Abstract
Aerobic exercise may represent a useful intervention for drug abuse in predisposed individuals. Exercise increases plasticity in the brain that could be used to reverse learned drug associations. Previous studies report that exposing mice to a complex environment including running wheels after drug conditioning abolishes conditioned place preference (CPP) for cocaine, whereas running can enhance CPP when administered before conditioning. The purpose of the present study was to test the hypothesis that timing of exercise relative to conditioning has opposing effects on cocaine CPP. Male C57BL/6J mice experienced 30 days of running or Sedentary treatments either before or after cocaine conditioning. Control animals always received saline and never cocaine but otherwise underwent the same conditioning and exercise treatments. Animals were administered BrdU injections at the onset of conditioning or exercise and euthanized at the end of the study to quantify survival of new neurons in the hippocampus as a marker of plasticity. Wheel running accelerated extinction of CPP when running occurred entirely after drug conditioning, whereas running delayed extinction when administered before conditioning. A single conditioning day after running was sufficient to abolish the accelerated extinction observed when all conditioning preceded running. Running approximately doubled adult hippocampal neurogenesis, whereas cocaine had no effect. Results suggest exercise-induced plasticity can facilitate learning that context is no longer associated with drug. However, if drug exposure occurs after exercise, running-induced plasticity may strengthen drug associations. Results provide insight into the interaction between exercise and drug conditioning that could have implications for drug abuse treatments.
Keywords: BrdU, hippocampal neurogenesis, exercise, CPP
Introduction
Additional treatments for cocaine abuse are critically needed. Compliance with current treatment programs is weak, and abstinence is typically ephemeral. For example, in a recent study, fifty-two percent of cocaine users dropped out of a National Institute for Drug Abuse treatment trial within three months (Ghitza et al., 2010). Studies have suggested that aerobic exercise could be useful as an intervention for maintaining abstinence in individuals willing to substitute exercise for drug reward (Sinyor et al., 1982). Exercise promotes brain plasticity and activates some of the same brain structures as those involved in reward and addiction. In rodents, the neural plasticity marker ΔFosB is upregulated in striatum and nucleus accumbens after wheel running to a comparable degree as after chronic cocaine exposure (Brene et al., 2007). In addition, mice bred for high levels of voluntary wheel running display increased brain activity marker c-Fos in brain structures implicated in reward. Among these are the lateral hypothalamus, medial frontal cortex, and striatum (Rhodes et al., 2003). Taken together, this evidence suggests that exercise may serve as a substitute reward, but whether substituting exercise for drugs helps to ameliorate addiction is still unknown.
Plasticity from exercise could be used to modify associations between drug reward and contextual cues. For example, exercise increases adult hippocampal neurogenesis, and new neurons have been hypothesized to display greater ability to mold to new experiences than pre-established neurons (van Praag et al., 1999a). In addition to being a site where exercise exerts effects on the brain, the hippocampus may influence reward circuitry because of its anatomical connections. The hippocampus receives input from the nucleus accumbens and ventral tegmental area and sends output to the nucleus accumbens (Eisch & Harburg, 2006). Manipulations of hippocampal dentate gyrus granule cells have been shown to influence dopaminergic signaling in the nucleus accumbens and ventral tegmental area, areas important to drug reward (Eisch & Harburg, 2006).
Consistent with the hypothesis that exercise can enhance plasticity and facilitate extinction of conditioned place preference (CPP), a recent study using C57BL/6 mice found that environmental enrichment including running wheels completely abolished CPP for cocaine when the enrichment was administered after conditioning (Solinas et al., 2008). The same group found that rearing C57BL/6 from weaning in an enriched environment abolished cocaine CPP when the animals were conditioned and tested as adults (Solinas et al., 2009). Similarly, in Lewis rats, forced treadmill running during adolescence weakened cocaine CPP when conditioning occurred after running (Thanos et al., 2010). On the other hand, wheel running strengthened cocaine CPP when adult Long-Evans rats were given wheel access before conditioning (Smith et al., 2008). To the best of our knowledge, no other studies have investigated effects of running on cocaine CPP. The purpose of this study was to determine (1) whether the influence of wheel running on cocaine CPP depends critically on the timing of wheel access relative to drug conditioning and (2) whether changes in CPP correlate with changes in adult hippocampal neurogenesis in response to the exercise and cocaine treatments.
Materials and methods
Animals
Ninety male C57BL/6J mice were obtained at 5 weeks of age (The Jackson Laboratory, Bar Harbor, ME) and housed 4 per cage in a climate-controlled environment on a 12 h light/dark cycle (lights off at 9:00 a.m.) for 12 days. Dimensions of cages without running wheels were 29 × 19 × 13 cm (L × W × H). Mice were individually housed for 1 week before starting the experimental procedures. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and adhered to NIH guidelines. All measures were taken to minimize the number of mice used as well as the pain and suffering of the animals.
Drugs
Cocaine hydrochloride (Sigma Aldrich, St. Louis, MO) was dissolved in 0.9% saline and was administered at a dose of 10 mg/kg via intraperitoneal (i.p.) injections in a volume of 10 ml/kg. Dose was chosen based on the literature and was prepared according to the salt not the base form (Zombeck et al., 2008).
Place conditioning chambers
The place conditioning chambers were modeled after Cunningham et al. (2006) and were the same as in previous studies from our laboratory (Zombeck et al., 2008; Johnson et al., 2010). They consisted of 20 identical black acrylic boxes (30 cm × 15 cm × 15 cm), with removable clear plastic tops. The floors were interchangeable and consisted of three types of distinct textures: HOLE, GRID, and HOLE/GRID. The HOLE/GRID floor consisted of half GRID and half HOLE textures. Distance traveled and location of mice within CPP boxes were recorded by TopScan video tracking software (Clever Sys Inc., Vienna, VA) (Zombeck et al., 2008; Johnson et al., 2010).
