Abstract
The regulation of innate immune responses during viral infection is a crucial step to promote anti-viralreactions. Recent studies have drawn attention to a strong relationship of pathogen associated molecular patterns (PAMP) recognition with autophagy for activation of APC function. Our initial observations indicated that autophagosomes formed in response to RSV infection of DC. To further investigate whether RSV-induced DC activation and innate cytokine production was associated with autophagy, we utilized several methods to block autophagosome formation. Using 3-MA,siRNA inhibition of LC3,or Beclin +/- mouse derived DC,studies establisheda relationship between RSV-induced autophagy and enhanced type I IFN, TNF, IL-6, and IL-12p40expression. Moreover, autophagosome formation induced by starvation also promoted innate cytokine expression in DC. The induction of starvation-induced autophagy in combination with RSV infection synergistically enhanced DC cytokine expressionthat was blocked by an autophagy inhibitor. The latter synergistic responses were differentially altered in DC from MyD88-/- and TRIF-/-mice supporting the concept of autophagy-mediated TLR signaling. In addition, blockade of autophagy in RSV-infected DC inhibited the maturation of DCs as assessed by MHC Class II and co-stimulatory molecule expression. Subsequently, we demonstrated that inhibition of autophagy in DCsused to stimulateprimary ovalbumin-induced and secondary RSV-infected responses significantly attenuatedcytokine production by CD4+ T cells. Thus, these studies have outlined that autophagy in DC afterRSV infection isa crucial mechanism for driving innate cytokine productionleading to alteredacquired immune responses.
Introduction
The autophagy system is a critical cellular response that facilitates self-digestion of misfolded or unused protein and cellular debris that impacts development, aging, and normal function of cellular processes (1-3). The classic induction of autophagy as a stress response can be induced by amino acid starvation that entails the sequential activation of Atgproteins that direct assembly of autophagosomesandcapture cytoplasmic components (4). The autophagy associated proteins appear to have important activation properties through the interaction with important signaling pathways, such as Beclin 1 interaction with BCL-2 family members, and are often targeted for subversion (5-7). Defective autophagogenic responses have been identified in numerous diseases including Alzheimer's and Parkinson's. However, more recent identification of the role of autophagy in Crohn's disease as well as in bacterial clearance has highlighted an important activation pathway in innate immune responses (8-10). Thus, this conserved and relatively well-defined process has a role for anti-pathogen responses in all cells, including a role for alerting the immune system during pathogen invasion. Induction of autophagy can directly impact the immune system by optimizing the activation of antigen presenting cells (APC) and other innate immune cells for immediate cytokine responses as well as for activation of T cells through increased antigen presentation (11-13). In fact, autophagy has been identified not only in the delivery of antigens for MHC class II presentation to CD4 T cells, but also for cross presentation of antigens for CD8 T cell activation. In addition, immune responses differentially regulate autophagy with Th1 promoting and Th2 inhibiting the process for activation in innate immune cells (14). Overall, autophagy may be one mechanism of delivery of pathogen-derived nucleic acid components to the TLR activation machinery in the lysosome compartment of APCs.
Recent data has suggested clear links between autophagy and toll-like receptor (TLR) as well as other pathogen receptor recognition (PRR) activation, such as RIG-I (15-20). Studies have established that PRR/TLR-mediated activation can induce autophagy and directly impact effector responses. Autophagy may especially be important for intracellular bacteria as well as during viral infections, especially those that do not enter the cell via receptor-mediated endosomes (21). One such virus that has significant pathology associated with it is respiratory syncytial virus (RSV). RSV is anomnipresent virus that is the most common cause of hospitalization in children under the age of 2(22-24). In addition, RSV can also adversely affect the elderly and immunocompromised individuals, causing severe lower respiratory tract infection and pneumonia(25). The pathology is associated with inefficient activation of innate immunity and skewing of the acquired immune responses toward a Th2 phenotype. Thus, the proper and efficient activation of innate and acquired immune responses is essential for avoiding pathology. RSV infects cells via a mechanism of cell membrane fusion and cytoplasmic entry that does not utilize an endosomic pathway (26). The initial route of innate cytokine generation may be via a RIG-I activation followed by TLR activation after delivery of RSV components to the lysosome from the cytoplasmic pool (27). Thus, autophagy would be one mechanism of delivery of RSV nucleic acid components to the TLR activation machinery in the lysosomalcompartment.
The present studies have examined the role of autophagy for RSV-induced dendritic cell activation for crucial cytokine production as well as subsequent T cell activation. The data clearly identify autophagy as a relevant mechanism for activation of the most appropriate anti-viral responses and further examine the relationship of specific TLR pathways with autophagy-mediated events.
Materials and Methods
Respiratory Syncytial Virus
Our laboratory utilizes the antigenic subgroup A strain of RSV, referred to as Line 19. This isolate was obtained from a sick infant at the University of Michigan (28), and has been demonstrated in animal models to mimic human infection by stimulating mucus production (29).
Real-time Taqman PCR
RNA was isolated as described (Invitrogen), and 5μg was reverse-transcribed to assess gene expression.Detection of cytokine mRNA in lung samples was determined using pre-developedprimer/probe sets (PE Biosystems, Foster City, CA) and analyzed using an ABI Prism 7500 Sequence Detection System(Applied Biosystems, Foster City, CA). GAPDH was analyzed as aninternal control and gene expression was normalized to GAPDH. Fold changes in gene expression levels were calculated by comparisonof the gene expression in unchallenged cells, which were assigned an arbitrary value of 1 and a fold increase calculated.
Generation of bone marrow dendritic cells
Bone marrow harvested from C57BL6 WT and/or Beclin+/- mice was seeded in tissue culture flasks in RPMI 1640 based complete media with 20 ng GM-CSF/ml (R&D Systems, Minneapolis, MN). Beclin +/- mice were generously provided by Dr. ZhenyuYue and expanded in our animal facilities (30). Cells were fed every 3 days and loosely adherent cells were collected after 10 days. The majority of the cells, >85%, were CD11c+ BMDCs and subsequently were infected with RSV (moi=1.0) or starved in Hank's Balanced Buffered Salt solution. The cells were then assessed for gene and protein expression as well as used for flow cytometric analysis
Confocal analysis of autophagy in DC
Isolated DC were infected with RSV or cultured under amino acid starvation conditions for the identified amount of time with or without 3-MA. Immunofluorescence analysis for LC3 was performed. Cells were fixed in 4% paraformaldehyde for 20 minutes. The cells are blocked for 1 hour in 5% NGS in PBS containing permeabilization agent 0.1% Tween 20. Cells were incubated in primary Ab Rabbit polyclonal anti-LC3B Cat. NB600-1384 Novus Biologicals for 2 hours at 37C and then incubated with secondary Ab goat anti-rabbit Alexafluor 568 Cat. A11011 Invitrogen. Antifade agent containing DAPI was added. Cells were imaged using an Olympus Fluoview 500 confocal microscope. A confocal Z stack was performed to obtain a stack of these two-dimensional images from successive focal planes.
