Abstract
Background & Aims
Chronic infection with hepatitis B or C virus (HBV or HCV) is a leading cause of cirrhosis, by unknown mechanisms of pathogenesis. Translocation of gut microbial products into the systemic circulation might increase because of increased intestinal permeability, bacterial overgrowth, or impaired clearance of microbial products by Kupffer cells. We investigated whether the extent and progression of liver disease in patients with chronic HBV or HCV infection are associated with microbial translocation and subsequent activation of monocytes.
Methods
In a retrospective study, we analyzed data from 16 patients with minimal fibrosis, 68 with cirrhosis, and 67 uninfected volunteers. We analyzed plasma levels of soluble CD14 (sCD14), intestinal fatty acid binding protein (I-FABP), and interleukin (IL)-6 by ELISA, and lipopolysaccharide (LPS) by the limulus amebocyte lysate assay, at presentation and after antiviral treatment.
Results
Compared with uninfected individuals, HCV- and HBV-infected individuals had higher plasma levels of LPS, I-FABP (indicating enterocyte death), sCD14 (produced upon LPS activation of monocytes), and IL-6. Portal hypertension, indicated by low platelet counts, was associated with enterocyte death (P=.045 at presentation, P<.0001 after therapy). Levels of sCD14 correlated with markers of hepatic inflammation (P=.02 for AST, P=.002 for ferritin) and fibrosis (P<.0001 for gamma-glutamyl transpeptidase, P=.01 for alkaline phosphatase, P<.0001 for alpha-fetoprotein). Compared to subjects with minimal fibrosis, subjects with severe fibrosis at presentation had higher plasma levels of sCD14 (P=.01) and more hepatic CD14+ cells (P=.0002); each increased risk for disease progression (P=.0009 and P=.005, respectively).
Conclusions
LPS-induced local and systemic inflammation are associated with cirrhosis and predict progression to end-stage liver disease in patients with HBV or HCV infection.
Keywords: Microbial translocation, soluble CD14, hepatitis, intestinal fatty acid binding protein, chronic liver disease, histology, immune response, microbiota
Introduction
Approximately 350 million people worldwide live with chronic HBV infection1 and over 200 million with chronic HCV infection2. Twenty-five percent of patients with chronic HBV infection die from chronic active hepatitis, cirrhosis or cancer. Twenty percent of patients with HCV infection progress to cirrhosis, of whom 20–25% progress to liver failure and death. The determinants of progression to cirrhosis and liver failure are poorly defined.
One contributor to chronic inflammation and fibrosis in chronic HBV and HCV infection is microbial translocation. Immunostimulatory microbial products from the intestine, such as lipopolysaccharide (LPS), translocate into the portal system where they are sensed and cleared by Kupffer cells. In the setting of liver disease, however, bacterial overgrowth, intestinal edema, altered hepatic architecture and Kupffer cell dysfunction increase entry of LPS into the peripheral circulation3,4. Increased plasma LPS levels have been reported in patients with chronic active hepatitis and cirrhosis due to viral hepatitis, alcohol and non-alcoholic fatty liver disease5–9.
LPS binding protein binds LPS, facilitating its binding to membrane CD14 (mCD14) on myeloid cells or to circulating soluble CD14 (sCD14)10. sCD14 transfers LPS to mCD14 and both sCD14 and mCD14 transfer LPS to the myeloid differentiation-2 (MD-2)/TLR4 complex, or to the MD-2/TLR4 complex11–14. The MD-2/TLR4/LPS complex activates NF-κb, inducing inflammatory cytokine production15,16. LPS activation of myeloid cells causes the shedding and secretion of sCD14, making sCD14 a marker of LPS bioactivity17–19.
While chronic liver disease may impair the clearance of translocating microbial products, LPS may also contribute to liver injury. LPS injections augment liver damage caused by carbon tetrachloride, choline deficiency and alcohol in animal models20. LPS mediates Kupffer cell production of tumor necrosis factor (TNF), IL-6 and IL-18, neutrophil recruitment, reactive oxygen species production and platelet aggregation in the hepatic sinusoids, leading to hepatic necrosis3. Kupffer cells produce transforming growth factor β (TGFβ), activating hepatic stellate cells (HSCs) to synthesize extracellular matrix proteins, resulting in fibrosis21,22. HSCs express TLR4, and LPS may activate HSCs by TLR4-mediated downregulation of a TGFβ decoy receptor23,24. Thus, LPS may induce fibrosis indirectly by inducing Kupffer cells to produce TGFβ and directly by sensitizing HSCs to TGFβ.
Because of the significant morbidity and mortality associated with progression to cirrhosis and liver failure and the potential association of LPS with liver pathology, we sought to investigate the relationship of microbial translocation and its downstream effects with clinical outcome in chronic HBV and HCV infections. Thus, we evaluated enterocyte death, microbial translocation, monocyte activation, liver function and liver pathology in a cohort of adults chronically infected with HBV or HCV.
Results
Study population
Eighty-four subjects with chronic HBV or HCV infection and 67 uninfected volunteers were included (Table 1). Chronic HBV infection is defined by HBsAg positivity for >6 months, serum HBV DNA >20,000 IU/ml, persistent or intermittent elevation in ALT or AST levels, and liver biopsy showing chronic hepatitis with moderate or severe necroinflammation25. Chronic HCV infection is defined as persistence of HCV RNA in serum 6 months after infection26. The median age for all subjects was 49.9 years, and 30 subjects (36%) were female. There were no significant associations between age or sex and any markers. Twenty-one had chronic HBV infection and 63 had chronic HCV infection, 40 (63.5%) with genotype 1. None of the subjects had underlying intestinal disease. Subjects were selected to compare subjects with severe fibrosis to those without fibrosis. Ishak scores were 0 or 1 in 16 subjects and 5 or 6 in 68 subjects27. Eighty-three subjects were classified as Child’s Class A, and the median MELD score was 8. Thus, even subjects with severe fibrosis and cirrhosis by liver biopsy were compensated cirrhotics. Baseline samples were drawn prior to antiviral treatment. Follow-up samples were drawn at least 7 months after enrollment when possible. Eight subjects were lost to follow-up.
Table 1.
Baseline characteristics at enrollment. The numbers indicate either median value (range) or # subjects (percentage).
