Abstract
The discovery of the T1R family of Class C G protein-coupled receptors in the peripheral gustatory system a decade ago has been a tremendous advance for taste research, and its conceptual reach has extended to other organ systems. There are three proteins in the family, T1R1, T1R2, and T1R3, encoded by their respective genes, Tas1r1, Tas1r2, and Tas1r3. T1R2 combines with T1R3 to form a heterodimer that binds with sugars and other sweeteners. T1R3 also combines with T1R1 to form a heterodimer that binds with L-amino acids. These proteins are expressed not only in taste bud cells, but one or more of these T1Rs have also been identified in the nasal epithelium, gut, pancreas, liver, kidney, testes and brain in various mammalian species. Here we review current perspectives regarding the functional role of these receptors, concentrating on sweet taste and feeding. We also discuss behavioral findings suggesting that a glucose polymer mixture, Polycose, which rodents avidly prefer, appears to activate a receptor that does not depend on the combined expression of T1R2 and T1R3. In addition, although the T1Rs have been implicated as playing a role in glucose sensing, T1R2 knock-out (KO) and T1R3 KO mice display normal chow and fluid intake as well as normal body weight compared with same-sex littermate wild type (WT) controls. Moreover, regardless of whether they are fasted or not, these KO mice do not differ from their WT counterparts in their Polycose intake across a broad range of concentrations in 30-min intake tests. The functional implications of these results and those in the literature are considered.
Keywords: gustatory system, T1R1, T1R2, T1R3, sweet taste, Polycose, glucose sensing, nutrient sensing
INTRODUCTION
Over the last decade, the field of gustatory science has benefited greatly from the discovery of the receptor proteins that bind with sugars, synthetic sweeteners, some sweet-tasting proteins, and amino acids. For many years, it was known that such compounds generate activity in taste receptor cells, which in turn stimulate peripheral afferents projecting to the central gustatory pathway, but exactly how these responses were initiated remained unknown. It is now clear that in humans and the other mammals studied (mostly rodents), the receptor proteins responsible for the generation of the signals that are ultimately interpreted as “sweetness” and those leading to the so called “umami” taste associated with some L-amino acids, especially L-glutamate, are members of the T1R family consisting of a group of three Class-C G protein-coupled receptors: T1R1, T1R2, and T1R3. Each of these proteins is encoded by their respective genes: Tas1r1, Tas1r2, and Tas1r3 [4, 37, 46, 60–61, 67, 83]. In the apical membrane of taste receptors cells, T1R3 combines with T1R1 to form a heterodimer (T1R1+3) that binds with L-amino acids and it combines with T1R2 to form a heterodimer (T1R2+3) that binds with sugars, a subset of D-amino acids, artificial sweeteners, and certain sweet-tasting proteins [54, 67, 116]. The exact binding profiles vary somewhat across the mammalian species studied and, as expected, these differences correlate with behavioral responses. For example, rats do not prefer the artificial sweetener aspartame nor treat it as sucrose-like [72, 90], and aspartame does not bind with the rat T1R2+3 [54, 67]. In the domestic cat the Tas1r2 is an unexpressed pseudogene, a finding that likely explains the lack of preference for sweeteners by this species [53].
Although it is uncontested that the T1R proteins play a large role in sweet and umami taste, it has been questioned whether these receptors are both necessary and sufficient for the generation of normal qualitative taste perception to their respective ligands. In other words, are there other taste receptors that bind with these same ligands and contribute to perception as well? Moreover, it has now been shown that the T1R proteins are expressed in non-gustatory tissue such as the nasal epithelium, the gut, the pancreas, and the brain. What is the functional role of these proteins in these other tissues? The following pages will review the literature in the context of addressing these questions.
T1R LIGAND BINDING PROFILES
Heterologous Expression System: T1R2+3
Most of what is known about ligand selectivity of the T1R heterodimers is based on data from heterologous expression systems [54, 67, 116]. When cells from the Human Embryonic Kidney-293 line (HEK-293; or from the variant HEK293T) expressing promiscuous G-proteins are transfected with both rat T1R2 and T1R3, the application of stimuli from a panel of mono-and disaccharides, artificial sweeteners, and a subset of D-amino acids that are considered sweet-tasting by humans [85] and that rodents prefer [3, 5, 76] results in intracellular calcium responses [54, 66–67]. Co-expression of human T1R2 and T1R3 leads to similar results, except that responses to sweet-tasting proteins (e.g., monellin and thaumatin) and to some artificial sweeteners (including aspartame and cyclamate), not seen in cells transfected with rat receptors, were observed [54]. With respect to the stimuli that were effective at stimulating calcium responses from cells transfected with the human, but not rat, T1R2+3 receptors, as one might predict, humans find those compounds sweet, but rodents do not treat them as sucrose-like [eg. 5, 71, 90–91]. Similarly, lactisole, a compound that inhibits sweetness in humans [42] but appears to be ineffective in rodents [94, 115], inhibits responses to sucrose in cells transfected with human, but not rat, T1R2 and T1R3. Importantly, transfection of only a single T1R subunit does not lead to calcium responses [67]. Cells transfected with human or rat T1R2 and T1R3 are not activated by bitter-tasting compounds or L-amino acids, but they are stimulated by the achiral amino acid glycine, which is sweet-tasting [54].
Heterologous Expression System: T1R1+3
HEK-293 cells transfected with both mouse T1R1 and T1R3 are stimulated by L-amino acids as well as glycine; D-amino acids are generally ineffective (except for perhaps D-alanine) [54, 67]. The mouse T1R1+3 appears to bind generally with common L-amino acids and responsiveness by cells transfected with the heterodimer are enhanced by the application of the 5’-ribonucleotide, inosine monophosphate (IMP) [54, 67], which is known to amplify, in vivo, behavioral and neural responses to these same stimuli in rodents [e.g. 16, 66, 117–118]. Interestingly, the human T1R1+3 appears to be much more narrowly tuned than its rodent counterpart and only binds with L-glutamate and L-aspartate and the responses of T1R1+3-transfected cells are enhanced by addition of both IMP and guanosine monophosphate (GMP) [54]. The difference between the rodent and human in the ligand binding specificity of the T1R1+3 receptor raises the possibility that the taste perceptions generated by various L-amino acids may not be completely concordant across these specific mammalian species. Transfection of only one of the T1R subunits in all the aforementioned studies with heterologous expression systems was ineffective, at least with the ligands tested [54, 67], supporting the view that these proteins function in heterodimeric form.
Ligand Binding Domains
It has been proposed that there are different ligand binding domains for the T1Rs which may explain how a single receptor can interact with structurally diverse compounds that humans label as sweet. For example, the T1R2 N-terminal extracellular domain appears to be the binding site for aspartame and neotame and G protein coupling requires the transmembrane segment of T1R2 [116]. It appears the T1R3 transmembrane domain interacts with cyclamate, lactisole [42, 116], and the sweetener, neohesperidin dihydrochalcone [114]. Neoculin and brazzein, compounds described as sweet by humans, are thought to bind to the N-terminal domain of human T1R3 [48] and the cysteine-rich region of human T1R3 [43] respectively. Furthermore, in a study in which purified recombinant proteins were used, conformational changes in both the T1R2 and T1R3 N-terminal domains were observed in response to sucralose, sucrose and glucose, suggesting the binding of these ligands involves both subunits [68]. Less has been reported regarding the binding domains of the T1R1+3 heterodimer thought to mediate umami taste. It has been proposed that glutamate binds to a site on the Venus flytrap domain of T1R1 and 5’-purine nucleotides such as IMP and GMP bind to an adjacent site and stabilizes the closed conformational form [121].
LOCALIZATION OF T1R EXPRESSION
Gustatory Tissue
Although T1R1, T1R2, and T1R3 can be found in taste buds of the anterior tongue, posterior tongue and palate, the relative expression of each T1R subunit varies across taste receptor fields [37, 45–46, 51, 60–61, 83]. In an initial study of expression patterns of T1R1 and T1R2 that combined mouse and rat tissues, T1R1 was found to be expressed in taste bud cells from fungiform papillae, which are found in the anterior two-thirds of the tongue and palate, and expressed to a lesser extent in the taste bud cells of the circumvallate and foliate papillae in the posterior tongue. Distribution of T1R2 was more densely expressed in the circumvallate and foliate papillae and only moderately in the geschmacksstreifen of the palate and less so in the fungiform papillae [37]. In the rat, the relative lack of T1R2 expression in taste bud cells of the fungiform papillae is consistent with the relatively low response to sucrose of the chorda tympani (CT) nerve, a branch of cranial nerve VII that innervates the anterior tongue [e.g.,34, 65, 75]. Unlike in the rat, however, the CT in the mouse responds relatively well to sucrose and other sweeteners [eg. 14, 39, 123], which contrasts with the relative lack of T1R2 expression in fungiform tissue; in another study, however, a denser T1R2 expression was observed in the fungiform taste buds of mice [45]. In palatal taste buds of mice, T1R2 and T1R3 positive cells have been found [107], but it would also be instructive to examine T1R expression specifically in the taste buds in the rat incisive papilla at the opening of the nasoincisor ducts, because sucrose placed on this palatal field is particularly effective at stimulating neural activity in the gustatory system [109–110].
In the mouse, T1R3 has also been shown to be expressed in both the fungiform and circumvallate papillae [45–46, 60–61, 67, 83]. To our knowledge, the relative degree of expression of the T1R proteins in the taste buds of the laryngeal epithelium, which are innervated by the superior laryngeal nerve and thought to be critical in the protection of the airways, has yet to be examined. From a stimulus coding perspective, it is of interest to ask to what degree are these T1R subunits co-expressed with each other, as well as with other receptor proteins serving transduction mechanisms for stimuli associated with other taste qualities. Consistent with the view that the T1R proteins function in heterodimeric form, there is a high degree of co-expression of T1R3 with either T1R1 or T1R2 in taste receptor cells [46, 60–61, 67]. In the mouse circumvallate, a high proportion of taste receptor cells that express T1R3 also express T1R2 or T1R1, but this proportion is smaller in the fungiform papillae [45]. This raises the possibility that, at least in the anterior tongue, there may be a subset of T1R3 expressing cells that do not express other T1R proteins, supporting the hypothesis that the population of T1R receptor types may include T1R3 acting as a homodimeric receptor complex [67]. Importantly, the proportion of cells expressing all three T1R subunits is very low and thus there appears to be a segregation of signals for L-amino acids vs. common sweeteners at the first stage of taste stimulus processing in the periphery. All of the T1Rs appear to be selectively expressed in Type II cells in taste buds [24], which do not possess conventional synapses with afferent fibers, which are a characteristic of Type III cells [119], but appear to stimulate intragemmal axons by releasing ATP [25].
Double-labeling experiments using probes for T2Rs, which bind with bitter-tasting ligands, and T1Rs show no overlap in the expression of these receptor families [2, 67]. Similarly, there appears to be no coexpression of T1R3 and PKD2L1, an ion channel implicated in taste transduction of acid stimuli [38]. The segregation of these receptor populations in non-overlapping sets of taste bud cells suggests that the peripheral gustatory system selectively channels at least some neural signals generated by stimuli that are associated with different perceptual taste qualities [see 11, 103].
