- and cue-induced craving were reduced in individuals dependent upon opioids or cocaine following treatment with an α2 agonist, supporting a role of adrenergic signaling in both processes(16-17).
Here, we tested a role for NA signaling in cue-induced reinstatement of cocaine-seekingusingtheα2 agonist clonidine, as well as α1 orβreceptor antagonists, to determine the contributions of pre- and post-synaptic adrenergic receptors.Given that clonidine acts at both α2 and imidazoline-1 (I1) receptors (18), we also administered agonists with varying affinities for α2 and I1receptors. I1 receptors are implicated in the central regulation of blood pressure and are the primary target of second-generation antihypertensive agents such as moxonidine and rilmenidine, which lack clonidine-like sedation due to their low affinity for α2 receptors (19-21).In addition to reducing hypertension, I1 receptor agonists may have the advantage of reducing conditions associated with metabolic syndrome X, including insulin resistance and glucose intolerance(21-25).A potential role for I1 receptor signaling in addiction is supported by recent findings that I1 agonists reduced opiate and ethanol withdrawal effects in rats (26-29). However, the possible useof I1 receptor agonists as anti-relapse therapeutics has been less explored. Studies presented here reveal that stimulation ofα2 and I1receptors prevents relapse of cocaine-seeking, indicating new pharmacologic approaches foraddiction treatment.
Methods and Materials
Animals
Male Sprague Dawley rats (initial weight 250-300 g; Charles River, Raleigh, NC) were single- or pair-housed in a temperature- and humidity-controlled, AAALAC-accredited animal facility at MUSC. Rats were housed under a reversed 12-hr light/dark cycle (lights off at 6 a.m.), with ad libitum food and water (except for food self-administration study, described below). All experiments were approved by the Institutional Animal Care and Use Committee at MUSC and conducted according to specifications of the National Institutes of Health as outlined in the Guide for the Care and Use of Laboratory Animals.
Catheter surgery
Following acclimation to the animal facility, rats to be given cocaine self-administration were anesthetized with ketamine/xylazine (and equithesin in some cases), given nonsteroidal anti-inflammatory analgesics, and implanted with intravenous catheters. Silastic tubing was inserted into and secured to the right jugular vein, while the other end passed subcutaneously over the shoulder and exited the back via either a chronic indwelling cannula (30) or an external infusion harness (31), as previously described. Beginning 3 days after surgery, catheters were flushed once daily with 0.1 ml each of the antibiotic cefazolin (100 mg/ml) and heparin (100 U/ml). Self-administration sessions began after 1 week of recovery from surgery.
Cocaine self-administration
Operant chambers were housed in sound-attenuating cubicles. Stimulus presentations, cocaine infusions, and data recording were controlled via MED-PC IV software (Med-Associates, St. Albans, VT). During 2-hr daily sessions, presses on an active lever resulted in a cocaine infusion (fixed ratio-1; 0.2 mg in 50 μl via motorized pump) paired with tone and light cues (78 dB, 2900 Hz; white stimulus light above the active lever), followed by a 20-sec timeout. Presses on an inactive lever had no consequence. Rats were given 10 self-administration sessions in which they earned at least 10 infusions per session.Rats then were given daily extinction sessions, during which lever presses had no consequence (no drug or cues). Prior to reinstatement testing, rats were required to meet an extinction criterion of ≤ 25 active lever presses for 2 consecutive days (at least 7 extinction sessions prior to the first reinstatement session, and 2 extinction sessions prior to subsequent reinstatement sessions).
For cue-induced reinstatement, active lever presses resulted in presentation of tone and light cues in the same manner as during self-administration, but no drug was delivered. For cocaine-induced reinstatement, rats were injected with a priming injection of cocaine (10 mg/kg, i.p.) immediately prior to the reinstatement test session. Animals undergoing abstinence prior to extinction remained in their home cages and were periodically handled. NA and CRF receptor ligands were administered 30 min prior to reinstatement or extinction sessions (except RS-79948, which was administered 60 min prior).In a within-subject design, each animal received 1 reinstatement session with vehicle pretreatment and 1-2 reinstatement sessions with a NA or CRF receptor ligand (1-2 doses of a single ligand), for which the order of treatments was counterbalanced among animals.
Food self-administration
Rats were food-restricted throughout the study, so that each rat received 10 g of rat chow daily in the home cage at the end of the day; in combination with the food earned during the self-administration session, this was sufficient for each animal to maintain its weight (range among rats was 350-400 g). During 1-hr daily sessions in operant chambers similar to those used for cocaine self-administration, presses on an active lever resulted in the delivery of a single food pellet into a hopper (fixed ratio-1; 45-mg grain-based pellets from Bio-Serv, Frenchtown, NJ) with no cue pairings and no timeout period. Presses on an inactive lever had no consequence. Once rats acquiredfood self-administration, they were given 6 test sessions for which different NA ligands were administered in a counterbalanced order 30 min prior to food self-administration sessions, in a within-subject design. At least one intervening self-administration session was given between test sessions to ensure that rates re-stabilized.
Locomotor testing
Rats were tested for spontaneous locomotion during a 60-min test session in clear acrylic chambers (40 × 40 × 30 cm) equipped with Digiscan monitors (AccuScan Instruments, Inc., Columbus, OH) that monitored horizontal (16 × 16 photobeam array) and vertical activity (16 photobeams). NA receptor ligands were administered 30 min prior to locomotor testing. Each rat was given 2 locomotor tests, for which they were randomly assigned to receive 2 different test ligands, for a between-subjects design.
Drugs
Cocaine HCl (National Institute on Drug Abuse) was dissolved in 0.9% sterile saline. NA and CRF receptor ligands(Table 1) were administered in a volume of 1 ml/kg (i.p.) 30 min prior to reinstatement testing, except RS-79948 (60 min prior to test).Vehicles and receptor affinities are reported on Table 1(18, 20, 32). All NA and CRF receptor ligands were purchased through Tocris Bioscience (Ellisville, MO), except clonidine (Sigma-Aldrich, St. Louis, MO).UK-14,304 tartrate dosing was based on free weight.Moxonidine was dissolved in a few drops of 1M HCl prior to adding saline (and then brought to pH 7).Prazosin was dissolved in its vehicle by heating and vortexing. Vehicles for the CRF antagonists were prepared by heating and stirring (and then adding to the antagonist).