Conditioned place preference (CPP) procedure
We followed Cunningham et al. (2003; 2006). Runners and Sedentary animals were counterbalanced with respect to the conditioned stimulus, and experienced cocaine on GRID (CS+GRID) or cocaine on HOLE (CS+HOLE) texture and saline on the alternate texture. During testing, animals explored the same size chamber as during conditioning except with the HOLE/GRID floor type. Hence the animals were forced to spend time on either HOLE or GRID side, and duration on HOLE is equivalent to the total duration of the test (30 min) minus duration on GRID (see Place Conditioning Chambers above). Conditioned place preference was determined by comparing the duration spent on HOLE (or GRID, statistics would be the same) between groups, CS+HOLE versus CS+GRID. The design ensures that any difference in duration spent on textures between groups (CS+GRID versus CS+HOLE) is due to drug-to-context learning, as this is the only variable that differs between the two groups. Biases in baseline preference for textures cannot produce false positives with this method because duration spent on one texture (HOLE or GRID) is compared between subgroups CS+GRID and CS+HOLE, both of which would be expected to display the bias if one developed. Hence, when the difference score is computed, any bias is subtracted out. The two groups are also matched for drug exposure which is important because drug exposure itself could affect the development of biases in preference. Moreover, each group serves as the other group’s learning control, because both groups learned to associate one texture with cocaine and the alternate texture with saline. This is important because as compared to using a control in which all animals receive saline on both textures, the experience of learning itself could bias preferences for the textures (Cunningham et al., 2003; Cunningham et al., 2006).
On the days of CPP habituation, pretesting, conditioning, testing, and cocaine priming (see below), mice were moved to a testing room, where lights were turned off at 9:00 A.M. Mice were kept in the room for one hour before testing began. Between sessions, the chambers were cleaned with disinfectant. Animals were returned to home cages with or without wheels immediately after testing. Hence, Runners had continuous access to running wheels throughout behavioral testing except when in the conditioning chambers.
Habituation
To familiarize the mice with the place conditioning chambers, animals were placed on a flat surface without a texture in the conditioning chambers in the morning (900 h; for 30 min) and in the afternoon (1500 h; for 30 min) for one day without any injection treatment (Cunningham et al., 2006).
Pretesting
To determine individual biases in preference for the textures prior to drug pairing, animals were weighted, received a 10 ml/kg saline injection, and were immediately placed in the apparatus with HOLE/GRID floor in the morning (900 h; for 30 min) and afternoon (1500 h; for 30 min) (Cunningham et al., 2006).
Conditioning
Four conditioned stimulus (CS+) trials (i.e., cocaine paired with one floor texture: HOLE or GRID) and four CS− trials (i.e. vehicle paired with the alternate floor texture) were administered over four days. Each day, one CS+ trial and one CS− trial was administered in the morning and afternoon. The order of exposure to CS+ and CS− was counterbalanced within each group. Experimental animals were weighed, received an injection of 10 mg/kg cocaine (CS+ trial) or vehicle (CS− trial), and were immediately placed on the appropriate floor texture. Control animals underwent the identical procedure, except that they always received vehicle (saline) on both floor textures.
Testing
Testing took place twice daily: in the morning (900 h; 30 min) and in the afternoon (1500 h; 30 min) for four consecutive days. Prior to each testing session, each mouse was weighed, injected i.p. with 10 ml/kg saline, and placed into the center of the HOLE/GRID conditioning chamber.
Cocaine priming
Cocaine priming began two days after the final CPP test session and consisted of two daily (morning and afternoon) 30 min exposures to the HOLE/GRID texture for four consecutive days. Experimental animals were weighed and injected with 10 mg/kg cocaine immediately before being place into the HOLE/GRID conditioning chamber, whereas control animals received a saline injection before all priming sessions.
Running wheels and sedentary treatment
Dimensions of running wheel cages were 36 × 20 × 14 cm (L × W × H) with a 23 cm diameter wheel mounted in the cage top. Running wheel rotations were monitored continuously in 1 min increments throughout the experiments via magnetic switches interfaced to a computer. Mice assigned to the Sedentary group were deliberately not housed in cages with locked wheels because mice climb in locked wheels and we intended to keep physical activity to a minimum in the Sedentary group (Koteja et al., 1999; Rhodes et al., 2000; Rhodes et al., 2003).
Experimental design
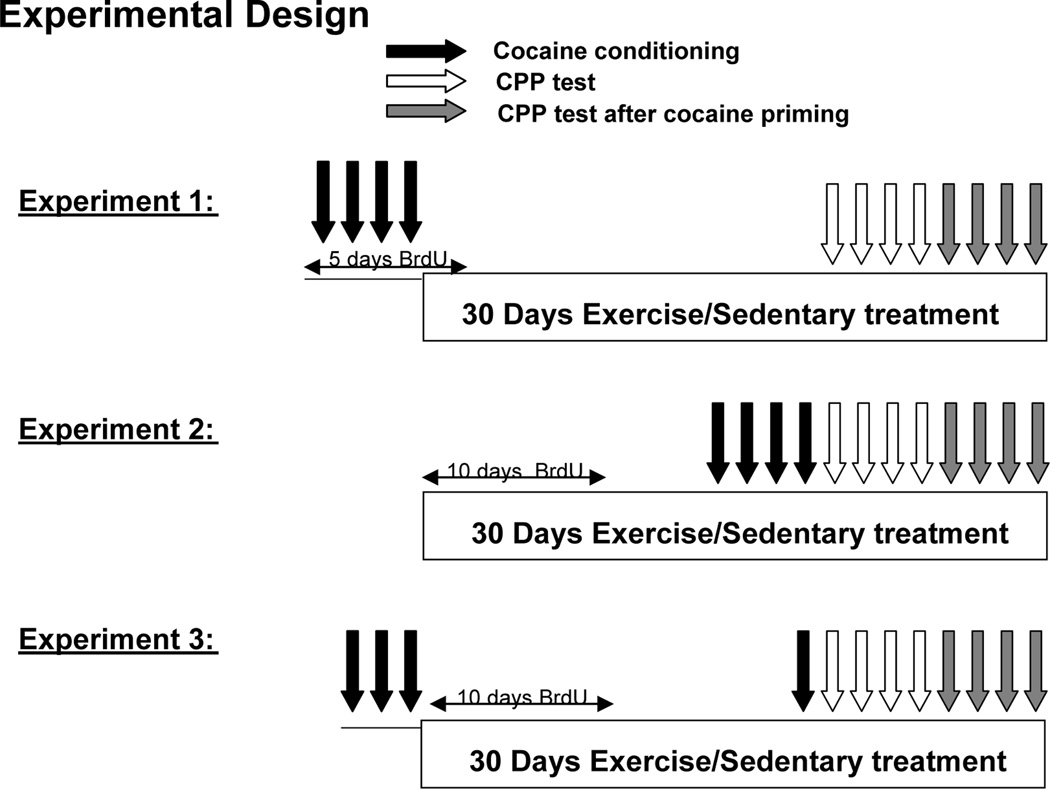
Experiment 1
At 54 days of age, animals (n = 30 total) underwent habituation, pretesting, and cocaine CPP (n = 20, Cocaine group) or CPP without cocaine, (n = 10, Control group) as detailed above (Fig. 1). During the four conditioning days, mice received daily injections of 50 mg/kg Bromodeoxyuridine (BrdU) to label dividing cells. The day after the four conditioning days, mice were placed individually in cages either without (Sedentary, n = 10 Cocaine and 5 Control) or with running wheels (Runner, n = 10 Cocaine and 5 Control) for 30 days. On the second day of Sedentary or Runner treatment, mice received one additional BrdU injection. After 30 days, mice underwent four consecutive days of CPP testing, followed one day later by four consecutive days of CPP testing after cocaine priming. After testing each day, mice were returned to their cages with or without running wheels so that Runners had continuous access to wheels throughout the testing period.
