Abstract
Stimulation with LPS induces tyrosine phosphorylation of numerous proteins involved in the TLR signaling pathway. In this study, we demonstrate that MD-2 is also tyrosine phosphorylated following LPS stimulation. LPS-induced tyrosine phosphorylation of MD-2 is specific, it is blocked by the tyrosine kinase inhibitor, Herbimycin A, and by an inhibitor of endocytosis, Cytochalsin-D, suggesting that MD-2 phosphorylation occurs during trafficking of MD2 and not on cell surface. Furthermore, we identify two possible phospho-accepting tyrosine residues at positions 22 and 131. Mutant proteins in which these tyrosines were changed to phenylalanine have reduced phosphorylation and significantly diminished ability to activate NF-κB in response to LPS. In addition, MD2 co-precipitates and colocalizes with Lyn kinase, most likely in ER. A Lyn-binding peptide inhibitor abolished MD2 tyrosine phosphorylation, suggesting that Lyn is a likely candidate to be the kinase required for MD-2 tyrosine phophorylation. Our study demonstrates that tyrosine phosphorylation of MD-2 is important for signaling following exposure to LPS and underscores the importance of this event in mediating an efficient and prompt immune response.
Introduction
Myeloid differentiation-2 (MD-2) is an essential component of the signaling receptor complex that recognizes and initiates an innate immune response to bacterial LPS (1). At the receptor level, LPS binding protein (2), CD14 (3) and TLR4(4–6) are the also required in this signaling event, as is evidenced by the fact that mice deficient in either of these proteins or MD-2 (1) display a similar hyporesponsiveness to LPS challenge. For signaling to occur, LPS is first extracted from the bacterial membrane by LBP, which is then transferred to CD14 in its monomeric form. CD14 subsequently delivers LPS to MD-2, which is a secreted glycoprotein that belongs to the MD-2-related lipid recognition (ML) family (4), the signature sequence of which is a secretion signal. MD-2 may be present in a soluble form or bound to the ectodomain of TLR4 (5, 6). Upon LPS binding, a receptor multimer composed of two copies of the TLR4-MD-2-LPS complex is formed (7), which triggers a downstream signaling cascade, culminating in the activation of transcription factors such as nuclear factor-κB (NF-κB) and the interferon regulatory factors (IRFs), which in turn induce various immune and inflammatory genes.
We have recently identified and described a novel alternatively spliced isoform of human MD-2, which lacks the region encoded by exon 2 of the MD-2 gene and showed that it is upregulated by IFN-γ , IL-6 and TLR4 stimulation, is a negative regulator of LPS-mediated TLR4 signaling and competitively inhibits binding of full length MD-2 to TLR4 (8).
Upon ligand binding, the TLR signaling pathway initiates a cascade of serine, threonine and tyrosine phosphorylation events. Interestingly, several members of the TLR family are also tyrosine phosphorylated, including TLR2 (9), TLR3 (10–12), and TLR4 (13, 14). To date the identity of the kinases involved have yet to be elucidated, however, in the case of TLR4, the Src kinase Lyn has been implicated in this posttranslational modification (13). In addition to TLRs, the TLR adapter proteins, MyD88 (15), MyD88-adapter like (16), TRIF (17) and TRAM (18) have also been shown to be phosphorylated.
In this study, we have identified that MD-2 is also tyrosine phosphorylated upon LPS binding. This phosphorylation event is inhibited by the tyrosine kinase inhibitor herbimycin A. Furthermore, an endocytosis inhibitor, cytochalasin D, could block the tyrosine phosphorylation of MD-2 in cells stimulated with LPS. We have identified two residues, located at positions 22 and 131, as possible phospho-accepting tyrosines. Mutant proteins in which these tyrosines were altered to phenylalanine have less phosphorylation and a significantly reduced ability to activate LPS-induced NF-κB and IL-8. In addition, we determined that Lyn interacts with MD2 and that a Lyn-binding peptide inhibitor specifically abolishes MD-2 tyrosine phosphorylation, indicating that Lyn is the likely kinase required for MD-2 tyrosine phophorylation. Our study is the first to identify MD-2 as a phosphoprotein and demonstrates the importance of this posttranslational event as a mechanism required for MD-2-TLR4-LPS signaling.
Materials and Methods
Cell culture and biological reagents
The HEK293 cell line was cultured in Dulbecco’s modified Eagle’s medium, supplemented with 10% heat-inactivated FBS and 2 mM glutamine. LPS (TLRGrade) was from (Alexis). Protein tyrosine kinase inhibitor herbimycin A was purchased from Sigma-Aldrich. Lyn peptide inhibitor was purchased from Tocris Cookson. 4G10 anti-phosphotyrosine Ab was purchased from Upstate. Anti-Flag agarose affinity gel and anti-Flag Ab were from Sigma Aldrich. Anti-Calnexin, anti-Myc, anti-Lyn and anti-HA Abs were from Santa Cruz Biothecnology and Zymed Labs respectively. Alexa Fluor 594 anti-rabbit, Alexa Fluor 488 anti-mouse and Alexa Fluor 647 anti-goat IgG were from Invitrogen.
Immunoprecipitation
HEK293 cells were seeded into 100 mm dishes (1.5x106) 24 h prior to transfection. Transfections were performed using lipofectamine 2000 (Invitrogen). For co-immunoprecipitations, 4 μg of each construct was transfected. The total amount of DNA in each sample was kept constant by using empty vector cDNA. Cells were harvested 24 h following transfection in 600 μl of lysis buffer (50 mM HEPES, pH 7.5, 100 mM NaCl, 1 mM EDTA, 10% glycerol, 0.5% NP-40 containing protease inhibitor cocktail, and 1 mM sodium orthovanadate). For immunoprecipitations, anti-Flag M1 agarose affinity gel or anti-Myc Ab with TrueBlot anti-mouse Ig beads (eBioscience) was incubated with the cell lysates overnight at 4° C. The immune complexes were then washed and the associated proteins were eluted from the beads by boiling in 35 μl of sample buffer, and then fractionated by SDS-PAGE.
