Abstract
Peptide loading of MHC class II (MHCII) molecules is catalyzed by the non-classical MHCII-related molecule, H2-M. H2-O, another MHCII-like molecule, associates with H2-M and modulates H2-M function. The MHCII presentation pathway is tightly regulated in dendritic cells (DCs); yet how the key modulators of MHCII presentation, H2-M and H2-O, are affected in different DC subsets in response to maturation is unknown. Here we show that H2-O is markedly downregulated in vivo in mouse CD8α− DCs in response to a broad array of TLR agonists. In contrast, CD8α+ DCs only modestly downregulated H2-O in response to TLR-agonists. H2-M levels were slightly down-modulated in both CD8α− and CD8α+ DCs. As a consequence, H2-M:H2-O ratios significantly increased for CD8α− but not CD8α+ DCs. The TLR-mediated downregulation was DC-specific, as B cells did not show significant H2-O and H2-M downregulation. TLR4 signaling was required to mediate DC H2-O downregulation in response to LPS. Finally, our studies showed that the mechanism of H2-O downregulation was likely due to direct protein degradation of H2-O as well as down regulation of H2-O mRNA levels. The differential H2-O and H2-M modulation after DC maturation support the proposed roles of CD8α− dendritic cells in initiating CD4-restricted immune responses by optimal MHCII presentation and CD8α+ DCs in promoting immune tolerance via presentation of low levels of MHCII-peptide.
Introduction
The initiation of the adaptive immune response requires the presentation of peptides derived from foreign antigens bound to MHC class II (MHCII3) on the surface of antigen presenting cells to CD4 T cells. MHCII assembly begins in the ER where the MHCII αβ heterodimers form a complex with the invariant chain, which prevents the association of nascent peptides with MHCII and also contains trafficking signals necessary for MHCII to travel to endosomal compartments, where antigen loading occurs (1). In protease-rich endosomes the invariant chain is selectively cleaved until only small remnants of the invariant chain, called class II associated invariant chain peptides (CLIP) remain bound in the MHCII peptide binding grove. In order for loading of peptides derived from antigens to occur, MHCII-bound CLIP must be exchanged for self- and foreign-derived peptides. Two MHCII homologues, H2-M (HLA-DM in humans; DM) and H2-O (HLA-DO in humans; DO) control this process (2, 3). H2-M facilitates the exchange of CLIP for antigen-derived peptides, prolongs the half-life of peptide receptive MHCII molecules and also ensures that only high affinity MHCII-peptide complexes are presented at the cell surface (4–8). The molecular mechanisms by which H2-M exerts these various functions remain poorly understood.
Identifying the in vivo function of H2-O/DO has proven to be more difficult. H2-O relies on association with H2-M for transport from the ER to endosomes where H2-M:H2-O complexes accumulate (9). In vitro, DM complexed with DO is incapable of catalyzing peptide loading on to MHCII molecules and thus, functions as an inhibitor of antigen presentation (10–12). The in vivo function of H2-O/DO is more nuanced; the consensus being that H2-O modulates loading of MHCII by H2-M/DM. Overall, the data support an inhibitory role for DO in MHCII Ag presentation (13). Recent evidence has indicated that H2-O/DO modulation of MHCII peptide presentation can have important biological consequences. H2-O expression has been shown to reduce the ability of B cells to gain T cell help and participate in the germinal center reaction potentially setting a threshold for B cell entry into germinal centers (14). Additionally, modulation of antigen presentation by DO expression in NOD DCs results in the prevention of the autoimmune disease, type 1 diabetes (15). Thus, it is now clear that H2-O/DO expression can have profound consequences in vivo and understanding the molecular mechanisms by which H2-O/DO functions is an important goal.
For many years, the expression of H2-O was thought to be limited to B cells and to thymic epithelial cells (2). However, it is now clear that that H2-O is also highly expressed in mouse and human dendritic cells (DCs) (16–18). DCs are vital for initiating an immune response, being mechanistically optimized to activate naïve T cells (19). Exposure of DCs to pathogens, inflammatory stimuli, or other stimuli that activate TLRs leads to both morphological and functional changes in the DCs referred to as maturation or activation. DC maturation results in essential changes in the levels of co-stimulatory, adhesion, and MHCII molecules that enhance the ability of DCs to activate naïve CD4 T cells and induce an immune response to the offending pathogen (20).
How DC maturation alters MHCII expression and function has been studied in detail (21). Upon DC maturation, MHCII molecules undergo a transient increase in expression, decreased amount of re-cycling from the cell surface, and decreased ubiquitination, all of which facilitate increased MHCII cell surface expression and efficient CD4 T cell activation (21, 22). Indeed the effect of maturation on MHCII expression and function has fueled a concept known as “antigenic memory”, whereby antigen presentation is enhanced and prolonged for the foreign peptides that the DC acquires when it first encounters a pathogen (23). Very little is known, however, about how DC maturation affects the key modulators of the MHCII pathway, H2-M and H2-O.
Previous studies have shown that H2-O is differentially expressed in both human and mouse DC subsets (16–18). At steady state, mouse CD8α− DCs express higher levels of H2-M and lower levels of H2-O, which should support presentation on MHCII. CD8α+ DCs, on the other hand, express lower levels of H2-M and higher levels of H2-O which should dampen presentation on MHCII (16, 17). Plasmacytoid DCs also express H2-O but at much lower levels than CD8− and CD8+ DCs (17). Interestingly, recent studies from the Nussenzweig laboratory have shown that CD8α− DCs are more efficient at MHCII presentation than CD8α+ DCs, which nicely correlates with the higher H2-M:H2-O ratio in CD8α− DCs and an overall inhibitory role for H2-O (24, 25). In vivo DC activation by LPS injection results in H2-O downregulation in total splenic DCs (16). Collectively, these studies support an overall inhibitory role for H2-O in MHCII presentation. At steady-state DCs express H2-O, but upon DC activation, H2-O levels are downregulated to allow for more efficient MHCII presentation. Such a mechanism could contribute to DC “antigenic memory” (23).
Here we examine how DC maturation induced by TLR agonists alters H2-M and H2-O expression in mouse splenic DC subsets. Collectively our results show that H2-O was preferentially downregulated by CD8α− DCs compared to CD8α DCs following in vivo DC activation with multiple TLR agonists. H2-M was also downregulated but to a lesser extent. H2-O downregulation in CD8α− DCs resulted in a high H2-M:H2-O ratio that correlates with an optimally active MHCII peptide loading pathway. Our studies, therefore, identify another factor that likely contributes to the dampening of the ability of immature DCs to optimally process and present peptides on MHCII and further highlight how important the control of MHCII processing is for immunity and tolerance.
Materials and Methods
Mice and in vivo DC activation
C57BL/6 (B6) and C3H/HeJ mice were purchased from the Jackson Laboratory. Transgenic mice expressing human DO in mouse DCs (B6.DO mice) were generated by placing the human DOA and DOB genes under the control of the DC-specific CD11c promoter and have been previously described and characterized (26). All mice were bred and maintained under specific pathogen-free conditions in the Memorial Sloan-Kettering Cancer Center’s (MSKCC) animal facility. Use of animals was in accordance with MSKCC’s Institutional Animal Care and Use Committee guidelines.
For in vivo DC activation, 6–8 wk old male B6, C3H/HeJ or B6.DO mice were injected with the following TLR agonists in 200 μl of saline: 1 μg LPS (Escherichia coli 0111:B4; Sigma-Aldrich or Invivogen), 2 μg Bacterial Flagellin (BAC) (Salmonella Typhimurium FLA-ST; Invivogen), 5 μg R848 (Invivogen), 50 μg Polyinosinic-polycytidylic acid sodium salt (Poly I:C) (Sigma-Aldrich) or 50 μg anti-CD40 (clone FGK4.5) or rat IgG as an isotype control.
