Abstract
Cholangiocytes, the epithelial cells lining intrahepatic bile ducts, express multiple toll-like receptors (TLRs) and thus have the capacity to recognize and respond to microbial pathogens. In previous work we demonstrated that TLR4, which is activated by gram-negative lipopolysaccharide (LPS), is upregulated in cholangiocytes in response to infection with Cryptosporidium parvum in vitro and contributes to NFkB activation. Here, using an in vivo model of biliary cryptosporidiosis we addressed the functional role of TLR4 in C. parvum infection dynamics and hepatobiliary pathophysiology. We observed that C57BL mice clear the infection by three weeks post-infection. In contrast, parasites were detected in bile and stool in TLR4 deficient mice at four weeks post-infection. The liver enzymes, alanine transaminase (ALT) and aspartate transaminase (AST), and the proinflammatory cytokines Tumor Necrosis Factor (TNF)-α, Interferon (IFN)-γ, and Interleukin (IL)-6 peaked at one to two weeks postinfection and normalized by four weeks in infected C57BL mice. C57BL mice also demonstrated increased cholangiocyte proliferation (PCNA staining) at one-week post-infection, which was resolved by two weeks post-infection. In contrast, TLR4 deficient mice showed persistently elevated serum ALT and AST, elevated hepatic IL-6 levels, and histological evidence of hepatocyte necrosis, increased inflammatory cell infiltration, and cholangiocyte proliferation through four weeks post-infection. These data suggest that a TLR4-mediated response is required for efficient eradication of biliary C. parvum infection in vivo, and lack of this pattern recognition receptor contributes to an altered inflammatory response and an increase in hepatobiliary pathology.
Cholangiocytes, the intrahepatic biliary epithelial cells, have been increasingly recognized as significant contributors to the hepatobiliary innate immune response. While bile is considered a sterile environment, cholangiocytes are subjected to insult by potentially pathogenic microorganisms, or bioactive molecules derived from pathogenic microorganisms (e.g. LPS) that directly ascend into the biliary tract through the duodenum or, alternatively, are brought from sites of primary infection via the portal vein (Sung et al., 1992). Therefore, cholangiocyte recognition and response to microorganisms is a crucial first line of defense against invading pathogens in the intrahepatic bile ducts. Upon pathogen recognition, cholangiocytes secrete and respond to cytokines and chemokines (reviewed in (Lazaridis et al., 2004; Selmi et al., 2007)), and release antimicrobial peptides such as beta-defensins (Harada et al., 2004; Chen et al., 2005). Additionally, cholangiocytes interact with professional immune cells and may serve as antigen presenting cells (Morita et al., 1994; Scholz et al., 1997; Cruickshank et al., 1998). Therefore, cholangiocytes are at the interface between exogenous, potentially pathogenic insults, and the host immune system. The precise mechanisms and pathophysiological consequences of cholangiocyte responses to microbial pathogens are not fully understood.
The protozoan parasite, Cryptosporidium parvum, is a causative agent of zoonotic human cryptosporidiosis. A member of the phylum Apicomplexa, C. parvum is spread via a fecal-oral route by ingestion of oocysts. Once ingested, C. parvum oocysts excyst in the gastrointestinal tract to release four sporozoites that invade the apical domain of epithelial cells and form a parasitophorous vacuole in which the organism remains intracellular but extracytoplasmic. Although a predominantly intestinal pathogen, these parasites also infect cholangiocytes of immunocompromised individuals, causing a potentially life threatening disease in patients with AIDS or individuals with hyper-IgM syndrome (Chen et al., 2002). Various animal models of cryptosporidiosis have provided insight into the host response to intestinal infection, yet our knowledge of biliary cryptosporidiosis is restricted primarily to cell culture models.
Wild type mice are usually resistant to disease when C. parvum oocysts are given via oral inoculation, yet a persistent C. parvum infection develops in MyD88-(Rogers et al., 2006), and IFN-γ-(Theodos et al., 1997; Lacroix et al., 2001) deficient mice. Additionally, both human and murine models of cryptosporidiosis indicate that CD4+ T-cells play a critical role in the immune response to infection. While acquired resistance to cryptosporidial infection requires T-cells with the α/β type T-cell receptor, it is proposed that TLR-mediated innate immune responses by epithelial cells are critical to the initiation and regulation of host anti-C. parvum defense. Early studies demonstrated that C. parvum infection of intestinal epithelial cells correlates with the activation of NF-κB target genes (Laurent et al., 1997; Laurent et al., 1998; Seydel et al., 1998). Indeed, the invasion of intestinal and biliary epithelial cells by C. parvum in vitro activates TLR4/MyD88/NF-kB signaling resulting in the production and secretion of various cytokines and chemokines (e.g., IL-8 and IL-6)(Laurent et al., 1997; Chen et al., 2001), PGE2 (which stimulates mucin production) (Laurent et al., 1998), and anti-microbial peptides (e.g., β-defensins) which can kill C. parvum or inhibit parasite growth (Chen et al., 2005). These responses not only provide the first and rapid defense against the pathogen but also mobilize immune effector cells to the infection site to activate the adaptive immune response.