Fluorescent Microscopy
HEK293 cells were transiently transfected with plasmids expressing Flag-MD-2. After 24 hrs, cells were fixed with 4% paraformaldehyde (Electron Microscopy Science) in PBS, permeabilized using 0.2% triton-X 100 (Sigma), and blocked with DakoCytomation protein block (Dako). Cells were then incubated with primary antibodies for overnight at 4°C and with secondary antibodies for 1h at room temperature. Cells were mounted on slides using Prolong with DAPI (Invitrogen). Images were acquired in independent channels with a Zeiss ApoTome-equipped fluorescence microscope.
Immunoblotting
For immunoblotting, primary Abs were incubated overnight and detected using horseradish peroxidase–conjugated secondary antibodies, followed by enhanced chemiluminescence (Amersham Biosciences).
Reporter gene assays
HEK293 cells were transiently transfected with the expression vectors with constructs encoding the NF-κB and IL-8-luciferase reporter gene, and the phRL-TK reporter gene to normalize for transfection efficiency. In all cases, total DNA concentration was kept constant by supplementation with empty vector control. Following overnight incubation, cells were stimulated for 6 h with 50 ng/ml LPS, lysed and then luciferase activity was measured. Data are shown as mean ± S.D. of three or more independent experiments and are reported as a percentage of LPS-stimulated NF-κB and IL-8 promoter activity.
ELISA
HEK 293 cells, overexpressing TLR4, mutant MD-2 proteins and CD14 were pretreated with or without the Lyn peptide inhibitor for two hours prior to LPS stimulation, and supernatants collected. Levels of IL-8 were determined by ELISA (BD Biosciences).
Results
MD-2 is tyrosine phosphorylated upon stimulation with LPS
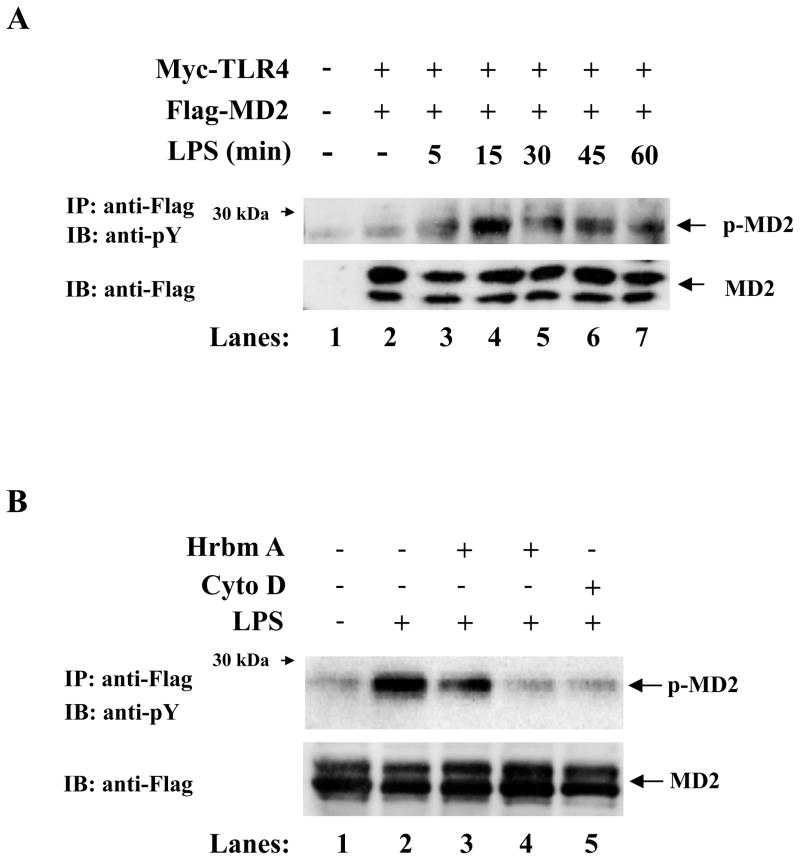
Tyrosine phosphorylation of numerous proteins is induced upon stimulation with LPS and indeed the LPS receptor, TLR4, is itself tyrosine phosphorylated. Given the important role of MD-2 following LPS stimulation, we investigated if it is post-translationally modified as well. HEK293 cells were transiently transfected with plasmids expressing Myc-TLR4, Flag-MD-2 and CD14 or mock transfected. 24 hrs later, cells were left untreated or stimulated with LPS for various time points. Proteins were immunoprecipitated from cell extracts with an anti-Flag Ab and analyzed by immunoblotting with an Ab that detects proteins phosphorylated on phosphotyrosine residues. Upon stimulation with LPS, MD-2 is tyrosine phosphorylated (Fig. 1A). The presence of 5 mM phosphotyrosine, a competitive inhibitor, completely abrogated the immunoreactivity detected by the anti-phosphotyrosine Ab, in contrast, phosphoserine or phosphothreonine had no effect on MD-2 tyrosine phosphorylation (data not shown), which confirms the specificity of this result. In addition, MD-2 tyrosine phosphorylation was not observed after stimulation with IL-1β or TNFα or RsDPLA, a biologically inactive analogue of lipid A (data not shown). To further confirm that MD-2 is posttranslationally modified, we pre-treated HEK 293 cells, overexpressing TLR4 and MD-2, with the tyrosine kinase inhibitor herbimycin A for two hours prior to LPS stimulation and observed that herbimycin A significantly inhibited LPS-induced MD-2 tyrosine phosphorylation in a dose dependent manner (Fig. 1B, compare lanes 3 and 4 to lane 2). We next hypothesized that MD-2 phosphorylation must occur during trafficking and not on the cell surface, therefore we investigated the role of receptor or ligand internalization and endocytosis on the phosphorylation status of MD-2. Since cytochalasins effectively block LPS internalization and signaling for cytokine release (19), we pre-treated HEK293 cells, overexpressing TLR4 and MD-2, with Cytochalasin-D for one hour prior to LPS stimulation. We observed that LPS-induced MD-2 tyrosine phosphorylation was significantly inhibited following pretreatment with cytochalasin D (Fig. 1B, lane 5). This suggests that MD-2 tyrosine phosphorylation most likely occurs intracellularly during trafficking and not on the cell surface.