Antibodies
Anti-mouse antibodies used for FACS analyses were from BD Pharmingen unless otherwise noted and were as follows: CD11c-PE (clone HL3), MHCII-Alexa 488 (I-A, 212.A1; MSKCC mAb Core Facility), MHCII-Alexa 488 (I-Ak, 10-2-16), B220-APC-Cy7 (RA3-6B2), streptavidin-PerCPCy5.5, CD8α-eFluor 450 (53-6.7, Ebiosciences), CD86-biotin (GL1), CD205-PE-Cy7 (205yekta, Ebiosciences), H2-M-Alexa 633 (2C3A, MSKCC mAb Core Facility), H2-O-Alexa 647 (Mags.Ob3 (17), MSKCC mAb Core Facility), DO-Alexa 647 (Mags. DO5 (27), MSKCC mAb Core Facility).
Flow cytometry
Flow cytometric analysis of splenic DCs was performed following digestion of spleens in 400 U/ml of collagenase D (Roche) and 100 μg/ml DNase I (Roche) for 30 min at 37°C. Cells were stained and analyzed as described (28); analysis was performed on a LSRII cytometer (BD Biosciences). For intracellular staining, samples were incubated with mAbs specific for surface proteins, fixed and permeablilized with Cytofix/Cytoperm (BD Pharminigen) and then stained according to the manufacturer’s protocol. Data was analyzed using FlowJo software and cell doublets were excluded from the analysis.
Immunoblot Analysis and Quantitation
DCs were purified from the spleens of B6 mice treated 16 hr earlier with either 1μg LPS in 200 μl saline or 200 μl saline (control). CD11c+ DCs were purified by positive selection with CD11c MACs beads (Miltenyi Biotech). DCs from control and LPS injected mice were ~90% and ~70% pure as judged by flow cytometry. Cell numbers for each DC preparation were normalized based upon purity and extracted in 20 mM Tris, 130 mM NaCl pH 8.0 containing 1% Triton X-100 and Complete Protease Inhibitor Cocktail (Roche). Following the removal of nuclear material by centrifugation, lysates were mixed with 10x Laemmli sample buffer containing 20 mM DTT and incubated at 95°C for 5 min prior to separation by 12% SDS-PAGE and transfer to PVDF membrane (Millipore). Membranes were incubated with rabbit Abs to the cytoplasmic tail of H2-Oβ (R.Ob/c; (17)) or with a mAb specific for H2-Mα (YoDMA.1; (26)) followed by detection with horseradish peroxidase (HRP)-conjugated goat anti-rabbit or goat anti-mouse IgG (Jackson ImmunoResearch Laboratories). Blots were developed with SuperSignal West Pico chemiluminescent peroxidase substrate (Pierce Biotechnology) followed by exposure to film. To ensure equal loading of the DC lysates, membranes were reprobed with anti-actin Abs (Sigma). Cell numbers used for each blot are indicated in the figure legend. For quantification of H2-Oβ and H2-Mα levels, films were scanned and bands were quantitated using QuantityOne software (Bio-Rad). The relative amount of H2-Oβ or H2-Mα was obtained by dividing pixel density obtained for the H2-Oβ or H2-Mα bands for LPS-activated DCs with that obtained for control DCs.
Quantitative real-time PCR
DCs were purified from the spleens of 2 to 3 B6 or B6.DO mice treated 16 hr earlier with either 1 μg LPS in 200 μl saline or 200 μl saline (control) were purified by positive selection with CD11c MACs beads (Miltenyi Biotech). The resultant CD11c+ DCs were stained with mAbs (as described in Fig. 1) and separated into CD8α+ and CD8α− DCs by FACS on a BD FACS Aria II.
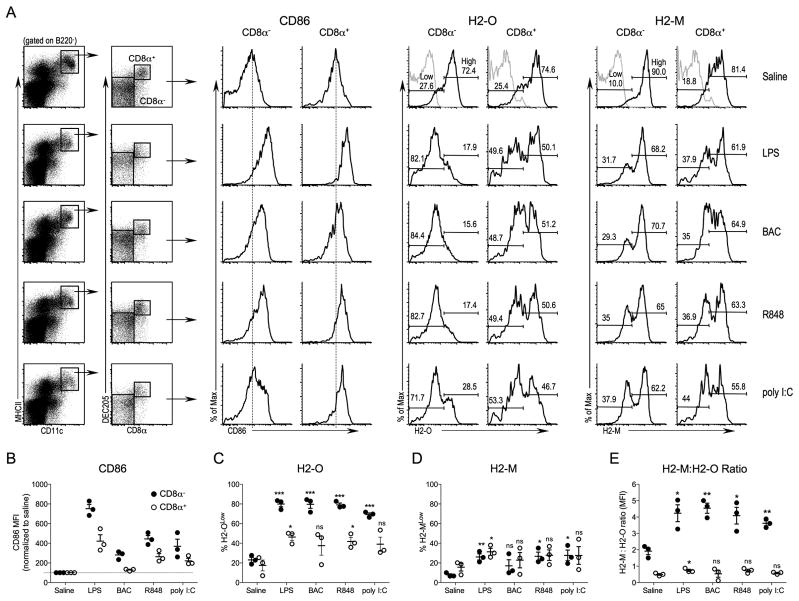
FIGURE 1.
Preferential downregulation of H2-O following in vivo DC activation by multiple TLR agonists. A, B6 mice were injected intravenously with saline, or the indicated TLR agonist, and 16 hr later spleens were harvested and subjected to flow cytometric analysis. Cells were gated for lack of B220 expression and then plotted as CD11c versus MHCII. DCs were defined as MHCII+CD11C+ as indicated by the black box. DCs were further separated based upon CD8α and DEC205 expression to define CD8α− DEC205− (CD8α−) and CD8α+DEC205+ (CD8α+) DC subsets as indicated. Histograms show the levels of CD86, H2-M, and H2-O (black lines) for CD8α− and CD8α+ DCs. Grey lines on top histograms (Saline) for H2-O and H2-M represent the level of non-specific staining. Numbers on plots for H2-O and H2-M represent the percent of cells falling within the Low and High gates as indicated. Vertical dashed lines on CD86 histograms are provided to aid comparison of CD86 levels. B, Quantification of the CD86 mean fluorescence intensity (MFI) for CD8α− and CD8α+ DCs after in vivo DC activation. The MFI for each condition is normalized to that observed for control mice injected with saline to allow for comparisons between experiments. C and D, H2-O and H2-M levels were separated into low and high expressing populations as indicated in A on the histograms for Saline injected control mice and the % of H2-OLow (C)and H2-MLow (D) among the CD8α− and CD8α+ DCs after in vivo activation with TLR agonists were quantitated for multiple experiments. E, Ratio of intracellular H2-M to H2-O MFI. Data is compiled from three independent experiments. The statistical significance of potential differences between the percent H2-OLow DCs, the percentage of H2-MLow DCs and the H2-M:H2-O ratio obtained for DCs obtained from saline injected compared to TLR agonist injected mice was determined by the Student’s T test. ns, nonsignificant.