Transfection of DC and inhibition of MAP1LC3 and infection with RSV
BMDC grown from bone marrow normal mice were plated at 1 × 106/ml and transfected with MAP1LC3 specific or control siRNA using the Amaxa DC transfection kit and the Amaxanucleofector system (LonzaCologene AG, Cologne, Germany) as directed. The siRNAs were purchased from Thermo Scientific Dharmacon as a pool of 4 with target sequences
CGGUGAUCAUCGAGCGCUA,
UGAGCGAGUUGGUCAAGAU,
GUAAGGAGGUGCAGCAGAU,
CCUUCUUCCUGCUGGUCAA
The transfection efficacy of the cells was between 60 and 80% for all experiments, and viability of cells after 48 hrs in culture after transfection maintained in DC growth media was ~80% and not different from the transfection control cells. Analysis of MAP1LC3 knockdown was assessed by mRNA analysis and by induction of autophagosome formation by localizing LC3 by confocal microscopy. After 48 hrs of transfection the DC were infected with RSV followed by harvest of cells and/or supernatants at various times post-infection for analysis of autophagosome formation and cytokine induction.
Flow cytometric analysis
Cells were stained with the indicated antibodies (BD Pharmingen, San Diego, CA) that were specific for costimulatory molecules and analyzed using a FACS Calibur and Cell Quest software (BD Biosciences, San Jose, CA). Isotype control antibodies were used to demonstrate specificity of our staining and to establish the criteria for our flow cytometry populations.
T cell restimulation
Animals were infected with RSV (1 × 105 PFU/mouse) by intratracheal administration as previously described using the Line 19 strain of virus (31, 32). Mediastinal and cervical lymph nodes were harvested from mice infected with RSV for 8 days and single cell suspensions obtained by passing nodes through a 40μm nylon mesh filter. CD4+ T lymphocytes were isolated by magnetic bead selection using a negative selection protocol yielding a >95% pure CD4 T cell population (Miltenyl). Samples were counted and plated in triplicate at 1×106 cells per well followed by restimulation with BMDC (1 × 105/well; 1:10 ratio) that had been infected overnight with RSV (MOI-1.0) with or without 3-MA treatment (10 uM) to block autophagy during APC activation. Cells were incubated at 37°C for 24 hours and supernatants collected for analysis on the BioRadBioplex 200 system according to the manufacturer's protocol. Kits (Biorad) containing antibody-coated beads to specific for IL-17 and IFNγwere used to assay for cytokine production in each of the samples.
Statistics
Data were analyzed using Prism GraphPad software. Unless otherwise specified, data shownsarerepresentative of two or more experiments. Statistical significance in all experiments was determined by One-way ANOVA, followed by a Newman-Keulspost test. Significant differences were regarded as p < 0.05.
Results
RSV infection induces cytokine production and maturation of DCs via Autophagy
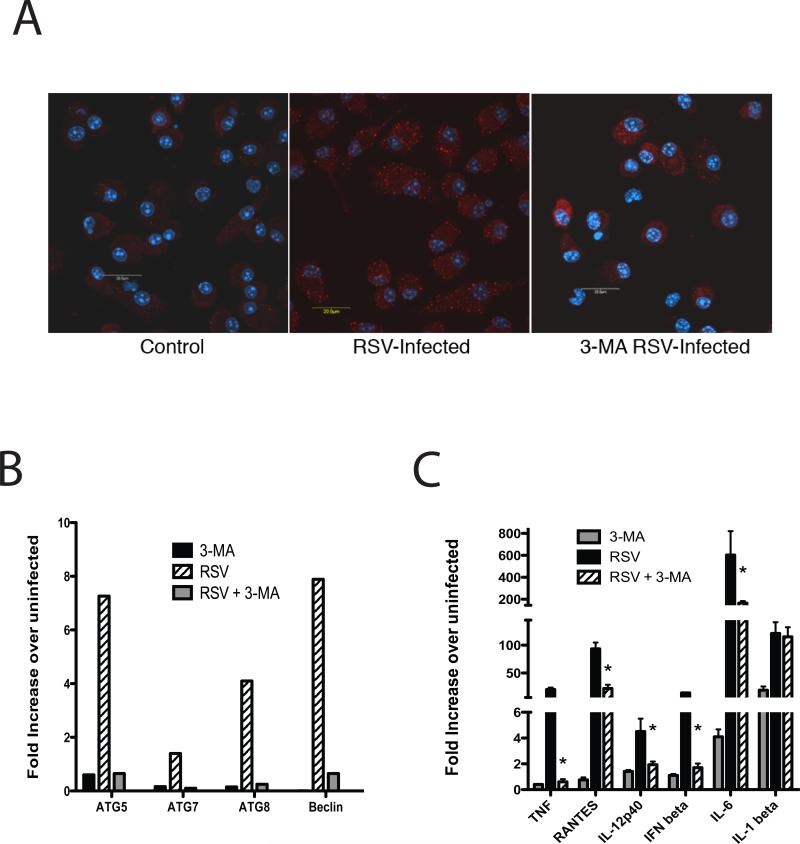
Recent evidence has identified autophagy as an efficient mechanism for delivery of potential cytoplasmic antigens to the endosome for degradation, processing, activation and presentation by APCs.RSV is a virus that infects cells via membrane fusion leading to a cytoplasmic entry mechanism. Previous data have demonstrated that RSV-induced cytokine production from DCs is partially or completely dependent on activation of TLRs found predominately in the endosome compartment (33-38). Thus, our hypothesis for these studies was that APCs utilize autophagy for delivery of RSV antigens for endosomic-mediated functions, such as TLR activation and APC functions. The initial studies utilizing LC3 staining and confocal microscopy have identified that RSV infection induces autophagy in bone marrow-derived DCs (Figure 1A). These events were similar to the response induced by starvation by amino acid deprivation of the cells. A traditional way to assess whether autophagy was occurring utilizes 3-MA to block autophagosome formation. As indicated in Figure 1A the induction of autophagosomes mediated by RSV infection could be inhibited by addition of 3-MA (1mM) to the cultures. Next we also assessed the induction of classic autophagy-associated genes known to be involved in promoting the assembly and formation of the autophagosome (Figure 1B). RSV significantly upregulated the expression of ATG5, ATG7, ATG8 as well as beclin-1 during the infectious process and their expression was completely regulated by the addition of the inhibitor 3-MA. Thus, RSV not only induces the assembly of the autophagosome but also upregulates expression of genes related to continual cytoplasmic capture via autophagosome formation.
Figure 1.
RSV infection induces autophagosome formation and 3-MA sensitive innate cytokine production in BMDC. A) Dendritic cells were infected withRSV for 2 hrs and preserved on glass slides with 4% paraformaldehyde, followed by fluorescent anti-LC3 antibody staining for identifying autophagosome formation by confocal microscopy.Dendritic cells were pre-treated with 1 uM of 3-MA 30 minutes prior to RSV infection. B) RSV-infected BMDC infected with RSV upregulate a number of autophagy protein genes at 24 hrs post-infection and are blocked by inhibiting autophagy with 3-MAby qPCR. C) Innate cytokine gene expression was highly upregulated in RSV-infected BMDC after 24 hrs of infection and was inhibited by 3-MA, which blocks autophagy. Data represent mean ± SEM from triplicate wells with similar results repeated in 3 different experiments.