Figure 1.
MD-2 is tyrosine phosphorylated and this phosphorylation is inhibited by herbimycin A and cytochalasin D. (A) HEK293 cells were transiently transfected with Myc-TLR4, Flag-MD-2 and CD14 constructs (lanes 2–7) or mock transfected (lane 1). 24h later cells were left untreated (lanes 1 and 2) or stimulated with LPS (lanes 3–7). Flag-tagged proteins were immunoprecipitated with an anti-Flag Ab in cell lysates and analyzed by SDS-PAGE and immunoblotted with an anti-phosphotyrosine Ab (top panel), or an anti-Flag Ab (middle and lower panels). (B) HEK293 cells were transiently transfected with Flag-TLR4 and Flag-MD-2 (lanes 1–5). 24 hrs later, cells were pretreated for 2hrs with herbimycin A at 0.5μg/ml (lane 3) or 2.5μg/ml (lane 4), or for 1hr with 2μM cytochalasin D (lane 5) prior to stimulation with LPS for 5 mins.
Identification of Tyr-22 and Tyr-131 as Possible Phospho-acceptors
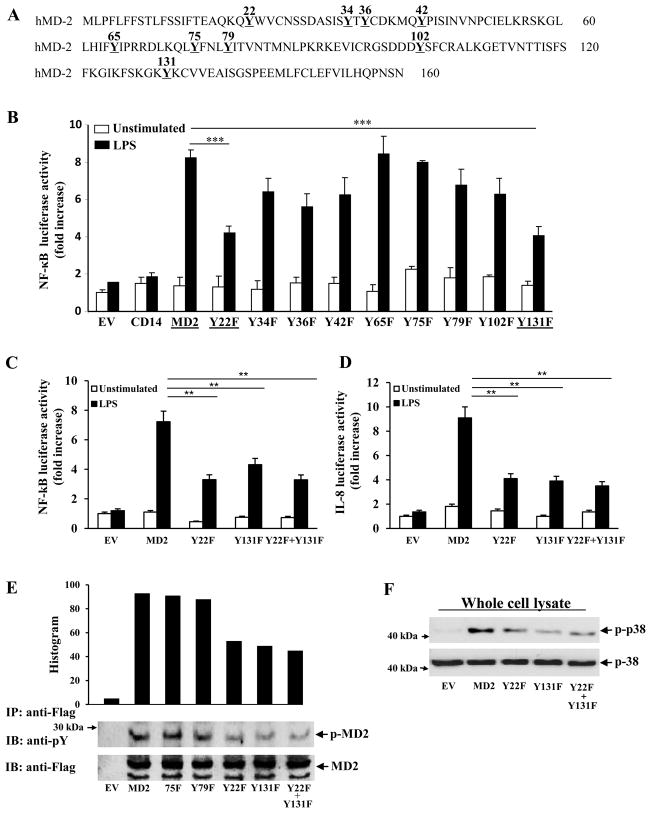
Human MD-2 contains nine tyrosine residues (Fig. 2A). We mutated all these residues conservatively to phenylalanine and tested their ability to respond to LPS. HEK293 cells stably transfected with a NF-κ B reporter gene and TLR4 were transiently transfected with plasmids encoding wild-type MD-2, Y22F, Y34F, Y36F, Y42F, Y65F, Y75F, Y79F, Y102F, or Y131F. 24 hrs later, cells were stimulated with LPS. As can be seen in Fig. 2B, upon LPS stimulation the mutant proteins Y22F and Y131F are significantly less potent in their ability to activate NF-κ B compared to wild-type MD-2. Analysis of a double mutant protein, Y22F+Y131F, further confirmed that these residues are important for MD-2 to signal NF-κ B activation in response to LPS (Fig. 2C). We also determined that the mutant proteins lacking tyrosine residues located at positions 22 and 131 had a diminished ability to activate IL-8 (Fig. 2D). We further characterized these mutant MD-2 proteins by analyzing their phosphorylation status after LPS stimulation. Supporting our prediction that the sites we mutagenized were phosphorylation sites, the mutant proteins, Y22F and Y131F, were less phosphorylated compared to wild-type MD-2 (Fig. 2E). Importantly, the unaffected mutants, Y75F and Y79F, had similar amounts of phosphorylation compared to WT MD-2 (Fig. 2E). Additionally, we measured the amount of phosphorylated p38 after LPS stimulation. Cells transfected with the mutant MD-2 proteins had less phospho-p38 compared to the normal MD-2 transfected cells, indicating diminished signaling in these cells (Fig. 2F). In addition, we also confirmed that these mutant proteins were secreted (data not shown), and displayed a similar glycosylation pattern as wild-type MD-2 upon SDS-PAGE analysis, and were expressed at similar levels (Fig. 2E).
Figure 2.
The mutant proteins Y22F, Y131F, and Y22F+Y131F do not activate NF-κ B as strongly as wild-type MD-2. (A) Schematic diagram showing the location of the tyrosine residues of MD-2. (B) HEK293 cells stably transfected with a NF-κ B reporter gene and TLR4 were transiently transfected with wild-type MD-2, Y22F, Y34F, Y36F, Y42F, Y65F, Y75F, Y79F, or Y131F constructs, for 24 h. Cells were left untreated or stimulated with LPS for 6 hours and luciferase activity measured in cell lysates and expressed as fold induction relative to mock-transfected cells (EV). (C) and (D) HEK293 cells stably transfected with a NF-κ B and IL-8 reporter gene and TLR4 were transiently transfected with wild-type MD-2, Y22F, Y131F, or Y22F+Y131F constructs. Cells were left untreated or stimulated with LPS for 6 hours and luciferase activity measured in cell lysates and expressed as fold induction relative to mock-transfected cells (EV). (E–F) HEK293 cells were transfected with plasmids expressing the indicated mutant Flag-MD-2 proteins, wild type Flag-MD-2 or empty vector, plus Myc-TLR4 and CD14 constructs. The cells were stimulated with LPS for 15 minutes. (E) Flag-tagged proteins were immunoprecipitated with an anti-Flag Ab in cell lysates and analyzed by SDS-PAGE and immunoblotted with an anti-phosphotyrosine Ab, or an anti-Flag Ab. (F) Cell lysates were prepared and samples were analyzed by immunoblotting with an p38 or an anti-phospho-p38 antibody. *** P<0.05.