We previously demonstrated that C. parvum infection induces TLR-dependent NF-kB activation in cultured human cholangiocytes. Furthermore, we demonstrated that C. parvum infection increases TLR4 protein expression in cholangiocytes (Chen et al., 2007). In the current study we address the role of TLR4 in murine biliary cryptosporidiosis in vivo by utilizing an approach of direct gallbladder injection of C. parvum oocysts. We demonstrate that TLR4 deficient mice display more persistent biliary cryptosporidiosis and fecal oocyst shedding, while the wild type C57BL mice cleared the infection by 3-weeks post-infection. In addition to the prolonged presence of parasite in the stool and bile, mice deficient in TLR4 had a deficient INFγ, and prolonged TNFα response, cytokines that others have shown are elevated in murine models of intestinal infection, and essential for eradication and may have protective roles against this parasite, respectively. Interestingly, C57BL mice exhibited a transient increase in IL-6, a known proinflammatory cytokine implicated in a variety of liver pathologies, while TLR4-deficient mice exhibited persistent IL-6 elevation. Moreover, TLR4 deficient mice exhibited persistent elevation of serum aspartate transaminase (AST), and alanine transaminase (ALT), persistent portal tract inflammation and increased cholangiocyte proliferation. In all, we demonstrate a requirement of TLR4 in the efficient eradication of C. parvum from the biliary tract, while lack of this receptor promotes an overwhelming and destructive biliary proinflammatory response.
MATERIALS AND METHODS
Mice
Control C57BL/6 and TLR4 deficient (C57BL/10ScNJ; Tlr4lps-del/Tlr4lps-del geneotype) were obtained from Jackson Laboratories and housed in the animal care facilities located on the Mayo Clinic-Rochester campus, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. The protocol for infection and sacrifice of mice was approved by the Mayo Clinic Institutional Animal Care and Use Committee according to National Institute of Health guidelines. The in vivo induction of biliary cryptosporidiosis used in these mice was previously published (Verdon et al., 1998). Briefly, C. parvum oocysts were treated with a 10% bleach solution, washed twice with PBS, and injected directly into the gallbladder of 16 wild type C57BL and 16 TLR4 deficient mice through a small abdominal incision. As a control, 4 wild type C57BL and 4 TLR4 deficient mice were injected with sterile phosphate buffered saline (PBS). Mice were sacrificed at 1, 2, 3, and 4 week post-infection time points, and livers were initially perfused with a 0.9% sodium chloride solution, and small sections were removed and placed in PBS containing Complete® mini protease inhibitors (Roche) for protein analysis. The livers were then perfused with a 4% paraformaldehyde solution to preserve liver samples for histological sectioning, staining, and paraffin embedding which were performed by the Mayo Clinic histology laboratory. In addition, gallbladders were removed to isolate bile, which was cytospun onto slides and analyzed by immunofluorescence for the presence of parasite. Serum samples were also taken and sent to the Department of Laboratory Medicine and Pathology at Mayo Clinic-Rochester for analysis of serum chemistries alkaline phosphatase, aminotransferase, and alanine transaminase. Stool samples were taken at each time point and analyzed by acid fast Ziehl Neelsen staining for the presence of oocysts.
Acid Fast Ziehl Neelsen staining of stool
Stool samples were collected from wild type C57BL and TLR4 deficient mice for each time point, weighed, diluted 1/1000 in PBS, and homogenized. Homogenized stool samples were smeared onto slides and allowed to dry. Slides were then methanol fixed and stained in carbol fuscin for 5 min. The slides were washed in PBS, decolorized in 1% acid alcohol for 30 s, washed again, and counterstained in methyl blue for 30 s. Slides were then analyzed by light microscopy, and the number of oocysts was counted per 40× field.
Immunofluorescent detection of C. parvum in bile
Bile was obtained from gallbladders removed from wild type C57BL and TLR4 deficient mice as follows. Gallbladders were placed in microcentrifuge tubes containing 100 ul of PBS plus Complete® mini protease inhibitor cocktail. The samples were sonicated for 30 s and allowed to rest on ice for 1 min. This was repeated 3 times. Sonicated bile samples were added to cryospin slides fitted with cardboard filters and centrifuged in a Shandon cytospin at 100×g for 5 min. The slides were allowed to dry, and subsequently fixed in methanol and incubated with a rabbit monoclonal antibody to the C. parvum Cp2 protein (O'Hara et al., 2004) for 1 hr. The slides were washed and incubated with a goat anti-rabbit alexa fluor 488 antibody (Invitrogen) for 1 hr. Immunofluorescence imaging was performed using a Zeiss 510 confocal microscope (Zeiss), and the presence of C. parvum was quantitated by counting the number of parasite/40× field.