While the mere identification of autophagy occurring during this infection is interesting, these studies also examined the role of autophagy for induction of several important anti-viral cytokines from RSV-infected DC. Following infection with RSV (MOI-1.0) as above with or without 3-MAthe DCs were processed for mRNA isolation and subjected to QPCR analysis. The data indicated that while RSV infection clearly drives IFNβ, TNF, RANTES (CCL5), IL-6, and IL-12p35, the addition of 3-MA significantly blocked the expression of all of these cytokines in RSV infected DCs (Figure 1C). In contrast, when we examined IL-1β, we found that 3-MA blockade of autophagy did not reduce the activation of IL-1β.
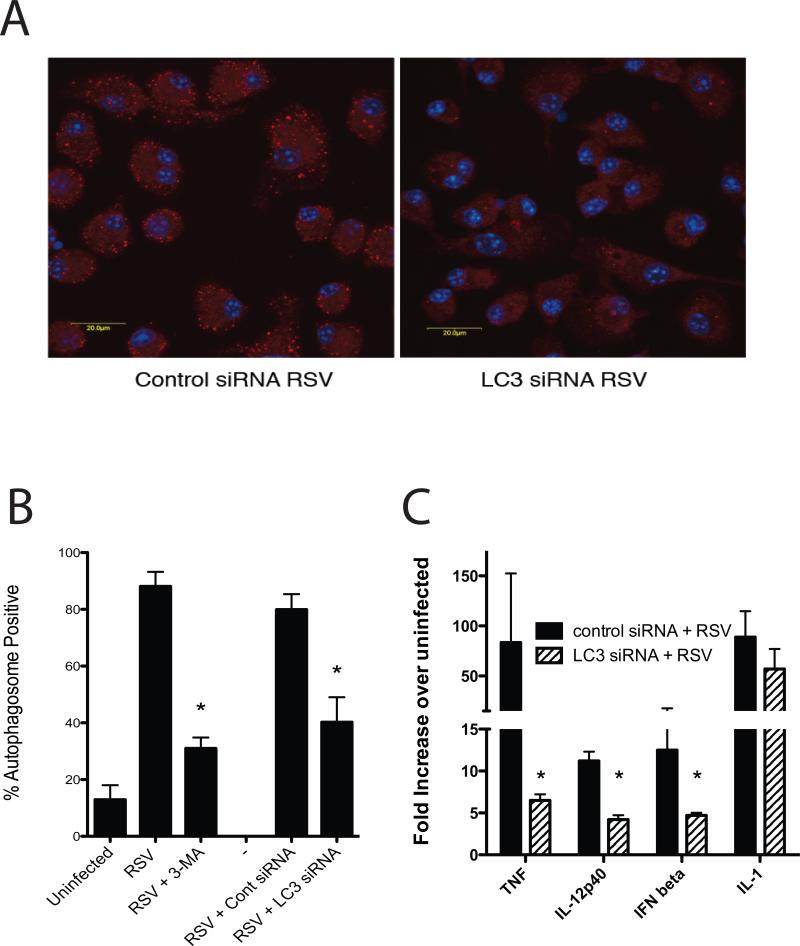
To more specifically determine whether RSV-induced responses were dependent upon autophagy, siRNA specific for MAP1LC3 was used to transfect the DC and block autophagic responses. Using an Amaxa electroporation DC were specifically transfected with siRNA and after 48hrs infected with RSV. The fluorescently labledsiRNA indicated that our transfection efficacy was approximately 70% (data not shown). The expression of LC3 (MAP1LC3) was assessed after RSV infection and verified that the siRNA specifically “knocked down” the increased mRNA expression of LC3 by qPCR (Control RSV- 2.6 fold increase, nontargetsiRNA + RSV- 4.6 fold, LC3 siRNA + RSV- 0.59 fold). More importantly, when we examine the induction of autophagosomes by confocal staining of LC3 we observed significantly reduced RSV-induced autophagosome formation (Figure 2A). This reduction in autophagosome formation was similar to that observed with 3-MA treatment, as indicated by quantifying the percentage (%) of DC that were autophagosome positive by confocal microscopy (Figure 2B). When we subsequently examined the ability of the siRNA transfected cells to induce cytokines in response to RSV, a significant reduction in IFNβ, TNF and IL-12p40 was observed, with little effect on IL-1 (Figure 2C).
Figure 2.
SiRNA inhibition of LC3 reduces RSV-induced autophagy induction in BMDC as well as induced cytokine expression. A) Inhibition of autophagosome formation in RSV-infected DC was verified using confocal microscopy of LC3 specific antibody stained DCs (red). B) The percent of DC that were positive for autophagosome formation after RSV infection were assessed using confocal microscopy assisted quantification. The data represents mean ± SE from 4-6 different replicates for each group. C) Expression of innate cytokine mRNAby control and LC3 specific siRNA-treated cellswas assessed at 24hrs post RSV infection by qPCR. Data represents mean ± SEM from 3 replicate treatments and was repeated in a separate experiment with similar results.
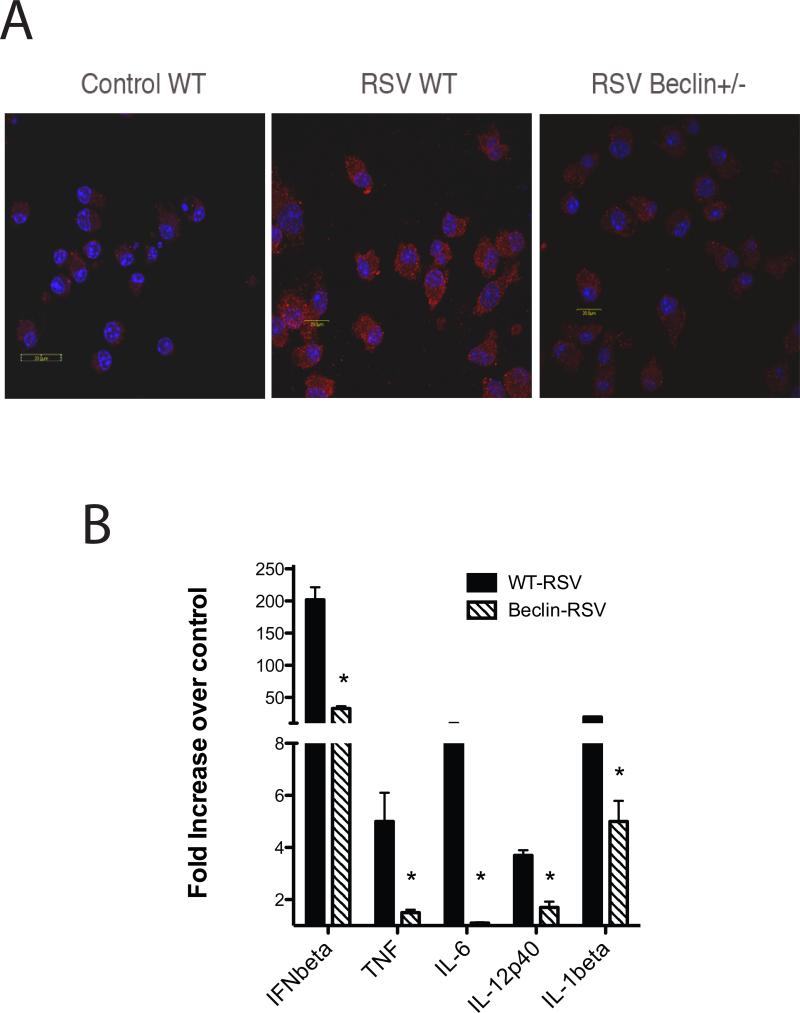
In a final experiment to verify that an increase in autophagy during RSV infection was necessary for induction of innate cytokines by DC, bone marrow DC from Beclin +/- mice, which have a deficit in autophagosome formation, were obtained (30). When DC from the Beclin+/- animals were subjected to RSV infection they did not efficiently form autophagosomes as indicated by confocal microscopy (Figure 3A). Furthermore, when the induction of innate cytokines was assessed they demonstrated a significant decrease in the induction of IFNβ, TNF, IL-12p40, and IL-6 (Figure 3B). Interestingly, the induction of IL-1b was also reduced after RSV infection of DC from Beclin +/- mice unlike the previous two experiments when autophagy was altered during RSV infection. Together these data demonstrate that induction of innate cytokines during RSV infection appears to be dependent upon autophagy-related events in DC.