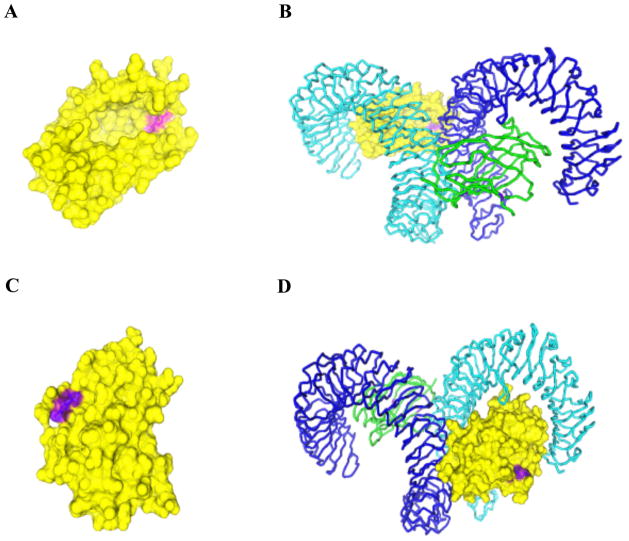
Given that we have shown that Tyr 22 and Tyr 131 are likely phospho-accepting residues, we next determined the location of these residues with respect to the published crystal structure of MD-2. As shown in Figure 3A, and 3C, the hydroxyl groups of both residues appear to be surface exposed, thereby allowing phosphorylation of these tyrosine residues to occur. Although Tyr 131 is located at the hydrophobic pocket of MD-2, neither Tyr 22 nor 131 are involved in the main dimerization interface of the TLR4-MD-2-LPS complex (Fig 3. B and D).
Figure 3.
Structure of the TLR4-MD-2 complex. All figures are based on the published crystal structure of the TLR4-MD-2 receptor complex. The structure with PDB ID: 3FXI was modified with 3-D molecule viewer (a component of vector NTI Advance 11.0-Invitrogen). The surface was calculated using the Conolly method. (A) The structure of MD-2 (yellow) with the location of Y131 depicted in purple. (B) The structure of MD-2 as shown in (A) in complex with TLR-4 (Cyan) and with one more copy of the TLR-4 (blue)-MD-2 (green) complex. (C) Y131 is shown in purple, the overall structure of MD-2 (yellow) with the location of Y22 depicted in purple. (D) The structure of MD-2 as shown in (C) in complex with TLR-4 (Cyan) and with one more copy of TLR-4 (blue)-MD-2(green) complex. The position of Y22 is shown in purple.
MD-2 interacts with Lyn
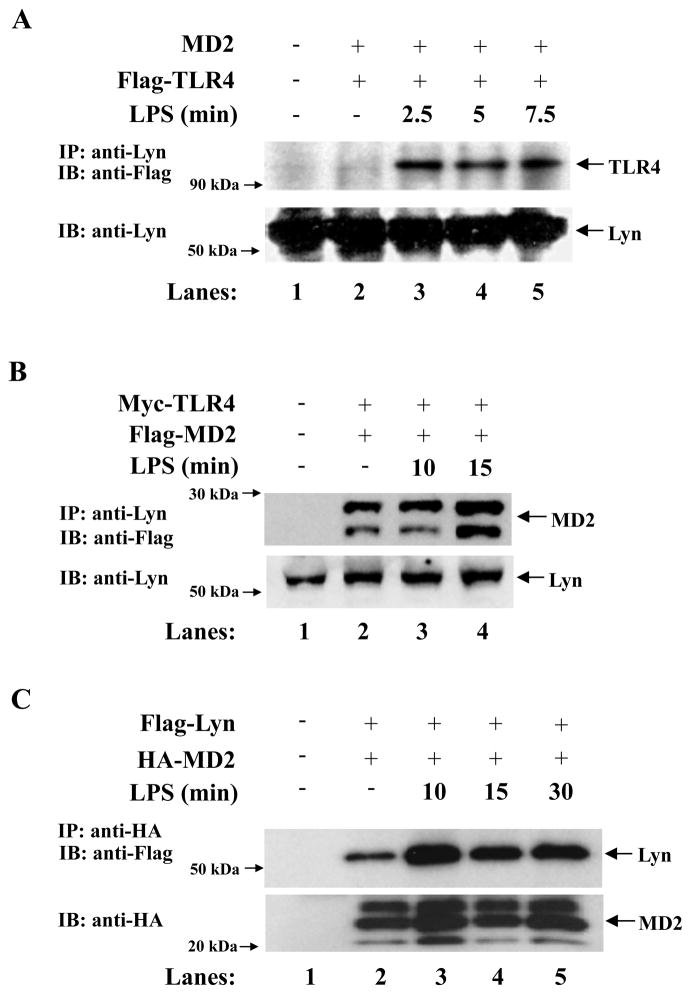
Prior studies have shown that the Src kinase, Lyn, is recruited to TLR4 (13) as well as to CD14 (20). Furthermore it has been suggested that Lyn may be involved in TLR4 tyrosine phosphorylation. Similar to previous results we found that TLR4 immunoprecipitated with Lyn upon LPS stimulation (Fig. 4A). We therefore investigated if Lyn was also involved in the phosphorylation of MD-2, given that MD-2 tyrosine phosphorylation was abolished following pretreatment with herbimycin A (Fig. 1B), a potent Src kinase inhibitor, which has been shown to inhibit Lyn activity (21). HEK293 cells were transiently transfected with Myc-TLR4, Flag-MD-2 and CD14 constructs. Lyn was immunoprecipitated from cell lysates with an anti-Lyn antibody and immunoblotted with an anti-Flag antibody. We observed that MD-2 immunoprecipitated with Lyn (Fig. 4B). However, given that Lyn also immunoprecipitates with TLR4 we wanted to confirm that this was due to a direct interaction with MD-2. We therefore, transiently transfected HEK293 cells with plasmids expressing HA-MD-2 and Flag-Lyn and immunoprecipitated HA-tagged proteins and immunoblotted with an anti-Flag antibody. We saw that even without the presence of TLR4, MD-2 immunoprecipitated with Lyn, confirming that Lyn and MD-2 could directly interact (Fig. 4C), suggesting that Lyn is most likely the kinase that phosphorylates MD-2.