Total RNA was extracted from the FACS purified DCs with Trizol (Invitrogen) according to manufacturer’s protocol. RNA quantity and quality was evaluated with a NanoDrop ND-1000 spectrophotometer (Thermo Scientific). cDNA was synthesized from total RNA using Oligo(dT) and the SuperScript III First-Strand Synthesis SuperMix (Invitrogen). Quantitative real-time PCR was carried out using iQ SYBR Green supermix (Bio-Rad) on a CFX96 real time system (Bio-Rad). Assays were carried out in duplicate. Real-time PCR products were analyzed for incorporation of SYBR Green and raw data (Ct, threshold cycle) were obtained with the CFX96 Real-time system software (Bio-Rad). The comparative Pfaffl method was used to determine fold change in expression between for DCs from LPS or saline (control) injected mice. mRNA leves for β-actin were used as the housekeeping reference gene. Relative mRNA levels were calculated by the 2ΔCt(target gene)/2ΔCt(β-actin) method (ΔCt = Ctsaline − CtLPS). Analyses were derived from DCs pooled from 2–3 mice/condition from 3–4 independent experiments. IL-6 message levels were used as a positive control for DC activation (29).
PCR primers used for the analyses were as follows: H2-Oβ F2: TCAGGCAAAGGCGGACTGTTAC, H2-Oβ R: TCCTCTCTGGATACACTGTCACCTC H2-Mα F1: TGAAGGTCAAATCCCAGTGTCC, H2-Mα R: AGCGGTCAATCTCGTGTGTCAC, I-Abα F1: TCTGGATGCTTCCTGAGTTTGG, I-Abα R1: CGTCTGCGACTGACTTGCTATTTC, HLA-DOβ F2: GGGCTAATCTTCCTTCTGGTGG, HLA-DOβ R2: AATCAGTTCGGGCTCCTCCAAGβ-actin F:TGCGTGACATCAAAGAGAAG, β-actin R:CGGATGTCAACGTCACACTT IL-6F: GGACTGATGCTGGTGACAAC and IL-6R: CCTCCGACTTGTGAAGTGGT.
Statistics
All statistical analyses were performed using GraphPad Prism version 5.0 using an unpaired or paired 2-tailed Student’s t test as appropriate. Values of P≤0.05 were considered significant and levels of significance are as follows: single asterisk indicates P = 0.01 – 0.05; double asterisk indicates P = 0.001 – 0.01; triple asterisk indicates P < 0.001.
Results
H2-O is downregulated in vivo by multiple TLR agonists, preferentially in CD8α− DCs
Previous studies have shown that H2-O levels are downregulated in activated splenic DCs following LPS injection in vivo (16). However, the impact of LPS-mediated DC activation on individual DC subsets was not evaluated. Furthermore, TLR agonists other than LPS were not evaluated. To address these questions B6 mice were injected intravenously with TLR4 (LPS), TLR5 (Bacterial flagellin; BAC), TLR7/8 (R848) or TLR3 (poly I:C) agonists to promote in vivo DC maturation. Saline was used as a control. Splenic DCs were harvested 16 hr later and analyzed by flow cytometry for the cell surface expression of CD86 and for the intracellular levels of H2-M and H2-O in the B220− CD11c+MHCII+CD8α− DEC205− and B220− CD11c+MHCII+CD8α+DEC205+ DC subsets (hereafter referred to as CD8α− and CD8α+ DCs, respectively) (Fig. 1).
Upregulation of CD86 following TLR agonist injection confirmed DC maturation and an activated phenotype for both CD8α− and CD8α+ DC subsets following injection with LPS, R848 and poly I:C (Fig. 1A, B). In vivo activation of DCs by BAC injection resulted in increased CD86 expression on CD8α− DCs but only a small increase in CD86 was observed for CD8α+ DCs (Fig. 1A, B). This was expected since CD8α+ DCs express only low levels of TLR5 (30).
H2-O expression levels in CD8α− and CD8α+ DC in saline-injected control mice separated into two distinct populations of DCs that expressed either H2-OHigh or H2-OLow levels (Fig. 1A). CD8α− and CD8α+ DCs from saline-injected mice displayed similar percentages of H2-OLow (~25%) and H2-OHigh (~75%) populations. However, upon in vivo activation of DCs by TLR agonists, CD8α− DCs displayed a striking and significant downregulation of H2-O protein, with 70–80% of the CD8α− DCs expressing low H2-O levels (Fig. 1A, C). H2-O was also downregulated in CD8α+ DCs, but to a much lesser extent than for CD8α− DCs, with the H2-O low populations being only 30–40% of the total. H2-O was also downregulated following BAC injection, despite CD8α+ DCs expressing only low levels of TLR5. It is not clear if this is due to the residual signaling via TLR5 in the CD8α+ DCs, or another indirect effect of BAC injection on other cells of the immune system. H2-O downregulation was also observed for both DC populations when mean fluorescence intensities (MFI) were used to measure H2-O levels (Supplemental Fig. 1).
Downregulation of H2-M was also apparent in both CD8α− and CD8α+ DCs (Fig. 1A, D), however, H2-M was downregulated to a much lesser extent than H2-O was. As for H2-O, H2-M was down modulated to a greater degree in the CD8α− DCs, but the preferential down modulation of H2-O in this DC subset was much larger. Once again, similar results were observed if MFI was used to measure H2-M levels in the DC subsets (Supplemental Fig. 1). The ability of H2-M to promote MHCII peptide loading is directly proportional to the overall H2-M to H2-O ratio (10, 11, 18, 27, 31). The marked H2-O downregulation but minimal H2-M downregulation in CD8α− DCs resulted in a significantly increased H2-M:H2-O ratio after DC activation with TLR agonists (Fig. 1E). In contrast, the H2-M:H2-O ratio for CD8α+ DCs only slightly increased post DC activation. The preferential downregulation of H2-O in CD8α− DCs should result in more active MHCII peptide loading, an idea supported by functional data showing that CD8α− DCs are better at activating naïve CD4 T cells (24, 25).
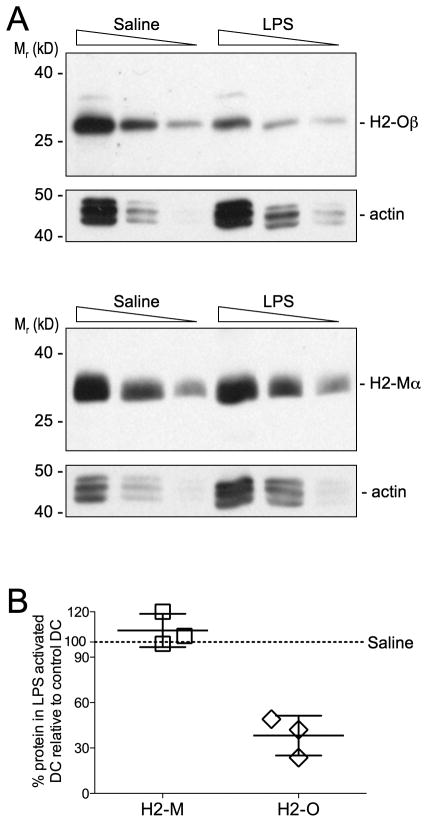
We next to confirmed H2-O downregulation using an independent assay. H2-Oβ and H2-Mα protein levels were determined by western blotting whole cell lystates of CD11c+ splenic DCs that had been purified from mice injected 16 hr earlier with saline (control) or LPS. We were unable to obtain sufficient purified CD8α+ and CD8α− DCs to blot the individual DC populations and thus, total CD11c+ DCs were analyzed. Results of these studies showed that DCs from saline and LPS injected mice had detectable levels of H2-Oβ and H2-Mα, however the level of H2-Oβ but not H2-Mα was clearly reduced for DCs purified from LPS injected mice (Fig. 2A). Quantification of multiple experiments showed that the level of H2-Oβ for DCs from LPS injected mice was approximately 40% of that obtained for saline (control) injected mice (Fig. 2B). Since this value represents the average protein level for CD8α+ and CD8α− DCs together, this value likely under estimates the magnitude of H2-Oβ preferential downregulation in CD8α− DCs (Fig. 1). Flow cytometric analyses of H2-M levels showed a small decrease in H2-M following TLR-mediated DC activation (Fig. 1). However, the analysis of H2-Mα levels by Western blotting did not show H2-Mα protein downregulation. This discrepancy is likely due to the insensitivity of the Western blotting approach, which is not suitable to detect small changes (<2-fold) in protein levels.