| Characteristic | F0–F1 | F5–F6 | |
|---|---|---|---|
| Age (years) | 48.0 | 50.3 | |
| Gender (Male) | |||
| Virus: HBV | 3 | 18 | |
| HCV | 13 | 50 | |
| HCV Genotype: 1/4 | 3 | 38 | |
| 2/3 | 10 | 7 | |
| Unknown | 3 | 22 | |
| Duration of follow-up (years) | 1.7 | 5.0 | |
| HCV patients with SVR | 11 | 16 | |
| Patients who progressed (other than non-responders) | 0 | 29 | |
| Enrollment | Aspartate aminotransferase (U/L) | 36 | 87 |
| Alanine aminotransferase (U/L) | 48 | 113 | |
| Gamma-glutamyl transferase (U/L) | 26 | 81 | |
| Ferritin (mcg/L) | 128 | 193 | |
| Alpha fetoprotein (ng/ml) | 3 | 10 | |
| Alkaline phosphatase (U/L) | 62 | 87 | |
| Direct bilirubin (mg/dL) | 0.1 | 0.2 | |
| Albumin (g/dL) | 4.0 | 3.8 | |
| INR | 1.0 | 1.2 | |
| Platelet (K/uL) | 235 | 125 | |
| Creatinine (U/L) | 0.8 | 0.8 | |
| ISHAK: 0 | 10 | ||
| 1 | 6 | ||
| 5 | 33 | ||
| 6 | 35 | ||
| MELD | 7 | 9 | |
| Child's Class: A | 16 | 67 | |
| B | 1 | ||
| C | |||
| Follow-up | Aspartate Aminotransferase (U/L) | 22 | 47 |
| Alanine Aminotransferase (U/L) | 28 | 47 | |
| Gamma-glutamyl Transferase (U/L) | 21 | 41 | |
| Ferritin (mcg/L) | 82 | 148 | |
| Alpha fetoprotein (ng/ml) | 2 | 4.6 | |
| Alkaline phosphatase (U/L) | 67 | 83 | |
| Direct bilirubin (mg/dL) | 0 | 0.2 | |
| Albumin (g/dL) | 4 | 3.7 | |
| INR | 1.0 | 1.1 | |
| Platelet (K/uL) | 240 | 134 | |
| Creatinine (U/L) | 0.8 | 0.8 | |
| ISHAK: 0 | 3 | 1 | |
| 1 | 1 | 2 | |
| 2 | 2 | ||
| 3 | 6 | ||
| 4 | 6 | ||
| 5 | 1 | ||
| 6 | 12 | ||
| MELD | 7 | 9 | |
| Child’s Class: A | 14 | 49 | |
| B | 0 | 10 | |
| C | 0 | 3 |
Plasma sCD14 levels distinguish subjects with severe liver fibrosis from those with minimal fibrosis
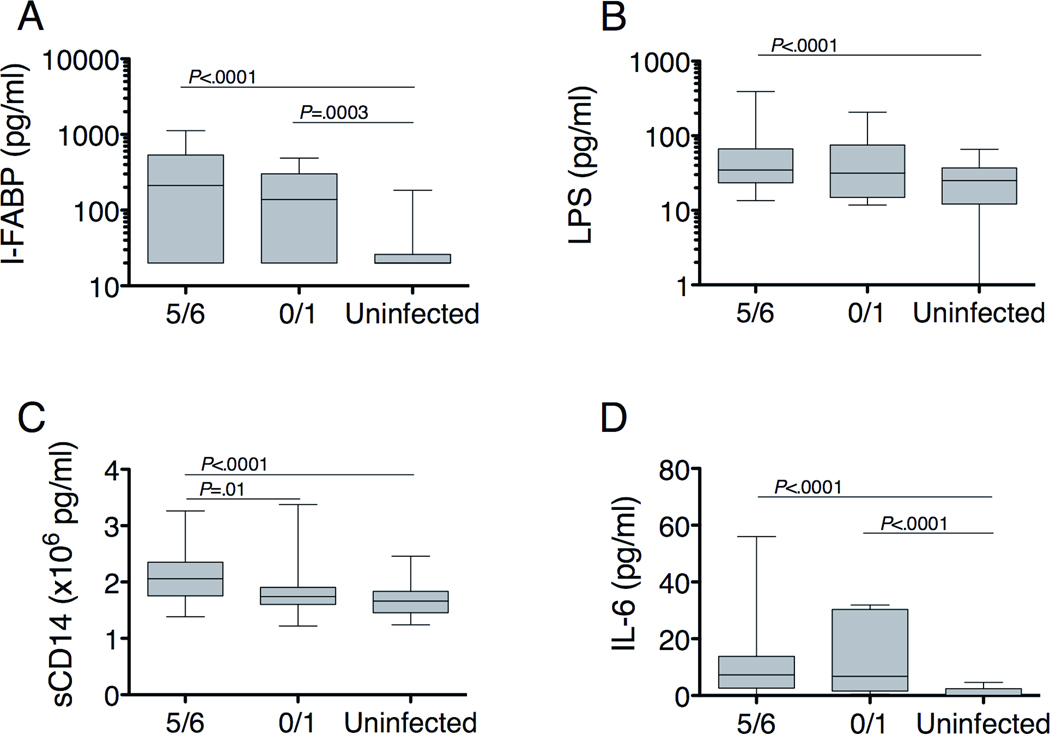
To assess for enterocyte loss, microbial translocation and the subsequent host response to microbial products, we measured baseline levels of LPS, sCD14, IL-6 and intestinal fatty acid binding protein (I-FABP), made exclusively by enterocytes and released into the bloodstream upon enterocyte necrosis28–30, and compared values between subjects with severe fibrosis and without fibrosis. sCD14 levels, reflective of LPS-induced monocyte activation, were significantly higher in subjects with severe fibrosis (2.06 × 106 pg/ml; Figure 1) compared to those without fibrosis (1.74 × 106 pg/ml, P=0.01) and healthy volunteers (1.66 × 106 pg/ml, P<0.0001), but there was no significant difference between subjects without fibrosis and healthy volunteers. I-FABP, LPS and IL-6, which may facilitate hepatic healing and increase upon LPS-induced monocyte activation31,32, were elevated regardless of fibrosis.
Figure 1.
(A,B) Baseline levels of markers of enterocyte death and microbial translocation are elevated in both minimal and severe fibrosis. For I-FABP, n=66 for F5/F6, n=14 for F0/F1, and n=41 for uninfected. For LPS, n=68 for F5/F6, n=16 for F0/F1 and n=67 for uninfected. (C) LPS-induced monocyte activation, reflected by sCD14, is higher in subjects with severe fibrosis (n=68) compared to those without fibrosis (n=16) or uninfected individuals (n=65). (D) IL-6 levels are elevated regardless of fibrosis. n=66 for F5/F6, n=14 for F1/F2, and n=24 for uninfected. Horizontal lines indicate the median value and 5-95% range. P-values were calculated with the Mann-Whitney U test. The number subjects varied based on the volume of plasma available and the amount needed for each assay.
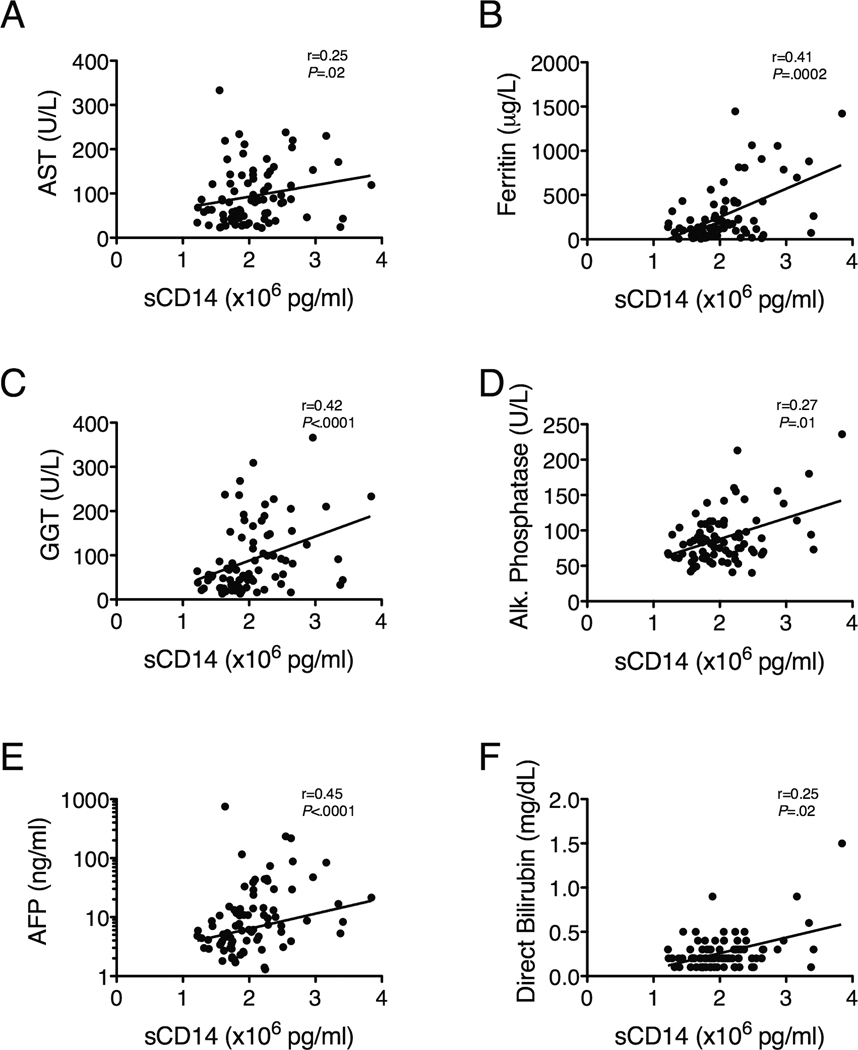
To further explore the relationship between LPS-induced monocyte activation and liver pathology, we compared sCD14 levels to markers of hepatic inflammation, fibrosis and synthetic function. sCD14 correlated with markers of hepatic inflammation, including AST and ferritin (Figure 2A-B) and the histology activity index (HAI; r=0.27, P=0.01); hepatic fibrosis, including gamma glutamyl-transpeptidase (GGT), alkaline phosphastase (ALP; Figure 2C-D), and the AST to platelet ratio index (APRI) (Supplementary Table 1); and hepatic regeneration, specifically AFP (Figure 2E). Direct bilirubin also correlated with sCD14, although the limited dynamic range of the direct bilirubin complicates this analysis (Figure 2F).