Non-gustatory Tissue
Interestingly, the T1R proteins are expressed in a variety of non-gustatory tissues as well, provoking questions as to what other functional role(s) they serve. Given their ligand binding characteristics, they are well suited to provide information about the presence of sugars (and other sweeteners), in the case of the T1R2+3 heterodimer, and the presence of L-amino acids, in the case of the T1R1+3 heterodimer, in the local environment of the cell. If the oral cavity is considered an extension of the gastrointestinal tract, it is not surprising that T1Rs have been shown to also be expressed in gastrointestinal tissue. The expression of T1R1 and T1R3 has been detected in the stomach of mouse and human [6, 35]. All three T1R members have been found expressed in the duodenum of mouse and human [6, 20, 41, 59], the jejunum of the rat [57], mouse and human [6, 20], and the ilium of the mouse [20]. Expression of T1R2 and T1R3 was found in pig intestinal epithelium [47, 63] and T1R3 has been found expressed in human intestinal endocrine cell lines [82]. In colon cells of mouse and human T1R1, T1R2 and T1R3 have been detected [6, 82]. Expression of T1R3 has also been detected in kidney of the pig [47], as well as in human liver and pancreas [108]. Pancreatic islet cells in mice as well as the beta cell line MIN6 have been shown to express T1R2 and T1R3 [64]. Additionally, moderate levels of T1R3 have been found in the testis of mouse and pig [46–47, 60]. Also, T1R3 appears to be expressed in solitary chemoreceptor cells (a taste receptor cell-like chemosensory cell) of the nasal epithelium in mouse [74]. Interestingly, these same T1R3-expressing solitary chemoreceptor cells co-expressed T2R5 and T2R8, receptors respectively binding with the bitter-tasting ligands propylthiouracil and cycloheximide [74]; co-expression of T1Rs with T2Rs does not occur in the gustatory system [2, 37, 67].
To our knowledge, quantitative data regarding the level of co-expression of T1R subunits in non-gustatory tissue is lacking, which leaves open the question as to whether these proteins form the same type of functional heterodimer receptors as in the oral cavity. In human duodenal tissue, some co-expression of T1R2 and T1R3 has been shown in enteroendocrine L-cells [41]. Measures taken from duodenum and colon tissue indicate some co-expression of taste signal transduction proteins, but there is heterogeneity across the cells [6]. In the rat jejunum, T1R1 and T1R3 are found colocalized with transducin more frequently than with α-gustducin [57] raising the possibility that these proteins may form receptors that function differently than those in gustatory tissue. For example, perhaps T1R3 can effectively function as a homodimer in gastrointestinal tissue.
In the mouse brain, all three T1R members have been detected, with the highest levels found in the hypothalamus, notably in the arcuate nucleus and the paraventricular nucleus, compared with cortex and hippocampus [81]. Furthermore, fasting appears to affect expression levels of T1R1 and T1R2 in the mouse hypothalamus [81]. Rats maintained on a high-fat diet over a period of 6 weeks displayed decreased T1R3 expression in circumvallate taste buds compared with rats on a control diet [12]. These recent examples in the literature suggest a possible link between nutritional status and T1R expression (see below for more discussion).
TASTE FUNCTION
Neural Signaling in the Gustatory System
The generation of knock-out (KO) mice in which the genes encoding for specific T1R proteins have been deleted has been the primary means by which the functional role of these receptors has been assessed. Such experiments do not yield data on the sufficiency of the T1R proteins in taste function, but they do provide a test of their necessity, notwithstanding the caveat of potential ontogenetic compensation associated with genetic knock-out preparations. Electrophysiological recordings from the chorda tympani nerve (CT), which innervates the anterior tongue, and the glossopharyngeal nerve (GL), cranial nerve IX, which innervates the posterior tongue, show that T1R2 KO and T1R3 KO mice are entirely unresponsive to artificial sweeteners including, Na-saccharin, SC45647 and sucralose [13, 122]. It is true that the GL in T1R3 KO mice displays some degree of responsiveness to AceK [13], but this is evident at concentrations for which the ligand could possibly activate T2R receptors (see below) or for which the potassium cation could affect the response.
Although responses to natural sugars including glucose, maltose, fructose and sucrose are severely reduced in the CT and GL of T1R2 KO and T1R3 KO mice compared with WT mice, there is evidence that these nerves have weak responses to the sugars at higher concentrations [13, 73, 122–123]. Furthermore, while CT responses to natural sugars are severely attenuated, those to Polycose, a mixture of glucose polymers with an average molecular weight of 1000, are very similar between T1R3 KO and WT mice [123]. This latter finding suggests that longer chain glucose polymers might bind with a yet to be identified taste receptor different from those that sugars activate, as initially hypothesized by Sclafani several decades ago [88].
The findings from T1R2 and T1R3 KO preparations match electrophysiological measures taken from the CT and GL of mice from the 129.B6-Tas1r3 segregating congenic strain. These mice have the allele of the Tas1r3 gene from 129 mice, a strain which appears to be less sensitive to low concentrations of sweeteners, placed on a C57BL/6 (B6) genetic background. The allelic variation of the Tas1r3 gene has been shown to influence responsiveness to many compounds that humans describe as sweet, but does not appear to contribute to Polycose taste responses [39]. In contrast to the residual responses to sugars seen in single T1R KOs, the CT in mice for which both T1R2 and T1R3 are genetically deleted appears to be entirely unresponsive to all sweeteners (sugars and artificial sweeteners) tested but responds normally to compounds representing other basic taste qualities (quinine, NaCl and citric acid representing tastes that humans describe as “bitter”, “salty” and “sour,” respectively) [122]. The latter finding supports the view that T1R2 and T1R3 can possibly be forming homodimers that are maintaining some degree of function to some ligands, albeit in a highly compromised fashion.
Very little work has been conducted to date examining taste responses in the central gustatory system of T1R KO mice. In the only study of which we are aware, taste responses of neurons in the nucleus of the solitary tract (NST), the first relay of ascending gustatory information in the brainstem, were significantly lower in T1R3 KO mice to all sweeteners presented relative to WT mice [52]. Responses to sugars (sucrose, fructose and glucose) were near zero, but substantial residual activity was observed to artificial sweeteners (Na-saccharin and AceK) and glycine at the concentrations tested. Given that both Na-Saccharin and AceK have been shown to bind with human T2Rs, the receptors which mediate responses to ligands that are bitter tasting to humans [50, 72, 78, 96], and also contain cations that can potentially stimulate salt taste transduction pathways, it is quite possible that the residual responsiveness of NST neurons to these artificial sweeteners is mediated by receptor mechanisms that are not associated with sucrose-like (i.e., “sweet”) taste. The significant residual responsiveness to glycine, however, is somewhat unexpected. Glycine, an achiral amino acid, has been shown to activate both the T1R1+3 and the T1R2+3 receptors in heterologous expression systems [66]. It then follows that the deletion of the T1R3 subunit should inactivate both receptors and leave glycine tasteless. It could be, however, that glycine is capable of binding with homodimers involving either the T1R1 or T1R2 in the absence of T1R3. Humans describe glycine as having a sweet component [86–87] and rodents treat it as having a sucrose-like quality [72, 77], but its affective potency in mice is not particularly impressive especially compared with common sugars or artificial sweeteners [5, 19]. Thus, it remains possible that glycine is stimulating some other taste receptors associated with other taste qualities that could be responsible for the partial responsiveness to this amino acid in NST neurons of T1R3 KO mice. Indeed, in general, glycine-responsive neurons in T1R3 KO mice were also responsive to NaCl, HCl, and quinine. These findings highlight a caveat in interpreting outcomes from tests based on physiological or behavioral responsiveness to amino acids and artificial sweeteners in T1R KO preparations. Some T1R ligands cannot be considered to generate single pure taste sensations and likely activate non-T1R receptors.
Taste-Related Behavioral Responses to T1R Ligands
In most, but not all, cases, the electrophysiological findings discussed above correspond with behavioral measures. The differences depend on the behavior being measured, the stimuli presented, and the experimental design.
24-h two-bottle intake tests
Perhaps the most common measure of behavioral responsiveness to taste stimuli is the 24-h two-bottle intake test in which a test compound is pitted against water and the relative intake is recorded. When T1R3 KO mice have been tested in this fashion with artificial sweeteners such as AceK, SC45647, or sodium saccharin, they display no preference for these compounds over water, in contrast to very clear preferences observed in WT mice [13, 67, 122]. At higher concentrations the T1R3 KO mice avoid these sweeteners in favor of water, supporting the notion that, at least at high concentrations, artificial sweeteners bind with T2Rs (sometimes referred to as “bitter” receptors) [50, 78, 96]. In contrast to the absolute lack of preference for and even avoidance of artificial sweeteners, T1R3 KO mice will display some degree of preference for glucose and higher concentrations of sucrose solutions in these tests. Experienced KO mice that have been previously tested show a preference at lower sucrose concentrations compared with naïve KO mice suggesting that some degree of learning is involved [122–123]. It has long been known that intake during long-term two-bottle preference tests can be influenced by postingestive events [eg. 62, 93, 113]. Moreover, there is ample evidence that rodents can associate motivationally neutral orosensory cues with positive postingestive nutritional consequences and develop a flavor preference with repeated exposure [eg. 1, 21, 89, 93]. Zukerman et al. [124] has shown that the increase in sucrose preference by T1R3 KO mice with repeated exposure is partially dependent on an intact olfactory system, suggesting that the mice might be using smell as a cue. Importantly, given the presence of T1R3 in the gut, it is noteworthy that this protein is not necessary for the conditioning of flavor preferences via intragastric sucrose infusions [92].
Brief access taste tests
In efforts to overcome some of the interpretive limitations of long-term intake tests, investigators have used procedures in which licking responses to a given taste stimulus are assessed in very brief trials on the order of seconds [eg. 8, 15, 31, 98, 99, 120]. A panel of taste compounds or various concentrations of a single compound can be tested in a single session (although often several sessions are tested to increase the number of trials) in a specialized testing apparatus that allows individual presentations of more than one stimulus in a randomized fashion [eg. 98]. These so-called brief access tests minimize the ability of the animal to associate any postingestive consequence with a given stimulus or specific concentration presented during the session. Initial application of the brief access test to assess responsiveness of T1R KO mice to sweeteners indicated that T1R2 KO and T1R3 KO mice were completely unresponsive to artificial sweeteners and displayed entirely flat concentration-response functions, in striking contrast to the typically sigmoidally rising curves characterizing the responses of WT mice [111]. When tested with the sugars sucrose, glucose, and maltose, the T1R2 KO and T1R3 KO mice had severely blunted licking responses, but displayed some small elevation in licking at higher concentrations which was somewhat more pronounced in the T1R2 KO mice [122]. Double KO mice missing both the T1R2 and the T1R3 subunits had completely flat sugar concentration-response functions similar to that observed with the single KOs tested with artificial sweeteners [122]. These behavioral outcomes emulated the pattern of electrophysiological results obtained with nerve recordings from these various KO preparations and buttressed the view that T1R2 and especially T1R3 could potentially form homodimers that are capable of maintaining some degree of function to at least high concentrations of sugars, but not artificial sweeteners.