Figure 1.
Schematic diagram of the experimental design. The black arrows indicate when CPP conditioning sessions were administered. The white arrows indicate when CPP testing took place. The grey bars indicate when CPP testing took place after a cocaine priming dose was administered. The boxes indicate when the Runner/Sedentary conditions were administered relative to conditioning and CPP testing. Each experiment 1–3 contained 30 animals (20 conditioned with cocaine and 10 saline controls divided equally into Runner and Sedentary groups). In all 3 experiments, animals experienced 1 day of habituation to reduce novelty effects and 1 day of CPP pretesting to establish baseline texture preferences over the 2 days immediately preceding the conditioning (experiments 1 and 3) or Runner/Sedentary treatments (experiment 2). Animals in all 3 experiments experienced 30 days of uninterrupted running or sedentary treatment and a total of 4 days of CPP conditioning. The 3 experiments differed in the timing of the 4 CPP conditioning sessions relative to the Runner/Sedentary treatment phase. Animals were returned to cages with or without running wheels immediately after conditioning and testing to avoid the potential confound of animals experiencing withdrawal from running during the testing procedures.
Experiment 2
At 54 days of age, animals underwent habituation and pretesting (n = 30 total). The next day, mice were individually placed into cages either without (Sedentary, n = 15) or with running wheels (Runner, n = 15) for 30 days. During the first 10 days of Sedentary or Runner treatment, mice received daily injections of 50 mg/kg BrdU. After 30 days, the mice underwent cocaine CPP (n = 20, Cocaine group, 10 Runners and 10 Sedentary) or CPP without cocaine (n = 10, Control group, 5 Runners and 5 Sedentary) for four days. Mice then underwent four consecutive days of CPP testing, followed one day later by four consecutive days of CPP testing after cocaine priming. After conditioning or testing each day, mice were returned to their cages with or without running wheels so that Runners had continuous access to wheels throughout the testing period.
Experiment 3
At 54 days of age, animals (n = 30 total) underwent habituation, pretesting, and cocaine CPP (n = 20, Cocaine group) or CPP without cocaine (n = 10, Control group) for three consecutive days. The next day, mice were placed individually in cages either without (Sedentary, n = 10 Cocaine and 5 Control) or with running wheels (Runner, n = 10 Cocaine and 5 Control) for 30 days. The first 10 days of Sedentary or Runner treatment, mice received daily injections of 50 mg/kg BrdU. After 30 days, mice underwent one additional day of CPP conditioning to simulate a relapse episode, followed by four consecutive days of CPP testing, and then, one day later, by four consecutive days of CPP testing with cocaine priming. After the last conditioning or testing session each day, mice were returned to their cages with or without running wheels so that Runners had continuous access to wheels throughout the testing period.
Immunohistochemistry
Tissue preparation
Following behavioral testing, all the mice (n = 90) were anesthetized with 100 mg/kg sodium pentobarbital (i.p.) and then perfused transcardially with 4% paraformaldehyde in phosphate buffer solution (PBS; 0.287% sodium phosphate monobasic anhydrous, 1.102% sodium phosphate dibasic anhydrous, 0.9% sodium chloride in water). Brains were postfixed overnight and then transferred to 30% sucrose in PBS. Brains were sectioned using a cryostat into 40 µm thick coronal sections. Sections were placed into tissue cryoprotectant (30% ethylene glycol, 25% glycerin, 45% PBS) in 24 well plates and stored at −20 °C. Two separate one-in-six series of these sections (i.e. series of sections throughout the rostro-caudal extent of the brain with 240 µm increments separating each section, approximately nine sections) were stained in the following way.
BrdU-DAB
Purpose
To detect BrdU-positive (newly divided) cells in the dentate gyrus. Free floating sections were washed in tissue buffering solution (TBS; 1.3% Trizma hydrochloride, 0.19% Trizma base, 0.9% sodium chloride) and then treated with 0.6% hydrogen peroxide in TBS for 30 min. To denature DNA, sections were treated for 120 min with a solution of 50% de-ionized formamide and 2X SSC buffer, rinsed for 15 min in 2X SCC buffer, then treated with 2 N hydrochloric acid for 30 min at 37 °C, then 0.1 M boric acid in TBS (pH 8.5) for 10 min at room temperature. Sections were then treated (blocked) with a solution of 0.3% Triton-X and 3% goat serum in TBS (TBS-X plus) for 30 min, and then incubated in primary antibody against BrdU made in rat (AbD Serotec, Raleigh, NC, USA, catalog number OBT0030) at a dilution of 1:100 in TBS-X plus for 72 h at 4 °C. Sections were then washed in TBS, blocked with TBS-X plus for 30 min and then incubated in biotinylated secondary antibody against rat made in goat (Vector, Burlingame, CA, USA, catalog number BA-9400) at 1:250 in TBS-X plus for 100 min at room temperature. Sections were then treated using the ABC system (Vector, Burlingame, CA, USA, catalog number PK-6100) and stained using a diaminobenzidine kit (Sigma, St. Louis, MO, USA, catalog number D4418-505ET).
Double-fluorescent label
Purpose
To determine the proportion of BrdU-positive cells in the dentate gyrus that differentiated into neurons. Sections were treated as for BrdU-DAB except a cocktail was used for the primary antibody step. Rat anti-BrdU (1:100; AbD Serotec, Raleigh, NC, USA, catalog number OBT0030) was combined with mouse anti-neuronal nuclear protein (NeuN) (1:50; Millipore, Billerica, MA, USA, catalog number MAB377) for 72 h at 4 °C. Secondary antibodies were conjugated with fluorescent markers (Cy2-green anti-mouse, and Cy3-red anti-rat; Jackson ImmunoResearch, West Grove, PA, USA, catalog numbers 115-225-166 and 112-165-167, respectively) at dilution of 1:250 and also delivered as a cocktail.