Figure 2.
Baseline sCD14 levels correlate with plasma markers of hepatic inflammation (AST, ferritin), fibrosis (GGT, ALP, AFP) and poor synthetic function (direct bilirubin), n=84 for all analyses. Correlations among variables were evaluated using Spearman’s rank correlation.
To evaluate whether elevations of these markers were different in the two viral infections, we compared values between the HBV- and HCV-infected subjects. sCD14 levels were increased in subjects with either HBV or HCV infection, but only statistically significant with HCV (Supplementary Figure 1). I-FABP, LPS and IL-6 levels were also higher in subjects with either HBV or HCV infection compared to healthy volunteers. Of note, sCD14 and IL-6 levels were significantly higher in subjects with HCV than those with HBV infection. Thus, while markers of enterocyte necrosis, microbial translocation and monocyte activation are increased in subjects with HBV or HCV infection, the degree of the host response to LPS, as reflected by the level of sCD14, correlates with hepatic inflammation and distinguishes subjects with severe fibrosis.
Enterocyte death is associated with portal hypertension
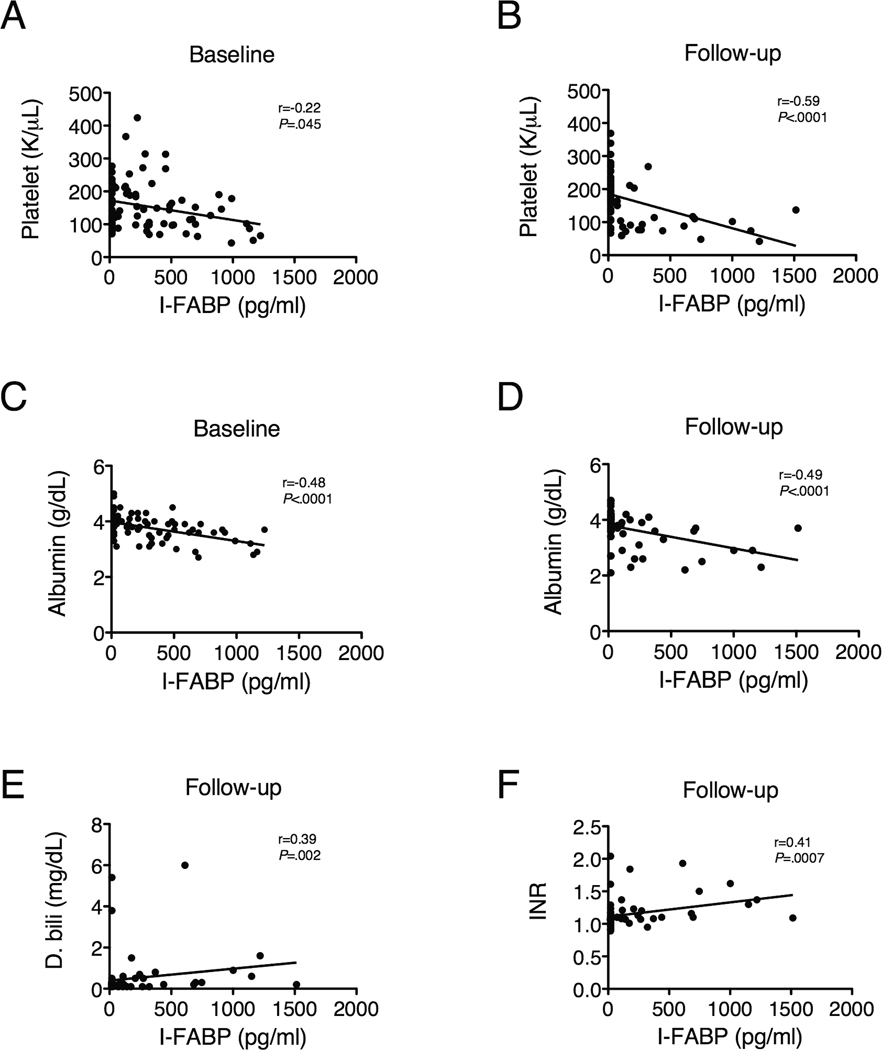
To further explore which processes are involved in enterocyte death, which may facilitate microbial translocation, we correlated baseline I-FABP levels to markers of liver pathology. High baseline I-FABP levels correlated with high Ishak scores (r=0.23, P=0.04) and low baseline platelet counts, a consequence of portal hypertension (r=−0.22, P=0.045; Figure 3A). Furthermore, after antiviral treatment, almost all subjects with detectable I-FABP levels had platelet counts lower than 150,000/µl (Figure 3B), suggesting that portal hypertension is associated with increased enterocyte death.
Figure 3.
Enterocyte death (reflected by I-FABP) is associated with portal hypertension (indicated by low platelet counts) and poor synthetic function (indicated by low albumin and high direct bilirubin and INR) at baseline (n=72) and after antiviral treatment (median 4.2 years, n=64). Correlations among variables were evaluated using Spearman’s rank correlation.
I-FABP levels were correlated negatively with albumin at baseline (Figure 2C) and positively with direct bilirubin (see Supplementary Table 1). At follow-up, I-FABP levels were correlated with albumin, direct bilirubin and international normalized ratio (INR) (Figure 2D-F), supporting a relationship between enterocyte death and decreased hepatic function. I-FABP also correlated with AST, ALT and alpha-fetoprotein (AFP) and with the AST to platelet ratio index (APRI; see Supplementary Table 2) at follow-up, suggesting a tight relationship between continued hepatic inflammation and fibrosis, portal hypertension and enterocyte death.
I-FABP and IL-6 decrease with successful treatment of HCV infection
All 21 patients infected with HBV infection and 60 of the 63 patients with HCV infection were treated according to standard of care, while two were never treated and one dropped out of the study. Twenty-seven HCV-infected subjects had a sustained virological response (SVR), defined by undetectable HCV RNA in serum at 6 months after completing therapy. All HBV-infected patients remained on therapy for the duration of their follow-up.