Protein isolation and ELISA
To extract protein, liver samples were placed in sterile PBS containing Complete® mini protease inhibitors and subjected to sonication for 30 sec. followed by resting on ice for 1 min. This was repeated four times, and then samples were centrifuged, and cleared supernatant was transferred to a new tube. The expression of mouse TNFα, INFγ, and IL6 protein levels were analyzed by ELISA. Protein samples were diluted 1:1000 in PBS and added to 96-well plates containing capture antibodies specific to mouse TNFα (Alpha Diagnostics), INFγ (Abcam), and IL-6 (R&D Systems), or human IL-6 (R&D Systems). The plates were incubated for 1 hr at room temperature, washed, and then incubated with the appropriate secondary antibody for 1 hr at room temperature. Plates were washed again, and a substrate solution was added to each well and incubated for 30 minutes. After incubation with substrate solution, a stop solution was added to each well, and the plates were read at 450 nm (reference filter: 630 nm).
H69 cell line and C. parvum in vitro infection
H69 cells are SV40-transformed normal human cholangiocytes originally derived from normal liver harvested for transplant. These cholangiocytes continue to express biliary epithelial cell markers, including cytokeratin 19, γ-glutamyl transpeptidase and ion transporters consistent with biliary function and have been extensively characterized (1, 2). C. parvum oocysts of the Iowa strain were purchased from a commercial source (Bunch Grass Farm, Deary, ID). Oocysts were excysted to release infective sporozoites as previously described (O'Hara et al., 2009), and H69 cells were infected with a 1:1 ratio of sporozoite/cell. H69 cells were transfected with a TLR4 dominant negative (DN) plasmid using Fugene HD transfection reagent (Roche) following manufacturer protocol. Stably transfected cells were selected with 2 µg/µl of G418 sulfate (CellGro) to culture media, and selected cells were maintained in culture media containing 1 µg/µl G418 sulfate. TLR4 DN plasmids are kinase-inactive dominant negative mutants of TLR4 and were kindly provided by Dr. M.F. Smith (University of Virginia, Charlottesville, VA). Normal H69 and H69 cells stably transfected with a TLR4-DN cells were grown in 6-well plates, and cultured in the presence or absence of C. parvum for 4, 12, or 24 hr. After each time point, cells were washed twice with PBS and treated with M-PER (Pierce) containing Complete® mini protease inhibitors.
Proliferating Cell Nuclear Antigen (PCNA) Staining
PCNA staining was performed on slides containing paraffin embedded liver sections from wild type C57BL and TLR4 deficient mice. Paraffin slides were deparaffinized for 2×15 min in 100% xylene, and hydrated by washing in increasing ethanol dilutions. The slides were subjected to boiling in antigen unmasking solution for 30 min and allowed to cool to room temp for 20 mins. Slides were incubated in a blocking solution and then incubated with a rabbit polyclonal antibody against PCNA (Abcam) for 1 hr. at room temp. Slides were then washed, incubated with a goat anti-rabbit alexa fluor 488 secondary antibody (Invitrogen) for 1 hr. at room temperature, and then analyzed by confocal microscopy for the presence of proliferation.
Statistical Analysis
All values are given as mean +/− SE. Means of groups were compared with Student’s t test (unpaired) or ANOVA test when appropriate. P values <0.05 were considered statistically significant.