Figure 3.
RSV infection of DC from Beclin+/- have altered autophagosome formation and an attenuated innate cytokine response. DC obtained from bone marrow of Beclin +/- and wildtype control B6 animals were infected with RSV and then subjected to confocal microscopy after staining for LC3 to identify autophagosomes (A). Expression of innate cytokine expression was assessed by quantitative PCR in the DC from wildtype and Beclin +/- mice after 24hrs of infection (B). Data represents mean ± SE from 2 repeat experiments.
Innate cytokine expression during cellular starvation-induced autophagy and exacerbation of RSV-induced cytokines are associated with MyD88 and TRIF-mediated mechanisms
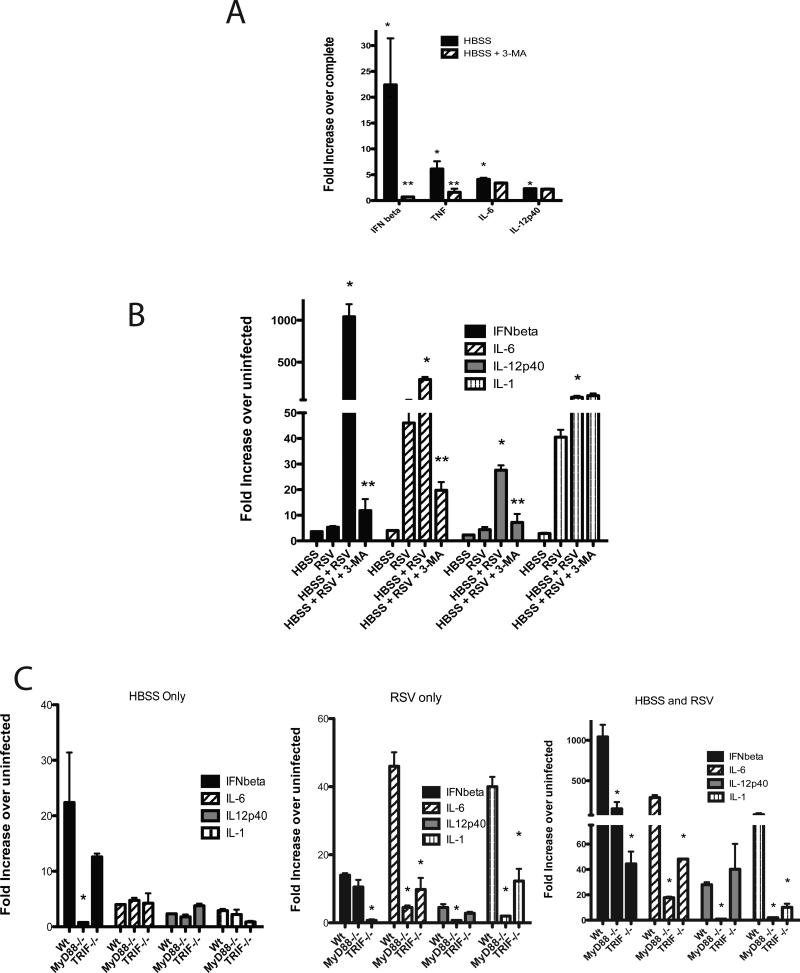
To further understand the initial steps of autophagy and whether the innate cytokines are activated by the development of the autophagosome in general, the cytokines were examined in the context of amino acid starvation (Figure 4). The data demonstrate 2 important and novel findings. Firstly, starvation alone induces significant cytokine gene expression, including type I IFN, IL-6, and IL-12p40. Secondly, the cytokines are not all inhibited by blocking autophagy with 3-MA (Figure 4A), as IL-12 and IL-6 were not reduced. However, the overall level of the cytokine induction during starvation alone did not reach the level that was observed in RSV infection. Thus, the mere induction of starvation-induced autophagy led to increased cytokine expression that may influence an inflammatory response.
Figure 4.
Starvation-induced autophagy significantly upregulates cytokine production and synergistically enhances RSV-induced responses via MyD88 and TRIF associated pathways. A) BMDC amino acid starved in HBSS for 2 hrswere treated with and without 3-MA (10 uM) to block autophagy, then expression of innate immune cytokines was assessed by quantitative PCR of Isolated mRNA . B) BMDC were starved for 2 hrs then reconstituted with complete media, infected with RSV or both treatments performed together. After 24 hrs of culture the cells were harvested and isolated mRNA assessed for induction of cytokine expression by quantitative PCR. C) DC from MyD88-/- and TRIF-/- mice were used to examine the relative contribution of the two pathways for starvation, RSV or the combined treatment groups for cytokine produciton.Experiments for 4C were performed in parallel with those displayed in 4A and 4B. Data represents mean ± SE from 2 repeat experiments.
An important question for these studies was whether the starvation-induced autophagy responses altered a RSV-induced response. Isolated BMDC were infected with RSV after an initial 2 hr amino acid starvation and left in culture 22 hours or infected with only RSV or starved for 2 hours followed by 22 hours in complete media.The data in Figure 4B indicate that if DC were amino acid starved there was a synergistic increase in the production of the critical innate cytokines, IFNβ, IL-12p40 and IL-6, during RSV infection that was inhibited by blocking autophagosome formation with 3-MA. As was previously observed, IL-1 was induced and synergistically elevated by starvation during RSV infection, but was not reduced by blockade of autophagy by 3-MA. These striking data further reflect the importance of autophagosome formation for innate cytokine production during the initial stages of APC activation.
The studies next addressed whether this process would rely on similar endosomic-related TLR mechanisms that have been described previously for autophagy (18). When examining HBSS alone (2 hr of starvation) or RSV alone (24 hrs of infection), the MyD88 and TRIF deficient DC displayed differential effects upon RSV infection (Figure 4C). The primary cytokine induced by HBSS was IFNbetathat was significantly reduced in the MyD88-/- derived but not the TRIF-derived DC. Subsequently, when the starvation-induced RSV exacerbation identified above was examined with DC from MyD88-/- or TRIF-/- mice the production of cytokines overall involved both of these adapter pathways similar to RSV only(Figure 4C), further establishing an intrarelationship between the endosomic PAMP recognition and autophagy systems. Interestingly, although type I IFN, IL-1 and IL-6 all were significantly reduced in DCs from both MyD88 and TRIF-/- mice, IL-12p40 relied fully upon MyD88-mediated mechanisms.These data further verify that the targeted pathway for the autophagy-mediated activation was related to the TLR-induced events and the two stimuli together significantly augmented the cytokine expression patterns.