Figure 4.
Lyn interacts with MD-2. (A) HEK293 cells were transiently transfected with MD-2, Flag-TLR4 and CD14 construct. 24h later, cells were left untreated (lanes 1 and 2) or stimulated with LPS (lanes 3–5). Lyn proteins were immunoprecipitated with an anti-Lyn antibody in cell lysates and analyzed by SDS-PAGE and immunoblotted with an anti-Flag Ab (top panel), or an anti-Lyn Ab (lower panel). (B) HEK293 cells were transiently transfected with Myc-TLR4 and Flag-MD-2 and CD14 constructs. 24h later, cells were left untreated (lanes 1 and 2) or stimulated with LPS (lanes 3–4). Lyn was immunoprecipitated with an anti-Lyn Ab in cell lysates, and analyzed by SDS-PAGE and immunoblotted with an anti-Flag Ab. (C) HEK293 cells were transiently transfected with Flag-Lyn and HA-MD-2 construct. 24h later, cells were left untreated (lanes 1 and 2) or stimulated with LPS (lanes 3–5). HA-tagged proteins were immunoprecipitated with an anti-HA Ab in cell lysates and analyzed by SDS-PAGE and immunoblotted with an anti-Flag Ab.
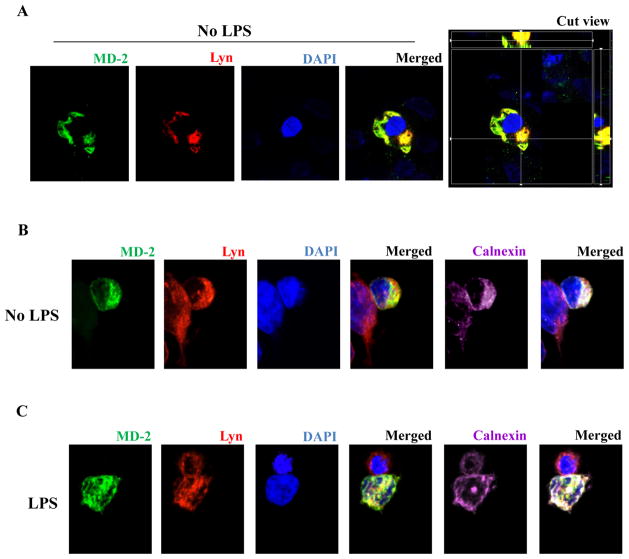
Colocalization of endogenous Lyn and MD-2 in ER
Since we found that Lyn kinase and MD-2 could directly interact with each other in HEK293 cells (Fig. 4B–C), we also investigated whether endogenous Lyn and transfected MD-2 would colocalize as determined by confocal microscopy. HEK293 cells were transiently transfected with plasmids expressing Flag-MD-2 and Myc-TLR4. After 24 hrs, cells were fixed and stained with anti-Lyn (red), anti-Flag (green) antibodies and nuclear stain DAPI (blue). Yellow staining in the merged image indicates colocalization between Lyn kinase and MD-2 (Fig. 5A). Further investigations revealed that the colocalization of endogenous Lyn with MD-2 was most likely in the endoplasmic reticulum (Fig. 5B). Finally, the addition of LPS to the cells did not alter the colocalization of Lyn kinase with MD-2 (Fig. 5C).
Figure 5.
Colocalization of endogenous Lyn with MD-2. HEK293 cells were transiently transfected with plasmids expressing Flag-MD-2. After 24 hrs, cells were immunostained with anti-Lyn (red), anti-Flag (green) antibodies, and nuclear stain DAPI (blue). Some samples were also stained with anti-calnexin (far-red). (A) Colocalization of endogenous Lyn with MD-2. HEK293 cells were transiently transfected with plasmids expressing Flag-MD-2 (yellow-merged image). (B) Lyn and MD-2 colocalize with ER (white-merged image). (C) Lyn and MD-2 colocalize with ER (white-merged image) in the presence of LPS.