FIGURE 2.
Biochemical analyses of H2-O downregulation following in vivo DC activation by LPS. A Titrated amounts of splenic DC detergent lystates (4, 2 and 1 × 105 DC equivalents/lane) from mice injected 16 hr earlier with LPS or saline (control) were separated by SDS-PAGE, transferred to membranes and probed with Abs specific for the cytoplasmic tail of H2-Oβ (top) or H2-Mα (bottom). To demonstrate proportional loading, the blots were also probed with a mAb specific for actin. Data are representative of three independent experiments. B Quantification of the level of H2-Oβ and H2-Mα for DC from LPS injected mice relative to the level from control (saline) injected) mice. Each symbol represents an individual experiment and small horizontal bar indicates the mean ± SD.
TLR agonist-induced H2-O downregulation is DC-specific
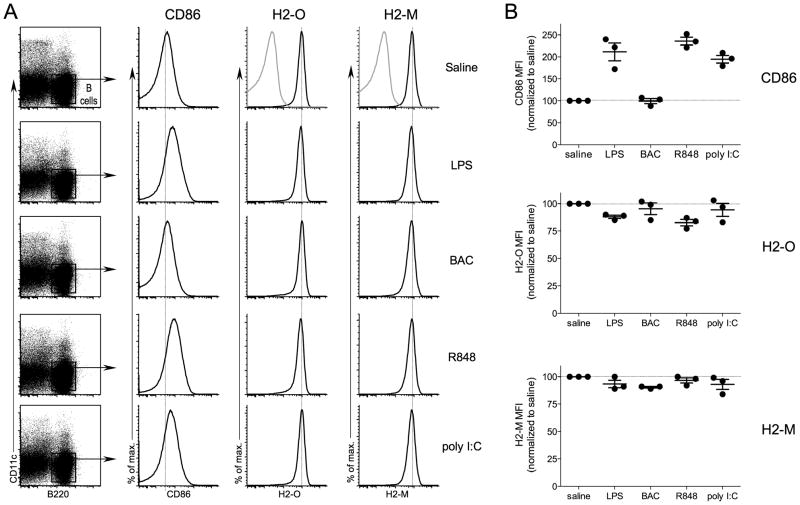
H2-O/DO is highly expressed in naïve B cells and is downregulated upon B cell activation (27, 31, 32). Naïve and memory B cells, both of which express high levels of H2-O/DO, have been shown to express and to be responsive to numerous TLR agonists (33). Thus, we wanted to determine whether TLR ligation in vivo could also induce H2-O downregulation in B cells. As before, mice were injected intravenously with either saline or TLR agonists and 16 hr later splenic B cells were analyzed by flow cytometry for cell surface CD86 and intracellular H2-O and H2-M expression levels (Fig. 3). B cells were defined as CD11c− B220+ to exclude DCs from the analyses. LPS, R848 and poly I:C induced B cell activation as evident from increased CD86 levels. BAC injection did not induce CD86 expression on the B cells, consistent with a lack of TLR5 expression in B cells (33). H2-O and H2-M levels remained high in the TLR agonist injected mice, independent of TLR-agonist induced B cell activation. Only very small changes occurred in expression levels, with no apparent H2-OLow B cells after TLR agonist injection. These results are in clear contrast of what was observed for CD8α− DCs which displayed a distinct H2-OLow population after TLR agonist induced maturation in vivo. This demonstrates that the DC and B cell compartments respond quite differently to the intravenous injection of a TLR agonist, with marked H2-O downregulation being confined only to the DCs.
FIGURE 3.
Minimal B cell H2-O downregulation following in vivo TLR agonist injection. A, B6 mice were injected intravenously with saline, or the indicated TLR agonist, and 16 hr later spleens were harvested and subjected to flow cytometric analysis. B cells were defined as B220+CD11c− to exclude DCs from the analyses as indicated by the black box. Histograms show the levels of CD86, H2-M, and H2-O (black lines) for B cells. Grey lines on top histograms (Saline) for H2-O and H2-M represent the level of non-specific staining. Vertical dashed lines on histograms are provided to aid comparison of levels of CD86, H2-O and H2-M. B, Quantification of the CD86, H2-O and H2-M mean fluorescence intensities (MFI) for B cells after in vivo activation. The MFI for each condition is normalized to that observed for control mice injected with saline to allow for comparisons between experiments. Data were compiled from three independent experiments.
Maturation by non-TLR pathways results in only modest H2-O down modulation
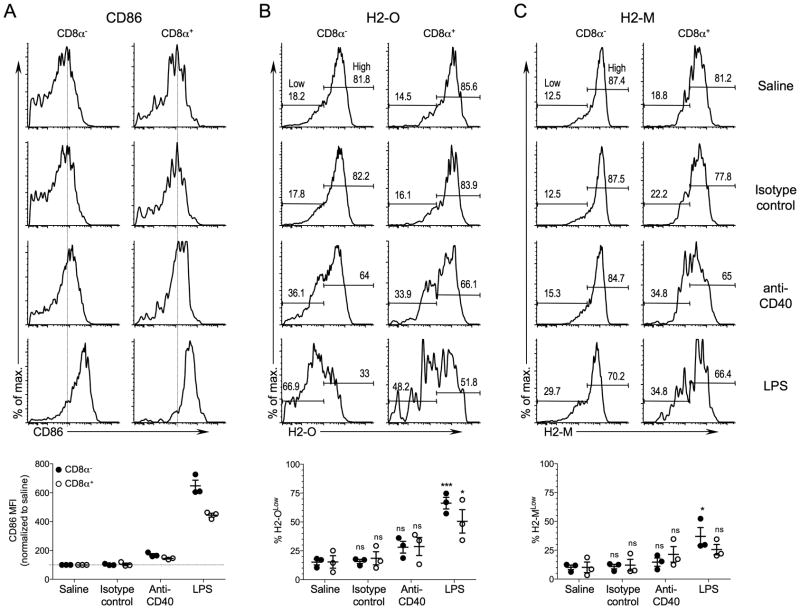
Next, we wanted to determine whether the downregulation of H2-O occurred only after DC activation by pathogen-specific signals such as the TLR agonists. DC:T cell interactions via CD40 can induce DC activation and maturation, and provides a way to activate DCs independent of TLR ligation (34). Thus, DCs were activated in vivo by the injection of anti-CD40 activating antibody (FGK45; (35)). An isotype-matched antibody was also used as a control, and LPS injected mice were included as a positive control for TLR-mediated H2-O downregulation. After 16 hr, splenic DC subsets were examined as in previous experiments.