RESULTS
TLR4 deficient were inefficient at clearing C. parvum infection
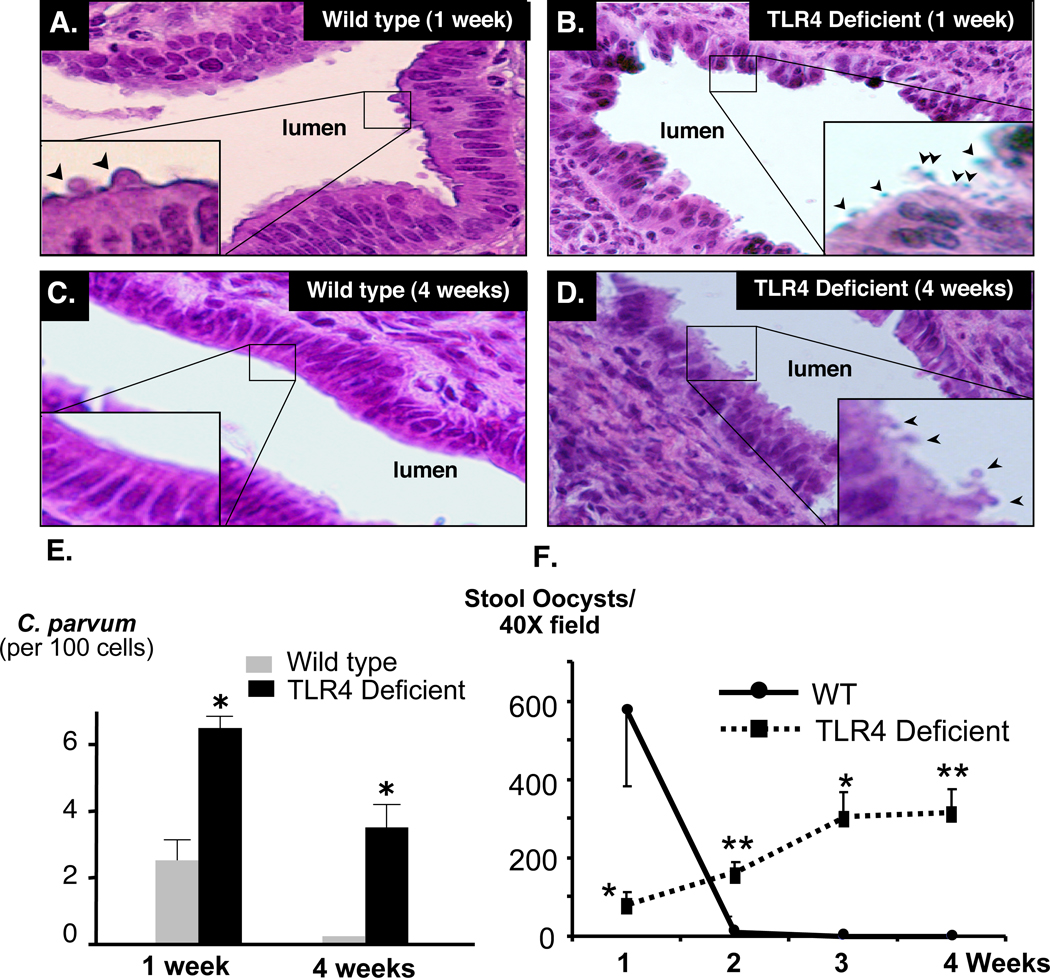
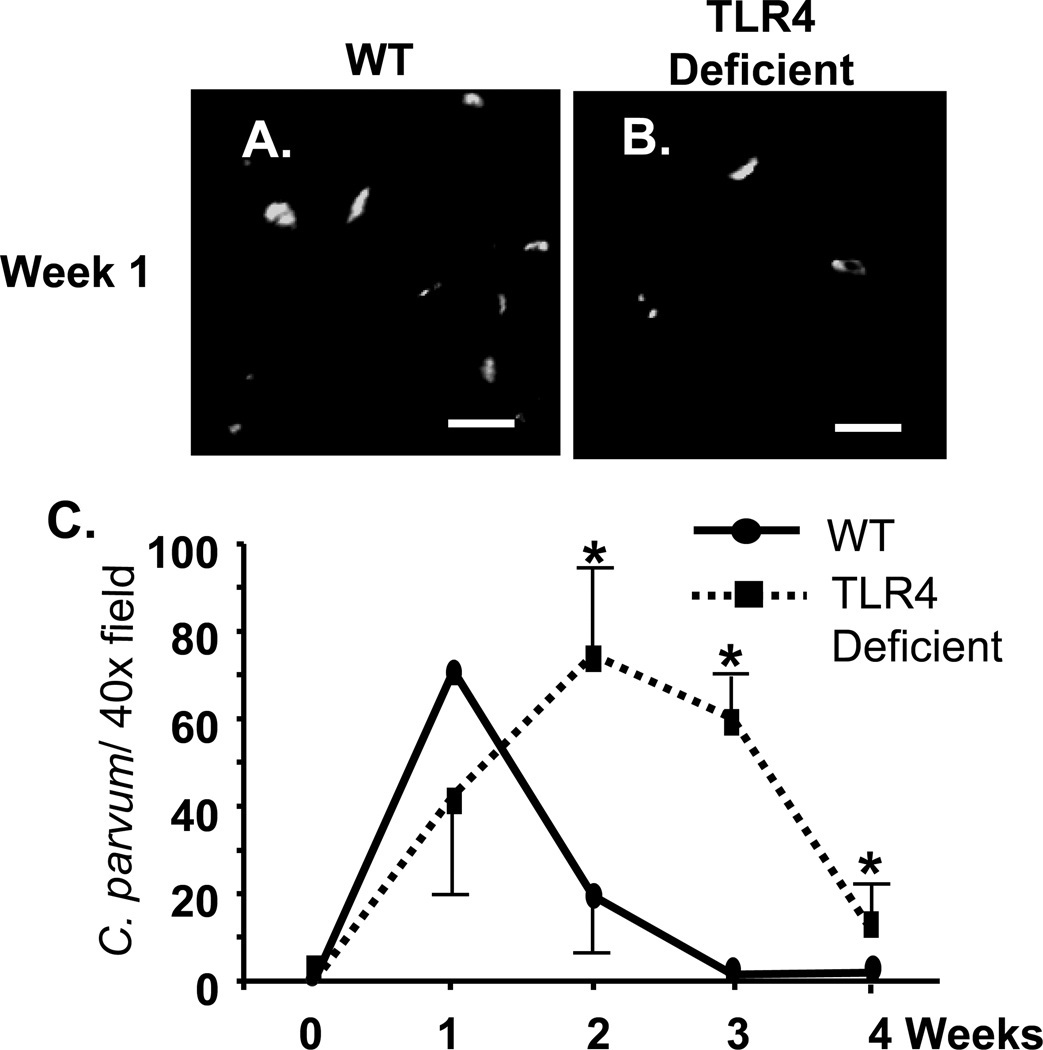
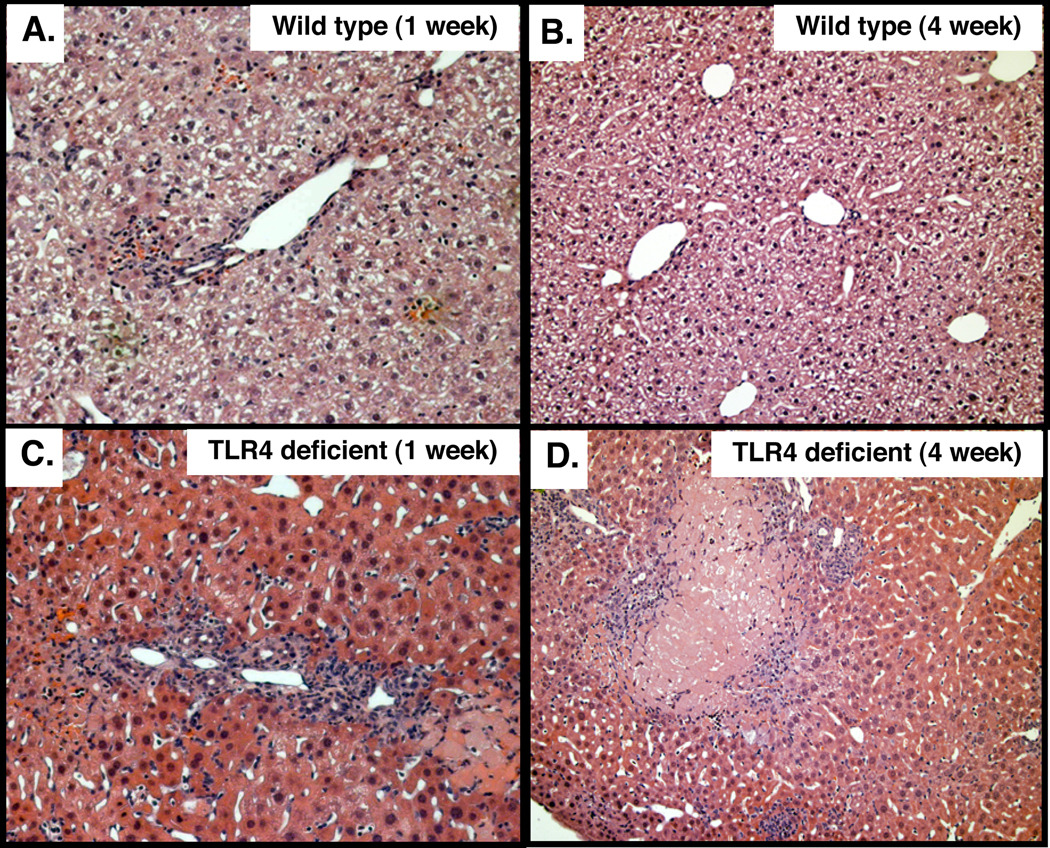
Wild type and TLR4 deficient C57BL mice were infected by direct injection of C. parvum oocysts into the gallbladder. Hematoxylin and Eosin (H&E) staining of liver samples was performed to confirm infection of the biliary epithelium (Fig. 1A–D). Wild type mice showed presence of C. parvum parasites in the intrahepatic bile ducts at week 1 post-infection (Fig. 1A), but no parasites were detected by week 4 post-infection (Fig. 1C). In TLR4 deficient mice, parasites were detected in the intrahepatic bile ducts at week 1 post-infection (Fig. 1B); however, parasites were also observed on the apical surface of cholangiocytes through 4-weeks post-infection (Fig. 1D). Quantitative analysis of parasites identified in H&E stained sections (number of identifiable infection sites/ 100 cells) in wild type and TLR4 deficient mice at weeks 1 and 4 post-infection showed significantly greater parasite burden in TLR4 deficient mice compared to wild type (Fig. 1E). The course of infection was also monitored by quantifying oocyst shedding in the stool of wild type and TLR4 deficient mice (Fig. 1F). Wild type mice exhibited a greater number of oocysts shed at 1 week post-infection with a significant drop in oocyst shedding at week 2; by the third week post-infection oocysts were undetected in stool samples. Conversely, stool samples from TLR4 deficient mice showed an increase in oocyst shedding from week 1 to 4 post-infection with a significantly greater number of oocysts shed at weeks 2, 3, and 4 