Image Analysis
BrdU-DAB
The entire granule layer (bilateral), represented in the 1-in-6 series, was photographed by systematically advancing the field of view of the Zeiss brightfield light microscope and taking multiple photographs, via camera interfacted to computer, under 10× (total 100×) magnification. Positively labeled cells in these photographs were counted to generate estimates of total number of labeled cells. The total volume of the dentate gyrus represented in the series was measured so that the counts could be expressed per µm3 dentate gyrus sampled.
Double label
A Leica SP2 laser scanning confocal microscope (using a 40× oil objective, pinhole size 81.35 µm diameter) was used to determine the proportion of dentate gyrus BrdU-positive cells that differentiated into neurons (NeuN+). Dentate gyrus BrdU-positive cells were identified as either co-expressing NeuN or not. Each BrdU-positive cells in the granular layer (represented in the 1-in-6 series) was analyzed by focusing through the tissue in the z-axis to establish co-labeling with NeuN. The number of new neurons per µm3 per mouse was calculated as the number of BrdU cells per µm3 multiplied by average proportion BrdU cell co-expressing NeuN for the designated group.
Statistical analysis
Data were analyzed using SAS (version 9.1) or R (version 2.7.2) statistical software. In all analyses, P < 0.05 was considered statistically significant. Each experiment (1, 2, and 3) was analyzed separately. Conditioned place preference data were analyzed the following way. Control animals that never received cocaine were analyzed separately from cocaine-treated animals. First, the duration spent on the HOLE texture was analyzed by three-way repeated measures ANOVA with conditioned stimulus (CS+HOLE versus CS+GRID; between-subjects), exercise history (Runner versus Sedentary; between subjects), day of testing (1–4; within-subjects) and all interactions entered as factors. Testing session, whether at 9:00 h or 15:00 h, was also included as a factor in initial models but was never significant and therefore was removed from the final linear models. In addition, the CPP data were analyzed separately within each group using standard methods (Cunningham et al., 2006). Within each group, the duration spent on the HOLE texture was compared between the CS+HOLE and CS+GRID groups (Cunningham et al., 2006). Number of new neurons in the granule layer of the dentate gyrus were analyzed by two-way ANOVA with exercise history (Runner versus Sedentary), cocaine treatment (cocaine versus vehicle) and all interactions entered as factors. The correlation between distance traveled and number of new neurons was estimated by simple linear regression. The proportion of BrdU-labeled cells in the granule cell layer that co-expressed NeuN was analyzed by logistic regression, where proportion (binomial response) was modeled as a linear function of drug treatment (cocaine versus saline), exercise group (Runner versus Sedentary), and all interactions entered as factors.
Results
Baseline preference
During the pretest, before the animals ever experienced cocaine, and before any of the animals ran on wheels, animals spent approximately 50% of their time on each side, an average of 15.1 minutes (± 0.32 SE) on the HOLE texture. Running for 30 days slightly changed the baseline preference of Control animals, as was evidenced at CPP testing, when Control animals that had never received cocaine but had run showed a bias toward the HOLE texture that they had not displayed during the initial pretest. Sedentary Control animals that never received cocaine spent approximately 50% of their time, an average of 14.8 minutes (± 0.74 SE), on the HOLE texture, whereas Runners spent approximately 60% of their time, 18.0 minutes (± 0.74 SE) on HOLE (F1,246 = 10.8, P = 0.001). This bias in preference observed in Runners does not compromise analysis of cocaine CPP because the bias was present in both groups of Runners (CS+ GRID and CS+ HOLE) being compared to establish CPP (see Methods, CPP procedure).
Locomotor activity in CPP chambers
During conditioning, animals administered cocaine traveled an average of 56.7 meters (± 1.26 SE) per conditioning session, whereas animals administered saline traveled 35.0 meters (± 1.26 SE) per conditioning session (F1,413 = 617.5, P < 0.0001). Runners moved a similar distance in the apparatus as compared to Sedentary animals after saline or cocaine administration.
Wheel running
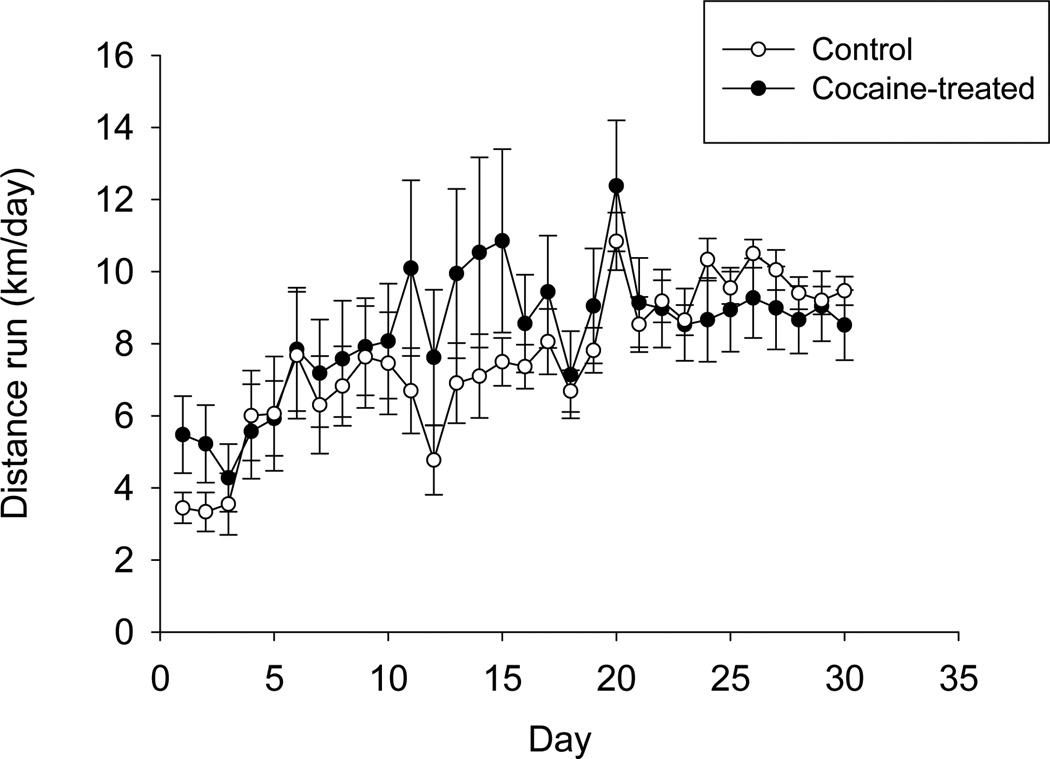
Running increased from days 1–20 and then maintained a plateau for remaining days (e.g., for experiment 1, day was significant, F29,1311 = 23.7, P < 0.0001) (Fig. 2). Cocaine had no influence on wheel running (Fig. 2). Average level of running across all animals in all experiments was 6.2 kilometers (± 0.41 SE).