To assess the effect of antiviral treatment on these markers, levels pre- and post-treatment were compared. High baseline sCD14 levels were associated with failure to respond to therapy for HCV, reflecting the connection between fibrosis and therapeutic failure. The median baseline sCD14 level of subjects with SVR was 1.81 × 106 pg/ml compared to 2.24 × 106 pg/ml in nonresponders (P=0.001). No other marker was associated with response to therapy.
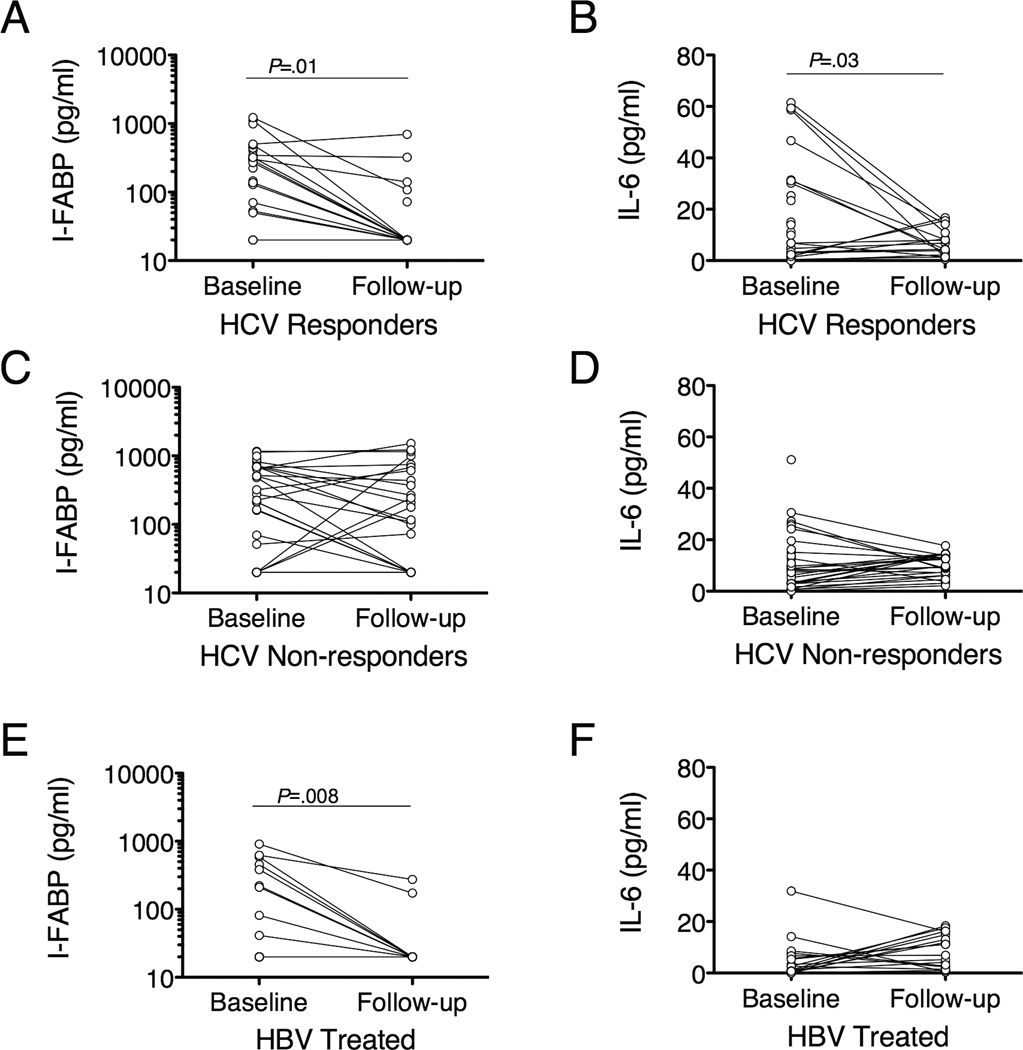
I-FABP levels in subjects with HCV infection and SVR fell from 295.4 pg/ml at baseline to undetectable at follow-up (P=0.01, Figure 4A) but did not change in non-responders (Figure 4C). IL-6 followed the same pattern (Figure 4B, 4D). In HBV-infected subjects I-FABP levels fell from 30.6 pg/ml to undetectable (P=0.008, Figure 4E), but IL-6 did not change significantly (Figure 4F). Levels of sCD14 and LPS did not change appreciably regardless of treatment response.
Figure 4.
I-FABP and IL-6 levels decline with successful treatment of HBV and HCV infections. Subjects with HCV infection with SVR have a significant decrease in I-FABP and IL-6 levels at follow-up (n=17 for both), but subjects who fail to respond to therapy have persistent elevations in IL-6 (n=25) and I-FABP (n=26). I-FABP levels fell in HBV-treated subjects but IL-6 levels did not (n=17 for both). P-values were calculated using a paired t test.
At follow-up, sCD14 levels were associated with direct bilirubin, INR, ALP, GGT, ALT, AST, AFP, APRI, total IgM, IL-6 and I-FABP and inversely associated with albumin and platelet counts (see Supplementary Table 2). IL-6 levels also correlated with LPS and markers of hepatic inflammation, fibrosis and poor synthetic function (see Supplementary Table 2). Together, these data suggest a persistent association between LPS bioactivity and hepatic inflammation, fibrosis, decreased synthetic function and portal hypertension. Thus, successful treatment of HBV and HCV infections may attenuate intestinal epithelial necrosis but does not decrease LPS or LPS-induced monocyte activation.
Baseline levels of sCD14 are associated with worse clinical outcome
The 43 subjects who responded to treatment and whose liver disease was stable had “no progression,” while the 31 with worsening cirrhosis based on clinical presentation, imaging or biochemical parameters, splenomegaly, portal hypertension, hepatic encephalopathy, hepatocellular carcinoma or need for liver transplant had “progression.” The remaining individuals had not been followed for a sufficient length of time to be classified. Four of the progressors had HBV infection, 26 were HCV-infected nonresponders with hepatic decompensation, and 1 had untreated HCV infection and hepatocellular carcinoma.