compared to wild type mice (Fig.1F). To determine if the increase in oocyst shedding in TLR4 KO mice correlated with parasite burden in the biliary tract, bile of infected mice was assessed for the presence of parasite (Fig. 2). C. parvum was detected in the bile of both wild type and TLR4 deficient mice at week 1 post-infection as demonstrated by immunofluorescence microscopy, (Fig 2A, B). Consistent with parasite clearance by 3 weeks post-infection, as demonstrated by oocyst shedding in stool, C57BL mice cleared the parasite from bile by week 3 post-infection. In contrast, C. parvum was detected in the bile of TLR4 deficient mice from week 1 through week 4. Parasite numbers were significantly higher than wild type mice beginning at week 2 post-infection and the number of parasites declined but was still detected at week 4 post infection (Fig 2C).
Figure 1.
TLR4 knock-out mice are less efficient at eradicating C. parvum biliary infection. 106 C. parvum oocysts were injected into the gallbladder of wild type C57BL or TLR4 knock-out mice. A. C. parvum infection in the intrahepatic ducts of wild type mice was detected by H&E staining one week post-injection. B. More parasites were found in the intrahepatic ducts of TLR4 knock-out mice one week post-infection compared with wild type. C. C. parvum parasites were not found in the intrahepatic bile ducts in the wild type mice four weeks post-infection, while parasites were detected in TLR4 knock-out mice (D). Insets in A – D are higher power of the boxed areas showing the parasite (arrowheads). E. Quantitative analysis showing parasite number in the intrahepatic bile ducts in the wild type and TLR4 knock-out mice one and four