Figure 2.
Wheel running over the course of the study. Average distance run (km/day) (± SE) for a representative experiment (experiment 1), shown separately for cocaine-treated (n = 10, solid circles) and Control mice (n = 5, open circles) that never experienced cocaine. Wheel running data for experiments 2 and 3 were similar (data not shown). Increased wheel running over the first 20 days is typical for C57BL/6J mice.
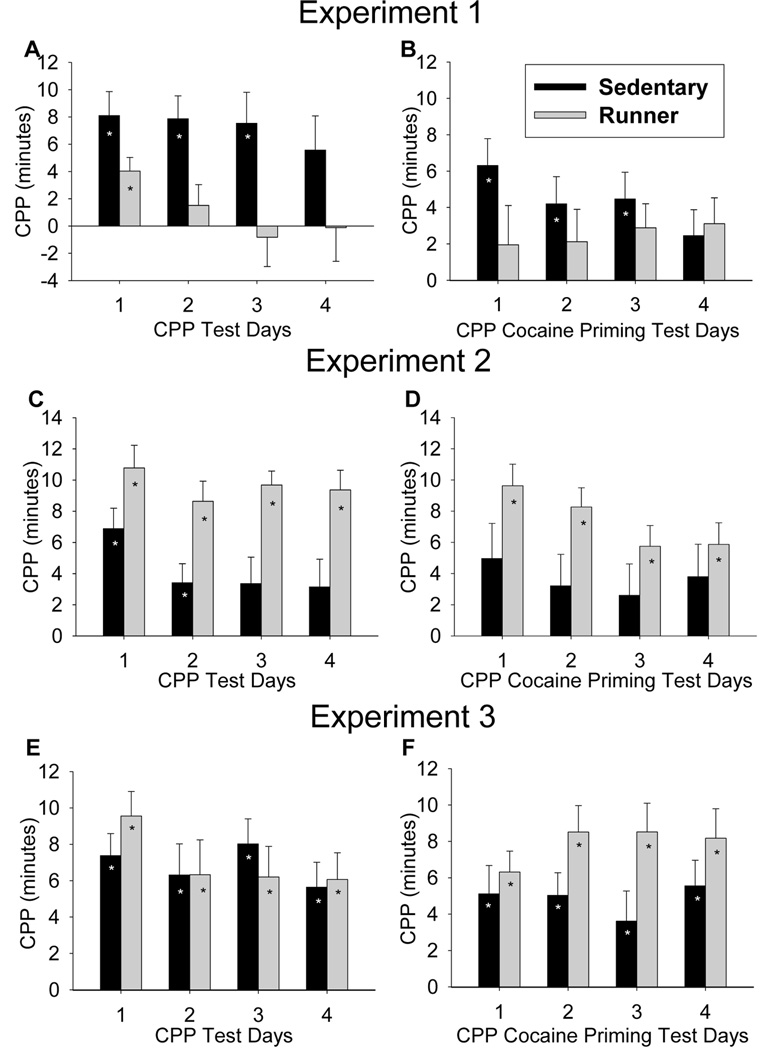
Experiment 1: Running after CPP
Sedentary animals displayed significantly stronger CPP than Runners, as indicated by a significant interaction between texture group (whether conditioned with cocaine on HOLE or GRID) and exercise group (Runner or Sedentary) (F1,128 = 7.7, P = 0.007). In addition, CPP extinguished faster in Runners as compared to Sedentary animals as indicated by a significant interaction between exercise group and day (F3,128 = 2.7, P = 0.046) (Fig. 3A). Posthoc analyses revealed that Sedentary animals displayed significant CPP on days 1–3 (all P < 0.05), whereas Runners displayed significant CPP only on day 1 (P < 0.05). The strength of preference on day 1 was slightly lower in Runners as compared to Sedentary animals (P = 0.08).
Figure 3.
Conditioned place preference for cocaine during testing and cocaine priming. Mean difference in duration (min) ± SE spent on the HOLE texture between animals receiving cocaine on HOLE texture (CS+HOLE) and animals receiving cocaine on GRID texture (CS+GRID) plotted separately for Runners and Sedentary animals. Each bar represents data for 10 animals (n = 5 CS+HOLE animals and n = 5 CS+GRID animals). A), C), and E) show testing data for experiments 1, 2 and 3, respectively whereas B), D), and F) show cocaine priming data for experiment 1, 2, and 3, respectively. The stars indicate significant place preference at P < 0.05.
During cocaine priming, Sedentary animals displayed significantly stronger CPP than Runners, as indicated by a significant interaction between exercise group and day (F3,114 = 4.4, P = 0.006) and a significant three-way interaction between exercise group, texture group and day (F3,114 = 3.0, P = 0.035) (Fig. 3B). Posthoc analyses indicated that Sedentary animals displayed significant CPP only on day 1 of cocaine priming (P < 0.05), whereas Runners never displayed significant CPP during cocaine priming, and the strength of preference on day 1 was significantly lower in Runners as compared to Sedentary animals (P = 0.03).
Experiment 2: Running before CPP
Runners displayed significantly stronger CPP than Sedentary animals, as indicated by a significant interaction between texture group and exercise group (F1,128 = 10.3, P = 0.002). In addition, CPP tended to extinguish faster in Sedentary animals as compared to Runners, as suggested by an interaction between exercise group and day that approaches significance (F3,128 = 2.5, P = 0.066) (Fig. 3C). Posthoc analyses revealed that Runners displayed significant CPP on all days 1–4 (all P < 0.05), whereas Sedentary animals displayed significant CPP only on days 1–2 (P < 0.05) and the strength of preference on day 2 was significantly lower in Sedentary animals as compared to Runners (P = 0.027).
During cocaine priming, Runners displayed significantly stronger CPP than Sedentary animals, as indicated by a significant interaction between exercise group and day (F3,128 = 5.2, P = 0.002) and a significant interaction between texture group and day (F3,128 = 5.8, P = 0.0009) (Fig. 3D). Posthoc analyses indicated that Runners displayed significant CPP on days 1–4 of cocaine priming (P < 0.05), whereas Sedentary animals displayed significant CPP only on the first day of cocaine priming, and the strength of preference on day 1 tended to be lower in Sedentary animals as compared to Runners (P = 0.07).