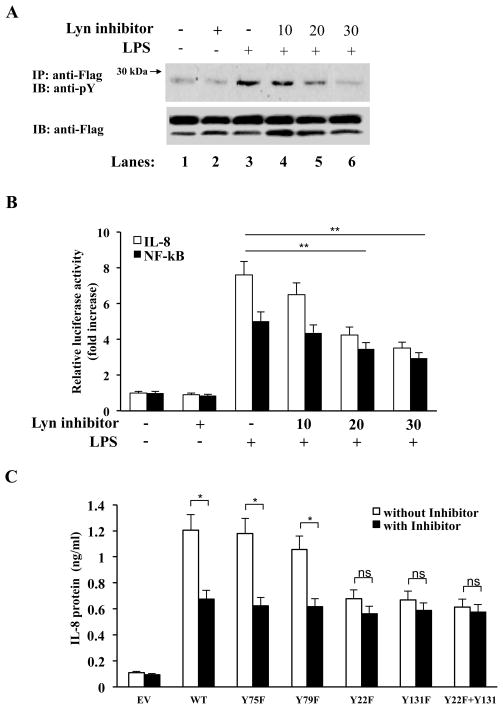
Lyn specific peptide inhibitor blocks MD-2 tyrosine phosphorylation
Given that MD-2 tyrosine phosphorylation was abolished following pretreatment with herbimycin A, and that MD-2 and Lyn were found to be in a complex together, we next wanted to further confirm that Lyn was required for MD-2 tyrosine phosphorylation. We therefore used a Lyn specific peptide inhibitor (22) to examine the effect of inhibiting Lyn activation on MD-2 phosphorylation. HEK 293 cells, overexpressing TLR4, Myc-MD-2 and CD14 were pretreated with the Lyn peptide inhibitor for two hours prior to LPS stimulation. As can be seen in Fig. 6A, the Lyn peptide inhibitor significantly abolished LPS-induced MD-2 tyrosine phosphorylation in a dose dependent manner (Fig. 6A, compare lanes 4-6 to lane 3). Furthermore, following pretreatment with the Lyn peptide inhibitor, LPS-induced activation of IL-8 and NF-kB was also abolished in HEK293 cells overexpressing TLR4, CD14 and MD-2 (Fig. 6B), Additionally, we measured the protein levels of IL-8 (Fig. 6C). HEK 293 cells, overexpressing TLR4, CD14, and wild type or mutant MD-2 proteins, were pretreated with or without the Lyn peptide inhibitor for two hours prior to LPS stimulation. IL-8 production was significantly diminished in cells transfected with wild-type MD-2, and the non-affected mutants Y75F and Y79F in the presence of Lyn peptide inhibitor, whereas there was no significant difference with and without Lyn peptide inhibitor in the phosphorylation mutants Y22F, Y131F, and Y22F, Y131F double mutant transfected cells, thus confirming the importantance of MD-2 tyrosine phosphorylation by Lyn kinase in mediating the signaling of the LPS-MD-2-TLR4-complex.
Figure 6.
MD-2 tyrosine phosphorylation is inhibited by Lyn peptide inhibitor. (A) HEK293 cells were transiently transfected with TLR4 and Myc-MD-2 (lanes 1–6). 24 hrs later, cells were pretreated for 2hrs with Lyn peptide inhibitor at 10, 20 or 30μM, prior to stimulation with LPS for 10 mins (lanes 3–6). Myc-tagged proteins were immunoprecipitated with an anti-Myc Ab in cell lysates, and analyzed by SDS-PAGE and immunoblotted with an anti-phosphotyrosine Ab (top panel), or an anti-Myc Ab (lower panel). (B) HEK293 cells stably transfected with a NF-κ B or IL-8 reporter gene and TLR4 were transiently transfected with wild-type MD-2 24 hrs later, cells were pretreated for 2hrs with Lyn peptide inhibitor (μM) at indicated doses. Cells were left untreated or stimulated with LPS for 6 hours and luciferase activity measured in cell lysates and expressed as fold induction relative to untreated cells. (C) HEK293 cells were transiently transfected with TLR4, wild-type MD-2, Y75F, Y79F, Y22F, Y131F, or Y22F+Y131F constructs. 24 hrs later, cells were pretreated for 2hrs with or without Lyn peptide inhibitor (20μM) and stimulated with LPS for 6 hours. IL-8 levels in the supernatants were measured by ELISA. * P<0.05.
Discussion
To date it is apparent that LPS requires both TLR4 and MD-2 to initiate signal transduction an innate immune responses, in addition to LPS-binding protein (2) and CD14 (3). Furthermore, crystal structural data for the TLR4-MD-2 complex bound to hexa-acyl lipid A has shown that MD-2 directly binds the lipid A portion of LPS with high avidity, as its hydrophobic pocket can accommodate up to five lipid A acyl chains (7). In this study we further clarify the role of MD-2 in TLR4 signaling and demonstrate that MD-2 undergoes tyrosine phosphorylation upon LPS stimulation and that this phosphorylation event is required for LPS-induced NF-κ B activation.
Excessive inflammation is the hallmark of a number of infectious pathologies, such as sepsis, acute respiratory distress syndrome and multiple organ failure (23), therefore tight regulation of the TLR4 signaling pathway at multiple levels is imperative in order to prevent an over activated immune response. Phosphorylation is a highly conserved mechanism that can be employed to regulate protein function. Indeed several studies have illustrated that the TLR signaling pathway is dependent on a series of phosphorylation events. Here we discovered that similar to TLR2, TLR3, and TLR4, MD-2 also undergoes tyrosine phosphorylation in response to LPS. Furthermore, our results suggest that phosphorylation of MD-2 on specific tyrosines are required for NF-κ B activation and may be a regulatory step employed to curtail an over exuberant host immune response.
LPS-TLR4-MD-2 complex is rapidly endocytosed upon initiation of signaling and can be located in endosomes, Golgi apparatus and lysosomes (24, 25). Cytochalasin D, a potent actin polymerization inhibitor that prevents LPS endocytosis (19), inhibited LPS-induced tyrosine phosphorylation of MD-2, suggesting that LPS-induced MD-2 phosphorylation occurs in intracellular compartments following internalization and trafficking of the LPS-TLR4-MD-2 complex and not on the cell surface. Furthermore, we confirmed that this MD-2 tyrosine phosphorylation was specific to LPS stimulation, as it did not occur following stimulation with IL-1β or TNFα.