weeks post-infection. F. WT and TLR4 KO stool samples were stained with carbol fuscin and analyzed by light microscopy. Wild type mice showed a greater number of oocysts shed in the stool at week 1 post-inoculation with a significant drop at week 2. By weeks 3 and 4 post-injection, oocysts were undetected indicating clearance of the C. parvum oocyst by the wild type mice. Conversely, oocyst shedding was detected in knock out mice from week 1 to week 4 post-inoculation indicating persistence of C. parvum infection. *, p < 0.05; **, p < 0.01 compared to wild type. *, P < 0.05 compared with wild type mice.
Figure 2.
Immunofluorescent detection of C. parvum in the bile of infected mice. Bile was collected from infected mice. 50 µl of bile was cytospun onto glass slides, and immunofluorescence was performed to detect the parasite (A, B). A. Multiple CP2 antibody positive parasites were detected in the bile of both wild type C57BL mice (A) and TLR4-deficient mice (B) one week post-infection, Bars = 10 µm. C. Quantification of parasites observed over the course of 4 weeks. The slides containing the immunofluorescently labeled parasites were used to quantify the number of parasites present in bile. Fifteen 40X fields were observed from each slide, and the number of parasites per 40X field (presented as mean ± SEM) was calculated. By 2 weeks post-infection, the number of parasites observed in the bile of B57BL mice drops precipitously and by 3 weeks post-infection, no parasites were observed. In contrast, while the numbers of parasites are decreased, parasites were detected in the bile of TLR4 deficient mice through 4 weeks post-infection. *, p < 0.05 compared to wild type.