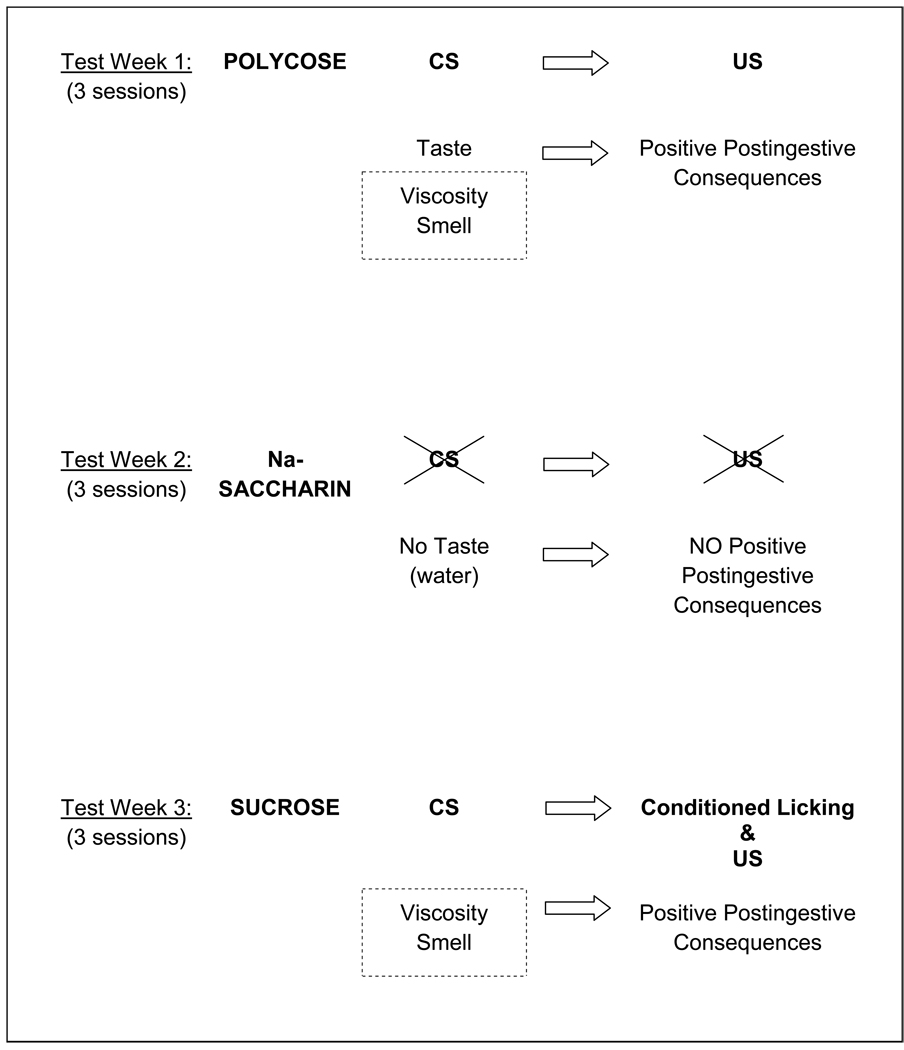
More recent applications of the brief access test have revealed some interesting characteristics of the behavioral responses of T1R KO mice to sucrose and the glucose polymer mixture Polycose. When the brief access test was used to assess the responsiveness of T1R2 KO and T1R3 KO mice and their WT controls to Polycose, sucrose, and saccharin, mice of both KO genotypes displayed concentration-dependent licking to Polycose that was just as vigorous if not more so then that displayed by WT mice [111]. As was reported in prior studies [122], the KO mice had completely flat concentration-response functions to saccharin, but both T1R2 and T1R3 KO mice displayed some degree of concentration-dependent responding at higher sucrose concentrations. Upon closer inspection of the data, an interesting pattern emerged based on the experimental design. All of the mice were tested with all 3 taste stimuli over the course of 3 weeks, with each taste stimulus tested for 3 days one week (Monday, Wednesday, Friday) and then a different stimulus tested the following week and so on. The order of presentation of the stimuli across the 3 weeks was counterbalanced within each genotype in a Latin Square manner. Accordingly there were 3 subgroups of animals for each genotype. As shown in Figure 1, the concentration-response functions for Polycose and saccharin for the KO mice did not depend on which week the compounds were tested. However, when sucrose was tested in the first or second week, before these mice had any experience with Polycose, most of the KO mice, regardless of genotype, showed very flat concentration-response curves and some KO mice did not even initiate enough trials to compose a curve. This is very different than what was observed in WT mice (Figure 2). When, however, sucrose was tested in week 3 after experience with Polycose testing in a prior week, those KO mice showed rather impressive concentration-dependent licking (Figure 1). We interpreted the latter result as reflecting a learning process in which some of the non-T1R–dependent orosensory properties of sucrose (e.g., smell, viscosity) were shared with Polycose and those sensory signals may have been associated with the positive nutritive benefits of Polycose ingestion during those test sessions (Figure 3). There are other examples in the literature in which KO mice, initially ageusic as a result of various taste-related gene deletions, display increased behavioral responsiveness to saccharides after previous exposure to caloric compounds [95, 122–123]. As an important methodological postscript, it appears that, under certain conditions, even responses in the brief-access test can be influenced by postingestive events through learning mechanisms, if animals are exposed to the same compound on repeated sessions.
Figure 1.
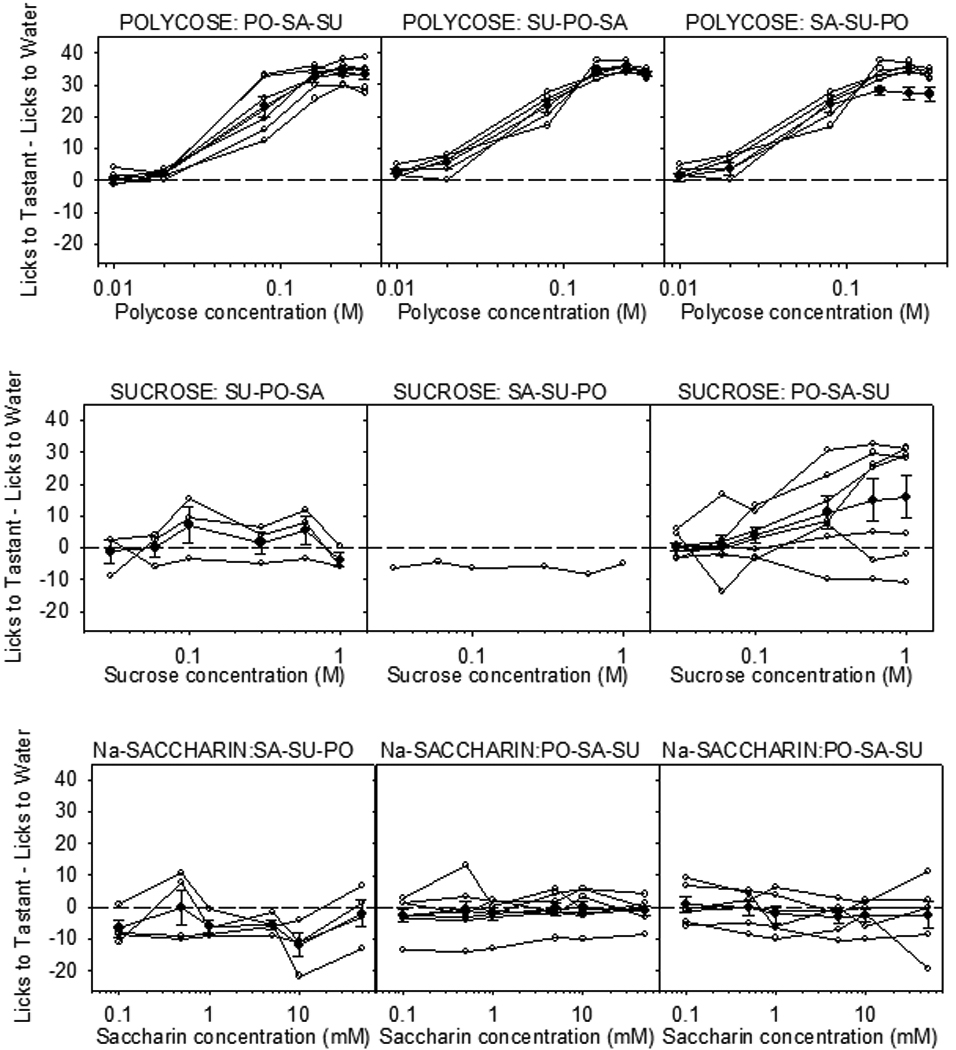
Licks (adjusted for water) to Polycose (top row), sucrose (middle row) and Nasaccharin (bottom row) for individual T1R3 KO mice (open circles) and mean ± SE (bold plot) for each stimulus. Animals were tested partially food and water restricted (1 g chow, 2 ml water 23 h before the test session) with a series of concentrations from a single compound on Monday, Wednesday and Friday for Week 1, and then were tested with the next compound on Week 2, and then with the last compound on Week 3. The order of presentation for compounds across weeks within each group was determined by a Latin Square. The trials were 5 s in duration and sessions were 25 min. Only animals that initiated at least 2 trials per concentration were included in the concentration-response analysis. Each row indicates the test stimulus and each column indicates the response of the subgroup that was tested with that stimulus in weeks 1, 2, and 3 respectively. The order of stimulus presentation for a given subgroup is presented above each graph (PO = Polycose; SA = Na-saccharin; SU = sucrose). All KO mice responded in a concentration dependent manner to Polycose and displayed virtually flat responses to Nasaccharin regardless of order of presentation. When sucrose was presented as the first or second test stimulus, KO mice did not generally show concentration-dependent responses, but some degree of concentration-dependent licking to sucrose was observed in KO mice presented sucrose in the third week after Polycose exposure in the first week. This experience-dependent increase in sucrose responsiveness is thought to be based on learning (see text and Figure 3). A similar series of results were obtained for T1R2 KO mice (not shown). Reprinted from Treesukosol et al. [111] with permission from the American Physiological Society.
Figure 2.
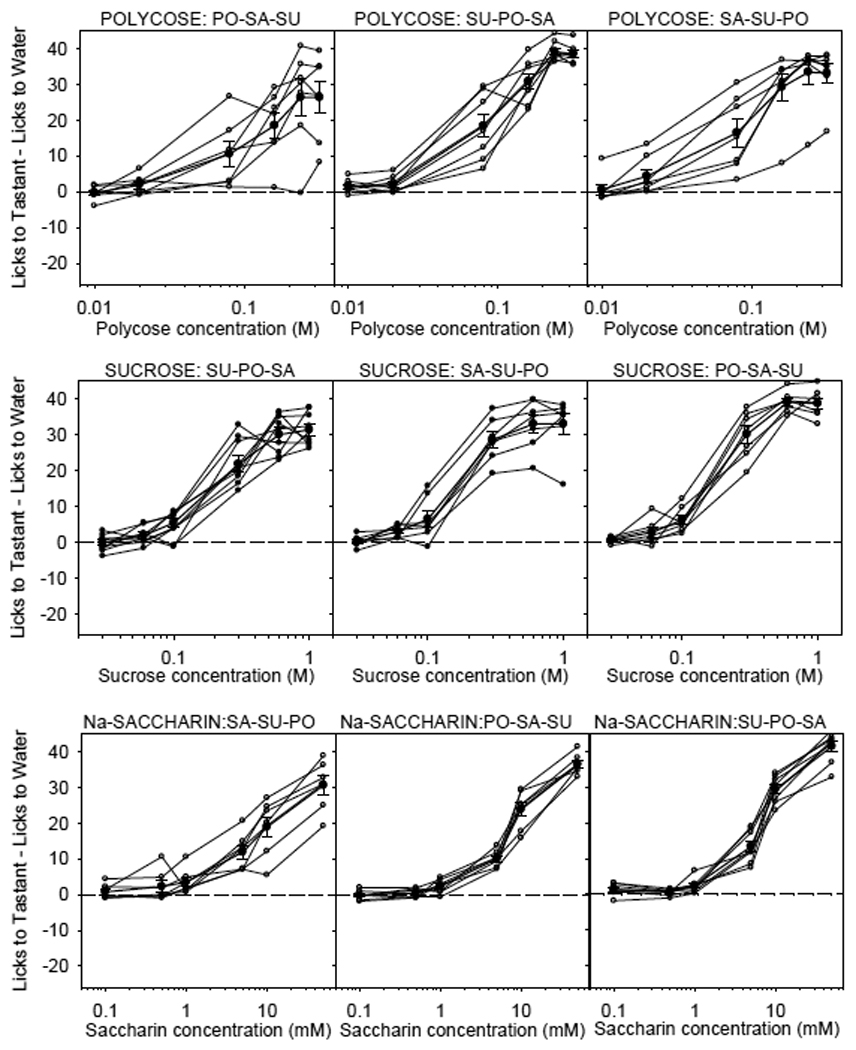
Licks (adjusted for water) to Polycose (top row), sucrose (middle row) and Nasaccharin (bottom row) for individual WT littermate controls of T1R3 KO mice (open circles) and mean ± SE (bold plot) for each stimulus. See Figure 1 caption for details of testing and analysis. Regardless of testing order, all WT mice showed concentration-dependent licking responses to Polycose, sucrose and Na-saccharin. A similar series of results was obtained for T1R2 WT littermate controls (not shown). Reprinted from Treesukosol et al. [111] with permission from the American Physiological Society.
Figure 3.
Hypothesis for why concentration-dependent licking of sucrose was observed in some KO mice tested (Week 3) after prior Polycose exposure (Week 1). Animals could potentially associate cues such as taste, viscosity, and smell of Polycose with the positive nutritive consequences of its ingestion. In this sense, these cues become conditioned stimuli (CS). It is thus possible that sucrose shares some of these cues such as viscosity and smell (dashed box). Consequently, the concentration-dependent licking of sucrose in Week 3 in mice that had prior experience with Polycose testing (Week 1) could be attributed to a conditioned response rather than to the unconditioned hedonic characteristics of the stimulus (see text for more elaboration).