Injection of the anti-CD40 antibody led to increased CD86 expression in both the CD8α− and CD8α+ DCs indicating that the anti-CD40-treatment resulted in DC activation (Fig. 4A). However, DC CD86 levels were only modestly increased when compared to CD86 levels for DCs from LPS challenged mice. CD86 levels on DCs from isotype control antibody injected mice remained nearly identical to those observed for mice injected with saline indicating that the observed DC activation in the anti-CD40 injected mice was specific to CD40 ligation. A slightly larger population of H2-OLow DCs was present for the CD8α− and CD8α+ DCs after in vivo DC activation with anti-CD40 compared to mice injected with saline or isotype control antibody. In this case both populations of DCs downregulated H2-O to similar levels, however. In contrast to injection with LPS, H2-O downregulation in response to anti-CD40 did not reach statistical significance for either DC population (Fig. 4B). H2-M levels also trended down but to a much lesser extent than H2-O (Fig. 4C). A lack of significant H2-O and H2-M downregulation after anti-CD40 DC activation in vivo was also observed when MFIs were used to monitor H2-O and H2-M downregulation (Supplemental Fig. 2). Thus, unlike DC activation with TLR agonists, activation of DCs by a non-pathogen stimulus did not result in significant downregulation of H2-O. Although anti-CD40 upregulated CD86 on DCs, it did not upregulate CD86 to the levels observed following LPS injection. Thus, it might appear that H2-O downregulation parallels the level of DC activation as measured by CD86 upregulation. However, that is clearly not the case since DCs derived from BAC injected mice showed CD86 upregulation to levels similar to those observed with anti-CD40 injection, yet promoted profound H2-O down modulation (Fig. 1A-C). Thus, we conclude that DC activation only by pathogen-specific signals such as TLR agonists efficiently promote H2-O down modulation.
FIGURE 4.
In vivo maturation of DCs with anti-CD40 antibody does not result in significant H2-O down-modulation. B6 mice were injected intravenously with saline, anti-CD40 Ab, isotype control Ab, or LPS, and 16 hr later spleens were harvested and subjected to flow cytometric analyses to determine CD86 (A), H2-O (B) and H2-M (C) levels for CD8α− and CD8α+ DCs. DCs were identified as CD8α− and CD8α+ DCs as shown in Figure 1. Numbers on histogram plots for H2-O and H2-M represent the percent of cells falling within the Low and High gates as indicated. Vertical dashed lines on CD86 histograms are provided to aid comparison of CD86 levels. Graphs at bottom show quantification of CD86 (mean fluorescence intensities for each condition relative to saline injected mice), H2-O and H-2M levels for CD8α− and CD8α+ DCs after in vivo DC activation for multiple mice and experiments. H2-O and H2-M levels were separated into Low and High expressing populations as indicated on the histograms for saline injected control mice and the % of H2-OLow (B)and H2-MLow (C) among the CD8α− and CD8α+ DCs after in vivo activation were plotted. Data were pooled from three independent experiments. The statistical significance of potential differences between the percent H2-OLow or H2-MLow DCs obtained for saline injected and anti-CD40 Ab, control Ab or LPS injected mice was determined by the Student’s T test. ns, nonsignificant.
LPS-mediated H2-O downregulation is dependent on TLR4
LPS mediates its effects on immune function through interaction with cell surface TLR4 (36). Thus, if LPS-induced downregulation of H2-O is downstream of TLR4 activation, then H2-O downregulation should be abrogated in LPS treated mice that are unable to signal through TLR4. To test this, we utilized the well-characterized C3H/HeJ mice, which possess an inactivating TLR4 mutation (36, 37). DCs from the C3H/HeJ mice were refractory to maturation 16 hr post LPS injection, as expected (Fig. 5A). Consistent with a lack of DC activation, H2-M and H2-O levels for CD8α− and CD8α+ were similar for LPS and control saline injected C3H/HeJ mice (Fig. 5B, C; Supplemental Fig. 3), indicating that TLR4 signaling is essential for LPS-induced activation and associated downregulation of H2-O and H2-M.
FIGURE 5.
Functional TLR4 signaling is required for LPS induced downregulation of H2-O. C3H/HeJ mice were injected intravenously with saline (control) or LPS, and 16 hr later spleens were harvested and subjected to flow cytometric analyses to determine CD86 (A), H2-O (B) and H2-M (C) levels for CD8α− and CD8α+ DCs. DCs were identified as CD8α− and CD8α+ DCs as shown in Figure 1. Numbers on histogram plots for H2-O and H2-M represent the percent of cells falling within the Low and High gates as indicated. Vertical dashed lines on CD86 histograms are provided to aid comparison of CD86 levels. Graphs at bottom show quantification of CD86 (mean fluorescence intensities for each condition relative to saline injected mice), H2-O and H-2M levels for CD8α− and CD8α+ DCs after in vivo DC activation for multiple mice and experiments. H2-O and H2-M levels were separated into Low and High expressing populations as indicated on the histograms for saline injected control mice and the % of H2-OLow (B) and H2-MLow (C) among the CD8α− and CD8α+ DCs after in vivo activation were plotted. Data were pooled from three independent experiments.
Ectopic expression of human DO in mouse DCs results in regulated protein expression
Previously, we generated transgenic mice that express human the DOA and DOB genes under the control of the CD11c promoter to drive expression in mouse DCs (26). Since the H2-O promoter was not used to drive DO expression in these transgenic mice (B6.DO), the DO genes are not subjected to the same transcriptional regulation as the endogenous H2-O genes. Thus, if the ectopically expressed DO protein was also downregulated during DC maturation, this would support that a protein degradative mechanism contributes to H2-O downregulation in activated DCs.
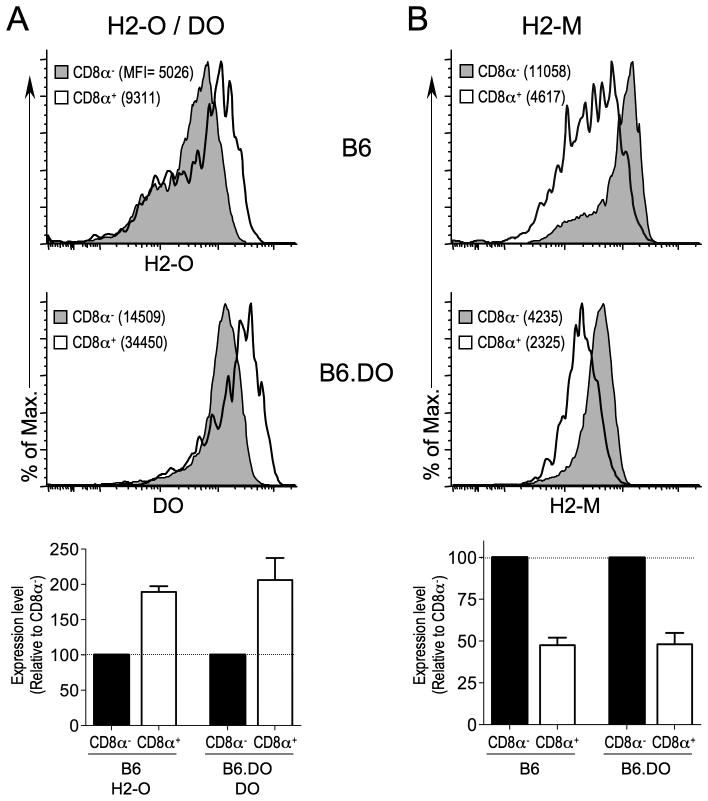
Previous studies have shown that CD8α− DCs expresses ~2-fold less H2-O than CD8α+ DCs (16, 26). Thus, we first asked if ectopically expressed DO protein levels were also differentially regulated in CD8α− and CD8α+ DCs. H2-O levels in B6.DO DCs are nearly undetectable, presumably due to more efficient assembly of DO with H2-M in the ER, which ultimately results in the accumulation of H2-M/DO complexes in B6.DO mice (data not shown). Thus, when considering the differential expression levels of DO in CD8α− and CD8α+, endogenous H2-O levels do not need to be considered. Flow cytometric analysis of B6.DO splenic DCs using a mAb specific for human DO that does not recognize H2-O (27) showed that DO protein levels were approximately 2-fold lower in CD8α− than CD8α+ DCs (Fig. 6A). This differential level of expression is similar to what is observed for H2-O levels in non-transgenic B6 CD8α− and CD8α+ DCs (Fig. 6A). Thus, DO protein levels mimicked endogenous H2-O levels for CD8α− and CD8α+ DCs. H2-O/DO stability and trafficking requires H2-M/DM (9, 26). Thus, an increase in DO and H2-O levels in CD8α+ DCs might simply be due to higher levels of H2-M in CD8α+ DCs. However, H2-M levels are ~2-fold higher in CD8α− DCs from B6 and B6.DO mice (Fig. 6B; (16, 26)). These data strongly support that a post-translational mechanism is in place that controls DO (and H2-O) expression levels in splenic DCs.