Table 1.
Noradrenergic (α, β), imidazoline (I1) and corticotropin-releasing factor (CRF R1) receptor ligands used in the current studies.
| Drug | Receptor action | Doses (mg/kg) | Vehicle |
|---|---|---|---|
| Clonidine | I1> α2 agonist (4-fold) | 0.005, 0.01, 0.02 | Saline |
| RS-79948 | α2 antagonist (selective) | 1.0 | Saline |
| UK-14,304 | α2> I1 agonist (100-fold) | 0.05, 0.1, 0.2 | Water |
| Guanfacine | α2A agonist (selective) | 0.2, 1.0 | Saline |
| Moxonidine | I1> α2 agonist (40-fold) | 0.2, 1.0 | Saline |
| Prazosin | α1 (and α2B) antagonist | 1.0, 3.0 | 10% DMSO in water |
| (S)-(-)-Propranolol | β antagonist | 5, 10 | Saline; (R)-(-)-Prop control |
| CP-154,526 | CRF R1 antagonist | 20 | 5% cremophor in saline |
| Antalarmin | CRF R1 antagonist | 10, 20 | 10% cremophor in water |
Experimental groups
NA and CRF receptor ligands were administered prior to cue-induced reinstatement (n=6-15 per group, within-subject comparisons), extinction (n=8 per group, between-subjects comparisons), locomotor testing (n=6-7 per group, between-subjects comparisons), or food self-administration (n=8, within-subject comparisons). Exact group numbers are presented in the figure legends.
Data analyses
All results are presented as means ± SEM. Animals were completely removed from data analyses if they lacked reinstatement (< 20 active lever presses) on allreinstatement tests, including vehicle sessions. Reinstatement with vehicle pretreatment was not significantly different among groups of rats receiving different doses of a given NA or CRF receptor ligand (using t-test or ANOVA, depending on number of groups), so data were pooled into a single bar for graphs. However, statistical analyses were run separately on each group of rats for within-subject comparisons of reinstatement effects.
Reinstatement data were analyzed using paired t-testsor one-way repeated-measures ANOVAs (with Tukey-Kramer’s post-hoc comparisons), depending on whether a given group received 2 or 3 reinstatement sessions (as described in cocaine self-administration methods); Bonferroni correction was employed when more than one statistical test was performed for analysis ofa given NA or CRF receptor ligand. Mixed-model ANOVAs with Bonferroni post-hoc comparisons were used to analyze extinction behavior across sessions (Figs. 1d, 2d) and the effects of RS-79948 alone (Fig. 1c). Locomotor and food self-administration data were analyzed using a one-way ANOVA (repeated measures for food self-administration) with Dunnett’s Multiple Comparison Test for post-hoc analyses against saline.
Figure 1.

Stimulation of α2 adrenergic receptors blocked cue-induced reinstatement of extinguished cocaine-seeking. (A) The mixed α2/ imidazoline-1 (I1) receptor agonist clonidine significantly attenuated cue-induced reinstatement of active lever pressing at 20 μg/kg (n=15), but not 5 μg/kg (n=8) or10 μg/kg (n=7), as compared to vehicle (***p<0.001). Extinction (ext) levels of responding prior to reinstatement are also shown. (B) Pretreatment with the selective α2 antagonist RS-79948 (1 mg/kg) blocked the effects of 20 μg/kg clonidine on cue-induced reinstatement (n=10; *p<0.05, as compared to 0/0 vehicle condition). (C) RS-79948 alone did not trigger reinstatement when administered prior to an extinction session (ext; n=8), and did not potentiate cue-induced reinstatement (cues; n=9). (D) Acute clonidine reduced cocaine-seeking on the first day of extinction (n=8 and 8; ***p<0.001). Clonidine- and vehicle-treated rats were not significantly different on subsequent extinction days when no pretreatment was given. (E) The selective α2 agonist UK-14,304 significantly attenuated cue-induced reinstatement at 200 μg/kg (n=6), but not 50 or 100 μg/kg (n=12), as compared to vehicle (**p<0.01). (F) The selective α2A agonist guanfacine significantly attenuated cue-induced reinstatement at 1 mg/kg, but not 0.2 mg/kg (n=9), as compared to vehicle (*p<0.05).
Figure 2.
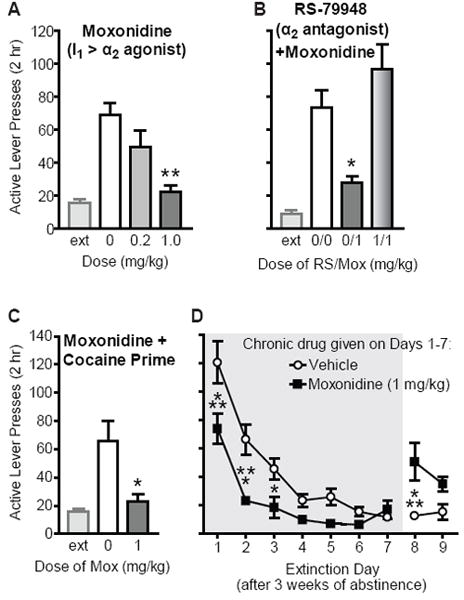
Moxonidine, an imidazoline-1 (I1) receptor-preferring agonist, reduced cue-induced reinstatement of extinguished cocaine-seeking, as well as cocaine-induced reinstatement and extinction responding. (A) Moxonidine significantly attenuated cue-induced reinstatement at 1 mg/kg, but not 0.2 mg/kg (n=10), as compared to vehicle (**p<0.01). Extinction (ext) levels of responding prior to reinstatement are also shown. (B) Pretreatment with the selective α2 antagonist RS-79948 (1 mg/kg) blocked the effects of 1 mg/kg moxonidine on cue-induced reinstatement (n=12; *p<0.05, as compared to 0/0 vehicle condition), indicating that the effects of moxonidine required actions at α2 receptors. (C) Moxonidine significantly attenuated reinstatement elicited by a cocaine prime (n=12), as compared to vehicle (*p<0.05). (D) Chronic moxonidine reduced cocaine-seeking on the first days of extinction following 3 weeks of abstinence from self-administration (n=8 and 8). There was a rebound of responding when moxonidine pretreatment was discontinued after 7 days (*p<0.05, ***p<0.001).