Autophagy regulates RSV-induced DC maturation and T cell activation
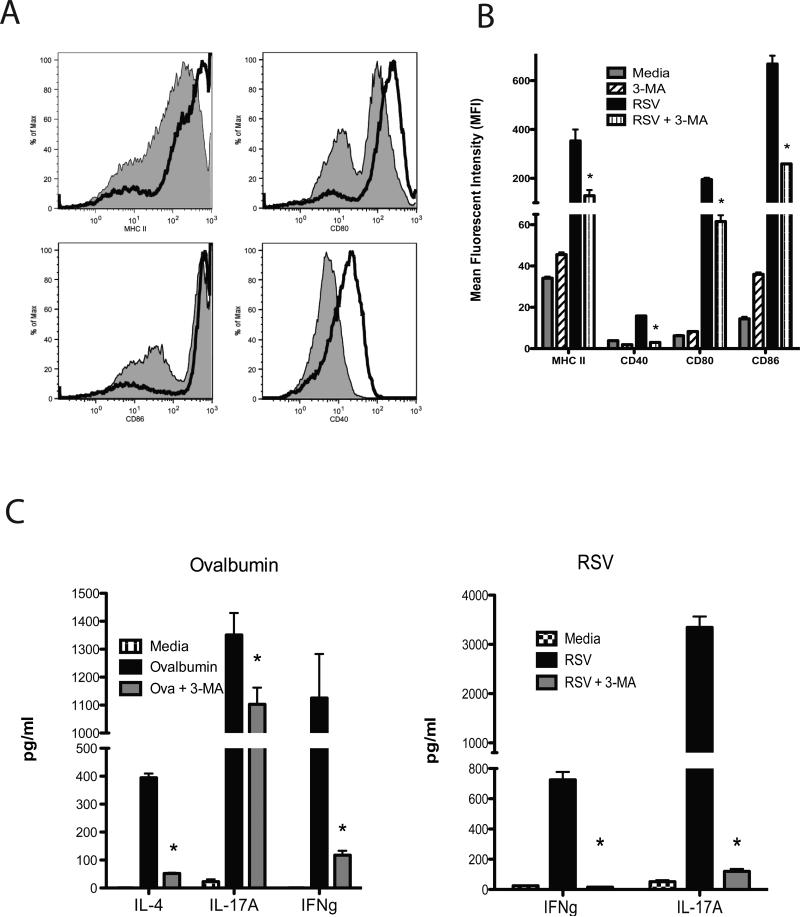
The APC function of DC during initiation of immune responses includesupregulation of MHC and co-stimulatory molecules that facilitate DC activation of T cells. To examine whether autophagy was involved in these processes RSV-infected DC were treated with 3-MA and examined for expression of MHC class II and co-stimulatory proteins CD40, CD80 and CD86 by flow cytometry (Figure 5). The flow plots illustrate that all 4 molecules are significantly upregulated on the surface of RSV-infected DC demonstrating maturation of cells, while treatment of DC with the autophagy inhibitor 3-MA significantly reduced the surface expression of all 4 maturation proteins (Figure 5A). To statistically assess the reduction in the response MFI (Mean Fluorescent Intensity) was assessed in individual samples. The data in Figure 5B illustrates that treatment of DC with 3-MA significantly attenuates the activation upon RSV infection. Thus, supporting the concept that autophagy has a role in the activation and maturation of DC during viral infection.
Figure 5.
Alteration of DC maturation and T cell activation from RSV-infected animals. A) Control (black line) and 3-MA treatment (grey area) of RSV infected BMDC were examined for expression fortheir maturation as assessed by flow cytometry of DC maturation markers at 24 hrs post-RSV infection.B) Multiple samples were quantitatively assessed based upon the mean fluorescent intensity (MFI) of each of the markers demonstrating a decreased expression level after 3-MA treatment. C) To assess the APC function of the RSV-infected DC, their capacity toreactivatie CD4 T cells isolated from splenic T cells from DO.11 ovalbumin TCR transgenic mice and lung draining lymph nodes of RSV-infected mice was assessed by quantifying IL-4, IFNγ, and IL-17 RNA/protein after 24 hrs of co-culture. Data represents mean ± SEM from repeat experiments. No increased IL-4 was detected in RSV-rechallenged T cells and therefore was not displayed.
In order to determine if the maturation and cytokine production from DC had an impact on subsequent primary and secondaryimmune responses in CD4+ T cells from either ovalbumin responsive DO.11 TCR transgenic mice or lung draining lymph nodes of RSV infected mice were isolated. Spleens from naïve DO.11 or lung draining lymph nodes from mice infected for 8 days with RSV were removed and CD4+ T cells isolated by magnetic bead cell sorting (MACS) were used for restimulation assays. Bone marrow-derived DC were incubated with ovalbumin (100 ug/ml) or infected with RSV (MOI-1.0) in the presence or absence of 3-MA for 2 hrs, washed to clear residual 3-MA and subsequently combined with the isolated CD4+ T cells. After 72 hrs supernatants were harvested and IL-4, IFNγ and IL-17 were assayed by Bioplex analysis (Figure 5C). The data demonstrate that when 3-MA was utilized a significant reduction of cytokineswas observed by blocking autophagosome formation during both a primary ovalbumin response and a secondary RSV infection response. Thus, these data confirm the importance of autophagy for driving the acquired immune responses.
Discussion
The recognition of pathogenic stimuli can be achieved by both cytoplasmic sensors and TLRs with the latter found in the endosomic compartment andrecognize pathogen associated molecular patterns (PAMPs). In these studies we report that autophagy modulates several underlying mechanisms in RSV-infected DC activation:i) Authophagy drives innate cytokine production, ii) autophagy is necessary for DC maturation, iii) RSV-induced DC activation is augmented by starvation-enhancedautophagy, and iv)autophagy facilitates APC:T cell interactions for cytokine production. While many viruses enter host cells via receptor-mediated endocytosis that are immediately shuttled to the endosome pathway, others may escape immediate recognition by the endosome-associated TLRs by directly depositing their nuclear contents into the cytoplasm(39). This is the case for RSV. Thoughthere are cytoplasmic pathogen recognition pathways that are crucial for RSV-induced activation (27, 40), the most effective responses still require endosomal TLR activation for maximal clearance with minimal pathology, including TLR3 and TLR7(36, 38, 41-44). The capacity of autophagyto clear pathogens has been most clearly established for intracellular bacterial infections(45-49) but has also been associatedwith anti-viral responsesas well as in development of pathogenesis of viral disease (50-55).
In the present study, results suggest that autophagy-mediated pathways may be one of the critical mechanisms for facilitating activation of cytoplasmic RSV PAMPs inthe endosome for TLR-mediated recognition and subsequent innate cytokine production. A seminal study utilizing a related virus, sendai virus, has demonstrated that autophagy was necessary for induction of TLR7-mediated type I IFN production in plasmacytoid DCs (21). An interesting codependent relationship between TLRsand autophagy has been established whereby initial TLR activation depends upon autophagosome delivery to the lysosome and optimal autophagosome development requires TLR activation(56, 57). In a separate study examining anti-viral pathways Atg5-Atg12 was shown to inhibit sendai virus-mediated RIG-I-induced type I IFN through interaction of the CARD domain, whereas endosome dependent HSV activation of type I IFN did not depend upon Atg5 and autophagy (58). Thus, several important innate recognition pathways may be influenced by autophagyincluding RIG-I that also has a significant role in activationduring early RSV infection(27, 59-63). Recent studies have demonstrated that autophagosome formation in macrophages can occur very quickly, peaking as early as 30-60 minutes (64). A striking observation in our studies was thatupregulation of autophagy in DC under amino acid starvation conditions could greatly sensitize the cell for a synergistic cytokine response upon RSV infection.