FIGURE 6.
Ectopic expression of human DO in mouse DCs results in regulated protein expression. Splenocytes from B6 or B6.DO mice were stained with mAbs to identify CD8α− and CD8α+ DCs as shown in Figure 1 and stained intracellularly for H2-O or DO(A) and H2-M (B) and analyzed by flow cytometry. The MFI obtained for H2-O, DO or H2-M staining is indicated in parenthesis on each histogram. Bar graphs below plots show the relative expression level of H2-O, DO or H2-M in CD8α− and CD8α+ DCs normalized to the expression level for CD8α− DCs for multiple mice. Data are representative of three independent experiments.
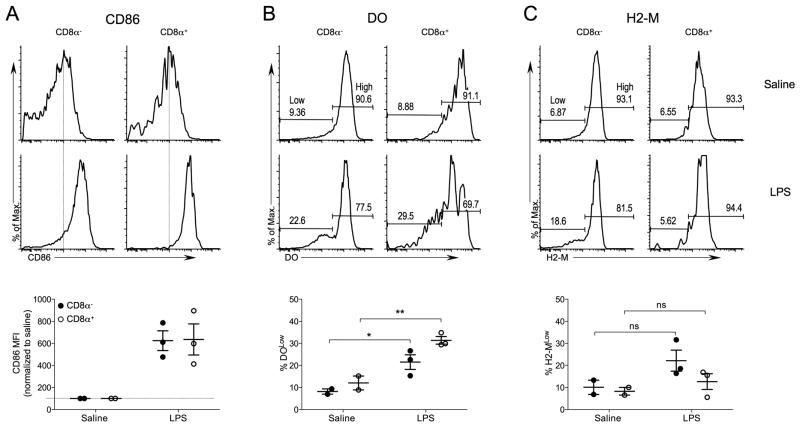
Next we determined if ectopically expressed DO, similar to H2-O, was downregulated in activated splenic DCs. B6.DO mice were challenged with LPS, or saline (control), and 16 hr later surface CD86 and intracellular H2-O and H2-M levels were analyzed by flow cytometry. Upregulation of CD86 on DCs from the LPS injected B6.DO mice confirmed that DC activation occurred (Fig. 7A). Activated CD8α− and CD8α+ DCs had a significantly higher percentage DOLow cells when compared to saline injected mice (Fig. 7B). H2-M levels also trended down, but the decrease in H2-M expression did not reach statistical significance (Fig. 7C). Taken together, the regulated DO expression in DCs from B6.DO mice support that post-translational regulatory mechanisms are operable in both basal and maturation-induced downregulation of H2-O in splenic DCs.
FIGURE 7.
DO is downregulated in B6.DO DCs after in vivo DC maturation. B6.DO mice were injected intravenously with saline (control) or LPS, and 16 hr later spleens were harvested and subjected to flow cytometric analyses to determine CD86 (A), DO (B) and H2-M (C) levels for CD8α− and CD8α+ DCs. DCs were identified as CD8α− and CD8α+ DCs as shown in Figure 1. Numbers on histogram plots for DO and H2-M represent the percent of cells falling within the Low and High gates as indicated. Vertical dashed lines on CD86 histograms are provided to aid comparison of CD86 levels. Graphs at bottom show quantification of CD86 (mean fluorescence intensities for each condition relative to saline injected mice), DO and H-2M levels for CD8α− and CD8α+ DCs after in vivo DC activation for multiple mice and experiments. DO and H2-M levels were separated into Low and High expressing populations as indicated on the histograms for saline injected control mice and the % of DOLow (B)and H2-MLow (C) among the CD8α− and CD8α+ DCs after in vivo activation were plotted. Data were pooled from two independent experiments. The statistical significance of potential differences between the percent DOLow or H2-MLow DCs obtained for saline injected and LPS injected mice was determined by the Student’s T test. ns, nonsignificant.
Transcriptional control of H2-O mRNA following TLR induced DC maturation
Expression of MHCII, H2-M and H2-O mRNA is controlled by the class II transactivator (CIITA) (38, 39). Previous studies have shown that DC maturation triggered by TLR agonists results in the rapid reduction of the synthesis of CIITA mRNA and protein, which in turn results in downregulation of MHCII mRNA and protein in mature DCs (40). Since CIITA also controls the transcription of H2-M and H2-O we asked if transcriptional control of H2-O mRNA might be an additional mechanism that contributes to TLR-induced downregulation of H2-O.
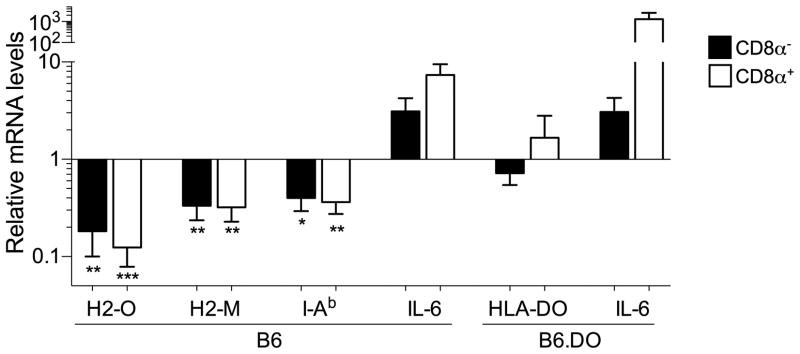
H2-O (beta), H2-M (alpha) and I-Ab (alpha) mRNA levels were determined by quantitative real time PCR for CD8α− and CD8α+ DCs that had been purified from mice injected 16 hr earlier with saline (control) or LPS (Fig. 8). As a control, the induction of IL-6 mRNA, which is upregulated upon TLR-induced DC maturation, was also measured (29). As expected, relative to the mRNA levels for DCs from saline injected mice, I-Ab mRNA levels were significantly decreased, while IL-6 levels were increased, in both DC subsets. Also, as anticipated, H2-M mRNA levels were also downregulated, similarly to I-Ab following LPS induced DC maturation. Interestingly, H2-O levels were decreased to an even greater extent than H2-M and I-Ab. The decrease in H2-M and H2-O mRNA levels was, however, similar for both CD8α− and CD8α+ DCs. As shown in Fig. 1, H2-O protein levels preferentially decreased in CD8α− DCs. Therefore, these data are also consistent with a model in which post-translational mechanisms contribute to the downregulation of H2-O preferentially in CD8α− DCs.
FIGURE 8.