Using a modified incarnation of the brief access test in which two solutions are simultaneously presented for 60-s (i.e., a brief access 2-bottle preference test), Zuckerman et al (2009) found that both WT and T1R3 KO mice (T1R2 KO mice were not tested) displayed preferences for Polycose over water that increased with concentration. The KO mice did have significantly lower Polycose preferences compared with WT, but the difference was slight, and both genotypes had monotonically rising concentration-response functions. When tested with sucrose, WT mice showed robust preferences for the disaccharide, whereas the KO mice did not. In principle, these findings match those found for CT recordings [123] and for the brief access test as described above [111] in that Polycose responses in T1R3 KO mice are near normal.
All of the findings in which taste-related responses to Polycose were measured in T1R2 or T1R3 KO mice collectively suggest that glucose polymers of a sufficient chain length may be stimulating a taste receptor that does not depend on the presence of T1R2 or T1R3. Alternatively, it could be that either T1R2 or T1R3 alone is sufficient, perhaps as a homodimer, to maintain normal taste-related responsiveness to Polycose. With respect to this latter possibility it would be informative to test Polycose responses in T1R2/T1R3 double KO mice; such experiments are in progress in our laboratory.
Psychophysical taste detection and discrimination tasks
The two-bottle preference and the brief access tests rely on the motivational properties (e.g., reward and aversion) of the taste stimuli to drive responding. Because the affective characteristics of a taste solution can vary independently from its taste quality (i.e., its discriminative characteristics), researchers have adopted the use of classical and operant conditioning procedures in which taste solutions serve as cues for subsequent events. Accordingly, the ability of the taste stimulus to serve as a signal can be assessed in psychophysically oriented experimental designs.
In one such study, a two-response discrimination procedure was used to determine whether B6 mice could discriminate sucrose from other sugars and putatively sweet-tasting amino acids. Thirsty mice were trained to sample a taste stimulus from a centrally positioned stimulus-delivery tube and then lick a left-positioned drinking spout if the stimulus was sucrose and right-positioned drinking spout if the stimulus was something else (the positions of the response spouts was counterbalanced across mice). If the mouse made a correct response, it received a water reward and if it made an incorrect response, it was punished with a 30-s time-out delaying the opportunity to obtain another water reinforcer. Although such mice were able to discriminate sucrose from NaCl, L-serine, and L-threonine, they were unable to discriminate it from glucose and fructose. The L-amino acids would be activating the T1R1+3 receptor, whereas the sugars would be activating the T1R2+3 heterodimer. Even if those signals converged centrally, as suggested by the fact that animals treat some L-amino acids as having a sweet component [18, 36, 72], the animals could potentially use the oral origin of the signal as a cue given that the relative distribution of the T1R1 and T1R2 proteins across the various taste bud fields varies. Moreover, it is possible that those amino acids also bind with some T1R–independent taste receptors. The fact that the mice were unable to discriminate sucrose from glucose and fructose strongly suggests that these three sugars produce a unitary qualitative perceptual experience (“sweetness”) and is in agreement with the findings in the literature showing that these three sugars activate the same receptor – the T1R2+3 heterodimer. It is worth emphasizing that in taste quality discrimination studies, failure to discriminate between compounds is always more compelling conceptually than success, provided an animal’s competence in the task can be demonstrated, because it suggests an identity exists between the neural signals generated by the two stimuli somewhere along the gustatory neuraxis.
The mice in the Dotson and Spector study [18] described above displayed some ability, albeit poor, discriminating sucrose from maltose. There have been other reports in the literature suggesting that maltose and sucrose, while perhaps sharing some perceptual features, are not qualitatively identical. Rats can be trained in a shock-avoidance paradigm to discriminate sucrose from maltose and performance is severely disrupted by transection of the gustatory branches of the facial nerve (chorda tympani and greater superficial petrosal nerves), confirming that normal competence in the task depends on orosensory signals in taste nerves [101]. If a taste aversion is conditioned to sucrose by pairing its ingestion with a LiCl injection, which causes temporary visceral malaise, then rats will avoid its ingestion on future presentations and will also avoid maltose, suggesting the two sugars have some shared perceptual features. However, the conditioned rats will not avoid maltose to the same extent, suggesting the two sugars have some discriminable perceptual features [70, 100]. Importantly, the converse is also true if maltose serves as the conditioned stimulus [100]. Conditioned aversions to sucrose do generalize well to other sugars, including fructose and glucose, in mice [69], rats [72], hamsters [72], and gerbils [40], in agreement with the results from the two-response discrimination procedure. It is interesting to note that, provided intensity cues are accounted for, humans appear to be unable to discriminate glucose from either fructose or sucrose in a two alternative forced choice discrimination procedure, but they can discriminate fructose (and therefore presumably sucrose) from maltose [9].
Maltose is a disaccharide of two glucose moieties. It is tempting to speculate that in addition to binding with the T1R2+3 heterodimer, it binds with the hypothesized polysaccharide taste receptor that is putatively activated by Polycose. Rats trained to avoid Polycose or sucrose in a conditioned taste aversion paradigm show no or very weak cross-generalization [70, 79, 84] supporting the view that these two carbohydrate solutions have different taste qualities. Rats conditioned to avoid Polycose show some generalization, however, to maltose [70]. Hamsters conditioned to avoid Polycose, sucrose, or a mixture of the two compounds display some degree of cross-generalization, but Polycose and sucrose appear to have characteristics that nonetheless make the compounds distinct from one another [27].
Support for the importance of the T1R3 protein in behavioral responsiveness to at least sucrose and glucose is provided by taste detection thresholds to these sugars measured in strains of mice that have different Tas1r3 alleles that appear to relate to preference for many sweeteners at low concentrations in 24-h two-bottle intake tests [e.g.,3, 5, 10, 29, 39, 55]. Mice from the C57BL/6 and SWR strains are considered “taster” mice because of their higher preference for sweeteners at low concentrations relative to the DBA/2 and 129 strains, which are considered “non-tasters.”1 When mice from these strains were trained to discriminate water from sucrose, glucose, and glycine in the two-response taste discrimination procedure, the detection thresholds, collapsed across strain, for sucrose and glucose were highly correlated (r=.81), with “taster” mice having lower thresholds (i.e., more sensitive) than “non-taster” mice (Figure 4). The correlation between detection thresholds for glycine and these two sugars was much more modest (r≤0.43). As mentioned in prior pages, glycine binds with both the T1R1+3 and the T1R2+3 receptor, possibly related to the fact that it is achiral, and the allelic variation of the Tas1r3 does not appear to affect the function of the T1R1+3 heterodimer. Accordingly, the latter receptor likely contributes to the lower correspondence between the detection thresholds for the sugars and those for glycine, because sucrose and glucose do not bind with T1R1+3.
Figure 4.
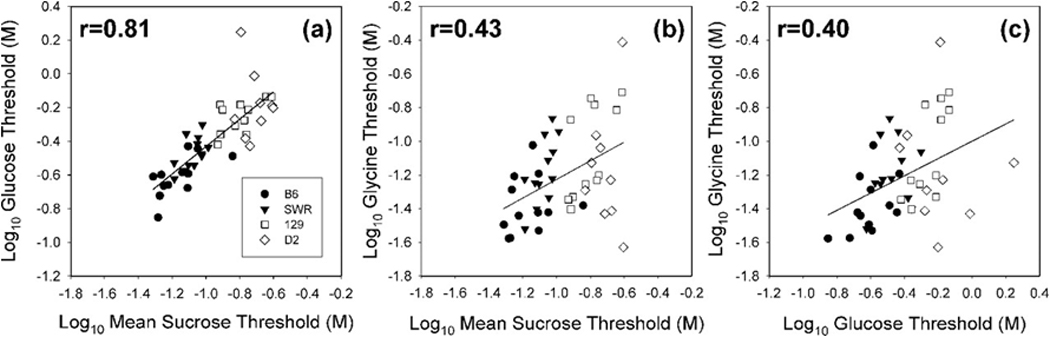
Correlation between taste detection thresholds of individual mice from 4 different strains: C57BL/6J (B6, circles), SWR/J (SWR, triangles); 129P3/J (129, squares); DBA/2J (DBA, diamonds) for sucrose vs. glucose (a), sucrose vs. glycine (b) and glucose vs. glycine (c). The B6 and SWR strains (so called “taster” strains) possess an allelic form of Tasr3 that has been shown to be responsible for the relatively greater preferences of these mice for low concentrations of many sweeteners compared with mice from the 129 and D2 strains (so called “non-taster” strains) which possess a different allele. The effect of the allelic variation could be seen in the ability of the mice to detect sucrose and glucose in a psychophysical task. As would be predicted, sucrose thresholds correlated highly with glucose thresholds. However, glycine thresholds did not correlate as well with those for the two sugars presumably because this amino acid binds with both the T1R2+3 and T1R1+3 heterodimers and activation of the latter is not affected by this particular allelic variation of Tas1r3. Reprinted from Eylam and Spector [23] with permission from Oxford University Press
To date, little work has been conducted using T1R KO mice as subjects in sensory detection or discrimination procedures. Such experiments are currently ongoing in our laboratory. However, there is one published study in which detection thresholds to sucrose and monosodium glutamate (MSG) were measured in T1R3 KO and WT mice through the use of a shock avoidance procedure. Generally, manipulations of the gustatory system that have major effects on neural responses to taste stimuli are reflected in shifts in sensory thresholds in animal models [e.g.,7, 22, 30, 97, 102, 104, 112], but T1R3 KO mice had normal detection thresholds for both sucrose and MSG compared with WT mice [17]. Considering the evidence in the literature supporting the role of T1R2 and T1R3 in mediating sucrose taste, it would be expected that T1R3 KO mice would at least have elevated, as opposed to normal, sucrose detection thresholds. Detection thresholds to sucrose and glucose were previously shown to be dependent on T1R3 allele status [23], thus it is surprising that the deletion of the gene would not yield large effects on threshold. This unexpected finding remains vexing and is worthy of further experimental scrutiny.
NUTRIENT SENSING
The T1R2 and T1R3 proteins are also expressed in a variety of non-taste tissues including the gut, pancreas, and even the brain, raising the question as to what their functional roles in addition to taste might be. Given their uncontested ability to bind with mono and disaccharides in heterologous expression systems as well as in the gustatory epithelium, the expression of these proteins in non-taste tissue has been hypothesized to contribute to glucose sensing. Such a hypothesis enjoys some support from certain experimental outcomes, but not without qualification.
Gastrointestinal Tract
The most compelling evidence to date deals with the expression of T1R2 and T1R3 in the enteroendocrine cells in the small intestine. For example, glucagon-like peptide-1(GLP-1), an incretin hormone synthesized and released by enteroendocrine L-cells which facilitates the action and release of insulin, is co-expressed with signaling proteins important in taste transduction of sweet-tasting ligands such as α-gustducin , Gβ3, Gγ13, PLCβ2, TRPM5, and, importantly, T1R3 in some cells in the human duodenum [6, 41, 59]. The T1R2 subunit is also co-expressed in at least some enteroendocrine cells with α-gustducin, GLP-1, and T1R3 [6, 41]. Although to our knowledge a comprehensive quantification of the degree of overlap of T1R2, T1R3, GLP-1, and other taste signaling elements remains to be conducted, there are at least some enteroendocrine cells which co-express these critical proteins [6, 41].