We next assessed the relative functional importance of the nine tyrosine residues in MD-2 by substituting each individual tyrosine with phenylalanine. Two of the substitutions, Y22F and Y131F, resulted in a 50% decrease in NF-κ B activity and IL-8 secretion upon LPS stimulation, indicating that these residues were critical for maximal NF-κ B activation and could possibly be phospho-accepting residues. In addition, by analyzing the published crystal structure of MD-2, we determined that the hydroxyl groups of both MD-2 tyrosine residues, located at positions 22 and 131, are surface exposed thereby permitting phosporylation of the aforementioned residues to occur. A very early event in LPS signaling in monocytes is activation of lyn kinase activity (26). A candidate kinase for MD-2 phosphorylation is also Lyn kinase. Lyn kinase is recruited to TLR4 as well as to CD14 (20) upon LPS stimulation and has also been implicated as the kinase involved in the tyrosine phosphorylation of TLR4 (13). In this study, we determined that MD-2 is also present in a complex with TLR4 and Lyn. Additionally, we observed that MD-2 could immunoprecipitate with Lyn in the absence of CD14 and TLR4 and colocalize by microscopy, most likely in the ER. Furthermore we determined that LPS-induced MD-2 tyrosine phosphorylation is strongly abolished following pre-treatment with a Lyn-binding peptide inhibitor. Importantly, the Lyn-binding peptide inhibitor had no effect on the phosphorylation mutants Y22F and Y131F. Since MD-2 and Lyn appear to directly interact with one another, and MD-2 tyrosine phosphorylation is diminished following Lyn kinase inactivation, we hypothesize that similar to TLR4, Lyn kinase is involved in the tyrosine phosphorylation of MD-2. We also show that Lyn is pre-associated with MD-2 prior to LPS stimulation, yet only associates with TLR4 after LPS stimulation. This observation raises the possibility that MD-2 may deliver Lyn to the TLR4 complex in order to facilitate TLR4 phosphorylation by Lyn as well as phosphorylating MD-2. Even though Lyn and MD-2 appear to be pre-associated, MD-2 phosphorylation does not occur until LPS/TLR4 signaling is initiated, perhaps triggering some conformational shift, which now allows Lyn to phosphorylate MD-2.
The mechanism by which Lyn kinase might interact and phosphorylate MD-2 is still unclear. MD-2 is a glycosylated protein that resides in the outer membrane or as a secrete form. While there is debate about this, several investigators have suggested that MD-2 may play a chaperon role and is required for proper localization of TLR4 to the surface of the cell (27). Lyn kinase is a myristoylated and palmitoylated protein tyrosine kinase and therefore is also located in the lipid rafts and in the caveolae, in the inner portion of the outer membrane, where it helps initiate signaling events and giving it access to the signaling machinery of TLR4 (28, 29). MD-2 can also likely become tyrosine phosphorylated upon LPS stimulation during trafficking after LPS internalization. Indeed our data showing that the endocytosis inhibitor, Cytochalasin-D inhibits LPS-induced phosphorylation of MD-2 (Fig. 1B) suggests this possibility. The concept of early vesicles that are designed to fuse with each other, the relationship of receptor signaling and trafficking and endosome signaling as it relates to generation of signal diversity and specificity is a field that has been evolving as older paradigms and dogmas are being questioned, modified or discarded. Indeed, evidence has emerged recently that uncovered a role for the endocytic pathway in the transduction of signal dispersal and transduction of signals through the endocytic pathway and bi-directional interplay between signaling and membrane transport networks (30-31). Progress against old dogma in this field is further supported by recent surprising findings of ER membranes forming the phagosome membranes, and ER-mediated phagocytosis (32), and the presence of endosomal and phagosome proteins that are phosphorylated by endogenous phagosome-associated kinases that may include tyrosine kinases (33). Additionally, a recent study have found that LPS can signal through an intracellular TLR4/MD-2 pathway that did not involve recycling of surface TLR4/MD-2 (34). This new study underscores the complexity of TLR4/MD-2 signaling and that what was previously thought of as dogma (TLR4 and surface signaling) can change with data. Furthermore this study showing that MD-2 can recognize and signal in response to LPS intracellularly makes our observation of MD-2 phosphorylation even more intriguing.
Previously, it was believed that the role of MD-2 was simply bringing together LPS and TLR4 and thus providing the framework for successful signaling (7). However, our data now suggests that MD-2 might play a more important role in the downstream signaling events, given that MD-2 is a phosphoprotein that undergoes tyrosine phosphorylation upon LPS stimulation and that this event is required for optimal LPS-induced NF-κ B activation. We therefore have defined an additional mechanism that may be involved in regulating the host innate immune response to invading pathogens and define a novel role for MD-2, further enhancing the importance of MD-2 in mounting a response to LPS. Further research into the mechanism of MD-2 phosphorylation and its role in TLR4 activation is required.
Acknowledgments
We thank K. Miyake (Tokyo University) for the plasmid encoding Flag MD-2.
Supported by the NIH Grants (HL-66436 and AI-058128 to MA).