Results
For all rats undergoing cocaine self-administration (n=189), the means (± SEM) for the last 2 days of self-administration were 38.5 (±0.9) and 39.6 (±1.0) infusions(appx.20 mg/kg per day), and 54.7 (±3.7) and 53.6 (±3.2) active lever presses. The mean (± SEM) active lever presses for Days 1 and 7 of extinction were 84.3 (±3.2) and 18.2 (±0.8), respectively, for animals that received no treatment on extinction days.Unless noted, there were no significant differences for responding on the inactive lever during testing.
Alpha2 agonists reduced cue-induced reinstatement
We first tested clonidine, an α2 agonist with comparable affinity for I1 receptors, to determine whether noradrenergic signaling might be involved in cue-induced reinstatement.Clonidine reduced cue-induced reinstatement of extinguished cocaine-seeking at 20 μg/kg (t14=4.50; p=0.0005), but not 5 μg/kg (t7=1.57; p=0.16) or10 μg/kg (t6=2.68; p=0.037, not sig. after Bonferroni correction), as compared to vehicle(Fig. 1a). Clonidine also reduced inactive lever pressing at 10 μg/kg (t6=3.33; p=0.016, sig.) and 20 μg/kg (t14=3.81; p=0.0019; not shown).
In a separate group of rats, pretreatment with the selectiveα2 antagonist RS-79948 (1 mg/kg) reversed the effects of 20 μg/kg clonidine on cue-induced reinstatement(F2,9=9.42; p=0.0016). Post-hoc analyses revealed that combined treatment with RS-79948/clonidine was different from vehicle/clonidine (p<0.01) but not from vehicle/vehicle (p>0.05), whereas vehicle/clonidine reduced reinstatement as compared to vehicle/vehicle (p<0.05; Fig. 1b).Importantly, administration of RS-79948 alone did not trigger reinstatement during extinction and did not potentiate cue-induced reinstatement (F1,15=1.66; p=0.22; Fig. 1c).
Clonidine (20 μg/kg) also reduced cocaine-seeking when given prior to the first day of extinction. Across the first 7 days of extinction, there was a significant effect for clonidine(F1,49=4.42; p=0.041) and an interaction between clonidine and extinction session (F6,49=2.53; p=0.033). Post-hoc analyses revealed that clonidine reduced active lever pressing on the first day of extinction only, when clonidine was administered (p<0.001; Fig. 1d).Clonidine also reduced inactive lever pressing on Day 1 of extinction(p<0.001; not shown).
We then tested ligands with more selective affinity forα2 receptors on cue-induced reinstatement. The selective α2 agonist UK-14,304 reduced cue-induced reinstatement at 200 μg/kg (t5=5.55; p=0.0026, sig. after Bonferroni correction), but not at 50 or 100 μg/kg (F2,11=3.10; p=0.065), as compared to vehicle (Fig. 1e). Additionally, the selective α2Areceptor agonist guanfacine reduced cue-induced reinstatement (F2,8=4.85; p=0.023) when administered at 1 mg/kg (post-hoc, p<0.05), but not 0.2 mg/kg (p>0.05), as compared to vehicle (Fig. 1f).
I1 receptor agonist reduced reinstatement
We next tested moxonidine on cue-induced reinstatement to evaluate whether actions at I1 receptors may contributeto clonidine’s effects on reinstatement. This compound has 40-fold selectivity for I1 over α2 receptors (18);more selective I1 agonists or antagonists are not readily available. Moxonidine reduced cue-induced reinstatement (F2,9=8.66; p=0.0023)at 1 mg/kg (post-hoc, p<0.01), but not 0.2 mg/kg (p>0.05), as compared to vehicle (Fig. 2a).
Pretreatment with the α2 antagonist RS-79948 (1 mg/kg) reversed the effects of moxonidine (1 mg/kg) on cue-induced reinstatement (F2,11=12.19; p=0.0003). Post-hoc analyses revealed that combined treatment with RS-79948/moxonidine was different from vehicle/moxonidine (p<0.001) but not from vehicle/vehicle (p>0.05), whereas vehicle/moxonidine reduced reinstatement as compared to vehicle/vehicle (p<0.05; Fig. 2b).Inactive lever pressing was also different across the sessions (F2,11=3.46; p=0.049), but there were no significant post-hoc effects.
Moxonidine (1 mg/kg) also reduced reinstatement elicited by a cocaine prime (t11=2.72; p=0.020; Fig. 2c). In a separate group of rats, moxonidine reduced first-day extinction responding, as compared to vehicle-treated rats,following 3 weeks of abstinence from cocaine self-administration(post-hoc, p<0.001; Fig. 2d). These rats continued to receive treatment with either moxonidine or vehicle chronically throughout the first 7 days of extinction, followed by2 days of extinction with no treatment. Analysis of these 9 days of extinctionrevealed a significant effect oftreatment (F1,63=15.40; p=0.0002) and an interaction between treatment and extinction session (F8,63=5.66; p<0.0001), such that moxonidine- and vehicle-treated rats were different on Days 1-3 of extinction (p<0.001, 0.001, 0.05, respectively) and on Day 8, when all rats received no treatment for the first time (p<0.001; Fig. 2d).Responding on the inactive lever during these 9 days of extinction was different only on Day 1(post-hoc, p<0.01; not shown).In these same rats, we then tested for potential carry-over effects of chronic moxonidine on subsequent reinstatement conductedin the absence of any treatment (i.e., no moxonidine or vehicle injections).There were no differencesin cue- or cocaine-induced reinstatementfor animals that previously received chronic moxonidine during extinction, as compared to vehicle animals (p>0.05; not shown).