More importantly, manipulations that are known to affect the function of T1R3, in turn, also influence the function of enteroendocrine cells. In a mouse enteroendocrine cell line, GLUTag, the application of sucralose elicits an increased release of GLP-1 and glucose-dependent insulinotropic peptide (GIP) [80] Gurmarin, thought to inhibit activity by acting on the T1R2+3 heterodimer [59] blocks sucralose-stimulated release of both GLP-1 and GIP from GLUTag cells [59]. In NCI-H716 enteroendocrine L cells, the application of sucrose, glucose, or sucralose elicits a release of GLP-1 [41]. Lactisole, which suppresses human taste responses to a range of sweeteners by targeting the T1R3 subunit, inhibits sucralose-stimulated release of GLP-1 from NCI-H716 cells [41]. Likewise, siRNA targeting mRNA for α-gustducin, a G-protein implicated in the taste transduction of sweet-tasting ligands, transfected into the NCI-H716 cells leads to outcomes similar to lactisole administration. The application of 2-deoxyglucose, which is metabolically inactive, was ineffective at triggering the release of the incretin hormones from the NCI-H716 cells. It thus appears that compounds that are sweet to humans and preferred by animals regardless of whether they have nutritional value (e.g., sucralose) are capable of stimulating enteroendocrine cells and that this ability is mediated through a process that depends on the T1R3 protein.
In an in vivo study conducted in mice, glucose was infused directly into the duodenum which was isolated by ligation from the rest of the gut but with circulatory contact maintained. In WT mice, plasma GLP-1 levels peaked 10 min after glucose infusion, but in T1R3 KO mice and α-gustducin KO mice, GLP-1 plasma concentrations did not increase [49]. It has been suggested that one of the actions of GIP and/or GLP-1 is to signal the presence of sugar in the gut to regulate the expression of glucose transporters [59]. Intestinal glucose absorption occurs via active transport mediated by the sodium-dependent glucose co-transporter-1 (SGLT1), or via facilitated diffusion mediated through the apical GLUT2 pathway [see 44]. In WT mice maintained on a high-carbohydrate (70% sucrose) diet, higher levels of intestinal SGLT1 mRNA and protein were expressed compared with mice maintained on a low-carbohydrate (1.9% sucrose) diet. In contrast, regardless of diet, the amount of SGLT1 mRNA expression in α-gustducin and T1R3 KO mice was similar to that of WT mice on the low-carbohydrate diet. Similarly, SGLT1 mRNA and protein expression was higher in WT mice given a sucralose solution and a low carbohydrate diet than in WT mice given the unsupplemented diet, but sucralose did not elicit an increase in SGLT1 mRNA expression in mice lacking α-gustducin and T1R3 [59]. In another study, glucose infused into the duodenum also induced an increase in SGLT-1 expression. A similar effect was observed after infusion of fructose which does not bind with SGLT-1, and with Na-saccharin which is thought not be absorbed or metabolized, but not with mannitol. These findings lend support to the hypothesis that the up-regulated expression of SGLT-1 involves the T1R2+3 receptors in the intestine and not simply osmolarity or luminal distension. Expression of SGLT-1 was higher in the duodenum and proximal jejunum compared to the distal jejunum which corresponded with relative mRNA expression of gustducin, T1R2 and T1R3 [105].
Along those lines, the artificial sweetener sucralose has also been shown to increase glucose absorption via an increase in apical GLUT2 trafficking in the rat intestine. This artificial sweetener-induced up-regulation of GLUT2 insertion into the apical membrane in enterocytes is thought to be mediated through a signaling pathway involving T1R2 and T1R3 as well as PLCβ2, an enzyme critical in sweetener taste transduction, and PKC β2, the enzyme that regulates intracellular trafficking of GLUT2 [57–58].
Pancreas
The expression of α-gustducin, T1R2 and T1R3 has also been found in the insulin-secreting pancreatic beta cells. In the presence of glucose, artificial sweeteners such as sucralose stimulate insulin release from cultured MIN6 cells (a beta cell line) in a concentration-dependent manner [64]. In pancreatic islet cells, sucralose appears to facilitate insulin release in the presence of a low, but not high, concentration of glucose. Although the functional ramifications of these findings remain to be understood, it is noteworthy that both T1R2 and T1R3 are expressed in pancreatic beta cells and that artificial sweeteners can have an influence on insulin secretion [64].
Brain
The expression of T1R1, T1R2, and T1R3 in various brain regions obviously leads to intriguing questions regarding their role. Some insight can be found in the fact that 24-h food deprivation has been shown to increase levels of T1R1 and T1R2 in the hypothalamus, an area well known to be involved with control of energy balance, but not in the cortex of C57BL/6 mice [81]. Similarly, hypothalamic expression levels of T1R1 and T1R2 in non-deprived C57BL/6 mice were higher than in obese hyperglycemic ob/ob mice which lack leptin. In cultured mouse embryonic hypothalamic N38 cells, which express all three T1R protein subunits, there was an up-regulation of T1R2 expression in a low glucose medium which was reversed by the application of higher glucose concentrations or sucralose; T1R1 and T1R3 expression levels were unaffected by the manipulations. These findings have been interpreted as implicating the T1R2+3 heterodimer as a candidate membrane-bound glucosensor. Such speculation is tempting based on the current set of findings but is not without its caveats. If T1R2 and T1R3 function normally as a heterodimer, then it remains to be explained why the expression level of only T1R2, but not T1R3, changed as a function of the manipulations above. Of course, one possibility is that these proteins can potentially form homodimers, but, in the gustatory system, even though some function can be maintained in the absence of one of the complementary subunits to the heterodimeric receptor, it is nonetheless in a severely compromised form at best. Ren et al., [81] hypothesized that because the T1R3 can form a functional receptor with either T1R1, which binds with L-amino acids, or with T1R2, which binds with sugars and other sweeteners, variations in T1R2 expression as a function of manipulations of glucose availability might determine how well it can compete with T1R1 for heterodimer formation with T1R3.
Functional Tests
Given the collection of findings indicating that the T1R2 and T1R3 subunits are expressed in a variety of tissues intimately involved with the regulation of feeding and energy balance as well as the physiological effects sugars and artificial sweeteners have, apparently triggered by T1R2+3 receptors expressed in cells outside the peripheral gustatory system, it is surprising that that deletion of neither the T1R2 or T1R3 protein in mice leads to discernable effects on body weight or feeding. We recently measured the body weight, chow intake, and water intake of adult T1R2 KO and T1R3 KO mice and their same-sex littermate controls over 10 days. Breeding pairs of KO mice were generously provided by Dr. Charles Zuker and were crossed with C57BL/6J mice (Jackson Laboratories) to produce heterozygous progeny, which, in turn, were crossed to produce same-sex WT and KO littermates (along with heterozygous mice). As shown in Figure 5 and Figure 6, T1R2 KO and T1R3 KO mice did not significantly differ from their WT counterparts in body weight, chow intake, or water intake.
Figure 5.
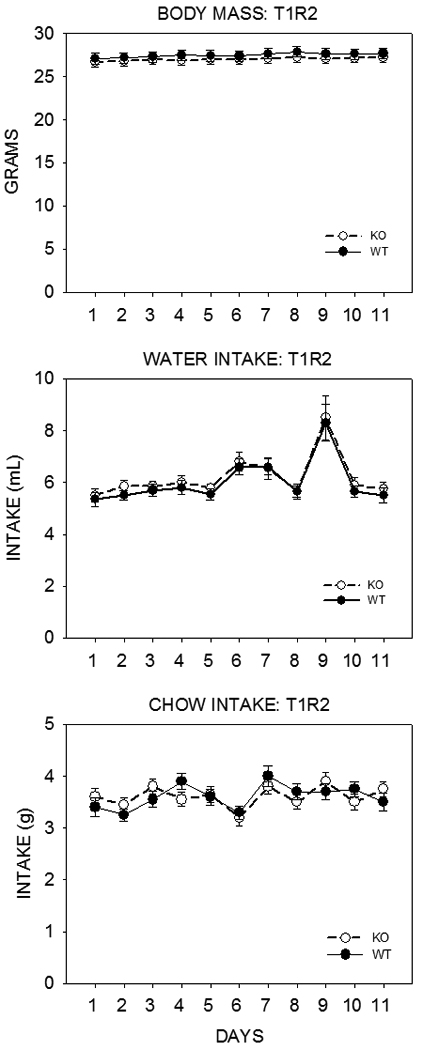
Mean ±SE body weight (top), daily water (middle) and chow (Purina Laboratory Chow 5001, St. Louis, MO) (bottom) intake of adult T1R2 KO mice (open symbols) and their same-sex WT littermate controls (closed symbols) for 11 days. The mice were housed individually in polycarbonate tub cages under a 12 h/12 h light/dark cycle in a room that had temperature and humidity automatically controlled. Each genotype group consisted of 12 males and 8 females. These mice had been previously tested in brief access tests with sugars, Na-saccharin, and Polycose. The mean age of the mice in each group was 29.3 ± 0.47 weeks. In a three-way ANOVA (genotype × sex × day) there was no significant main effect of genotype (F(1,36)=0.826, p=0.369) and no significant genotype × sex (F(1,36)=0.992, p=0.326), genotype × day (F(10,360)=0.780, p=0.648), or genotype × sex × day (F(10,360)=0.527, p=0.871) interactions for body weight; no significant main effect of genotype (F(1,36)=0.188, p=0.667) and no significant genotype × sex (F(1,36)=0.136, p=0.714), genotype × day (F(10,360)=0.112, p=1.000), or genotype × sex × day (F(10,360)=0.488, p=0.898) interactions for water intake; no significant main effect of genotype (F(1,36)=0.012, p=0.913) and no significant genotype × sex (F(1,36)=0.109, p=0.743), genotype × day (F(10,360)=1.379, p=0.188), or genotype × sex × day (F(10,360)=0.366, p=0.961) interactions for food intake. The perturbation in water intake on Day 9 is unexplained but when this data point was excluded, the statistical outcomes reported remained the same.
Figure 6.
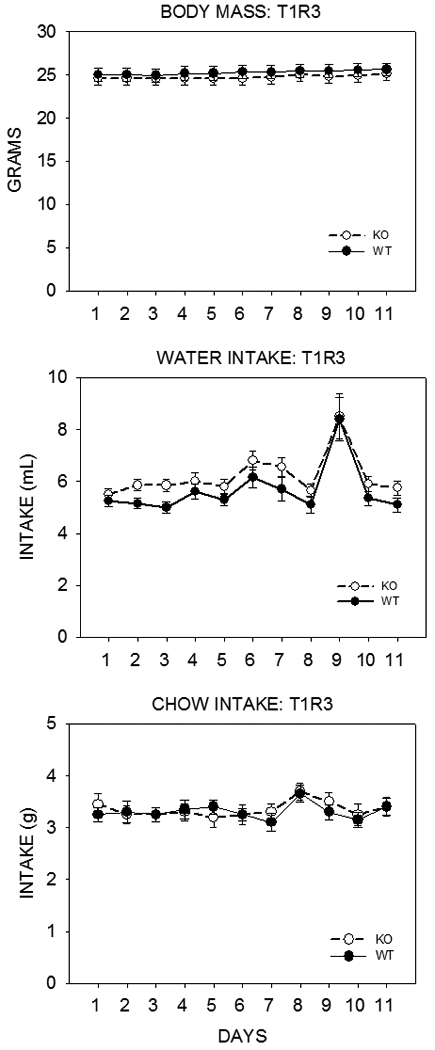
Mean ±SE body weight (top) and daily water (middle) and chow (Purina Laboratory Chow 5001, St. Louis, MO) (bottom) intake of adult T1R3 KO mice (open symbols) and their same-sex WT littermate controls (control symbols) for 11 days. The mice were housed individually in polycarbonate tub cages under a 12/12 light/dark cycle in a room that had temperature and humidity automatically controlled. Each genotype group consisted of 12 males and 8 females. These mice had been previously tested in brief access tests with sugars, Nasaccharin, and Polycose. The mean age of the mice in each group was 21.8 ± 0.73 weeks. The T1R2 groups were significantly older than the T1R3 groups in this study (F(1,72)=68.434, p<0.001). This likely accounted for the significant difference in body weight found between the T1R2 and T1R3 groups (F(1,72)=34.458, p<0.001). In a three-way ANOVA (genotype × sex × day) there was no significant main effect of genotype (F(1,36)=0.711, p=0.405) and no significant genotype × sex (F(1,36)=0.038, p=0.847), genotype × day (F(10,360)=0.862, p=0.569), or genotype × sex × day (F(10,360)=0.651, p=0.769) interactions for body weight; there was no significant main effect of genotype (F(1,36)=0.164, p=0.688) and no significant genotype × sex (F(1,36)=0.000, p=0.987), genotype × day (F(10,360)=0.479, p=0.903), or genotype × sex × day (F(10,360)=0.416, p=0.939) interactions for water intake; there was no significant main effect of genotype (F(1,36)=0.022, p=0.883) and no significant genotype × sex (F(1,36)=0.734, p=0.397), genotype × day (F(10,360)=0.341, p=0.969), or genotype × sex × day (F(10,360)=0.764, p=0.663) interactions for food intake. The perturbation in water intake on Day 9 is unexplained but when this data point was excluded, the statistical outcomes reported remained the same.