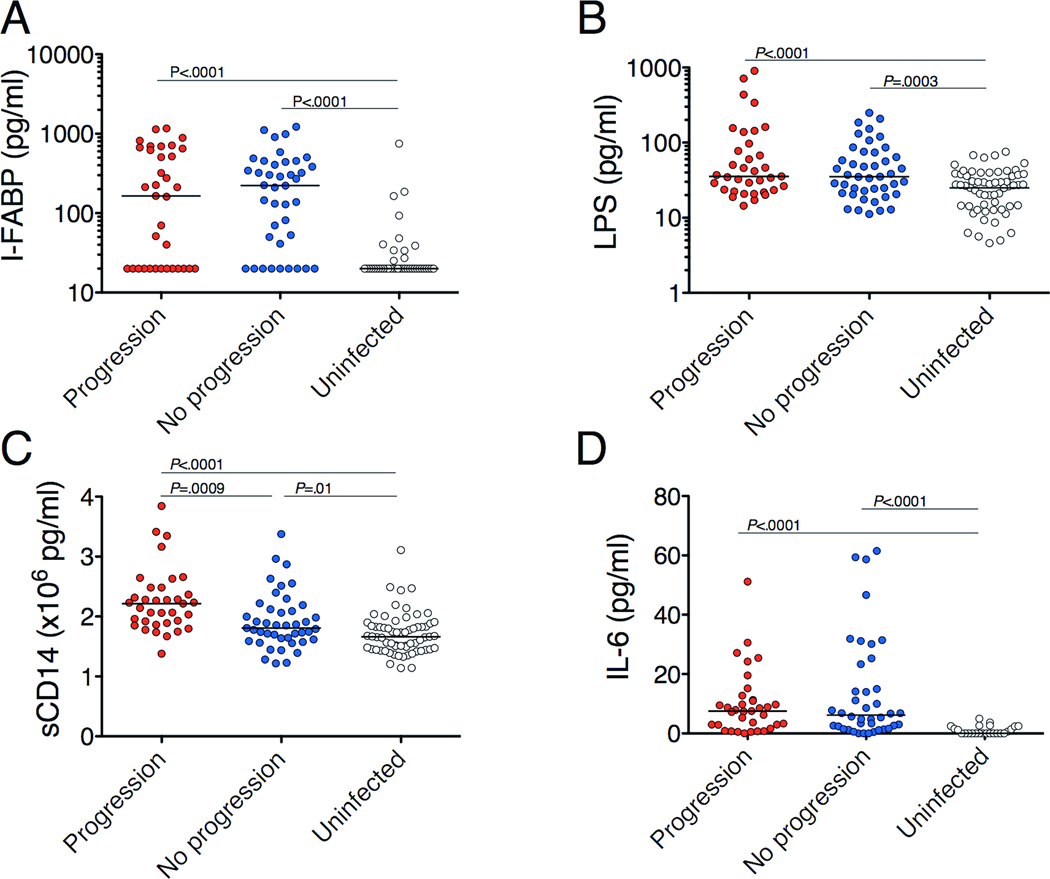
To determine whether these markers were associated with clinical outcome, subjects without progression were compared to progressors. Baseline plasma levels of IFABP, LPS, sCD14 and IL-6 were elevated in both progressors and non-progressors compared to uninfected individuals (Figure 5). However, sCD14 was significantly higher in progressors than non-progressors (2.14 × 106 pg/ml vs 1.81 × 106 pg/ml, P=0.003; Figure 5C). Furthermore, an increase in sCD14 of 1.0 × 106 pg/ml was associated with an odds ratio (OR) of disease progression of 3.7 (P=0.007) with no change after adjustment for Ishak score, AST, ALT, GGT, CRP, MELD score, APRI or HAI. sCD14 levels at follow-up were higher in progressors than non-progressors (2.37 vs 1.93 × 106 pg/ml, P=0.001). No other marker was associated with clinical outcome. Thus, high plasma levels of sCD14 predict poor outcomes in individuals with HBV or HCV infection independently of other markers of inflammation, disease progression and fibrosis.
Figure 5.
High baseline plasma sCD14 levels predict poor clinical outcome in patients with HBV and HCV infections. For LPS and sCD14, n=43 for non-progressors and n=31 for progressors. n=67 for LPS and n=65 for sCD14 for uninfected individuals. For I-FABP and IL-6, n=40 for non-progressors and n=31 for progressors. n=41 for I-FABP and n=24 for IL-6 for uninfected individuals. Horizontal lines indicate the median value. P-values were calculated with the Mann-Whitney U test.
A high density of hepatic CD14+ cells is associated with hepatic fibrosis and disease progression
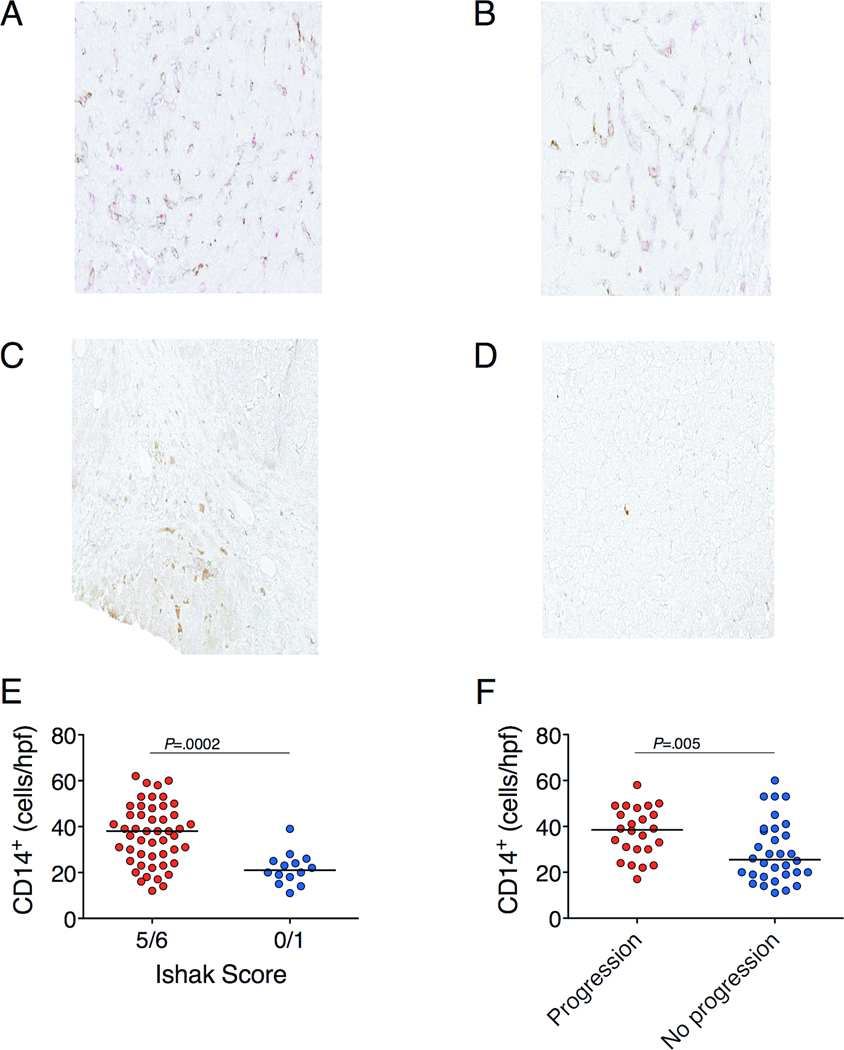
To assess whether Kupffer cell activation and the presence of bacterial products in the liver were associated with disease state and progression, we performed immunohistochemistry on liver biopsy sections taken at study enrollment. The CD14+ cells co-stained for CD68+ (Figure 6A), suggesting they were Kupffer cells. A greater number of CD14+ cells per high-powered field (hpf) was observed in subjects with severe (Figure 6A) than minimal (Figure 6B) fibrosis (median 38 vs 21, P=0.0002; Figure 6E). The number of CD14+ cells/hpf in the liver predicted which subjects would progress to end-stage liver disease (median 39 in those who progressed vs 26 in those who did not, P=0.01; Figure 6F). A high number of CD14+ cells/hpf was associated with poor synthetic function and portal hypertension, based on INR (r=0.26, P=0.04; Supplementary Figure 2A), direct bilirubin (r=0.33, P=0.008; Supplementary Figure 2B), albumin (r=−0.28, P=0.03; Supplementary Figure 2C) and platelet counts (r=−0.33, P=0.009; Supplementary Figure 2D). Furthermore, a greater number of CD14+ cells/hpf was associated with higher MELD scores (r=0.32, P=0.012; Supplementary Figure 2E) and with a higher APRI (r=0.30, P=0.018; Supplementary Figure 2F), suggestive of fibrosis. There was no difference in the number of CD14+ cells in subjects with HBV infection compared to HCV infection. Quantitative image analysis for the percentage area staining positive for CD14 did not reveal significant findings or correlate with the density of CD14+ cells, presumably because the density of CD14 staining differs among cells.
Figure 6.
(A) CD14/CD68 staining of a cirrhotic liver. Brown indicates CD14 and red CD68. (B) CD14/CD68 staining of a non-cirrhotic liver. E. coli staining of cirrhotic (C) and non-cirrhotic (D) liver is indicated by brown. (E,F) Subjects with cirrhosis (n=49) have more CD14+ cells than those without (n=14). Those who progressed (n=24) have more CD14+ cells than those who did not (n=32), regardless of fibrosis. Horizontal lines indicate the median value and 5–95% range. P-values for comparisons between groups were calculated with the Mann-Whitney U test.