The role of autophagy for activation of DCs appears to manifest itself at several levels, including cytokine production, DC maturation, as well as antigen presentation and activation of T cell responses. Numerous studies have outlined the role of cytokines for regulating autophagy in innate cells and activation of intracellular defense for bacterial removal, while only a couple have associated the role of autophagy in the activation of innate cytokines. In the present studies when BMDC were infected with RSV,induction of numerous innate cytokines associated with TLR activation were dependent upon autophagy, as treatment with 3-MA,siRNA knockdown of LC3, or use of Beclin +/- derived DC blocked their upregulation. The impact on type I IFN, TNF, IL-6, and IL-12 may be the most crucial, since these cytokines are not only important for early regulation of the infection, but they subsequently impact the intensity and progression of acquired immune responses. The maturation of DC to become functional antigen presenting cells (APC) after RSV infection depends primarily upon type I IFN and the observed dependency of autophagy on DC maturation is likely a manifestation of the regulation of type I IFN(37, 65, 66). This latter observation was revealed by the inability of DC to properly activate CD4 T cells from DO.11 and RSV-infected mice, thus reducing the production of T cell associated cytokines.The importance of the autophagy pathway for T cell activation during viral infection is supported by HSV infections using mutant viruses that do not block the autophagy pathways in APC and subsequently enhance T cell cytokine production (67, 68). Importantly, using Atg5-/- DCs it was demonstrated that there was a defect in presentation and processing during HSV infection and subsequent T cell activation, but no defect in DC cytokine production in this endosomal entering virus(69). Thus, the activation of DC via the autophagy-associated mechanisms may have a significant impact on the activation of the T cell associated anti-viral responses in general. Furthermore, since autophagy is down-regulated by Th2 responses (14), it may be that anti-viral responses would be dysregulated in the setting of chronic disease, such as in allergic asthma or COPD where persistent viral infection can be problematic and regulation of autophagy may occur.
While the blockade of critical pathogen-mediated pathways was observed, less of an effect on IL-1β, an inflammasome-associated cytokine, was observed when autophagy was inhibited during RSV infection.While others have demonstrated that proteosome activation can regulate autophagy in innate cells(70), the inverse may not be functioning. Interestingly, a recent study demonstrated that when atg16L1 was absent no autophagosome formation was observed in response to endotoxin and IL-1β levels were enhanced (71). Thus, there may be less efficient regulation of inflammasome-associated cytokine activation in the absence of autophagy.In fact, a recent study suggested that autophagy can control IL-1 by targeting pro-IL-1 for degradation and by regulating NLRP3 inflammasome activation (72). The altered IL-1 may also be linked to parallel activation pathways, such as reactive oxygen species (ROS), which have clearly been linked to RSV infection and influence subsequent cytokine production(61, 73-76). Indeed, these pathways are important during bacterial infections as well as in inflammatory diseases such as Crohn's. Given that some alteration of IL-1 was observed in the DC from the beclin +/- animals, the regulation of IL-1 in this setting is unclear but overall correlates with other studies suggesting that autophagy can enhance IL-1β responses (77).
Recent studies have observed that autophagy-mediated mechanisms have an expanding role for shaping the nature of immune responses. Autophagy has been implicated for facilitating presentation of cytosolic antigens via MHC II molecules, regulates central tolerance in the thymus, and may be involved in age-associated alterations in the immune system(78-83). Thus, the manipulation of autophagy responses may be useful for treating numerous inflammatory/immune diseases as well as optimizing and improving vaccination. A recent study demonstrated that by inducing autophagy with rapamycin in BCG vaccination an improved anti-tuberculoid response could be driven(84). This latter mechanism may be associated with more efficient APC function as autophagy not only impacts class II MHC presentation, but also increases cross-presentation of cytosolic antigens to CD8 T cells for enhanced cytotoxicity(11, 12). Together, the APC enhancement would be important in viral and intracellular bacterial responses and therefore might be a strategy to indeed enhance vaccine development. The present studies demonstrated that blocking autophagy altered the ability of DC to induce cytokine production from CD4+ T cells that would directly alteranti-viral responses.
The overall impact of autophagy during immune responses is becoming more clearly defined. However, further investigations are necessary to determine how the immune responses are altered and specifically interact with other critical innate and acquired immune functions. Future studies will help to determine if blocking or enhancing specific autophagy mechanisms will improve our ability to treat and/or prevent disease pathogenesis.
References
- 1.Kundu M, Thompson CB. Autophagy: basic principles and relevance to disease. Annu Rev Pathol. 2008;3:427–455. doi: 10.1146/annurev.pathmechdis.2.010506.091842. [DOI] [PubMed] [Google Scholar]
- 2.Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- 3.Swanson MS, Molofsky AB. Autophagy and inflammatory cell death, partners of innate immunity. Autophagy. 2005;1:174–176. doi: 10.4161/auto.1.3.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swanson MS. Autophagy: eating for good health. J Immunol. 2006;177:4945–4951. doi: 10.4049/jimmunol.177.8.4945. [DOI] [PubMed] [Google Scholar]
- 5.Vazquez CL, Colombo MI. Beclin 1 modulates the anti-apoptotic activity of Bcl-2: insights from a pathogen infection system. Autophagy. 2010;6:177–178. doi: 10.4161/auto.6.1.10743. [DOI] [PubMed] [Google Scholar]
- 6.Gannage M, Ramer PC, Munz C. Targeting Beclin 1 for viral subversion of macroautophagy. Autophagy. 2010;6:166–167. doi: 10.4161/auto.6.1.10624. [DOI] [PubMed] [Google Scholar]
- 7.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 8.Deretic V. Links between autophagy, innate immunity, inflammation and Crohn's disease. Dig Dis. 2009;27:246–251. doi: 10.1159/000228557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cario E. Innate immune signalling at intestinal mucosal surfaces: a fine line between host protection and destruction. Curr Opin Gastroenterol. 2008;24:725–732. doi: 10.1097/MOG.0b013e32830c4341. [DOI] [PubMed] [Google Scholar]
- 10.Delgado M, Singh S, De Haro S, Master S, Ponpuak M, Dinkins C, Ornatowski W, Vergne I, Deretic V. Autophagy and pattern recognition receptors in innate immunity. Immunol Rev. 2009;227:189–202. doi: 10.1111/j.1600-065X.2008.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Wang LX, Pang P, Twitty C, Fox BA, Aung S, Urba WJ, Hu HM. Cross-presentation of tumor associated antigens through tumor-derived autophagosomes. Autophagy. 2009;5:576–577. doi: 10.4161/auto.5.4.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uhl M, Kepp O, Jusforgues-Saklani H, Vicencio JM, Kroemer G, Albert ML. Autophagy within the antigen donor cell facilitates efficient antigen cross-priming of virus-specific CD8+ T cells. Cell Death Differ. 2009;16:991–1005. doi: 10.1038/cdd.2009.8. [DOI] [PubMed] [Google Scholar]