Combined α1and βadrenoceptorantagonist treatmentreduced cue-inducedreinstatement
Next we determined whether ligandsfor other adrenergic receptors would also decrease relapse to cocaine-seeking. The α1 antagonist prazosin had no effect on cue-induced reinstatement at 1 or 3 mg/kg (F2,14=0.64; p=0.53), as compared to vehicle (Fig. 3a). Administration of the β antagonist propranolol also had no effect on cue-induced reinstatement at 5 mg/kg (t10=1.00; p=0.34) or 10 mg/kg (t8=0.61; p=0.56), as compared to vehicle (Fig. 3b). However, a combination of prazosin (1 mg/kg) and propranolol (10 mg/kg) reduced reinstatement as compared to vehicle (t8=2.80; p=0.023; Fig. 3c).
Figure 3.
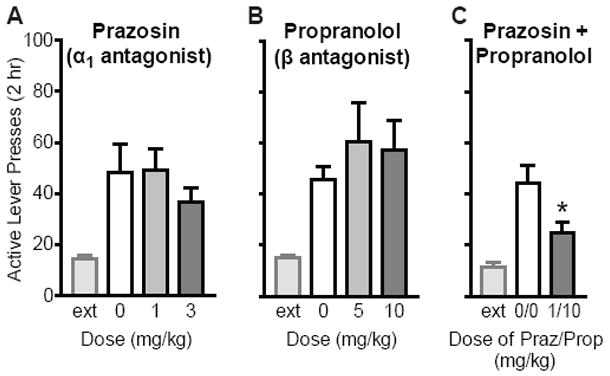
Prazosin plus propranolol reduced cue-induced reinstatement of extinguished cocaine-seeking. (A) The α1 antagonist prazosin had no significant effect on cue-induced reinstatement at 1 or 3 mg/kg (n=15). Extinction (ext) levels of responding prior to reinstatement are also shown. (B)The β antagonist propranolol had no effect on cue-induced reinstatement at 5 mg/kg (n= 11) or 10 mg/kg (n=9). (C) Combined pretreatment with prazosin (1 mg/kg) plus propranolol (10 mg/kg) significantly attenuated cue-induced reinstatement (n=9), as compared to vehicle (*p<0.05).
Most adrenergic ligands reduced activity in a novel locomotor chamber
Possible sedative effects of adrenergic ligands were tested in a novel locomotor chamber following administration of doses that reduced reinstatement, even if not significantly (20 μg/kg clonidine, 200 μg/kg UK-14,304, 1 mg/kg guanfacine, 1 mg/kg moxonidine, 3 mg/kg prazosin, or 1/10 mg/kg of prazosin/propranolol).There was a significant effect of drug administration on horizontal activity (F6,41=4.34; p=0.002), such that clonidine, UK-14,304, and guanfacine reduced activity as compared to saline (p<0.01; Fig. 4a). There was also a significant effect of drug administration on vertical activity (F6,41=4.69; p=0.001), such that clonidine, UK-14,304, guanfacine, prazosin, and prazosin/propranolol reduced activity as compared to saline (p<0.01 for all, except p<0.05 for prazosin/propranolol; Fig. 4a). Notably, moxonidine had no effect on horizontal or vertical activity (p>0.05).
Figure 4.
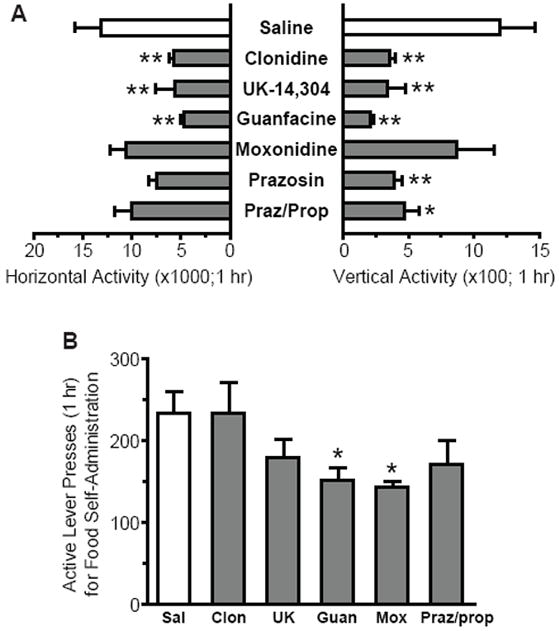
Most adrenergic ligands reduced activity in a novel locomotor chamberwith little effect on food self-administration.(A)Activity (number of beam breaks in 1 hr) was assessed in a locomotor chamber following pretreatment with saline (n=6), clonidine (20 μg/kg; n=7), UK-14,304 (200 μg/kg; n=7), guanfacine (1 mg/kg; n=7), moxonidine (1 mg/kg; n=7), prazosin (3 mg/kg; n=7), or prazosin plus propranolol (1+10 mg/kg; n=7). Horizontal activity was significantly reduced by clonidine, UK-14,304, and guanfacine, as compared to saline (**p<0.01). Vertical activity was significantly reduced by all ligands except moxonidine (*p<0.05, **p<0.01).(B) Food self-administration was tested following pretreatment with saline,clonidine (20 μg/kg), UK-14,304 (200 μg/kg), guanfacine (1 mg/kg), moxonidine (1 mg/kg), or prazosin plus propranolol (1+10 mg/kg) in a within-subject, counterbalanced design (n=8).Guanfacine and moxonidine reduced responding, as compared to saline (*p<0.05).