As LPS induces CD14 upregulation on Kupffer cells33, the liver biopsy sections were stained with an Escherichia coli (E. coli) lysate antibody (Figure 6C-D). An increased density of E. coli antigen staining was associated with increased levels of markers of hepatic inflammation and fibrosis, namely ALT (r=0.35, P=0.007), GGT (r=0.32, P=0.017;) and ferritin (r=0.33, P=0.013).
Discussion
The factors determining the degree of hepatic inflammation and progression to cirrhosis in chronic HBV and HCV infections are not well-defined. Microbial translocation has been suggested as a contributing factor in some human studies and mouse models5–9,20. However, the association of microbial translocation and its local and systemic effects with disease severity and prognosis has not been clearly established. We found that: (1) levels of sCD14, a marker of LPS bioreactivity, distinguished subjects with severe liver fibrosis and correlated with markers of hepatic inflammation and fibrosis; (2) enterocyte death, microbial translocation, LPS-induced monocyte activation and IL-6 levels were increased in HBV and HCV infections; (3) portal hypertension, reflected by low platelet counts, was associated with increased enterocyte necrosis; (4) levels of I-FABP, reflecting enterocyte necrosis, and IL-6, a marker of hepatic inflammation and regeneration, decreased with successful treatment of HBV or HCV infection; (5) high plasma sCD14 levels predicted disease progression in HBV and HCV infection independent of other markers of hepatic inflammation, fibrosis and disease progression; and (6) a high density of CD14+ cells in the liver was associated with hepatic fibrosis and predicted disease progression.
sCD14 levels correlated with ferritin, AST, GGT, AFP, APRI, Ishak score and HAI before and after antiviral therapy, suggesting an intimate association between LPS-induced monocyte activation and hepatic inflammation and fibrosis. Given the cross-sectional nature of these associations, the determination of cause and effect is difficult. Nonetheless, it is tempting to speculate and propose a model in which increased LPS in the portal circulation would activate Kupffer cells through CD14 and MD-2/TLR4 to produce cytokines such as IL-6, TNF and pro-fibrotic TGFβ, stimulating hepatic stellate cells to synthesize collagen, resulting in hepatic fibrosis. These effects would be enhanced in patients with more CD14+ cells and higher sCD14 levels. Hepatic fibrosis would result in portal hypertension, leading to enterocyte necrosis and further potentiating microbial translocation. Indeed, sickle cell disease patients with nodular regenerative hyperplasia (NRH) and portal hypertension (T.H., unpublished observations) have high sCD14 and LPS levels. In these patients, the hepatic venous pressure gradient, indicative of sinusoidal portal hypertension, correlated with low platelet counts and high sCD14 levels, which correlated with high ALP, a marker of NRH, suggesting that microbial translocation and LPS-induced monocyte or Kupffer cell activation perpetuate a cycle culminating in hepatic fibrosis and portal hypertension, even in the absence of viral infection. Thus, increased microbial translocation and the ensuing immune activation may be both a contributor to and a consequence of hepatic fibrosis.
Importantly, we found that high plasma levels of sCD14, reflective of the host response to LPS, rather than LPS itself, predict disease progression independent of Ishak score and markers of hepatic inflammation, fibrosis and disease progression. The association of sCD14 with disease progression is similar to observations in HIV/HCV co-infected patients, in whom increased sCD14 and LPS levels were associated with the development of cirrhosis34. The detection of LPS can be complicated by its rapid clearance and by inhibitory plasma proteins11,35–38, and not all bacteria produce bioactive LPS or LPS detectable by the Limulus assay39–41. Thus, sCD14 may be a more relevant biomarker of disease progression as it reflects the host response to products of microbial translocation. Higher sCD14 levels may reflect more cells responding to LPS or a genetic predisposition towards increased LPS responsiveness. Indeed, high sCD14 levels in the setting of alcohol abuse42 or HCV infection HCV infection43 have been associated with a polymorphism in the promoter region of the CD14 gene (−159C/T). LPS-induced monocyte activation may increase systemic cytokine production, particularly as both circulating CD14+CD16+ monocytes and hepatic macrophages are increased in cirrhosis44. High sCD14 levels may facilitate LPS-induced activation of endothelial14,45 and dendritic cells14 via the MD-2/TLR4 complex, leading to further production of pro-inflammatory cytokines. Thus, it is not the mere presence of the inflammatory stimulus that determines pathology but rather the host’s response to that stimulus. The association of sCD14 with worsening hepatic disease parallels findings in HIV infection in which sCD14, not LPS, predicts mortality, suggesting a common pathway in which activation of the immune system by microbial products accelerates the progression of a variety of diseases46.
While previous studies have demonstrated that LPS induces liver injury based on portal vein concentrations of LPS47, we detected E. coli proteins in the liver parenchyma, directly demonstrating that bacterial products drain into the liver in humans with chronic viral hepatitis infection. However, we found that the density of CD14+ cells in the liver, likely Kupffer cells which upregulate CD14 upon LPS stimulation20,33, but not the density of E. coli proteins was associated with severity of fibrosis and disease progression. This finding supports the concept that the host response to the stimulus, rather than the stimulus itself, is associated with pathology.
Taken together, our data show that LPS-induced activation of both circulating monocytes and resident Kupffer cells is associated with severe hepatic fibrosis and failure to respond to therapy and predicts progression to end-stage liver disease independent of the degree of fibrosis. Thus, attenuation of microbial translocation and its inflammatory consequences may improve clinical outcomes in HBV and HCV infections.
Methods
Study design
After informed consent, 84 patients with matched plasma and biopsy samples from enrollment with stored post-antiviral treatment plasma samples were selected from an IRB-approved study of a viral hepatitis cohort with chronic HBV (n=21) or chronic HCV (n=63) infection undergoing standard-of-care anti-viral treatment by the Liver Diseases Branch of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the National Institutes of Health (91-DK-0214). Liver biopsy specimens were evaluated and scored using the Ishak and HAI scoring system by one blinded hepatopathologist (DK). As the primary comparison to be explored was between patients with cirrhosis and those without, subjects were chosen with either minimal (Ishak 0 and 1) or severe (Ishak 5 and 6) liver fibrosis. To minimize sampling error, samples from subjects with Ishak 0 and 1 were evaluated together, as were subjects with Ishak 5 and 6. Based on a standard deviation of sCD14 of 0.30 × 106 pg/ml and a Type I error rate of 5% with 16 subjects in each arm, we would have 80% power to detect a difference as small as 0.31 × 106 pg/ml between groups. Similarly, we would have 80% power to detect a difference of I-FABP of 123 pg/ml, LPS of 18.4 pg/ml and IL-6 of 1.48 pg/ml.
No individuals were on therapy at the initial evaluation. Individuals with chronic HBV infection received interferon-based regimens and/or nucleoside or nucleotide analogues and were on a nucleoside or nucleotide analogue at follow-up. Individuals with chronic HCV infection received either interferon or PEG-interferon with or without ribavirin and were off therapy for at least six months at follow-up. Patients with decompensated liver disease underwent liver transplant soon after enrollment and were therefore not included in the study.
In order to establish reference values for the markers measured here, we recruited a cohort of 35 young, healthy volunteers not infected with HIV, HCV or HBV from the Vaccine Research Center Clinic at the National Institutes of Health and 32 individuals from the University of California San Francisco (for the 63 samples with accessible data, median 35 years old [IQR 27–43], 52% male).