TLR-induced DC maturation results in decreased H2-O, H2-M and MHCII mRNA transcript levels. The relative levels of H2-O (beta), H2-M (alpha), MHCII (I-Ab alpha), DO and IL-6 mRNA for purified CD8α− and CD8α+ DCs from B6 or B6.DO mice that had been injected 16 hr earlier with saline (control) or LPS were determined by quantitative real time PCR. mRNA levels were normalized to the value obtained for beta-actin and are presented as the fold change in mRNA for each DC subset relative to the value obtained for DCs from saline (control) injected mice. Data were derived from 3–4 independent experiments using DCs sorted from 2–3 mice injected with saline or LPS. The statistical significance of potential differences for each DC subset obtained for saline injected and LPS injected mice was determined by the Student’s T test.
To further test this model, we also examined transgene encoded DO mRNA levels in CD8α− and CD8α+ DCs from the B6.DO mice following either saline or LPS injection. Although DO protein levels are reduced (Fig. 7), DOB mRNA levels were essentially unaltered following LPS induced maturation (Fig. 8). Collectively, these studies show that the mechanism for preferential downregulation of H2-O in CD8α− DCs involved both transcriptional and post-translational mechanisms
Discussion
The presentation of pathogen-derived peptides bound to MHCII molecules on the surface of DCs is essential for the initiation of CD4-mediated adaptive immune responses. DC maturation induced by DC TLR ligation or other immune promoting stimuli results in a highly active MHCII processing and presentation pathway in mature DCs and promotes efficient CD4 T cell activation by mature DCs. Our studies show that H2-O is downregulated upon TLR-mediated DC activation. H2-M levels were also decreased following DC activation, but to a much smaller degree than H2-O. Agonism of both extracellular (TLR4, TLR5) and intracellular (TLR3, TLR7/8) TLRs, as well as MyD88 dependent (TLR4, TLR5, TLR7/8) and MyD88 independent (TLR3) TLRs all lead to the H2-O downregulation. The conservation among multiple TLR pathways strongly suggests that the downregulation of H2-O serves an important purpose in the MHCII presentation by mature DCs. H2-O inhibits or modifies H2-M mediated MHCII peptide loading, thus, the downregulation of H2-O likely contributes to efficient MHCII peptide presentation by mature DCs. Our studies also show that H2-O levels are downregulated to a greater degree in CD8α− than CD8α+ DCs resulting in a higher ratio of H2-M:H2-O and more active MHCII presentation by CD8α− DCs. These results are supported by functional studies showing that CD8α− DCs are more efficient than CD8α+ DCs in terms of priming naïve CD4 T cells and initiating immune responses (24, 25).
Our studies showed that DC maturation induced by pathogen-derived signals (TLR agonists) results in H2-O downregulation, however a non-pathogenic stimlus (anti-CD40) did not. DC maturation as measured by upregulation of CD86 following anti-CD40 injection in vivo was rather modest when compared to CD86 upregulation after LPS injection suggesting that the level of H2-O down modulation was related to DC maturation level. However, CD86 upregulation on CD8α− DCs following the injection of other TLR ligands (BAC, R848 and Poly I:C) was also rather modest, yet H2-O was profoundly down modulated for CD8α− DCs activated with these TLR ligands. Thus, the modulation of H2-O levels following DC activation is unlikely to be directly linked to DC activation status and more likely to be linked to the manner in which the DC is activated.
The precise molecular mechanisms downstream of TLR ligation that mediate H2-O (and H2-M) downregulation are unclear at this time. Maturation mediated degradation of H2-O within endosomes is an attractive hypothesis, because DC maturation induces changes in endosomal function and increases endosomal proteolytic activity (41). Further support for this mechanism came from our analysis of DO transgenic mice. Even when DO expression was controlled by a promoter and the 3′ untranslated regions (CD11c) that was not the endogenous H2-O promoter and regions, DO protein levels were regulated in a manner similar to endogenous H2-O both at steady state and following DC activation. H2-M and H2-O form a complex that localizes to endosomal compartments (9). Thus, our finding that H2-M is not downregulated to the same degree as H2-O following TLR-mediated DC activation demonstrates that the protein degradation is specifically targeted to H2-O. Targeted degradation of H2-O could be mediated by dissociation of H2-M and H2-O following TLR mediated DC activation and the targeting of the free H2-O for degradation. Finally, although our studies support that protein degradation contributes to the loss of H2-O following DC activation, our studies show that transcriptional control of H2-O mRNA is likely an additional mechanism. This is idea is further supported by studies from the Mellins laboratory showing that downregulation of DO in human Langerhans cells upon maturation with LPS, TNF or CD40L is mediated at least in part by transcription (18). Thus, we conclude that both protein degradation and transcriptional regulation are active mechanisms that contribute to ensuring the downregulation of H2-O in mouse DCs following maturation induced by TLR ligation.
The finding that the H2-O was downregulated in response to TLR agonists in DCs, but not in B cells is intriguing. Previous studies have shown that human and mouse germinal center B cells, which are highly activated B cells, also downregulate H2-O/DO and to a lesser degree H2-M/DM (17, 27, 31). The lack of H2-O down modulation in B cells following in vivo TLR agonist challenge was not due to lack of B cell activation since LPS, R848 and poly I:C induced clear upregulation of CD86. DCs, not B cells are APCs that are mainly responsible for priming naïve CD4 T cell responses (20). Thus, the maintenance of H2-O expression in B cells after challenge with TLR agonists would help to prevent B cells from presenting high levels of MHCII-peptide early in immune responses, thus promoting DC MHCII presentation and naïve CD4 T cell activation only by DCs. Support for this idea is provided by our recent studies showing that the presence of H2-O in naïve B cells reduces the ability of B cells to gain T cell help (14). Collectively, the studies presented here show the specific downregulation of H2-O in CD8α− DCs following activation and further highlight the importance of the developmental control of optimal MHCII presentation by DCs.
Supplementary Material
Acknowledgments
We thank Eric Pamer, Sergio Quezada, Bao Vuong and Desiree Ehleiter for sharing reagents and technical advice, and Derek Sant’Angelo and Nicole Draghi for helpful discussions and critical reading of the manuscript.
Footnotes
This work was supported by Public Health Service grants R01-AI061484 (to L.K. Denzin) and P30-CA08748 (to MSKCC).
Abbreviations used in this paper: BAC, bacterial flagellin; CIITA, class II transactivator; DC, dendritic cell; DM, HLA-DM; DO, HLA-DO; MHCII, MHC class II.