TLR4 deficient mice exhibit an altered proinflammatory cytokine response following C. parvum biliary infection
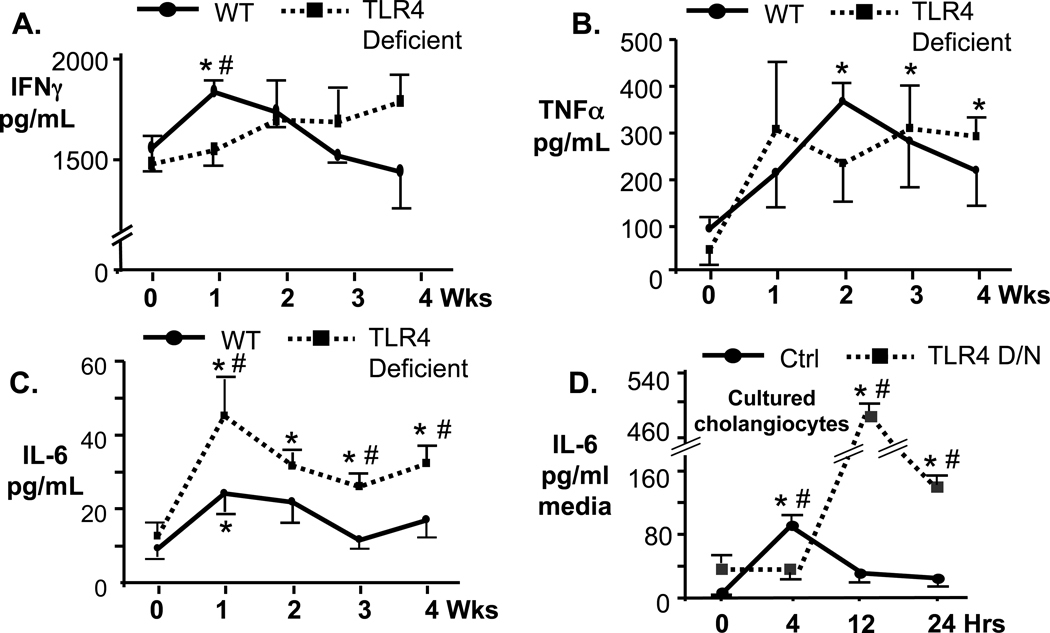
Given that TLR4 aggregates at sites of C. parvum internalization of cholangiocytes and contributes to NFkB activation in vitro, we addressed whether the lack of TLR4 alters the hepatic proinflammatory response. Protein levels of proinflammatory cytokines INFγ, TNFα, and IL-6 were analyzed in WT and TLR4 deficient mice from 0 to 4 weeks post-infection in homogenized infected livers. Protein levels of INFγ were slightly elevated in infected WT mice and peaked at week 1 post-infection (p<0.05 vs uninfected) but returned to uninfected control levels by week 2 (Fig. 3A). Conversely, INFγ protein levels in TLR4 deficient mice did not increase significantly over uninfected control at any time point (Fig. 3A). TNFα protein levels in the livers of WT mice peaked 2 weeks post-infection (p<0.05) and by week 3 post-infection were not significantly different from uninfected control. However, TNFα expression in the liver of TLR4 deficient mice was significantly elevated compared to uninfected controls by 3 weeks post-infection and remained elevated to week 4 post-infection (Fig. 3B). We next addressed the expression of IL-6, a proinflammatory cytokine associated with hepatobiliary diseases (Demetris et al., 2006). Hepatic IL-6 levels in C57 Black mice peaked at week 1 post-infection (p<0.05 compared to uninfected controls) and decreased thereafter to levels similar to those seen in uninfected mice (Fig. 3C). Interestingly, in TLR4-deficient mice, IL-6 protein levels peaked 1 week post-infection and remained elevated through week 4 post-infection. Overall, levels of IL-6 were significantly greater in TLR4 KO mice at week 1, 3 and 4 post-infection compared to WT mice (Fig. 3C), showing a greater disparity between the TLR4-deficient and wild type mice for this proinflammatory cytokine compared to that observed for INFγ and TNFα. Given that cholangiocytes secrete and respond to IL-6, we asked whether TLR4 deficiency in cultured human cholangiocytes resulted in increased IL-6 expression following C. parvum infection. H69 cells, an SV40 transformed cholangiocyte cell line originally obtained from a normal human liver harvested for transplant (Grubman et al., 1994), and H69 cells stably transfected with a plasmid expressing a kinase dead TLR4-DN were infected with C. parvum for 4, 12, and 24 hr and IL-6 present in the culture media was measured by ELISA (Fig. 3D). Similar to what was observed in vivo, IL-6 expression in cells expressing the TLR4-DN was significantly greater than control H69 cells (Fig. 3D). While IL-6 levels in control H69 cells peaked 4 hr post-infection and diminished by 12 hr post-infection, IL-6 levels in TLR4-DN cells peaked at 12 hr post-infection and remained elevated throughout the 24 hr period (Fig. 3D).