Biomarkers of microbial translocation, LPS bioactivity and enterocyte damage
I-FABP, LPS, sCD14 and IL-6 were measured in baseline plasma samples prior to the initiation of therapy and at follow-up after treatment for the underlying viral infection. All assays were performed blinded to type of infection, Ishak score, clinical laboratory values and clinical outcome; each test was determined in duplicate, and the average of each marker was calculated.
I-FABP (Cell Sciences, Canton, Massachusetts, USA), sCD14 (R&D Systems, Minneapolis, Minnesota, USA) and IL-6 (R&D Systems, Minneapolis, Minnesota, USA) were performed using commercially available ELISA assay kits according to the manufacturers’ protocols on plasma diluted to 50%, 0.5% and undiluted, respectively. For LPS measurement, the plasma was diluted to 10% in endotoxin-free water and quantified using the Limulus Amebocyte Lysate assay (Lonza, Basel, Switzerland), as previously described46.
Liver fibrosis and function
The degree of liver disease was defined based on a number of assays that are associated with hepatic inflammation, injury and/or fibrosis, e.g. ALT48, AST49, GGT50,51, ferritin52,53, AFP54 and ALP55; those traditionally associated with poor synthetic function, e.g. albumin, prothrombin time, INR55 and direct bilirubin56; and portal hypertension, e.g. platelets57.
Immunohistochemistry
Sixty-three patients had liver biopsy sections preserved in paraffin available for immunohistochemistry. After deparaffinizing the slides, antigen retrieval was performed followed by staining with Novocastra anti-CD14 (Leica Microsystems, Bannockburn, IL, USA) and anti-CD68 (Abcam, Cambridge, MA, USA) and developed with the EnVision G|2 Doublestain System using diaminobenzidine and Permanent Red (Dako, Glostrup, Denmark). In a different set of slides, antigen retrieval was performed followed by staining with a polyclonal rabbit antibody to an Escherichia coli lysate from Dako which recognizes a minimum of 80 antigens, and the slides were developed with DAB. All slides were counter-stained with Methyl Green (Vector Laboratories, Burlinghame, CA, USA). The number of CD14+ cells/hpf were counted in 10 different fields and averaged by the same blinded operator for all slides. The reliability of the staining was confirmed by comparison with positive and negative control tissues.
Quantitative image analysis was performed by scanning the slides using an Aperio ScanScope and eliminating edge effect by removing approximately 50 pixels around the perimeter of every section using Adobe Photoshop. An algorithm was established to identify CD14+ pixels and E. coli+ pixels in the respective slides as well as to differentiate areas of the slide containing tissue from those that are blank. The percentage of pixels CD14+ or E. coli+ in the entire slide was divided by the percentage of pixels of tissue to calculate the percentage of tissue that stained positively for the respective marker. All analyses were performed blinded to the origin of the sample.
Statistical Methods
Pairs of variables were compared using the Mann-Whitney U test, and correlations among variables were evaluated using Spearman’s rank correlation. These analyses were performed using GraphPad Prism, version 5.0b (GraphPad Software, Inc., La Jolla, California, USA). Power calculations and odds ratio of disease progression were calculated by logistic regression analysis using JMP version 7.0 (SAS Inc., Cary, North Carolina, USA).
Supplementary Material
Acknowledgements
We thank the members of the Cleveland Immunopathogenesis Consortium for their helpful discussions, Levelle Harris, Jason Brenchley, Jacob Estes and David Morcock for their assistance with quantitative image analysis, and Martha Nason for her assistance with statistical approaches.
Grant support: The intramural programs of NIAID, NIDDK and NCI (NIH), NIH grant AI-76174.
Abbreviations
- AFP
alpha-fetoprotein
- ALP
alkaline phosphatase
- APRI
AST:platelet ratio index
- CRP
C-reactive protein
- E. coli
Escherichia coli
- GGT
gamma-glutamyl transpeptidase
- HSC
hepatic stellate cells
- I-FABP
intestinal fatty acid binding protein
- IL
interleukin
- INR
international normalized ratio
- LPS
lipopolysaccharide
- mCD14
membrane CD14
- MD-2
myeloid differentiation-2
- sCD14
soluble CD14
- SVR
sustained virologic response
- TGFβ
transforming growth factor β
- TLR4
toll-like receptor 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
Author contributions:
NGS: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis
CK: study concept and design; acquisition of data
AR: acquisition of data; analysis and interpretation of data; statistical analysis
JLE: acquisition of data
RBS: analysis and interpretation of data
MD: acquisition of data
DEK: acquisition of data; analysis and interpretation of data
SGD: acquisition of data; study supervision; obtained funding; critical revision of the manuscript for important intellectual content
TJL: acquisition of data; study supervision; obtained funding
TH: study concept and design; acquisition of data; study supervision; obtained funding; critical revision of the manuscript for important intellectual content
DCD: study concept and design; obtained funding; drafting of the manuscript; critical revision of the manuscript for important intellectual content
References
- 1. http://www.cdc.gov/hepatitis/HBV/HBVfaq.htm.
- 2. http://www.who.int/vaccine_research/diseases/hepatitis_c/en/
- 3.Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol. 2002;8:961–965. doi: 10.3748/wjg.v8.i6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jirillo E, Caccavo D, Magrone T, et al. The role of the liver in the response to LPS: experimental and clinical findings. J Endotoxin Res. 2002;8:319–327. doi: 10.1179/096805102125000641. [DOI] [PubMed] [Google Scholar]
- 5.Dolganiuc A, Norkina O, Kodys K, et al. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology. 2007;133:1627–1636. doi: 10.1053/j.gastro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaeta GB, Perna P, Adinolfi LE, et al. Endotoxemia in a series of 104 patients with chronic liver diseases: prevalence and significance. Digestion. 1982;23:239–244. doi: 10.1159/000198756. [DOI] [PubMed] [Google Scholar]
- 7.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- 8.Urbaschek R, McCuskey RS, Rudi V, et al. Endotoxin, endotoxin-neutralizing-capacity, sCD14, sICAM-1, and cytokines in patients with various degrees of alcoholic liver disease. Alcohol Clin Exp Res. 2001;25:261–268. [PubMed] [Google Scholar]
- 9.Harte AL, da Silva NF, Creely SJ, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7:15. doi: 10.1186/1476-9255-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heumann D, Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clin Chim Acta. 2002;323:59–72. doi: 10.1016/s0009-8981(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 11.Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res. 2005;11:225–229. doi: 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- 12.Frey EA, Miller DS, Jahr TG, et al. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pugin J, Schurer-Maly CC, Leturcq D, et al. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci U S A. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verhasselt V, Buelens C, Willems F, et al. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919–2925. [PubMed] [Google Scholar]
- 15.Gioannini TL, Weiss JP. Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells. Immunol Res. 2007;39:249–260. doi: 10.1007/s12026-007-0069-0. [DOI] [PubMed] [Google Scholar]
- 16.Miyake K. Roles for accessory molecules in microbial recognition by Toll-like receptors. J Endotoxin Res. 2006;12:195–204. doi: 10.1179/096805106X118807. [DOI] [PubMed] [Google Scholar]