References
- 1.Wolf PR, Ploegh HL. How MHC class II molecules acquire peptide cargo: biosynthesis and trafficking through the endocytic pathway. Annu Rev Cell Dev Biol. 1995;11:267–306. doi: 10.1146/annurev.cb.11.110195.001411. [DOI] [PubMed] [Google Scholar]
- 2.Alfonso C, Karlsson L. Nonclassical MHC class II molecules. Annu Rev Immunol. 2000;18:113–142. doi: 10.1146/annurev.immunol.18.1.113. [DOI] [PubMed] [Google Scholar]
- 3.Alfonso C, Liljedahl M, Winqvist O, Surh CD, Peterson PA, Fung-Leung WP, Karlsson L. The role of H2-O and HLA-DO in major histocompatibility complex class II-restricted antigen processing and presentation. Immunol Rev. 1999;172:255–266. doi: 10.1111/j.1600-065x.1999.tb01370.x. [DOI] [PubMed] [Google Scholar]
- 4.Sloan VS, Cameron P, Porter G, Gammon M, Amaya M, Mellins E, Zaller DM. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995;375:802–806. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- 5.Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 6.Sherman MA, Weber DA, Jensen PE. DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity. 1995;3:197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 7.Kropshofer H, Vogt AB, Moldenhauer G, Hammer J, Blum JS, Hammerling GJ. Editing of the HLA-DR-peptide repertoire by HLA-DM. EMBO J. 1996;15:6144–6154. [PMC free article] [PubMed] [Google Scholar]
- 8.Denzin LK, Hammond C, Cresswell P. HLA-DM interactions with intermediates in HLA-DR maturation and a role for HLA-DM in stabilizing empty HLA-DR molecules. J Exp Med. 1996;184:2153–2165. doi: 10.1084/jem.184.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liljedahl M, Kuwana T, Fung-Leung WP, Jackson MR, Peterson PA, Karlsson L. HLA-DO is a lysosomal resident which requires association with HLA-DM for efficient intracellular transport. EMBO J. 1996;15:4817–4824. [PMC free article] [PubMed] [Google Scholar]
- 10.Denzin LK, Sant’Angelo DB, Hammond C, Surman MJ, Cresswell P. Negative regulation by HLA-DO of MHC class II-restricted antigen processing. Science. 1997;278:106–109. doi: 10.1126/science.278.5335.106. [DOI] [PubMed] [Google Scholar]
- 11.van Ham SM, Tjin EP, Lillemeier BF, Gruneberg U, van Meijgaarden KE, Pastoors L, Verwoerd D, Tulp A, Canas B, Rahman D, Ottenhoff TH, Pappin DJ, Trowsdale J, Neefjes J. HLA-DO is a negative modulator of HLA-DM-mediated MHC class II peptide loading. Curr Biol. 1997;7:950–957. doi: 10.1016/s0960-9822(06)00414-3. [DOI] [PubMed] [Google Scholar]
- 12.Liljedahl M, Winqvist O, Surh CD, Wong P, Ngo K, Teyton L, Peterson PA, Brunmark A, Rudensky AY, Fung-Leung WP, Karlsson L. Altered antigen presentation in mice lacking H2-O. Immunity. 1998;8:233–243. doi: 10.1016/s1074-7613(00)80475-6. [DOI] [PubMed] [Google Scholar]
- 13.Denzin LK, Fallas JL, Prendes M, Yi W. Right place, right time, right peptide: DO keeps DM focused. Immunol Rev. 2005;207:279–292. doi: 10.1111/j.0105-2896.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 14.Draghi NA, Denzin LK. H2-O, a MHC class II-like protein, sets a threshold for B-cell entry into germinal centers. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1004664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi W, Seth NP, Martillotti T, Wucherpfennig KW, Sant’Angelo DB, Denzin LK. Targeted regulation of self-peptide presentation prevents type I diabetes in mice without disrupting general immunocompetence. J Clin Invest. 2010;120:1324–1336. doi: 10.1172/JCI40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Reed-Loisel LM, Karlsson L, Jensen PE. H2-O expression in primary dendritic cells. J Immunol. 2006;176:3548–3556. doi: 10.4049/jimmunol.176.6.3548. [DOI] [PubMed] [Google Scholar]
- 17.Fallas JL, Yi W, Draghi NA, O’Rourke HM, Denzin LK. Expression patterns of H2-O in mouse B cells and dendritic cells correlate with cell function. J Immunol. 2007;178:1488–1497. doi: 10.4049/jimmunol.178.3.1488. [DOI] [PubMed] [Google Scholar]
- 18.Hornell TM, Burster T, Jahnsen FL, Pashine A, Ochoa MT, Harding JJ, Macaubas C, Lee AW, Modlin RL, Mellins ED. Human dendritic cell expression of HLA-DO is subset specific and regulated by maturation. J Immunol. 2006;176:3536–3547. doi: 10.4049/jimmunol.176.6.3536. [DOI] [PubMed] [Google Scholar]
- 19.Steinman RM. Dendritic cells and the control of immunity: enhancing the efficiency of antigen presentation. Mt Sinai J Med. 2001;68:160–166. [PubMed] [Google Scholar]
- 20.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 21.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 22.Shin JS, Ebersold M, Pypaert M, Delamarre L, Hartley A, Mellman I. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature. 2006;444:115–118. doi: 10.1038/nature05261. [DOI] [PubMed] [Google Scholar]
- 23.Villadangos JA, Schnorrer P, Wilson NS. Control of MHC class II antigen presentation in dendritic cells: a balance between creative and destructive forces. Immunol Rev. 2005;207:191–205. doi: 10.1111/j.0105-2896.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- 24.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 25.Kamphorst AO, Guermonprez P, Dudziak D, Nussenzweig MC. Route of Antigen Uptake Differentially Impacts Presentation by Dendritic Cells and Activated Monocytes. J Immunol. 2010 doi: 10.4049/jimmunol.1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fallas JL, Tobin HM, Lou O, Guo D, Sant’Angelo DB, Denzin LK. Ectopic expression of HLA-DO in mouse dendritic cells diminishes MHC class II antigen presentation. J Immunol. 2004;173:1549–1560. doi: 10.4049/jimmunol.173.3.1549. [DOI] [PubMed] [Google Scholar]
- 27.Glazier KS, Hake SB, Tobin HM, Chadburn A, Schattner EJ, Denzin LK. Germinal center B cells regulate their capability to present antigen by modulation of HLA-DO. J Exp Med. 2002;195:1063–1069. doi: 10.1084/jem.20012059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sant’Angelo DB, Cresswell P, Janeway CA, Jr, Denzin LK. Maintenance of TCR clonality in T cells expressing genes for two TCR heterodimers. Proc Natl Acad Sci U S A. 2001;98:6824–6829. doi: 10.1073/pnas.121179998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torri A, Beretta O, Ranghetti A, Granucci F, Ricciardi-Castagnoli P, Foti M. Gene expression profiles identify inflammatory signatures in dendritic cells. PLoS One. 2010;5:e9404. doi: 10.1371/journal.pone.0009404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33:827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Laur O, Kambayashi T, Li S, Bray RA, Weber DA, Karlsson L, Jensen PE. Regulated expression of human histocompatibility leukocyte antigen (HLA)-DO during antigen-dependent and antigen-independent phases of B cell development. J Exp Med. 2002;195:1053–1062. doi: 10.1084/jem.20012066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roucard C, Thomas C, Pasquier MA, Trowsdale J, Sotto JJ, Neefjes J, van Ham M. In vivo and in vitro modulation of HLA-DM and HLA-DO is induced by B lymphocyte activation. J Immunol. 2001;167:6849–6858. doi: 10.4049/jimmunol.167.12.6849. [DOI] [PubMed] [Google Scholar]
- 33.Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS One. 2007;2:e863. doi: 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Sullivan B, Thomas R. CD40 and dendritic cell function. Crit Rev Immunol. 2003;23:83–107. doi: 10.1615/critrevimmunol.v23.i12.50. [DOI] [PubMed] [Google Scholar]
- 35.Rolink A, Melchers F, Andersson J. The SCID but not the RAG-2 gene product is required for S mu-S epsilon heavy chain class switching. Immunity. 1996;5:319–330. doi: 10.1016/s1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- 36.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 37.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 38.Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome) Cell. 1993;75:135–146. [PubMed] [Google Scholar]
- 39.Harton JA, Ting JP. Class II transactivator: mastering the art of major histocompatibility complex expression. Mol Cell Biol. 2000;20:6185–6194. doi: 10.1128/mcb.20.17.6185-6194.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landmann S, Muhlethaler-Mottet A, Bernasconi L, Suter T, Waldburger JM, Masternak K, Arrighi JF, Hauser C, Fontana A, Reith W. Maturation of dendritic cells is accompanied by rapid transcriptional silencing of class II transactivator (CIITA) expression. J Exp Med. 2001;194:379–391. doi: 10.1084/jem.194.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–1403. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.