The abbreviations used are
- TLR
Toll-like receptor
- MD-2
Myeloid differentiation factor 2
- LPS
lipopolysaccharide
References
- 1.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, Tobias PS, Ulevitch RJ. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 3.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 4.Inohara N, Nunez G. ML -- a conserved domain involved in innate immunity and lipid metabolism. Trends Biochem Sci. 2002;27:219–221. doi: 10.1016/s0968-0004(02)02084-4. [DOI] [PubMed] [Google Scholar]
- 5.Kato K, Morrison AM, Nakano T, Tashiro K, Honjo T. ESOP-1, a secreted protein expressed in the hematopoietic, nervous, and reproductive systems of embryonic and adult mice. Blood. 2000;96:362–364. [PubMed] [Google Scholar]
- 6.Visintin A, Mazzoni A, Spitzer JA, Segal DM. Secreted MD-2 is a large polymeric protein that efficiently confers lipopolysaccharide sensitivity to Toll-like receptor 4. Proc Natl Acad Sci U S A. 2001;98:12156–12161. doi: 10.1073/pnas.211445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 8.Gray P, Michelsen KS, Sirois CM, Lowe E, Shimada K, Crother TR, Chen S, Brikos C, Bulut Y, Latz E, Underhill D, Arditi M. Identification of a novel human MD-2 splice variant that negatively regulates Lipopolysaccharide-induced TLR4 signaling. J Immunol. 2010;184:6359–6366. doi: 10.4049/jimmunol.0903543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar SN, Peters KL, Elco CP, Sakamoto S, Pal S, Sen GC. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat Struct Mol Biol. 2004;11:1060–1067. doi: 10.1038/nsmb847. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar SN, Elco CP, Peters KL, Chattopadhyay S, Sen GC. Two tyrosine residues of Toll-like receptor 3 trigger different steps of NF-kappa B activation. J Biol Chem. 2007;282:3423–3427. doi: 10.1074/jbc.C600226200. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar SN, Smith HL, Rowe TM, Sen GC. Double-stranded RNA Signaling by Toll-like Receptor 3 Requires Specific Tyrosine Residues in Its Cytoplasmic Domain. Journal of Biological Chemistry. 2003;278:4393–4396. doi: 10.1074/jbc.C200655200. [DOI] [PubMed] [Google Scholar]
- 13.Medvedev AE, Piao W, Shoenfelt J, Rhee SH, Chen H, Basu S, Wahl LM, Fenton MJ, Vogel SN. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J Biol Chem. 2007;282:16042–16053. doi: 10.1074/jbc.M606781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen LY, Zuraw BL, Zhao M, Liu FT, Huang S, Pan ZK. Involvement of protein tyrosine kinase in Toll-like receptor 4-mediated NF-kappa B activation in human peripheral blood monocytes. Am J Physiol Lung Cell Mol Physiol. 2003;284:L607–613. doi: 10.1152/ajplung.00116.2002. [DOI] [PubMed] [Google Scholar]
- 15.Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- 16.Gray P, Dunne A, Brikos C, Jefferies CA, Doyle SL, O'Neill LA. MyD88 adapter-like (Mal) is phosphorylated by Bruton's tyrosine kinase during TLR2 and TLR4 signal transduction. J Biol Chem. 2006;281:10489–10495. doi: 10.1074/jbc.M508892200. [DOI] [PubMed] [Google Scholar]
- 17.Bin LH, Xu LG, Shu HB. TIRP, a novel Toll/interleukin-1 receptor (TIR) domain-containing adapter protein involved in TIR signaling. J Biol Chem. 2003;278:24526–24532. doi: 10.1074/jbc.M303451200. [DOI] [PubMed] [Google Scholar]
- 18.McGettrick AF, Brint EK, Palsson-McDermott EM, Rowe DC, Golenbock DT, Gay NJ, Fitzgerald KA, O'Neill LA. Trif-related adapter molecule is phosphorylated by PKC{epsilon} during Toll-like receptor 4 signaling. Proc Natl Acad Sci U S A. 2006;103:9196–9201. doi: 10.1073/pnas.0600462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poussin C, Foti M, Carpentier JL, Pugin Jrm. CD14-dependent Endotoxin Internalization via a Macropinocytic Pathway. Journal of Biological Chemistry. 1998;273:20285–20291. doi: 10.1074/jbc.273.32.20285. [DOI] [PubMed] [Google Scholar]
- 20.Stefanova I, Corcoran ML, Horak EM, Wahl LM, Bolen JB, Horak ID. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J Biol Chem. 1993;268:20725–20728. [PubMed] [Google Scholar]
- 21.June CH, Fletcher MC, Ledbetter JA, Schieven GL, Siegel JN, Phillips AF, Samelson LE. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:7722–7726. doi: 10.1073/pnas.87.19.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adachi T, Stafford S, Sur S, Alam R. A novel Lyn-binding peptide inhibitor blocks eosinophil differentiation, survival, and airway eosinophilic inflammation. J Immunol. 1999;163:939–946. [PubMed] [Google Scholar]
- 23.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Micro. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 24.Husebye H, Halaas O, Stenmark H, Tunheim G, Sandanger O, Bogen B, Brech A, Latz E, Espevik T. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006;25:683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latz E, Visintin A, Lien E, Fitzgerald KA, Monks BG, Kurt-Jones EA, Golenbock DT, Espevik T. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem. 2002;277:47834–47843. doi: 10.1074/jbc.M207873200. [DOI] [PubMed] [Google Scholar]
- 26.Henricson BE, Carboni JM, Burkhardt AL, Vogal SN. LPS and Taxol activate Lyn kinase autophosphorylation in LPSn, but not in LPSd, macrophages. Molecular Medicine. 1995;1:428–435. [PMC free article] [PubMed] [Google Scholar]
- 27.Nagai Y, Akashi S, Nagafuku M, Gata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD2 in LPS responsiveness and TLR4 distribution. Nature Immunology. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 28.Kovarova M, Tolar P, Arudchandran R, Draberova L, Rivera J, Draber P. Structure-Function Analysis of Lyn Kinase Association with Lipid Rafts and Initiation of Early Signaling Events after Fce Receptor 1 Aggregation. Mol Cellular Biol. 2001;21:8318–8328. doi: 10.1128/MCB.21.24.8318-8328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbins SM, Quintrell NA, Bishop M. Myristoylation and Differential Palmitoylation of the HCK Protein Tyrosine Kinases Govern their Attachement to Membranes and Association with Caveolae. Mol cellular Biology. 1995;15:3507–3515. doi: 10.1128/mcb.15.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorkin A, von Zastrow M. Signal Transduction and Endocytosis: close encounters of many kinds. Nature Reviews, Mol Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Gaitan M. Signal Dispersal and Transduction through the Endocytic pathway. Nature Reviews, Mol Cell Biol. 2003;4:213–224. doi: 10.1038/nrm1053. [DOI] [PubMed] [Google Scholar]
- 32.Desjardins M. ER-Mediated phagocytosis; A new membrane for new functions. Nature Reviews Immunology. 2003;3:280–291. doi: 10.1038/nri1053. [DOI] [PubMed] [Google Scholar]
- 33.Emans N, Nzala NN, Desjardins M. Protein phosphorylation during phagosome maturation. FEBS Letters. 1996;398:37–42. doi: 10.1016/s0014-5793(96)01213-6. [DOI] [PubMed] [Google Scholar]
- 34.Shibata T, Motoi Y, Tanimura N, Yamakawa N, Akashi-Takamura S, Miyake K. Intracellular TLR4/MD-2 in macrophages senses Gram-negative bacteria and induces a unique set of LPS-dependent genes. Int Immunol. 2011;23:503–510. doi: 10.1093/intimm/dxr044. [DOI] [PubMed] [Google Scholar]