Most adrenergic ligands had little effect on food self-administration
When adrenergic ligands were administered prior to food self-administration, the effects were much less pronounced than those on cocaine-seeking. However, analysis of active lever-pressing following administration of adrenergic ligands at doses that reduced reinstatement of cocaine-seeking showed an overall significant effect(F5,35=3.27; p=0.016), and post-hoc analyses revealed that guanfacine and moxonidine were significantly different than saline ( p<0.05; Fig. 4b). CRF antagonists have previously been shown to have no effect on food self-administration(7, 33).
CRF antagonist reduced cue-induced reinstatement
A role for CRF in cue-induced reinstatement of cocaine-seeking was tested to further evaluate possible common mechanisms underlying cue- and stress-induced reinstatement. We tested two different CRF receptor-1 (R1) antagonists, CP-154,526 and antalarmin.Administration of CP-154,526 at 20 mg/kgsignificantly reduced reinstatement as compared to vehicle (t5=6.34; p=0.020; Fig. 5a).Antalarmin had no effect on reinstatement at 10 mg/kg (t11=0.39; p=0.71), but showed a trend for reducing reinstatement at 20 mg/kg (t10=1.50; p=0.16), as compared to vehicle(Fig. 5b).
Figure 5.
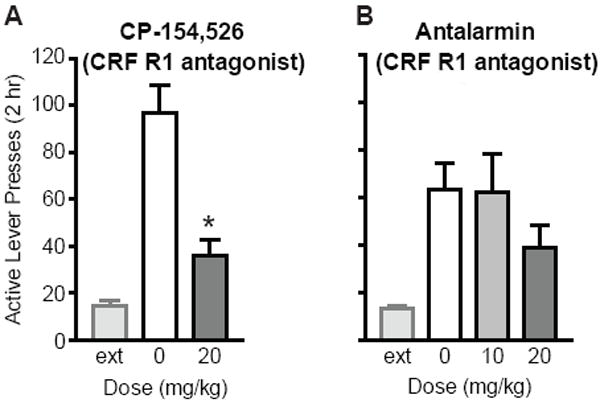
Corticotropin-releasing factor receptor-1 (CRF R1) antagonists reduced cue-induced reinstatement of extinguished cocaine-seeking. (A)CP-154,526 significantly attenuated cue-induced reinstatement at 20 mg/kg (n=6), as compared to vehicle (*p<0.05). Extinction (ext) levels of responding prior to reinstatement are also shown. (B)Antalarmin showed a trend for reducing cue-induced reinstatement at 20 mg/kg (n=11), but not 10 mg/kg (n=12), as compared to vehicle.
Discussion
Our results indicate that the I1 receptor-preferring agonist moxonidine is a potential therapeutic option for maintaining cocaine abstinence. Moxonidine reduced cocaine-seeking triggered by either drug-associated cues or cocaine prime, without signs of sedation.These reinstatement findings highlight the potential functional importance of the relatively-unknown imidazoline signaling system in drug addiction.In addition, the current studies revealed that α2 adrenergic receptor agonists and CRF R1 antagonists reduced cue-induced reinstatement, as previously observed for stress-induced reinstatement (4-11). These resultsindicate that neural pathways linked to stress-induced reinstatement of drug seeking also may be critically involved in the ability of cues to elicit drug craving and relapse.
Alpha2 and imidazoline receptor actions
We found that ligands with affinities for α2adrenoceptors and I1 receptors blocked cue-induced reinstatement of cocaine-seeking. The mixed α2 / I1 agonist clonidine attenuated cue-induced reinstatement as well as responding on the first day of extinction (Fig. 1), consistent with recent human studies showing that clonidine reduced cue- and stress-induced cocaine craving (17). The selective α2 agonists UK-14,304 and guanfacinealso reduced cue-induced reinstatement, showing that α2 receptor activation is sufficient to block reinstatement of cocaine-seeking (Fig. 1).This is in contrast to previous findings that the α2 agonist lofexidine did not block cue-induced reinstatement of drug-seeking for a cocaine-heroin combination (speedball) (34). Here, we found that the more specific I1 receptor agonist moxonidinereduced cue-induced reinstatement, cocaine-induced reinstatement, and extinction responding following 3 weeks of abstinence, when drug-seeking appeared to be elevated(Fig. 2D, as compared to Fig. 1D)(35).Taken together with previous findings that clonidine did not block cocaine-induced reinstatement of drug-seeking in self-administration or conditioned place preference paradigms (4, 36), these results indicate thatmoxonidine reduces drug-seeking behaviors more broadly than clonidine, highlighting its potential usefulness in addiction treatment.
The selective α2 antagonist RS-79948 blocked the reinstatement effects for both clonidine and moxonidine, indicating that at least some stimulation of α2 receptors is necessary for their actions (Fig. 1 and 2).It is unknown whether I1stimulation plays an equally important role, and there are no selective I1 receptor antagonists available to test this. However, we found that moxonidine did not causeclonidine-like reductions in locomotor activity (Fig. 4a)that have been linked to α2 receptor actions (19, 37-38). This indicates that moxonidine may be acting as a weak or partial agonist at α2 receptors as compared to clonidine, or that I1 receptor signaling requires coincident stimulation at α2 receptors. Previous studies also noted thatmoxonidine actions at α2 receptors are required for its hypotensive effects (39-40). Coincident stimulation of I1 and α2 receptors may play an important role in moxonidine’s unique ability to attenuate cue- and cocaine-induced reinstatement.
Reversal of reinstatement blockade by RS-79948 indicates that α2 receptors play a critical, although not necessarily sufficient, role in cocaine-seeking behavior. The α2 receptors involved in the reinstatement effect may be located on adrenergic neurons, where they act as autoreceptors, or located on non-adrenergic cells, where several actions have been described for α2receptors(41-43). As an important control, we showed that RS-79948 alone did not drive reinstatement of drug-seeking (Fig. 1), in contrast to previous observations for the non-selective α2 antagonist yohimbine(5, 44-45). Recent studies similarly found that RS-79948 does not trigger reinstatement, and indicate that yohimbine-induced reinstatement might not reflect adrenergic effects, but instead may be caused by dopamine and serotonin receptor actions (46-49).Notably, although selective α2 antagonists do not trigger reinstatement, norepinephrine itself does (47). Therefore, the recent finding that cue-induced cocaine-seeking was attenuated by the NA reuptake inhibitor atomoxetine(50) can most likely be attributed to specificstimulation of α2adrenoceptors (paralleling the effects seen here), and not due toenhanced NA signaling overall.