Experiment 3: Running before CPP with simulated relapse episode
Runners and Sedentary animals displayed significant CPP for all testing days, as indicated by a significant effect of texture group (F1,128 = 46.1, P < 0.0001) but no effects of exercise group or day or any interactions were significant (Fig. 3E). During cocaine priming, Runners displayed significantly stronger CPP than Sedentary animals, as indicated by a significant interaction between exercise group and day (F3,128 = 5.0, P = 0.003) (Fig. 3F). Posthoc analyses indicated that Runners displayed significant CPP on days 1–4 of cocaine priming (P < 0.05), whereas Sedentary animals displayed significant CPP only on the first day of cocaine priming, and the strength of preference on day 1 tended to be lower in Sedentary animals as compared to Runners (P = 0.07).
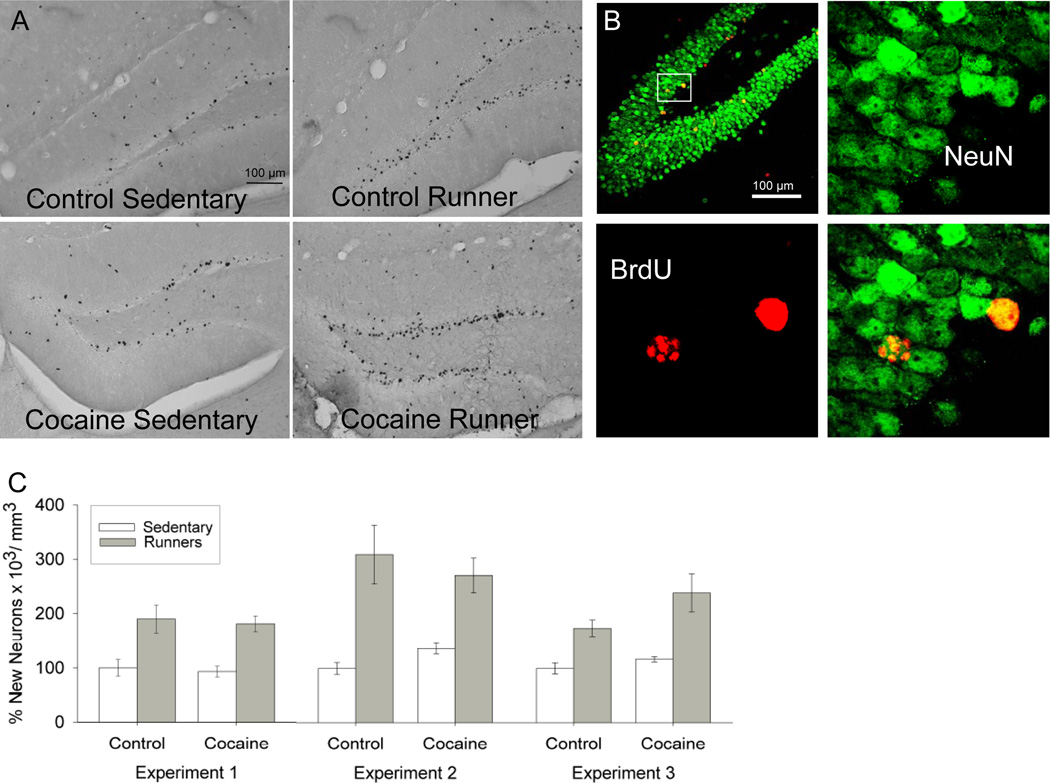
Hippocampal neurogenesis
Runners displayed approximately a 2-fold increase in the number of new neurons (BrdU cells co-labeled with NeuN) as compared to Sedentary animals in each of the 3 experiments (Experiment 1, F1,24 = 29.7, P < 0.0001; Experiment 2, F1,26 = 31.3, P < 0.0001; Experiment 3, F1,32 = 23.0, P < 0.0001) (Fig. 4). Average distance traveled in the wheel over the 30 days in Runners was significantly correlated with number of new neurons (F1,43 = 7.9, P = 0.007). Cocaine had no effect on neurogenesis (P > 0.05). Analysis of logistic regression revealed that running significantly increased the proportion of BrdU cells that differentiated into neurons in all three experiments (Deviance = 4.6, P = 0.03). The percentage of BrdU cells that differentiated into neurons as indicated by co-labeling with NeuN was 92% ± 1.2 SE in Sedentary animals and 94% ± 0.8 SE in Runners. Cocaine had no effect on proportion of BrdU cells that differentiated into neurons, and no interactions with cocaine or experiment were significant (P > 0.05). Given the high percentage of BrdU cells that differentiated into neurons, results were similar when total number of BrdU cells was analyzed instead of total number of new neurons (i.e., BrdU cells co-labeled with NeuN). Running approximately doubled the number of BrdU cells in the granule layer in each of the three experiments (Experiment 1, F1,24 = 16.7, P = 0.0004; Experiment 2, F1,26 = 22.6, P < 0.0001; Experiment 3, F1,32 = 32.1, P < 0.0001).
Figure 4.
Adult hippocampal neurogenesis. A) Photographs of the dentate gyrus stained for BrdU-DAB, showing representative sections from each of the four groups. Black dots are nuclei stained positive for BrdU indicating newly divided cells. B) Photographs of a representative section through the dentate gyrus of a Runner mouse double-stained green for NeuN (mature neuronal marker) and red for BrdU. Panels to the right show the tissue illuminated for each color separately and combined zoomed in around the BrdU cells, indicating two episodes of neurogenesis. C) Average number of new neurons per volume dentate gyrus shown as a percentage of the average Sedentary Control animal. Runners are shown as grey bars and Sedentary as white bars. Data are shown separately for Control animals (never treated with cocaine, n = 5 Sedentary and 5 Runners) versus Cocaine-treated (n = 10 Sedentary and 10 Runners) for each experiment. Standard error bars are shown.
Discussion
The main finding from the study is that wheel running has opposing effects on cocaine CPP depending on when running is administered relative to drug conditioning (Fig. 3). Results have implications for drug rehabilitation programs considering exercise as an intervention for maintaining abstinence (Sinyor et al., 1982). We speculate that when exercise is administered before drug conditioning, the plasticity engendered from running strengthens the learning of the drug-to-context association, such that when tested, the runners are resistant to extinction. This interpretation is consistent with many previous reports that running enhances learning and memory (e.g., van Praag et al., 1999b; Colcombe & Kramer, 2003; Eisenstein & Holmes, 2007; Clark et al., 2008; Griffin et al., 2009; Creer et al., 2010). On the other hand, when exercise is administered after drug conditioning, the plasticity engendered from running cannot strengthen drug learning because the plasticity was increased after the drug learning took place. However, the plasticity from running could be recruited later to facilitate the new learning that the context is no longer associated with the drug, and hence accelerated extinction of CPP was observed in runners. Taken together, results suggest that exercise could be a useful intervention to facilitate extinction of conditioned drug associations during abstinence. However, the benefit of exercise could be reversed if a relapse episode occurs after running primes the brain for plasticity.