We then reasoned that perhaps the expression of a functional deficit requires that the animals be challenged with a fast. Accordingly we tested a subset of these same mice for their intake of Polycose, in an ascending concentration series, during 30-min intake tests in a lickometer. The animals were tested after 23.5 h of food deprivation on one day and then food was replaced and the animals were tested in a nondeprived state the next day. This pattern was followed for four days at each concentration. We chose Polycose as the test stimulus because it is a mixture of glucose polymers and KO and WT mice treat it similarly in brief access tests. As depicted in Figure 7, there were significant concentration effects, and food restriction predictably increased licking, but there were no effects involving genotype – KO and WT mice licked the Polycose at similar levels regardless of deprivation state and concentration. Thus, to our knowledge, a discernable feeding or body weight phenotype has yet to be observed in T1R2 or T1R3 KO mice.
Figure 7.
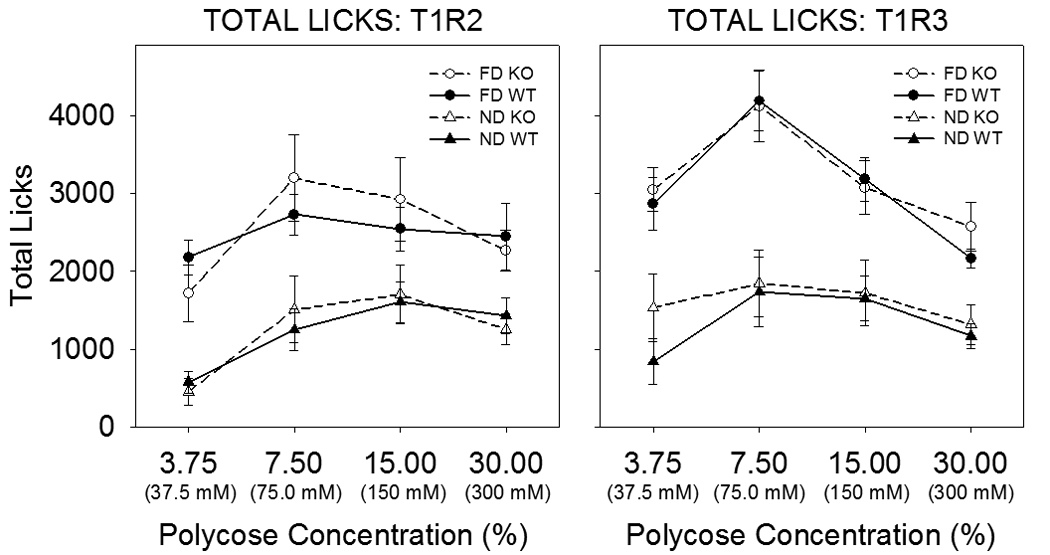
Mean ±SE total licks of Polycose as a function of concentration and fasting state by T1R2 (left panel) and T1R3 (right panel) KO mice (open symbols) and their WT littermate controls (closed symbols) in 30-min intake tests in a Davis Rig lickometer with a stationary drinking spout. The mean age of the mice at the start of testing for each T1R2 genotype (n=8) was 44.9 ± 1.48 weeks and for each T1R3 genotype (n=9) was 33.4 ± 0.69 weeks. Licking as opposed to intake was measured to avoid inaccuracies in volume measurement due to spillage, and it was assumed that the lick volumes were not different across the genotypes. The animals were tested after 23.5 h of food deprivation on one day (FD: circles) and then food was replaced and the animals were tested the next day in a non-deprived state (ND: triangles). This pattern was followed for four days (2 days deprived, 2 days non-deprived) at each concentration (ascending series). On 7 occasions involving 6 mice, data was lost due to a computer problem. For 5 of these mice, the licks from the complementary day were used instead of the mean of the two days in the analysis. For the sixth mouse, however, the computer problem happened on both days of the same condition and so this mouse had to be excluded from the analysis. In addition, one mouse was excluded from the analysis because it did not consistently sample the stimulus across sessions. In all cases when mice had to be removed from the analysis, their littermates were also excluded. All of these mice had been previously tested in brief access tests with sugars, Na-saccharin, and Polycose. In a three-way ANOVA (genotype × deprivation state × concentration) of total licks for T1R2 KO mice and their WT controls, there was no significant main effect of genotype (F(1,14)=0.008., p=0.93) and no significant genotype × concentration (F(3,42)=1.27, p=0.30), genotype × deprivation state (F(1,14)=0.043, p=.84), or genotype × deprivation state × concentration (F(3,42)=0.60, p=0.62) interactions. In a three-way ANOVA (genotype × deprivation state × concentration) of total licks for T1R3 KO mice and their WT controls, there was no significant main effect of genotype (F(1,16)=0.188, p=0.67) and no significant genotype × concentration (F(3,48)=0.928, p=0.43), genotype × deprivation state (F(1,16)=0.45, p=0.51), or genotype × deprivation state × concentration (F(3,48)=1.086, p=.36) interactions.
If T1R receptors are involved in sugar sensing in gut endocrine cells, it would follow that artificial sweeteners may be able to induce incretin release and ultimately lower blood sugar. However, oral gavage of artificial sweeteners did not reduce blood glucose elevation as tested in a intraperitoneal glucose tolerance test in rats, and, in Zuker diabetic fatty rats, neither sucralose nor stevia (a plant-derived sweetener) administered orally reduced blood glucose levels [28], suggesting sweeteners do not acutely enhance incretin hormone release in vivo. Furthermore, oral administration of glucose caused elevation of GIP and GLP-1 levels but this effect was not observed after oral administration of the artificial sweeteners tested [28].
In type 2 diabetic patients, during a test meal, stevioside supplementation did not significantly change insulin, GLP-1 or GIP levels, but, interestingly, did reduce blood glucose levels [32]. In another study, measures taken to evaluate blood glucose homeostasis did not reveal a significant difference between Type 2 diabetic patients given daily administration of sucralose (in capsules) for 3 months compared with those given a cellulose placebo [33]. The lack of effect on glucose homeostasis measures was despite the administration of sucralose at a dose three times the estimated maximum intake. Similarly, Ma et al., [56] found that the administration of sucralose or saline given by intragastric infusion did not have an effect on GLP-1, GIP, or insulin secretion, or on gastric emptying in healthy human subjects. In contrast, blood glucose, insulin, plasma GLP-1 and GIP increased, and gastric emptying rate decreased after administration of sucrose. In another study with human subjects, GLP-1 and PYY secretion was stimulated by intragastric infusion of glucose and, to a lesser extent, of fructose, but was not stimulated by infusion of the artificial sweeteners aspartame, acesulfame K or sucralose [106]. Similar findings were observed in a study in which sucralose ingestion did not elicit an increase in GLP-1 or PYY in fasted subjects [26]. Thus, the ability for artificial sweeteners to increase the release of incretin hormones and ultimately influence glucose homeostasis is equivocal in the literature. The disparities among these experimental findings await further resolution.
CONCLUDING REMARKS
On the whole, the available data clearly demonstrate that the T1R2 and T1R3 proteins are critical for normal qualitative perception of sugars and other compounds that humans describe as sweet and that rodent species find rewarding. There are nonetheless important nuances to these collective findings. Some T1R ligands have the potential to stimulate other receptors (e.g., the ability of some artificial sweeteners to bind with T2Rs) and this has to be considered in the interpretation of findings from T1R KO preparations and may explain why some degree of responsiveness to these compounds remains in these mice. This possibility notwithstanding, T1R2+3 double KO mice entirely lack neural or behavioral responsiveness to sugars and the artificial sweeteners tested. However, some degree of responsiveness is maintained in T1R2 or T1R3 KO mice, suggesting that single T1R subunits might be able to form functional homodimers in the absence of their complementary partner. Nevertheless, even in these cases, function is severely impaired. The one exception is that T1R3 KO mice reportedly have normal psychophysically assessed taste detection thresholds to sucrose and MSG despite the fact that this protein subunit is critical in the normal activation of the T1R2+3 and the T1R1+3 heterodimers. This finding invites further attention because it is difficult to reconcile with the data from the literature which would predict impairments in the sensitivity of T1R3 KO mice to these ligands. Finally, Polycose appears to activate a taste receptor that is not dependent on the combined presence of T1R2 and T1R3. This suggests that either glucose polymer mixtures can effectively stimulate T1R subunits forming homodimers or that they bind with a novel, yet to be identified taste receptor. A comparison of Polycose responsiveness in WT mice compared with single and double KO preparations would be useful in this regard and such experiments are in progress in our laboratory. Moreover, if such a receptor exists, it will be important to identify its optimal ligand.
The discovery of T1R expression in a variety of non-gustatory tissues, especially the gut, pancreas, and brain, leads to attractive hypotheses regarding the role of these receptors in nutrient sensing given their ligand-binding properties. Nevertheless, while the presence of these proteins has been linked to physiological consequences in certain experimental models, the ability of artificial sweeteners to influence the release of incretin hormones and modulate glucose homeostasis is equivocal in the literature and the disparities remain to be resolved. Indeed, although the deletion of the T1R2 or T1R3 proteins causes remarkable impairments in taste function in mice, it has no ostensible effect on feeding in a fasted or nonfasted state and does not affect body weight of mice maintained on a laboratory chow diet.
The identification of the T1R family of receptors has had a tremendous influence on taste research and has helped provide guidance in efforts to understand the functional organization of the gustatory system. The impact of this seminal discovery has extended to other organ systems and has provoked exciting hypotheses regarding the role of these proteins in nutrient sensing. As noted above, the developing story is not without challenging complexities, but it is nonetheless rife with significant conceptual insights.
ACKNOWLEDGMENTS
We would like to thank Dr. Charles Zuker for generously supplying the T1R2 and T1R3 knock-out breeding pairs. Supported by NIH R01-DC004574 (A.C.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yada Treesukosol, Email: yadatree@psy.fsu.edu.
Kimberly R. Smith, Email: ksmith@psy.fsu.edu.
Alan C. Spector, Email: spector@psy.fsu.edu.