- 13.Zhou D, Li P, Lin Y, Lott JM, Hislop AD, Canaday DH, Brutkiewicz RR, Blum JS. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22:571–581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Harris J, Master SS, De Haro SA, Delgado M, Roberts EA, Hope JC, Keane J, Deretic V. Th1-Th2 polarisation and autophagy in the control of intracellular mycobacteria by macrophages. Vet Immunol Immunopathol. 2009;128:37–43. doi: 10.1016/j.vetimm.2008.10.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tal MC, Iwasaki A. Autophagic control of RLR signaling. Autophagy. 2009;5:749–750. doi: 10.4161/auto.5.5.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci U S A. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeshita F, Kobiyama K, Miyawaki A, Jounai N, Okuda K. The non-canonical role of Atg family members as suppressors of innate antiviral immune signaling. Autophagy. 2008;4:67–69. doi: 10.4161/auto.5055. [DOI] [PubMed] [Google Scholar]
- 18.Shi CS, Kehrl JH. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J Biol Chem. 2008;283:33175–33182. doi: 10.1074/jbc.M804478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 21.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 22.Openshaw PJ, Dean GS, Culley FJ. Links between respiratory syncytial virus bronchiolitis and childhood asthma: clinical and research approaches. Pediatr Infect Dis J. 2003;22:S58–64. doi: 10.1097/01.inf.0000053887.26571.eb. discussion S64-55. [DOI] [PubMed] [Google Scholar]
- 23.Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr. 2003;143:S112–117. doi: 10.1067/s0022-3476(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 24.Everard ML. The role of the respiratory syncytial virus in airway syndromes in childhood. Curr Allergy Asthma Rep. 2006;6:97–102. doi: 10.1007/s11882-006-0046-z. [DOI] [PubMed] [Google Scholar]
- 25.Falsey AR. Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med. 2007;28:171–181. doi: 10.1055/s-2007-976489. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasakumar N, Ogra PL, Flanagan TD. Characteristics of fusion of respiratory syncytial virus with HEp-2 cells as measured by R18 fluorescence dequenching assay. J Virol. 1991;65:4063–4069. doi: 10.1128/jvi.65.8.4063-4069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herlocher ML, Ewasyshyn M, Sambhara S, Gharaee-Kermani M, Cho D, Lai J, Klein M, Maassab HF. Immunological properties of plaque purified strains of live attenuated respiratory syncytial virus (RSV) for human vaccine. Vaccine. 1999;17:172–181. doi: 10.1016/s0264-410x(98)00155-8. [DOI] [PubMed] [Google Scholar]
- 29.Lukacs NW, Moore ML, Rudd BD, Berlin AA, Collins RD, Olson SJ, Ho SB, Peebles RS., Jr. Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am J Pathol. 2006;169:977–986. doi: 10.2353/ajpath.2006.051055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukacs NW, Smit JJ, Mukherjee S, Morris SB, Nunez G, Lindell DM. Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J Immunol. 2010;185:2231–2239. doi: 10.4049/jimmunol.1000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tekkanat KK, Maassab HF, Cho DS, Lai JJ, John A, Berlin A, Kaplan MH, Lukacs NW. IL-13-induced airway hyperreactivity during respiratory syncytial virus infection is STAT6 dependent. J Immunol. 2001;166:3542–3548. doi: 10.4049/jimmunol.166.5.3542. [DOI] [PubMed] [Google Scholar]
- 33.Kallal LE, Schaller MA, Lindell DM, Lira SA, Lukacs NW. CCL20/CCR6 blockade enhances immunity to RSV by impairing recruitment of DC. Eur J Immunol. 2010;40:1042–1052. doi: 10.1002/eji.200939778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein Klouwenberg P, Tan L, Werkman W, van Bleek GM, Coenjaerts F. The role of Toll-like receptors in regulating the immune response against respiratory syncytial virus. Crit Rev Immunol. 2009;29:531–550. doi: 10.1615/critrevimmunol.v29.i6.40. [DOI] [PubMed] [Google Scholar]
- 35.Huang S, Wei W, Yun Y. Upregulation of TLR7 and TLR3 gene expression in the lung of respiratory syncytial virus infected mice. Wei Sheng Wu Xue Bao. 2009;49:239–245. [PubMed] [Google Scholar]
- 36.Murawski MR, Bowen GN, Cerny AM, Anderson LJ, Haynes LM, Tripp RA, Kurt-Jones EA, Finberg RW. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol. 2009;83:1492–1500. doi: 10.1128/JVI.00671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudd BD, Luker GD, Luker KE, Peebles RS, Lukacs NW. Type I interferon regulates respiratory virus infected dendritic cell maturation and cytokine production. Viral Immunol. 2007;20:531–540. doi: 10.1089/vim.2007.0057. [DOI] [PubMed] [Google Scholar]
- 38.Rudd BD, Schaller MA, Smit JJ, Kunkel SL, Neupane R, Kelley L, Berlin AA, Lukacs NW. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J Immunol. 2007;178:5820–5827. doi: 10.4049/jimmunol.178.9.5820. [DOI] [PubMed] [Google Scholar]
- 39.Saito T, Gale M., Jr. Principles of intracellular viral recognition. Curr Opin Immunol. 2007;19:17–23. doi: 10.1016/j.coi.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Bhoj VG, Sun Q, Bhoj EJ, Somers C, Chen X, Torres JP, Mejias A, Gomez AM, Jafri H, Ramilo O, Chen ZJ. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2008;105:14046–14051. doi: 10.1073/pnas.0804717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlender J, Hornung V, Finke S, Gunthner-Biller M, Marozin S, Brzozka K, Moghim S, Endres S, Hartmann G, Conzelmann KK. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J Virol. 2005;79:5507–5515. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol. 2005;79:3350–3357. doi: 10.1128/JVI.79.6.3350-3357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–10737. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 45.Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem. 2006;281:11374–11383. doi: 10.1074/jbc.M509157200. [DOI] [PubMed] [Google Scholar]
- 46.Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity. 2005;22:539–550. doi: 10.1016/j.immuni.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, Hamada S, Yoshimori T. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037–1040. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- 48.Dubuisson JF, Swanson MS. Mouse infection by Legionella, a model to analyze autophagy. Autophagy. 2006;2:179–182. doi: 10.4161/auto.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amer AO, Swanson MS. Autophagy is an immediate macrophage response to Legionella pneumophila. Cell Microbiol. 2005;7:765–778. doi: 10.1111/j.1462-5822.2005.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ito H, Aoki H, Kuhnel F, Kondo Y, Kubicka S, Wirth T, Iwado E, Iwamaru A, Fujiwara K, Hess KR, Lang FF, Sawaya R, Kondo S. Autophagic cell death of malignant glioma cells induced by a conditionally replicating adenovirus. J Natl Cancer Inst. 2006;98:625–636. doi: 10.1093/jnci/djj161. [DOI] [PubMed] [Google Scholar]
- 51.Prentice E, Jerome WG, Yoshimori T, Mizushima N, Denison MR. Coronavirus replication complex formation utilizes components of cellular autophagy. J Biol Chem. 2004;279:10136–10141. doi: 10.1074/jbc.M306124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Talloczy Z, Virgin H. W. t., Levine B. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy. 2006;2:24–29. doi: 10.4161/auto.2176. [DOI] [PubMed] [Google Scholar]