In humans, I1 agonistssuch as moxonidine and rilmenidineare preferred over α2 agonists for treatment of hypertension due to their ability to reduce blood pressure without the side effects associated with clonidine, such as dry mouth, decreased alertness, and sedation (51-52).However, moxonidine’s effects on reinstatement are most likely not related to its hypotensive properties, as other hypotensive agents tested here (e.g. prazosin and propranolol) did not reduce reinstatement.We found that most NA agents reduced activityin a novel locomotor chamber (Fig. 4a), which may indicate subtle sedative effects. Importantly, we found no profound effects of α2 / I1 agonistson lever-pressing during food self-administration (Fig. 4b), indicating that reinstatement blockade cannot be attributed to reductions in motivated behavior or operant ability (all rats showed > 100 presses/hr). However, although we found no reductions infood self-administration following most α2 agonists, corroborating previous observations for clonidine and UK-14,304 (6-7, 53), there were significant reductions following guanfacine and moxonidine treatment.Reductions in food intake following moxonidine might be related to satiety mechanisms, as moxonidine beneficially affects key metabolic signals involved in the regulation of feeding (21-25). Given that moxonidine appeared to have no clonidine-like sedative effects in the locomotor chamber and that food self-administration was largely intact following its administration, the current results favor moxonidine as a promising anti-relapse therapeutic.
Alpha1 and β receptor antagonists
Although α2 and I1 agonists reduced cue-induced reinstatement, the post-synapticα1 receptor antagonist prazosin or β receptor antagonist propranolol did not reduce cue-induced reinstatement of cocaine-seeking unless administered in combination (Fig. 3).The dosing used for each of these antagonists alone is known to be effectivein other behavioral tasks (53-55). The lack of effect with propranolol is particularly surprising in light of previous studies showing that localmicroinfusions ofβ antagonists into extended amygdala attenuate stress-induced reinstatement of cocaine-seeking(56). This indicates that β receptor signaling may be more involved in reinstatement elicited by stress than bycues. However, this discrepancy may also be due to differences in receptor selectivity with local and systemic dosing of β receptor antagonists.
The finding that reinstatement is only reduced by a prazosin/propranolol combination may indicate that cue-induced reinstatement involves NA, but that signaling through either α1 or β receptors is sufficient,so dual blockade is necessary to reduce reinstatement. However, reinstatement blockade following the prazosin/propranolol combination may also be explained by general effects onmemoryor cognition. Combined treatment with α1 and β receptor antagonistshas been shown to affect cognitive processing and memory retrieval (57-58).
Cue and stress commonalities
We found that systemic administration of the CRF R1 antagonist CP-154,526 (20 mg/kg) reduced cue-induced reinstatement (Fig. 5), as previously observed for stress-induced reinstatement(7-11). CP-154,526 has been shown to reduce cue-induced reinstatement of cocaine- and methamphetamine-seeking, as well as extinction responding for cocaine (14, 59-60). We found a trend for reduced reinstatement with another CRF R1 antagonist, antalarmin, at 20 mg/kg (Fig. 5). Antalarmin has been shown previously to reduce reinstatement elicited by yohimbine or footshock stress, as does CP-154,526 (9-10, 61-63).
As described above, we also found that α2 agonists reduced cue-induced reinstatement of cocaine-seeking (Fig. 1 and 2), as previously observed for stress-induced reinstatement of drug-seeking (4-7). These findings indicate that drug-associated cues and stress may share some neural mechanisms for driving relapse. This is supported by prior studies in rats and dependent humans, in which cocaine-associated cues increased anxiety and HPA axis activation (12-14, 64-65). Additionally, previous studies have shown that both cue- and stress-induced reinstatement are reduced by the orexin-1 receptor antagonist SB-334867, the corticosterone synthesis inhibitor ketoconazole, environmental enrichment, or manipulation of bed nucleus of striaterminalis(14-15, 30, 56, 66-69). Finally, cues have been shown to potentiate stress-induced reinstatement in rats(70-72).
Conclusions
We found that cue-induced reinstatement of cocaine-seeking is blocked by stimulation of α2adrenoceptors and I1 receptors, or antagonism of CRF R1s, as previously observed for stress-induced reinstatement of drug-seeking. These findings indicate that drug-associated cues and stress share at least some common mechanisms for driving relapse. Future experiments may further explore this overlap by evaluatingcue-induced reinstatement followingventral noradrenergic fiber bundle lesions or local microinfusions of NA or CRF receptor ligands.The current studies support the therapeutic use of I1 receptor agonists such as moxonidine as anti-relapse medicationsdue to theirability to reducecocaine-seeking behaviors triggered by a variety of factors without potential sedative side effects.
Acknowledgments
This work was supported by National Institutes of Health grants R37-DA06214, T32-DA007288, and P50-DA015369, and conducted in an animal facility constructed with support from National Institutes of Health grantC06-RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources.We thank Rebecca Fallon, Phong Do, EleniBucuvalas, and Paul Knackstedt for technical assistance.