One of the important findings from the study was that running after drug conditioning no longer accelerated extinction of CPP for cocaine when a single conditioning session was administered after running (Fig. 3E). The single additional conditioning trial abolished the benefits of exercise in facilitating extinction despite the fact that 75% of conditioning occurred prior to exercise exposure, when no new plasticity generated from running could help in acquisition of cocaine-to-context learning. In accordance with Smith et al.’s (2008) study, our results showed that even one drug conditioning day after exercise results in delayed CPP extinction, as compared to the results seen in studies where running combined with a complex environment precedes drug conditioning (Solinas et al., 2008; Chauvet et al., 2011). Moreover, the priming dose of cocaine elicited significant CPP across all cocaine priming days only in the animals that had run prior to cocaine conditioning (Fig. 3D, F). The fact that these animals’ CPP never extinguished even during cocaine priming suggests that their drug-to-context learning was especially strong, possibly due to enhanced plasticity that was present at the time of drug conditioning, e.g., BDNF, IGF-1, angiogenesis, synaptogenesis (Neeper et al., 1995; Carro et al., 2001; Dietrich et al., 2008; Gomez-Pinilla et al., 2008; Clark et al., 2009).
When administered before conditioning, exercise strengthened cocaine CPP and delayed extinction (Fig. 3C). As discussed above, this result is consistent with a vast literature demonstrating pro-cognitive effects of exercise on many different forms of learning and memory (e.g., van Praag et al., 1999b; Colcombe & Kramer, 2003; Eisenstein & Holmes, 2007; Clark et al., 2008; Griffin et al., 2009; Creer et al., 2010). Although there are relatively few studies examining effects of exercise on CPP for drugs of abuse, there is no a priori reason to believe effects of exercise or enrichment would be any different for drug learning as opposed to other forms of associative learning. Consistent with this proposition and our results (Fig. 3C), early studies found that housing Sprague-Dawley rats in an enriched environment (without running wheels) from weaning until adulthood strengthened CPP for amphetamine (Bowling & Bardo, 1994; Bardo et al., 1995), and a more recent study found that wheel running administered before conditioning strengthened CPP for cocaine in Long-Evans rats (Smith et al., 2008). However, two other studies found the exact opposite result (Solinas et al., 2009; Thanos et al., 2010). Solinas et al. (2009) recently reported that housing C57BL/6J mice from weaning until 2–3 months of age with toys and running wheels completely abolished cocaine CPP when conditioning and testing was administered after the enriched housing, as compared to mice housed in standard conditions, which displayed significant CPP. The Solinas et al. (2009) study is very similar to ours, with the same strain of mice and running wheels. The only difference between the Solinas et al. (2009) study and ours was that their mice were housed in cages with toys and running wheels from weaning to adulthood, whereas our animals were housed only with running wheels starting in adulthood. However, we would have expected the longer duration of enrichment to produce a greater enhancement in CPP learning and retention, and certainly not to abolish cocaine CPP. Solinas et al. (2009) argued that enrichment abolished CPP not by altering learning but by reducing the rewarding effects of cocaine, which may be a possibility. The other study that found that exercise before conditioning attenuated CPP for cocaine (Thanos et al., 2010) was conducted in adolescent rats and used forced treadmill running as the form of exercise. Hence, this study has numerous differences from our study, the most important of which is probably the forced running which can induce stress, and is not considered a rewarding form of exercise in animals (Greenwood et al., 2011).
Our speculation that plasticity from running facilitated learning in our experiments is consistent with our observation of increased adult hippocampal neurogenesis in Runners as compared to Sedentary animals in all three experiments (Fig. 4). A role for the hippocampus in associative learning has been established (Ferbinteanu & McDonald, 2001; Fuchs et al., 2005; Rudy & Matus-Amat, 2005). Moreover, several papers have provided direct evidence that new neurons can enhance associative learning (Winocur et al., 2006; Wojtowicz et al., 2008; Hernandez-Rabaza et al., 2009; Drew et al., 2010). New neurons have been hypothesized to display greater ability to mold to new experiences than older established neurons because their processes and connections are not yet solidified (van Praag et al., 1999b). Hence, it is conceivable that in the case where exercise is administered before conditioning, new neurons generated from running could be recruited during drug learning and lead to strengthening of associative learning, making the behavior more difficult to extinguish. Moreover, it is also conceivable that when exercise is administered after conditioning, new neurons could be recruited during extinction to more rapidly acquire the new association that the context is no longer paired with drug. However, at present, the connections between neurogenesis and the CPP outcomes are correlations only. Many other changes occur in the brain from exercise, including synaptogenesis, increases in trophic factors, growth factors, neurotransmitter concentrations, angiogenesis, and changes in dendritic morphology, among others that could account for our findings (Meeusen & De Meirleir, 1995; Neeper et al., 1995; Carro et al., 2001; Dietrich et al., 2008; Gomez-Pinilla et al., 2008; Clark et al., 2009). Moreover, exercise is known promote resistance to stress, which could contribute to the behavioral outcomes depending on how stressful the CPP testing was perceived to be by the animals (Greenwood & Fleshner, 2008). One way to directly test the role of new neurons in the behavioral outcomes is to repeat the study with animals that are unable to generate new neurons from exercise, through use of irradiation or transgenic mouse models (Saxe et al., 2006; Clark et al., 2008; Dupret et al., 2008; Deng et al., 2009).
Previous literature suggests that cocaine exposure reduces adult hippocampal neurogenesis (Noonan et al., 2010; Sudai et al., 2011). However, we did not observe such a result in any of the three experiments (Fig. 4). One explanation is the difference in duration of cocaine exposure between studies. In our study, animals were exposed to cocaine for a total of 8 days, 4 days during conditioning and 4 during cocaine priming, whereas cocaine exposure of up to 15 days is characteristic of studies that have found a significant cocaine-induced reduction in hippocampal neurogenesis. Moreover, most of the studies reporting a decrease in neurogenesis from cocaine employed operant conditioning methods, where the rats have to perform a lever press to receive an intravenous infusion of drug, as opposed to classical conditioning methods used here where animals are administered intraperitoneal injections (Noonan et al., 2010; Sudai et al., 2011).