REFERENCES
- 1.Ackroff K, Manza L, Sclafani A. The rat's preference for sucrose, Polycose and their mixtures. Appetite. 1993;21:69–80. doi: 10.1006/appe.1993.1037. [DOI] [PubMed] [Google Scholar]
- 2.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJP, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 3.Bachmanov AA, Beauchamp GK. Amino acid and carbohydrate preferences in C57Bl/6ByJ and 129P3/J mice. Physiol Behav. 2008;93:37–43. doi: 10.1016/j.physbeh.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses. 2001;26:925–933. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:905–913. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 7.Blonde GD, Garcea M, Spector AC. The relative effects of transection of the gustatory branches of the seventh and ninth cranial nerves on NaCl taste detection in rats. Behav Neurosci. 2006;120:580–589. doi: 10.1037/0735-7044.120.3.580. [DOI] [PubMed] [Google Scholar]
- 8.Boughter JD, Jr, St. John SJ, Noel DT, Ndubuizu O, Smith DV. A brief-access test for bitter taste in mice. Chem Senses. 2002;27:133–142. doi: 10.1093/chemse/27.2.133. [DOI] [PubMed] [Google Scholar]
- 9.Breslin PA, Beauchamp GK, Pugh EN. Monogeusia for fructose, glucose, sucrose, and maltose. Perception & Psychophysics. 1996;58:327–341. doi: 10.3758/bf03206809. [DOI] [PubMed] [Google Scholar]
- 10.Capeless CG, Whitney G, Azen EA. Chromosome mapping of Soa, a gene influencing gustatory sensitivity to sucrose octaacetate in mice. Behav Genet. 1992;22:655–666. doi: 10.1007/BF01066636. [DOI] [PubMed] [Google Scholar]
- 11.Chandrashekar J, Hoon MA, Ryba NJP, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 12.Chen K, Yan J, Suo Y, Li J, Wang Q, Lv B. Nutritional status alters saccharin intake and sweet receptor mRNA expression in rat taste buds. Brain Res. 2010;1325:53–62. doi: 10.1016/j.brainres.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 13.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 14.Danilova V, Hellekant G. Comparison of the responses of the chorda tympani and glossopharyngeal nerves to taste stimuli in C57BL/6J mice. BMC Neurosci. 2003;4:5. doi: 10.1186/1471-2202-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis JD. The effectiveness of some sugars in stimulating licking behavior in the rat. Physiol Behav. 1973;11:39–45. doi: 10.1016/0031-9384(73)90120-0. [DOI] [PubMed] [Google Scholar]
- 16.Delay ER, Beaver AJ, Wagner KA, Stapleton JR, Harbaugh JO, Catron KD, Roper SD. Taste preference synergy between glutamate receptor agonists and inosine monophosphate in rats. Chem Senses. 2000;25:507–515. doi: 10.1093/chemse/25.5.507. [DOI] [PubMed] [Google Scholar]
- 17.Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses. 2006 doi: 10.1093/chemse/bjj039. [DOI] [PubMed] [Google Scholar]
- 18.Dotson CD, Spector AC. Behavioral discrimination between sucrose and other natural sweeteners in mice: Implications for the neural coding of T1R ligands. J Neurosci. 2007;27:11242–11253. doi: 10.1523/JNEUROSCI.1227-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dotson CD, Spector AC. The relative affective potency of glycine, L-serine and sucrose as assessed by a brief-access test in inbred strains of mice. Chem Senses. 2004;29:489–498. doi: 10.1093/chemse/bjh051. [DOI] [PubMed] [Google Scholar]
- 20.Dyer J, Salmon KSH, Zibrik L, Shirazai-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 21.Elizalde G, Sclafani A. Flavor preferences conditioned by intragastric Polycose infusions: A detailed analysis using an electronic esophagus preparation. Physiol Behav. 1990;47:63–77. doi: 10.1016/0031-9384(90)90043-4. [DOI] [PubMed] [Google Scholar]
- 22.Eylam S, Spector AC. The effect of amiloride on operantly conditioned performance in an NaCl taste detection task and NaCl preference in C57BL/6J mice. Behav Neurosci. 2002;116:149–159. [PubMed] [Google Scholar]
- 23.Eylam S, Spector AC. Stimulus processing of glycine is dissociable from that of sucrose and glucose based on behaviorally measured taste signal detection in sac 'taster' and 'non-taster' mice. Chem Senses. 2004;29:639–649. doi: 10.1093/chemse/bjh068. [DOI] [PubMed] [Google Scholar]
- 24.Finger TE. Cell types and lineages in taste buds. Chem Senses. 2005;30 doi: 10.1093/chemse/bjh110. [DOI] [PubMed] [Google Scholar]
- 25.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone LM, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 26.Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, Frost GS, Bloom SR. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr. 2011 doi: 10.1038/ejcn.2010.291. [DOI] [PubMed] [Google Scholar]
- 27.Formaker BK, Kearns CE, Frank ME. The taste of polycose in hamsters. Chem Senses. 1998;23:675–682. doi: 10.1093/chemse/23.6.675. [DOI] [PubMed] [Google Scholar]
- 28.Fujita Y, Wideman RD, Speck M, Asadi A, King DS, Webber TD, Haneda M, Keiffer TJ. Incretin release from gut is acutely enhanced by sugar but not sweeteners in vivo. Am J Physiol Endocrinol Metab. 2009;296:E473–E479. doi: 10.1152/ajpendo.90636.2008. [DOI] [PubMed] [Google Scholar]
- 29.Fuller JL. Single-locus control of saccharin preference in mice. J Hered. 1974;65:33–36. doi: 10.1093/oxfordjournals.jhered.a108452. [DOI] [PubMed] [Google Scholar]
- 30.Geran LC, Guagliardo NA, Spector AC. Chorda tympani nerve transection, but not amiloride, increases the KCl taste detection threshold in rats. Behav Neurosci. 1999;113:185–195. doi: 10.1037//0735-7044.113.1.185. [DOI] [PubMed] [Google Scholar]
- 31.Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with abberant taste and oromotor function. Chem Senses. 2002;27:461–474. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- 32.Gregersen S, Jeppesen PB, Holst JJ, Hermansen K. Antihyperglycemic effects of stevioside in type 2 diabetic subjects. Metabolism. 2004;53:73–76. doi: 10.1016/j.metabol.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, Trout JR, Pi-Sunyer FX. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Assoc. 2003;103:1607–1612. doi: 10.1016/j.jada.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 34.Harada S, Yamamoto T, Yamaguchi K, Kasahara y. Different characteristics of gustatory responses between the greater superficial petrosal and chorda tympani nerves in the rat. Chem Senses. 1997;22:133–140. doi: 10.1093/chemse/22.2.133. [DOI] [PubMed] [Google Scholar]
- 35.Hass N, Schwarzenbacher K, Breer H. T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res. 2010 doi: 10.1007/s00441-009-0907-6. [DOI] [PubMed] [Google Scholar]
- 36.Heyer BR, Taylor-Burds CC, Tran LH, Delay ER. Monosodium glutamate and sweet taste: Generalization of conditioned taste aversion between glutamate and sweet stimuli in rats. Chem Senses. 2003;28:631–641. doi: 10.1093/chemse/bjg056. [DOI] [PubMed] [Google Scholar]
- 37.Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJP, Zuker CS. Putative mammalian taste receptors: A class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–551. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- 38.Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJP, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129.B6-Tas1r3 congenic mice. Physiol Genomics. 2007;32:82–94. doi: 10.1152/physiolgenomics.00161.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakinovich W., Jr Taste aversion to sugars by the gerbil. Physiol Behav. 1982;28:1065–1071. doi: 10.1016/0031-9384(82)90176-7. [DOI] [PubMed] [Google Scholar]
- 41.Jang H-J, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim B-J, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. PNAS. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang P, Cui M, Zhao B, Liu Z, Snyder LA, Benard LMJ, Osman R, Margolskee RF, Max M. Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J Biol Chem. 2005;280:15238–15246. doi: 10.1074/jbc.M414287200. [DOI] [PubMed] [Google Scholar]
- 43.Jiang P, Ji Q, Liu Z, Snyder LA, Benard LMJ, Margolskee RF, Max M. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J Biol Chem. 2004;279:45068–45075. doi: 10.1074/jbc.M406779200. [DOI] [PubMed] [Google Scholar]
- 44.Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Ann Rev Nutr. 2008;28:35–54. doi: 10.1146/annurev.nutr.28.061807.155518. [DOI] [PubMed] [Google Scholar]
- 45.Kim M-R, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun. 2003;312:500–506. doi: 10.1016/j.bbrc.2003.10.137. [DOI] [PubMed] [Google Scholar]
- 46.Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun. 2001;283 doi: 10.1006/bbrc.2001.4760. 263-242. [DOI] [PubMed] [Google Scholar]
- 47.Kiuchi S, Yamada T, Kiyokawa N, Saito T, Fujimoto J, Yasue H. Genomic structure of swine taste receptor family 1 member 3, TAS1R3, and its expression in tissues. Cytogenet Genome Res. 2006;115:51–61. doi: 10.1159/000094801. [DOI] [PubMed] [Google Scholar]
- 48.Koizumi A, Nakajima K-i, Asakura T, Morita Y, Ito K, Shmizu-Ibuka A, Misaka T, Abe K. Taste-modifying sweet protein, neoculin, is received at human T1R3 amino terminal domain. Biochem Biophys Res Commun. 2007;358:585–589. doi: 10.1016/j.bbrc.2007.04.171. [DOI] [PubMed] [Google Scholar]
- 49.Kokrashvili Z, Mosinger B, Margolskee RF. T1r3 and alpha-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann N Y Acad Sci. 2009;1170:91–94. doi: 10.1111/j.1749-6632.2009.04485.x. [DOI] [PubMed] [Google Scholar]
- 50.Kuhn C, Bufe B, Winnig M, Hofmann T, Frank O, Behrens M, Lewtchenko T, Slack JP, Ward C, Meyerhof W. Bitter taste receptors for saccharin and acesulfame K. J Neurosci. 2004;24:10260–10265. doi: 10.1523/JNEUROSCI.1225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kusakabe Y, Kim M-R, Miura H, Shindo Y, Ninomiya Y, Hino A. Regional expression paterns of T1r family in the mouse tongue. Chem Senses. 2005;30 doi: 10.1093/chemse/bjh094. [DOI] [PubMed] [Google Scholar]
- 52.Lemon CH, Margolskee RF. Contribution of the T1r3 taste receptor to the response properties of central gustatory neurons. J Neurophysiol. 2009;101:2459–2471. doi: 10.1152/jn.90892.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X, Li W, Wang H, Cao J, Maehashi K, Huang L, Bachmanov AA, Reed DR, Legrand-Defretin V, Beauchamp GK, Brand JG. Pseudogenization of a sweet-receptor gene accounts for cats' indifference toward sugar. PLoS Genet. 2005;1:27–35. doi: 10.1371/journal.pgen.0010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lush IE. The genetics of tasting in mice. VI. Saccharin, acesulfame, dulcin and sucrose. Genet Res. 1989;53:95–99. doi: 10.1017/s0016672300027968. [DOI] [PubMed] [Google Scholar]
- 56.Ma J, Bellon M, Wishard JM, Young R, Blackshaw LA, Jones KL, Horowitz MR, Christopher K. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2009;296:G735–G739. doi: 10.1152/ajpgi.90708.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mace OJ, Lister N, Morgan E, Shepherd E, Affleck J, Helliwell P, Bronk JR, Kellett GL, Meredith D, Boyd R, Pieri M, Bailey PD, Pettcrew R, David F. An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine. J Physiol. 2009;587:195–210. doi: 10.1113/jphysiol.2008.159616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Margolskee RF, Dryer J, Kokrashvili Z, Salmon KSH, Ilegems E, Daly K, Maillet E, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+ -glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- 61.Montmayeur J-P, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci. 2001;4:492–497. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- 62.Mook DG. Oral and postingestional determinants of the intake of various solutions. J Comp Physiol Psychol. 1963;56:645–659. [Google Scholar]
- 63.Moran AW, Al-Rammahi A, Arora DK, Batchelor DJ, Coulter EA, Daly K, Ionescu C, Bravo D, Shirazai-Beechey SP. Expression of Na+/glucose co-transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. Br J Nutr. 2010;104:637–646. doi: 10.1017/S0007114510000917. [DOI] [PubMed] [Google Scholar]
- 64.Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, Lohse MJ, Shigemura N, Ninomiya Y, Kojima I. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLos One. 2009;4:e5106. doi: 10.1371/journal.pone.0005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nejad MS. The neural activities of the greater superficial petrosal nerve of the rat in response to chemical stimulation of the palate. Chem Senses. 1986;11:283–293. [Google Scholar]