- 17.Landmann R, Knopf HP, Link S, et al. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64:1762–1769. doi: 10.1128/iai.64.5.1762-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiki N, Berger D, Prigl C, et al. Endotoxin binding and elimination by monocytes: secretion of soluble CD14 represents an inducible mechanism counteracting reduced expression of membrane CD14 in patients with sepsis and in a patient with paroxysmal nocturnal hemoglobinuria. Infect Immun. 1998;66:1135–1141. doi: 10.1128/iai.66.3.1135-1141.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bazil V, Strominger JL. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J Immunol. 1991;147:1567–1574. [PubMed] [Google Scholar]
- 20.Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256–G265. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- 21.Rivera CA, Bradford BU, Hunt KJ, et al. Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol. 2001;281:G200–G207. doi: 10.1152/ajpgi.2001.281.1.G200. [DOI] [PubMed] [Google Scholar]
- 22.Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 24.Schnabl B, Brandl K, Fink M, et al. A TLR4/MD2 fusion protein inhibits LPS-induced pro-inflammatory signaling in hepatic stellate cells. Biochem Biophys Res Commun. 2008;375:210–214. doi: 10.1016/j.bbrc.2008.07.150. [DOI] [PubMed] [Google Scholar]
- 25.Lok AS, McMahon BJ, Chronic hepatitis B. Update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 26.Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 28.Ockner RK, Manning JA. Fatty acid-binding protein in small intestine. Identification, isolation, and evidence for its role in cellular fatty acid transport. J Clin Invest. 1974;54:326–338. doi: 10.1172/JCI107768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohn SM, Simon TC, Roth KA, et al. Use of transgenic mice to map cis-acting elements in the intestinal fatty acid binding protein gene (Fabpi) that control its cell lineage-specific and regional patterns of expression along the duodenal-colonic and crypt-villus axes of the gut epithelium. J Cell Biol. 1992;119:27–44. doi: 10.1083/jcb.119.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelsers MM, Namiot Z, Kisielewski W, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. 2003;36:529–535. doi: 10.1016/s0009-9120(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 31.Hosel M, Quasdorff M, Wiegmann K, et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology. 2009;50:1773–1782. doi: 10.1002/hep.23226. [DOI] [PubMed] [Google Scholar]
- 32.Cressman DE, Greenbaum LE, DeAngelis RA, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 33.Matsuura K, Ishida T, Setoguchi M, et al. Upregulation of mouse CD14 expression in Kupffer cells by lipopolysaccharide. J Exp Med. 1994;179:1671–1676. doi: 10.1084/jem.179.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226–233. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cross AS, Opal SM, Warren HS, et al. Active immunization with a detoxified Escherichia coli J5 lipopolysaccharide group B meningococcal outer membrane protein complex vaccine protects animals from experimental sepsis. J Infect Dis. 2001;183:1079–1086. doi: 10.1086/319297. [DOI] [PubMed] [Google Scholar]
- 36.Kitchens RL, Thompson PA, Viriyakosol S, et al. Plasma CD14 decreases monocyte responses to LPS by transferring cell-bound LPS to plasma lipoproteins. J Clin Invest. 2001;108:485–493. doi: 10.1172/JCI13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ketchum PA, Novitsky TJ. Assay of Endotoxin by Limulus Amebocyte Lysate. Methods in Molecular Medicine. 2000;36:3–12. doi: 10.1385/1-59259-216-3:3. [DOI] [PubMed] [Google Scholar]
- 38.Eichbaum EB, Harris HW, Kane JP, et al. Chylomicrons can inhibit endotoxin activity in vitro. J Surg Res. 1991;51:413–416. doi: 10.1016/0022-4804(91)90143-a. [DOI] [PubMed] [Google Scholar]
- 39.Sveen K, Hofstad T, Milner KC. Lethality for mice and chick embryos, pyrogenicity in rabbits and ability to gelate lysate from amoebocytes of Limulus polyphemus by lipopolysaccharides from Bacteroides, Fusobacterium and Veillonella. Acta Pathol Microbiol Scand B. 1977;85B:388–396. doi: 10.1111/j.1699-0463.1977.tb01994.x. [DOI] [PubMed] [Google Scholar]
- 40.Hofstad T, Sveen K, Dahlen G. Chemical composition, serological reactivity and endotoxicity of lipopolysaccharides extracted in different ways from Bacteroides fragilis, Bacteroides melaninogenicus and Bacteroides oralis. Acta Pathol Microbiol Scand B. 1977;85:262–270. doi: 10.1111/j.1699-0463.1977.tb01972.x. [DOI] [PubMed] [Google Scholar]
- 41.Alhawi M, Stewart J, Erridge C, et al. Bacteroides fragilis signals through Toll-like receptor (TLR) 2 and not through TLR4. J Med Microbiol. 2009;58:1015–1022. doi: 10.1099/jmm.0.009936-0. [DOI] [PubMed] [Google Scholar]
- 42.Campos J, Gonzalez-Quintela A, Quinteiro C, et al. The -159C/T polymorphism in the promoter region of the CD14 gene is associated with advanced liver disease and higher serum levels of acute-phase proteins in heavy drinkers. Alcohol Clin Exp Res. 2005;29:1206–1213. doi: 10.1097/01.alc.0000171977.25531.7a. [DOI] [PubMed] [Google Scholar]
- 43.Meiler C, Muhlbauer M, Johann M, et al. Different effects of a CD14 gene polymorphism on disease outcome in patients with alcoholic liver disease and chronic hepatitis C infection. World J Gastroenterol. 2005;11:6031–6037. doi: 10.3748/wjg.v11.i38.6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmermann HW, Seidler S, Nattermann J, et al. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One. 2010;5:e11049. doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haziot A, Rong GW, Silver J, et al. Recombinant soluble CD14 mediates the activation of endothelial cells by lipopolysaccharide. J Immunol. 1993;151:1500–1507. [PubMed] [Google Scholar]
- 46.Sandler NG, Wand H, Roque A, et al. Plasma Levels of Soluble CD14 Independently Predict Mortality in HIV Infection. J Infect Dis. 2011 Mar 15;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Deventer SJ, Knepper A, Landsman J, et al. Endotoxins in portal blood. Hepatogastroenterology. 1988;35:223–225. [PubMed] [Google Scholar]
- 48.Gordon CP, Keller PA. Control of hepatitis C: a medicinal chemistry perspective. J Med Chem. 2005;48:1–20. doi: 10.1021/jm0400101. [DOI] [PubMed] [Google Scholar]
- 49.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 50.Silva IS, Ferraz ML, Perez RM, et al. Role of gamma-glutamyl transferase activity in patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2004;19:314–318. doi: 10.1111/j.1440-1746.2003.03256.x. [DOI] [PubMed] [Google Scholar]
- 51.Imbert-Bismut F, Ratziu V, Pieroni L, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 52.Metwally MA, Zein CO, Zein NN. Clinical significance of hepatic iron deposition and serum iron values in patients with chronic hepatitis C infection. Am J Gastroenterol. 2004;99:286–291. doi: 10.1111/j.1572-0241.2004.04049.x. [DOI] [PubMed] [Google Scholar]
- 53.Romagnuolo J, Andrews CN, Bain VG, et al. Simple clinical variables predict liver histology in hepatitis C: prospective validation of a clinical prediction model. Scand J Gastroenterol. 2005;40:1365–1371. doi: 10.1080/00365520500287400. [DOI] [PubMed] [Google Scholar]
- 54.Taketa K. Alpha-fetoprotein: reevaluation in hepatology. Hepatology. 1990;12:1420–1432. doi: 10.1002/hep.1840120625. [DOI] [PubMed] [Google Scholar]
- 55.Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. Cmaj. 2005;172:367–379. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bambha KM, Biggins SW. Inequities of the Model for End-Stage Liver Disease: an examination of current components and future additions. Curr Opin Organ Transplant. 2008;13:227–233. doi: 10.1097/MOT.0b013e3282ff84c7. [DOI] [PubMed] [Google Scholar]
- 57.Ito K, Shiraki K, Sakai T, et al. Portal hypertensive colopathy in patients with liver cirrhosis. World J Gastroenterol. 2005;11:3127–3130. doi: 10.3748/wjg.v11.i20.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.