Footnotes
Financial Disclosures The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lu L, Shepard JD, Scott Hall F, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 2.Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- 4.Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- 5.Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- 6.Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- 7.Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist d-Phe CRF((12-41)) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- 10.Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- 11.Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- 13.Berger SP, Hall S, Mickalian JD, Reid MS, Crawford CA, Delucchi K, et al. Haloperidol antagonism of cue-elicited cocaine craving. Lancet. 1996;347:504–508. doi: 10.1016/s0140-6736(96)91139-3. [DOI] [PubMed] [Google Scholar]
- 14.Goeders NE, Clampitt DM. Potential role for the hypothalamo-pituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology (Berl) 2002;161:222–232. doi: 10.1007/s00213-002-1007-4. [DOI] [PubMed] [Google Scholar]
- 15.Mantsch JR, Goeders NE. Ketoconazole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: relationship to the discriminative stimulus effects of cocaine. Psychopharmacology (Berl) 1999;142:399–407. doi: 10.1007/s002130050905. [DOI] [PubMed] [Google Scholar]
- 16.Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology (Berl) 2007;190:569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- 17.Jobes ML, Ghitza UE, Epstein DH, Phillips KA, Heishman SJ, Preston KL. Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ernsberger P, Damon TH, Graff LM, Schafer SG, Christen MO. Moxonidine, a centrally acting antihypertensive agent, is a selective ligand for I1-imidazoline sites. J Pharmacol Exp Ther. 1993;264:172–182. [PubMed] [Google Scholar]
- 19.Bousquet P, Feldman J. Drugs acting on imidazoline receptors: a review of their pharmacology, their use in blood pressure control and their potential interest in cardioprotection. Drugs. 1999;58:799–812. doi: 10.2165/00003495-199958050-00003. [DOI] [PubMed] [Google Scholar]
- 20.Ernsberger P, Friedman JE, Koletsky RJ. The I1-imidazoline receptor: from binding site to therapeutic target in cardiovascular disease. J Hypertens Suppl. 1997;15:S9–23. doi: 10.1097/00004872-199715011-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid JL. Update on rilmenidine: clinical benefits. Am J Hypertens. 2001;14:322S–324S. doi: 10.1016/s0895-7061(01)02239-7. [DOI] [PubMed] [Google Scholar]
- 22.Ernsberger P, Ishizuka T, Liu S, Farrell CJ, Bedol D, Koletsky RJ, et al. Mechanisms of antihyperglycemic effects of moxonidine in the obese spontaneously hypertensive Koletsky rat (SHROB) J Pharmacol Exp Ther. 1999;288:139–147. [PubMed] [Google Scholar]
- 23.Friedman JE, Ishizuka T, Liu S, Farrell CJ, Koletsky RJ, Bedol D, et al. Anti-hyperglycemic activity of moxonidine: metabolic and molecular effects in obese spontaneously hypertensive rats. Blood Press Suppl. 1998;3:32–39. [PubMed] [Google Scholar]
- 24.Velliquette RA, Ernsberger P. Contrasting metabolic effects of antihypertensive agents. J Pharmacol Exp Ther. 2003;307:1104–1111. doi: 10.1124/jpet.103.054221. [DOI] [PubMed] [Google Scholar]
- 25.Velliquette RA, Ernsberger P. The role of I(1)-imidazoline and alpha(2)-adrenergic receptors in the modulation of glucose metabolism in the spontaneously hypertensive obese rat model of metabolic syndrome X. J Pharmacol Exp Ther. 2003;306:646–657. doi: 10.1124/jpet.103.050468. [DOI] [PubMed] [Google Scholar]
- 26.Georges F, Aston-Jones G. Prolonged activation of mesolimbic dopaminergic neurons by morphine withdrawal following clonidine: participation of imidazoline and norepinephrine receptors. Neuropsychopharmacology. 2003;28:1140–1149. doi: 10.1038/sj.npp.1300161. [DOI] [PubMed] [Google Scholar]
- 27.Georges F, Caille S, Vouillac C, Le Moine C, Stinus L. Role of imidazoline receptors in the anti-aversive properties of clonidine during opiate withdrawal in rats. Eur J Neurosci. 2005;22:1812–1816. doi: 10.1111/j.1460-9568.2005.04356.x. [DOI] [PubMed] [Google Scholar]
- 28.Vandergriff J, Kallman MJ, Rasmussen K. Moxonidine, a selective imidazoline-1 receptor agonist, suppresses the effects of ethanol withdrawal on the acoustic startle response in rats. Biol Psychiatry. 2000;47:874–879. doi: 10.1016/s0006-3223(00)00229-8. [DOI] [PubMed] [Google Scholar]
- 29.Taksande BG, Kotagale NR, Patel MR, Shelkar GP, Ugale RR, Chopde CT. Agmatine, an endogenous imidazoline receptor ligand modulates ethanol anxiolysis and withdrawal anxiety in rats. Eur J Pharmacol. 2010;637:89–101. doi: 10.1016/j.ejphar.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 30.Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reichel CM, See RE. Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacology (Berl) 2010;210:337–346. doi: 10.1007/s00213-010-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlen S, Dambrova M, Nasman J, Schioth HB, Gu Y, Wikberg-Matsson A, et al. [3H]RS79948-197 binding to human, rat, guinea pig and pig alpha2A-, alpha2B- and alpha2C-adrenoceptors. Comparison with MK912, RX821002, rauwolscine and yohimbine. Eur J Pharmacol. 1998;343:93–101. doi: 10.1016/s0014-2999(97)01521-5. [DOI] [PubMed] [Google Scholar]
- 33.Goeders NE, Guerin GF. Effects of the CRH receptor antagonist CP-154,526 on intravenous cocaine self-administration in rats. Neuropsychopharmacology. 2000;23:577–586. doi: 10.1016/S0893-133X(00)00148-2. [DOI] [PubMed] [Google Scholar]
- 34.Highfield D, Yap J, Grimm JW, Shalev U, Shaham Y. Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology. 2001;25:320–331. doi: 10.1016/S0893-133X(01)00227-5. [DOI] [PubMed] [Google Scholar]
- 35.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bousquet P. Imidazoline receptors: from basic concepts to recent developments. J Cardiovasc Pharmacol. 1995;26(Suppl 2):S1–6. [PubMed] [Google Scholar]