- 53.Espert L, Denizot M, Grimaldi M, Robert-Hebmann V, Gay B, Varbanov M, Codogno P, Biard-Piechaczyk M. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J Clin Invest. 2006;116:2161–2172. doi: 10.1172/JCI26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossman JS, Lamb RA. Autophagy, apoptosis, and the influenza virus M2 protein. Cell Host Microbe. 2009;6:299–300. doi: 10.1016/j.chom.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 55.Jackson WT, Giddings TH, Jr., Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR, Kirkegaard K. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005;3:e156. doi: 10.1371/journal.pbio.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deretic V, Delgado M, Vergne I, Master S, De Haro S, Ponpuak M, Singh S. Autophagy in immunity against mycobacterium tuberculosis: a model system to dissect immunological roles of autophagy. Curr Top Microbiol Immunol. 2009;335:169–188. doi: 10.1007/978-3-642-00302-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corr SC, O'Neill LA. Listeria monocytogenes infection in the face of innate immunity. Cell Microbiol. 2009 doi: 10.1111/j.1462-5822.2009.01294.x. [DOI] [PubMed] [Google Scholar]
- 58.Jounai N, Takeshita F, Kobiyama K, Sawano A, Miyawaki A, Xin KQ, Ishii KJ, Kawai T, Akira S, Suzuki K, Okuda K. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc Natl Acad Sci U S A. 2007;104:14050–14055. doi: 10.1073/pnas.0704014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ling Z, Tran KC, Teng MN. Human respiratory syncytial virus nonstructural protein NS2 antagonizes the activation of beta interferon transcription by interacting with RIG-I. J Virol. 2009;83:3734–3742. doi: 10.1128/JVI.02434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoboua F, Martel A, Duval A, Mukawera E, Grandvaux N. Respiratory syncytial virus-mediated NF-kappa B p65 phosphorylation at serine 536 is dependent on RIG-I, TRAF6, and IKK beta. J Virol. 2010;84:7267–7277. doi: 10.1128/JVI.00142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jamaluddin M, Tian B, Boldogh I, Garofalo RP, Brasier AR. Respiratory syncytial virus infection induces a reactive oxygen species-MSK1-phospho-Ser-276 RelA pathway required for cytokine expression. J Virol. 2009;83:10605–10615. doi: 10.1128/JVI.01090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bitko V, Musiyenko A, Bayfield MA, Maraia RJ, Barik S. Cellular La protein shields nonsegmented negative-strand RNA viral leader RNA from RIG-I and enhances virus growth by diverse mechanisms. J Virol. 2008;82:7977–7987. doi: 10.1128/JVI.02762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sasai M, Shingai M, Funami K, Yoneyama M, Fujita T, Matsumoto M, Seya T. NAK-associated protein 1 participates in both the TLR3 and the cytoplasmic pathways in type I IFN induction. J Immunol. 2006;177:8676–8683. doi: 10.4049/jimmunol.177.12.8676. [DOI] [PubMed] [Google Scholar]
- 64.Swanson MS, Byrne BG, Dubuisson JF. Kinetic analysis of autophagosome formation and turnover in primary mouse macrophages. Methods Enzymol. 2009;452:383–402. doi: 10.1016/S0076-6879(08)03623-9. [DOI] [PubMed] [Google Scholar]
- 65.Boogaard I, van Oosten M, van Rijt LS, Muskens F, Kimman TG, Lambrecht BN, Buisman AM. Respiratory syncytial virus differentially activates murine myeloid and plasmacytoid dendritic cells. Immunology. 2007;122:65–72. doi: 10.1111/j.1365-2567.2007.02613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bartz H, Turkel O, Hoffjan S, Rothoeft T, Gonschorek A, Schauer U. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-gamma in naive T cells. Immunology. 2003;109:49–57. doi: 10.1046/j.1365-2567.2003.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.English L, Chemali M, Duron J, Rondeau C, Laplante A, Gingras D, Alexander D, Leib D, Norbury C, Lippe R, Desjardins M. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol. 2009;10:480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leib DA, Alexander DE, Cox D, Yin J, Ferguson TA. Interaction of ICP34.5 with Beclin 1 modulates herpes simplex virus type 1 pathogenesis through control of CD4+ T-cell responses. J Virol. 2009;83:12164–12171. doi: 10.1128/JVI.01676-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee HK, Mattei LM, Steinberg BE, Alberts P, Lee YH, Chervonsky A, Mizushima N, Grinstein S, Iwasaki A. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010;32:227–239. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C, Nunez G. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Travassos LH, Carneiro LA, Ramjeet M, Hussey S, Kim YG, Magalhaes JG, Yuan L, Soares F, Chea E, Le Bourhis L, Boneca IG, Allaoui A, Jones NL, Nunez G, Girardin SE, Philpott DJ. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 11:55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- 72.Harris J, Hartman M, Roche C, Zeng SG, O'Shea A, Sharp FA, Lambe EM, Creagh EM, Golenbock DT, Tschopp J, Kornfeld H, Fitzgerald KA, Lavelle EC. Autophagy controls IL-1{beta} secretion by targeting pro-IL-1{beta} for degradation. J Biol Chem. 2011 doi: 10.1074/jbc.M110.202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carpenter LR, Moy JN, Roebuck KA. Respiratory syncytial virus and TNF alpha induction of chemokine gene expression involves differential activation of Rel A and NF-kappa B1. BMC Infect Dis. 2002;2:5. doi: 10.1186/1471-2334-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hosakote YM, Liu T, Castro SM, Garofalo RP, Casola A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am J Respir Cell Mol Biol. 2009;41:348–357. doi: 10.1165/rcmb.2008-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Indukuri H, Castro SM, Liao SM, Feeney LA, Dorsch M, Coyle AJ, Garofalo RP, Brasier AR, Casola A. Ikkepsilon regulates viral-induced interferon regulatory factor-3 activation via a redox-sensitive pathway. Virology. 2006;353:155–165. doi: 10.1016/j.virol.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 76.Liu T, Castro S, Brasier AR, Jamaluddin M, Garofalo RP, Casola A. Reactive oxygen species mediate virus-induced STAT activation: role of tyrosine phosphatases. J Biol Chem. 2004;279:2461–2469. doi: 10.1074/jbc.M307251200. [DOI] [PubMed] [Google Scholar]
- 77.Petrovski G, Ayna G, Majai G, Hodrea J, Benko S, Madi A, Fesus L. Phagocytosis of cells dying through autophagy induces inflammasome activation and IL-1beta release in human macrophages. Autophagy. 2011;7 doi: 10.4161/auto.7.3.14583. [DOI] [PubMed] [Google Scholar]
- 78.Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Frohlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 79.Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 80.Deretic V. Multiple regulatory and effector roles of autophagy in immunity. Curr Opin Immunol. 2009;21:53–62. doi: 10.1016/j.coi.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Muller M, Kreymborg K, Altenberend F, Brandenburg J, Kalbacher H, Brock R, Driessen C, Rammensee HG, Stevanovic S. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci U S A. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, Munz C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 83.Nimmerjahn F, Milosevic S, Behrends U, Jaffee EM, Pardoll DM, Bornkamm GW, Mautner J. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. Eur J Immunol. 2003;33:1250–1259. doi: 10.1002/eji.200323730. [DOI] [PubMed] [Google Scholar]
- 84.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr., Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]