- 66.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJP, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 67.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJP, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 68.Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Current Biology. 2005;15:1948–1952. doi: 10.1016/j.cub.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 69.Ninomiya Y, Higashi T, Katsukawa H, Mizukoshi T, Funakoshi M. Qualitative discrimination of gustatory stimuli in three different strains of mice. Brain Res. 1984:322. doi: 10.1016/0006-8993(84)91183-1. [DOI] [PubMed] [Google Scholar]
- 70.Nissenbaum JW, Sclafani A. Qualitative differences in polysaccharide and sugar tastes in the rat: A two-carbohydrate taste model. Neuroscience and Biobehavioral Reviews. 1987;11:187–196. doi: 10.1016/s0149-7634(87)80025-8. [DOI] [PubMed] [Google Scholar]
- 71.Nowlis GH. Conditioned stimulus intensity and acquired alimentary aversions in the rat. J Comp Physiol Psychol. 1974;86:1173–1184. doi: 10.1037/h0037644. [DOI] [PubMed] [Google Scholar]
- 72.Nowlis GH, Frank ME, Pfaffman C. Specificity of acquired aversions to taste qualities in hamsters and rats. J Comp Physiol Psychol. 1980;94:932–942. doi: 10.1037/h0077809. [DOI] [PubMed] [Google Scholar]
- 73.Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. Multiple sweet receptors and transduction pathways reveal in knockout mice by temperature dependence and gurmarin sensitivity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R960–R971. doi: 10.1152/ajpregu.91018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ohmoto M, Matsumoto I, Yasuoka A, Yoshihara Y, Abe K. Genetic tracing of the gustatory and trigeminal neural pathways originating from T1R3-expressing taste receptor cells and solitary chemoreceptor cells. Mol Cell Neurosci. 2008;38:505–517. doi: 10.1016/j.mcn.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 75.Pfaffmann C. Gustatory nerve impulses in rat, cat and rabbit. Journal of Neurophysiology. 1955;18:429–440. doi: 10.1152/jn.1955.18.5.429. [DOI] [PubMed] [Google Scholar]
- 76.Pritchard TC, Scott TR. Amino acids as taste stimuli. I. Neural and behavioral attributes. Brain Res. 1982;253:81–92. doi: 10.1016/0006-8993(82)90675-8. [DOI] [PubMed] [Google Scholar]
- 77.Pritchard TC, Scott TR. Amino acids as taste stimuli. II. Quality coding. Brain Res. 1982;253:93–104. doi: 10.1016/0006-8993(82)90676-x. [DOI] [PubMed] [Google Scholar]
- 78.Pronin AN, Xu H, Tang H, Zhang L, Li Q, Li X. Specific alleles of bitter receptor genes influence human sensitivity to the bitterness of aloin and saccharin. Curr Biol. 2007:17. doi: 10.1016/j.cub.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 79.Ramirez I. Thresholds for starch and Polycose are lower than for sucrose in rats. Physiol Behav. 1991;50:699–703. doi: 10.1016/0031-9384(91)90005-9. [DOI] [PubMed] [Google Scholar]
- 80.Reimann F, Gribble FM. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes. 2002;51:2757–2763. doi: 10.2337/diabetes.51.9.2757. [DOI] [PubMed] [Google Scholar]
- 81.Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci. 2009:3. doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rozengurt N, Wu VS, Chen MC, Huang C, Sternini C, Rozengurt E. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G792–G802. doi: 10.1152/ajpgi.00074.2006. [DOI] [PubMed] [Google Scholar]
- 83.Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J Neurochemistry. 2001;77:896–903. doi: 10.1046/j.1471-4159.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- 84.Sako N, Shimura T, Komure M, Mochizuki R, Matsuo R, Yamamoto T. Differences in taste responses to Polycose and common sugars in the rat as revealed by behavioral and electrophysiological studies. Physiol Behav. 1994;56:741–745. doi: 10.1016/0031-9384(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 85.Schiffman SS. Taste quality and neural coding: Implications from psychophysics and neurophysiology. Physiology & Behavior. 2000:69. doi: 10.1016/s0031-9384(00)00198-0. [DOI] [PubMed] [Google Scholar]
- 86.Schiffman SS, Booth BJ, Losee ML, Pecore SD, Warwick ZS. Bitterness of sweeteners as a function of concentration. Brain Res Bull. 1995;36:505–513. doi: 10.1016/0361-9230(94)00225-p. [DOI] [PubMed] [Google Scholar]
- 87.Schiffman SS, Sennewald K. Comparison of taste qualities and thresholds of D- and L-amino acids. Physiol Behav. 1981;27:51–59. doi: 10.1016/0031-9384(81)90298-5. [DOI] [PubMed] [Google Scholar]
- 88.Sclafani A. Carbohydrate taste, appetite, and obesity: An overview. Neurosci Biobehav Rev. 1987;11:131–153. [PubMed] [Google Scholar]
- 89.Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81:773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 90.Sclafani A, Abrams M. Rats show only a weak preference for the artificial sweetener aspartame. Physiol Behav. 1986;37:253–256. doi: 10.1016/0031-9384(86)90228-3. [DOI] [PubMed] [Google Scholar]
- 91.Sclafani A, Bahrani M, Zukerman S, Ackroff K. Stevia and saccharin preferences in rats and mice. Chem Senses. 2010;35:433–443. doi: 10.1093/chemse/bjq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1643–R1650. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol. 2005;289:R712–R720. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- 94.Sclafani A, Perez C. Cypha [propionic acid, 2-(4-methoxyphenol) salt] inhibits sweet taste in humans, but not in rats. Physiol Behav. 1997;61:25–29. doi: 10.1016/s0031-9384(96)00316-2. [DOI] [PubMed] [Google Scholar]
- 95.Sclafani A, Zukerman S, Glendinning JI, Margolskee RF. Fat and carbohydrate preferences in mice: the contribution of alpha-gustducin and Trpm5 taste-signaling proteins. Am J Physiol Regul Integr Comp Physiol. 2007;239:R1504–R1513. doi: 10.1152/ajpregu.00364.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Slack JP, Brockhoff A, Batram C, Menzel S, Sonnabend C, Born S, Galindo MM, Kohl S, Thalmann S, Ostopovici-Halip L, Simons CT, Ungureanu I, Duineveld K, bologa CG, Behrens M, Furrer S, Oprea TI, Meyerhof W. Modulation of bitter taste perception by a small molecule hTAS2R antagonist. Curr Biol. 2010;20:1104–1109. doi: 10.1016/j.cub.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Slotnick BM, Sheelar S, Rentmeister-Bryant H. Transection of the chorda tympani and insertion of ear pins for stereotaxic surgery: equivalent effects on taste sensitivity. Physiol Behav. 1991;50:1123–1127. doi: 10.1016/0031-9384(91)90571-5. [DOI] [PubMed] [Google Scholar]
- 98.Smith JC. The history of the "Davis Rig". Appetite. 2001;36:93–98. doi: 10.1006/appe.2000.0372. [DOI] [PubMed] [Google Scholar]
- 99.Spector AC. The functional organization of the peripheral gustatory system: Lessons from behavior. Progress in Psychobiology and Physiological Psychology. 2003;18:101–161. [Google Scholar]
- 100.Spector AC, Grill HJ. Differences in the taste quality of maltose and sucrose in rats: issues involving the generalization of conditioned taste aversions. Chem Senses. 1988;13:95–113. [Google Scholar]
- 101.Spector AC, Markison S, St. John SJ, Garcea M. Sucrose vs. maltose taste discrimination by rats depends on the input of the seventh cranial nerve. Am J Physiol. 1997;272:R1210–R1218. doi: 10.1152/ajpregu.1997.272.4.R1210. [DOI] [PubMed] [Google Scholar]
- 102.Spector AC, Scalera G, Grill HJ, Norgren R. Gustatory detection thresholds after parabrachial nuclei lesions in rats. Behav Neurosci. 1995;109:939–954. [PubMed] [Google Scholar]
- 103.Spector AC, Travers SP. The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev. 2005;4:143–191. doi: 10.1177/1534582305280031. [DOI] [PubMed] [Google Scholar]
- 104.St. John SJ, Spector AC. Combined glossopharyngeal and chorda tympani nerve transection elevates quinine detection thresholds in rats (Rattus norvegicus) Behav Neurosci. 1996;110:1456–1468. doi: 10.1037//0735-7044.110.6.1456. [DOI] [PubMed] [Google Scholar]
- 105.Stearns AT, Balakrishnan A, Rohoads DB, Tavakkolizadeh A. Rapid upregulation of sodium-glucose transporter SGLT1 in response to intestinal sweet taste stimulation. Ann Surg. 2010;251:865–871. doi: 10.1097/SLA.0b013e3181d96e1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steinert RE, Frey F, Topfer A, Drewe J, Beglinger C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br J Nutr. 2011 doi: 10.1017/S000711451000512X. [DOI] [PubMed] [Google Scholar]
- 107.Stone LM, Barrows J, Finger TE, Kinnamon SC. Expression of T1Rs and gustducin in palatal taste buds in mice. Chem Senses. 2007;32:255–262. doi: 10.1093/chemse/bjl053. [DOI] [PubMed] [Google Scholar]
- 108.Taniguchi K. Expression of the sweet receptor protein, T1R3, in the human liver and pancreas. J Vet Med Sci. 2004;66:1311–1314. doi: 10.1292/jvms.66.1311. [DOI] [PubMed] [Google Scholar]
- 109.Travers SP, Norgren R. Coding the sweet taste in the nucleus of the solitary tract: Differential roles for anterior tongue and nasoincisor duct gustatory receptors in the rat. J Neurophysiol. 1991;65:1372–1380. doi: 10.1152/jn.1991.65.6.1372. [DOI] [PubMed] [Google Scholar]
- 110.Travers SP, Pfaffman C, Norgren R. Convergence of lingual and palatal gustatory neural activity in the nucleus of the solitary tract. Brain Res. 1986;365:305–320. doi: 10.1016/0006-8993(86)91642-2. [DOI] [PubMed] [Google Scholar]
- 111.Treesukosol Y, Blonde GD, Spector AC. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to polycose: implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R855–R865. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Treesukosol Y, Lyall V, Heck GL, DeSimone JA, Spector AC. A psychophysical and electrophysiological analysis of salt taste in Trpv1 null mice. Am J Physiol Regul Integr Comp Physiol. 2007:292. doi: 10.1152/ajpregu.00587.2006. [DOI] [PubMed] [Google Scholar]
- 113.Weingarten H, Watson S. Sham feeding as a procedure for assessing the influence of diet palatability on food intake. Physiol Behav. 1982;28:401–407. doi: 10.1016/0031-9384(82)90131-7. [DOI] [PubMed] [Google Scholar]
- 114.Winnig M, Bufe B, Kratochwil NA, Slack JP, Meyerhof W. The binding site for neohesperidin dihydrochalcone at the human sweet taste receptor. BMC Struct Biol. 2007;7:66. doi: 10.1186/1472-6807-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Winnig M, Bufe B, Meyerhof W. Valine 738 and lysine 735 in the fifth transmembrane domain of rTas1r3 mediate insensitivity towards lactisole of the rat sweet taste receptor. BMC Neurosci. 2005;6:22. doi: 10.1186/1471-2202-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu H, Straszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci U S A. 2004;101:14258–14263. doi: 10.1073/pnas.0404384101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yamamoto T, Matsuo R, Fujimoto Y, Fukunaga I, Miyasaka A, Imoto T. Electrophysiological and behavioral studies on the taste of umami substances in the rat. Physiol Behav. 1991;49:919–925. doi: 10.1016/0031-9384(91)90204-2. [DOI] [PubMed] [Google Scholar]
- 118.Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Taste effects of 'umami' substances in hamsters as studied by electrophysiological and conditioned taste aversion techniques. Brain Res. 1988;451:147–162. doi: 10.1016/0006-8993(88)90759-7. [DOI] [PubMed] [Google Scholar]
- 119.Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. "Type III" cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- 120.Young PT, Trafton CL. Activity countour maps as related to preference in four gustatory stimulus areas of the rat. J Comp Physiol Psychol. 1964:58. doi: 10.1037/h0044823. [DOI] [PubMed] [Google Scholar]
- 121.Zhang F, Klebansky B, Fine RM, Xu H, Pronin A, Liu H, Tachdjian C, Li X. Molecular mechanism for the umami taste synergism. Proc Natl Acad Sci U S A. 2008;105:20930–20934. doi: 10.1073/pnas.0810174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 123.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol. 2009;296:R866–R876. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zukerman S, Touzani K, Margolskee RF, Sclafani A. Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem Senses. 2009;34:686–694. doi: 10.1093/chemse/bjp055. [DOI] [PMC free article] [PubMed] [Google Scholar]