- 38.Gyires K, Zadori ZS, Torok T, Matyus P. alpha(2)-Adrenoceptor subtypes-mediated physiological, pharmacological actions. Neurochem Int. 2009;55:447–453. doi: 10.1016/j.neuint.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Tan CM, Wilson MH, MacMillan LB, Kobilka BK, Limbird LE. Heterozygous alpha 2A-adrenergic receptor mice unveil unique therapeutic benefits of partial agonists. Proc Natl Acad Sci U S A. 2002;99:12471–12476. doi: 10.1073/pnas.122368499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urban R, Szabo B, Starke K. Involvement of alpha 2-adrenoceptors in the cardiovascular effects of moxonidine. Eur J Pharmacol. 1995;282:19–28. doi: 10.1016/0014-2999(95)00297-x. [DOI] [PubMed] [Google Scholar]
- 41.Gilsbach R, Roser C, Beetz N, Brede M, Hadamek K, Haubold M, et al. Genetic dissection of alpha2-adrenoceptor functions in adrenergic versus nonadrenergic cells. Mol Pharmacol. 2009;75:1160–1170. doi: 10.1124/mol.109.054544. [DOI] [PubMed] [Google Scholar]
- 42.Shields AD, Wang Q, Winder DG. alpha2A-adrenergic receptors heterosynaptically regulate glutamatergic transmission in the bed nucleus of the stria terminalis. Neuroscience. 2009;163:339–351. doi: 10.1016/j.neuroscience.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delaney AJ, Crane JW, Sah P. Noradrenaline modulates transmission at a central synapse by a presynaptic mechanism. Neuron. 2007;56:880–892. doi: 10.1016/j.neuron.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 44.Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- 45.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 46.Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas JP, et al. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35:79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 47.Brown ZJ, Tribe E, D’Souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- 48.Nair SG, Adams-Deutsch T, Pickens CL, Shaham Y. Neuroscience Meeting Planner Online, Program No 55118. Chicago, IL: Society for Neuroscience; 2009. Role of dopamine receptors, but not 2 adrenoceptors, in yohimbine-induced reinstatement of high-fat food seeking. [Google Scholar]
- 49.Dzung Le A, Funk D, Harding S, Juzytsch W, Fletcher PJ. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology (Berl) 2009;204:477–488. doi: 10.1007/s00213-009-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Economidou D, Dalley JW, Everitt BJ. Selective norepinephrine reuptake inhibition by atomoxetine prevents cue-induced heroin and cocaine seeking. Biol Psychiatry. 2011;69:266–274. doi: 10.1016/j.biopsych.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 51.Ernsberger P. Pharmacology of moxonidine: an I1-imidazoline receptor agonist. J Cardiovasc Pharmacol. 2000;35:S27–41. doi: 10.1097/00005344-200000004-00005. [DOI] [PubMed] [Google Scholar]
- 52.Ziegler D, Haxhiu MA, Kaan EC, Papp JG, Ernsberger P. Pharmacology of moxonidine, an I1-imidazoline receptor agonist. J Cardiovasc Pharmacol. 1996;27(Suppl 3):S26–37. doi: 10.1097/00005344-199627003-00005. [DOI] [PubMed] [Google Scholar]
- 53.Wee S, Mandyam CD, Lekic DM, Koob GF. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang XY, Kosten TA. Prazosin, an alpha-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol Psychiatry. 2005;57:1202–1204. doi: 10.1016/j.biopsych.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Harris GC, Aston-Jones G. Beta-adrenergic antagonists attenuate withdrawal anxiety in cocaine- and morphine-dependent rats. Psychopharmacology (Berl) 1993;113:131–136. doi: 10.1007/BF02244345. [DOI] [PubMed] [Google Scholar]
- 56.Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berridge CW, Espana RA. Synergistic sedative effects of noradrenergic alpha(1)- and beta-receptor blockade on forebrain electroencephalographic and behavioral indices. Neuroscience. 2000;99:495–505. doi: 10.1016/s0306-4522(00)00215-3. [DOI] [PubMed] [Google Scholar]
- 58.Petrasek T, Doulames V, Prokopova I, Vales K, Stuchlik A. Combined administration of alpha1-adrenoceptor antagonist prazosin and beta-blocker propranolol impairs spatial avoidance learning on a dry arena. Behav Brain Res. 2010;208:402–407. doi: 10.1016/j.bbr.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 59.Gurkovskaya O, Goeders NE. Effects of CP-154,526 on responding during extinction from cocaine self-administration in rats. Eur J Pharmacol. 2001;432:53–56. doi: 10.1016/s0014-2999(01)01465-0. [DOI] [PubMed] [Google Scholar]
- 60.Moffett MC, Goeders NE. CP-154,526, a CRF type-1 receptor antagonist, attenuates the cue-and methamphetamine-induced reinstatement of extinguished methamphetamine-seeking behavior in rats. Psychopharmacology (Berl) 2007;190:171–180. doi: 10.1007/s00213-006-0625-7. [DOI] [PubMed] [Google Scholar]
- 61.Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse rodel: a role of CRF(1) receptors. Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, et al. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, et al. The CRF(1) receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007 doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- 64.DeVries AC, Pert A. Conditioned increases in anxiogenic-like behavior following exposure to contextual stimuli associated with cocaine are mediated by corticotropin-releasing factor. Psychopharmacology (Berl) 1998;137:333–340. doi: 10.1007/s002130050627. [DOI] [PubMed] [Google Scholar]
- 65.DeVries AC, Taymans SE, Sundstrom JM, Pert A. Conditioned release of corticosterone by contextual stimuli associated with cocaine is mediated by corticotropin-releasing factor. Brain Res. 1998;786:39–46. doi: 10.1016/s0006-8993(97)01328-0. [DOI] [PubMed] [Google Scholar]
- 66.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–2778. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: Effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology (Berl) doi: 10.1007/s00213-010-2008-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buffalari DM, See RE. Footshock stress potentiates cue-induced cocaine-seeking in an animal model of relapse. Physiol Behav. 2009;98:614–617. doi: 10.1016/j.physbeh.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 72.Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