The present study is the first to show an opposing effect of wheel running on cocaine CPP that depends critically on the timing of exercise relative to drug conditioning. The mechanisms by which running facilitates or extinguishes CPP are not known. One possible explanation is that plasticity generated from running, including new hippocampal units, could facilitate learning of drug-to context associations or learning that drug is no longer associated with context. A better understanding of the mechanisms that mediate effects of exercise on drug-to-context learning could lead to improved treatments for drug addiction.
Acknowledgements
This work was supported by NIH grants MH 083807 and DA027487. Special thanks to the Beckman Institute Animal Care staff. Thanks also to the Beckman Institute Imaging Technology Group and David Sohn and Elizabeth Wojcik for their assistance with the microscopic imaging. Thanks to Peter Clark for comments on previous versions of the manuscript.
References
- Bardo MT, Bowling SL, Rowlett JK, Manderscheid P, Buxton ST, Dwoskin LP. Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol. Biochem. Behav. 1995;51:397–405. doi: 10.1016/0091-3057(94)00413-d. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Bardo MT. Locomotor and rewarding effects of amphetamine in enriched, social, and isolate reared rats. Pharmacol. Biochem. Behav. 1994;48:459–464. doi: 10.1016/0091-3057(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Brene S, Bjornebekk A, Aberg E, Mathe AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiol. Behav. 2007;92:136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J. Neurosci. 2001;21:5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Jaber M, Solinas M. Brain regions associated with the reversal of cocaine conditioned place preference by environmental enrichment. Neuroscience. 2011;184:88–96. doi: 10.1016/j.neuroscience.2011.03.068. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19:937–950. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol. Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology. 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat. Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Andrews ZB, Horvath TL. Exercise-induced synaptogenesis in the hippocampus is dependent on UCP2-regulated mitochondrial adaptation. J. Neurosci. 2008;28:10766–10771. doi: 10.1523/JNEUROSCI.2744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav. Neurosci. 2010;124:446–454. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus. 2006;16:271–286. doi: 10.1002/hipo.20161. [DOI] [PubMed] [Google Scholar]
- Eisenstein SA, Holmes PV. Chronic and voluntary exercise enhances learning of conditioned place preference to morphine in rats. Pharmacol. Biochem. Behav. 2007;86:607–615. doi: 10.1016/j.pbb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, McDonald RJ. Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus. 2001;11:187–200. doi: 10.1002/hipo.1036. [DOI] [PubMed] [Google Scholar]
- Fuchs KA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Preston KL, Epstein DH, Kuwabara H, Endres CJ, Bencherif B, Boyd SJ, Copersino ML, Frost JJ, Gorelick DA. Brain mu-opioid receptor binding predicts treatment outcome in cocaine-abusing outpatients. Biol. Psychiatry. 2010;68:697–703. doi: 10.1016/j.biopsych.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur. J. Neurosci. 2008;28:2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Med. 2008;10:81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav. Brain Res. 2011;217:354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampal-dependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus. 2009;19:973–980. doi: 10.1002/hipo.20631. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Llorens-Martin M, Velazquez-Sanchez C, Ferragud A, Arcusa A, Gumus HG, Gomez-Pinedo U, Perez-Villalba A, Rosello J, Trejo JL, Barcia JA, Canales JJ. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159:59–68. doi: 10.1016/j.neuroscience.2008.11.054. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Revis AA, Burdick MA, Rhodes JS. A similar pattern of neuronal Fos activation in 10 brain regions following exposure to reward- or aversion-associated contextual cues in mice. Physiol. Behav. 2010;99:412–418. doi: 10.1016/j.physbeh.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koteja P, Garland T, Jr, Sax JK, Swallow JG, Carter PA. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Anim. Behav. 1999;58:1307–1318. doi: 10.1006/anbe.1999.1270. [DOI] [PubMed] [Google Scholar]
- Meeusen R, De Meirleir K. Exercise and brain neurotransmission. Sports Med. 1995;20:160–188. doi: 10.2165/00007256-199520030-00004. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J. Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Garland T, Jr, Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav. Neurosci. 2003;117:1243–1256. doi: 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Koteja P, Swallow JG, Carter PA, Garland T. Body temperatures of house mice artificially selected for high voluntary wheel-running behavior: repeatability and effect of genetic selection. J. Therm. Biol. 2000;25:391–400. doi: 10.1016/s0306-4565(99)00112-6. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Matus-Amat P. The ventral hippocampus supports a memory representation of context and contextual fear conditioning: implications for a unitary function of the hippocampus. Behav. Neurosci. 2005;119:154–163. doi: 10.1037/0735-7044.119.1.154. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinyor D, Brown T, Rostant L, Seraganian P. The role of a physical fitness program in the treatment of alcoholism. J. Stud. Alcohol. 1982;43:380–386. doi: 10.15288/jsa.1982.43.380. [DOI] [PubMed] [Google Scholar]
- Smith MA, Gergans SR, Iordanou JC, Lyle MA. Chronic exercise increases sensitivity to the conditioned rewarding effects of cocaine. Pharmacol. Rep. 2008;60:561–565. [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M. Reversal of cocaine addiction by environmental enrichment. Proc. Natl. Acad. Sci. U .S .A. 2008;105:17145–17150. doi: 10.1073/pnas.0806889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, El Rawas R, Lardeux V, Jaber M. Environmental enrichment during early stages of life reduces the behavioral, neurochemical, and molecular effects of cocaine. Neuropsychopharmacology. 2009;34:1102–1111. doi: 10.1038/npp.2008.51. [DOI] [PubMed] [Google Scholar]
- Sudai E, Croitoru O, Shaldubina A, Abraham L, Gispan I, Flaumenhaft Y, Roth-Deri I, Kinor N, Aharoni S, Ben-Tzion M, Yadid G. High cocaine dosage decreases neurogenesis in the hippocampus and impairs working memory. Addict. Biol. 2011;16:251–260. doi: 10.1111/j.1369-1600.2010.00241.x. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Tucci A, Stamos J, Robison L, Wang GJ, Anderson BJ, Volkow NDPK. Chronic forced exercise during adolescence decreases cocaine conditioned place preference in Lewis rats. Behav. Brain Res. 2010;215:77–82. doi: 10.1016/j.bbr.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999a;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. U. S. A. 1999b;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur. J. Neurosci. 2008;27:1494–1502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Chen GT, Johnson ZV, Rosenberg DM, Craig AB, Rhodes JS. Neuroanatomical specificity of conditioned responses to cocaine versus food in mice. Physiol. Behav. 2008;93:637–650. doi: 10.1016/j.physbeh.2007.11.004. [DOI] [PubMed] [Google Scholar]