Figure 3.
Analysis of proinflammatory cytokine expression (ELISA) in homogenized livers of C. parvum infected wild type (solid lines) and TLR4 deficient (dashed lines) C57BL mice. A. IFNγ expression in wild type mice peaked at one week post-infection and was significantly greater than control uninfected mice (*, p<0.05) and TLR4 deficient mice (#, p<0.05). The expression of IFNγ in TLR4 deficient mice was not significantly increased compared to matched, uninfected controls (0 wks) at any time point. B. TNFα expression in wild type C57BL mice peaked at 2 weeks post-infection and was significantly different than matched, uninfected controls (*, p<0.05). The expression of TNFα in TLR4 deficient mice was significantly greater than matched uninfected controls at 3 and 4 weeks post-infection (*, p<0.05). Expression of TNFα was not significantly different between the wild type and TLR4 deficient C57BL mice at any time point. C. IL6 expression in wild type mice peaked at one week post-infection and was significantly increased compared to matched uninfected controls at this time point (*, p<0.05). In contrast, IL-6 expression in TLR4 deficient mice was significantly increased compared to matched uninfected controls (0 wks) at each time point (*, p<0.05). Furthermore, IL-6 expression was significantly increased compared to wild type C57BL mice at weeks 1, 3, and 4 (#, p<0.05). D. IL-6 expression was measured by ELISA in cultured human cholangiocytes (H69, solid line) and H69 cells stably expressing the TLR4-DN (dashed line). IL-6 expression peaked at 4 hrs post-infection in the H69 cells and was significantly elevated compared to uninfected controls (*, p<0.05) and cells expressing the TLR4-DN (#, p<0.05) and diminished by 12 hrs post-infection. IL-6 expression in cells expressing the TLR4-DN peaked at 12 hr post-infection and was significantly elevated compared to matched uninfected controls (*, p<0.01) and H69 cells (#, p<0.01) at 12 and 24 hr post-infection. Values are presented as mean +/− SE.
TLR4 deficient mice exhibit elevated liver enzymes following C. parvum infection
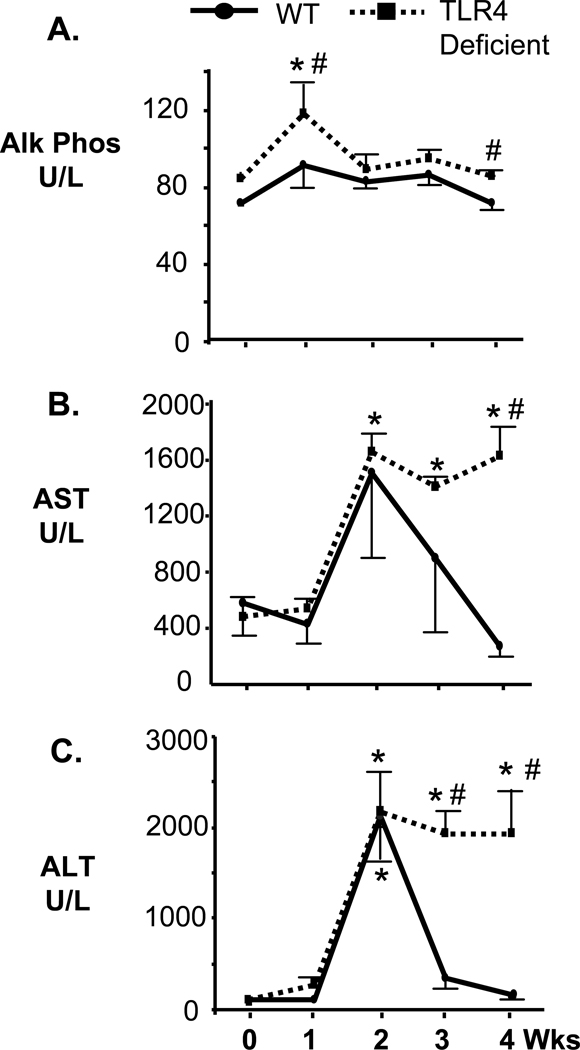
We next addressed whether TLR4 deficient mice exhibited altered liver enzyme biochemistries compared to the wild-type C57BL mice. The levels of alkaline phosphatase, aspartate transaminase (AST), and alanine transaminase (ALT) levels were analyzed from serum samples taken from C57BL and TLR4 deficient mice at 1, 2, 3, and 4 weeks post-infection and from uninfected C57BL and TLR4 deficient control mice (Fig. 4). High levels of alkaline phosphatase are frequently associated with inflamed bile ducts or cholestasis while elevated AST and ALT could indicate liver injury/damage. Alkaline phosphatase serum levels, while remaining at a similar level in C57BL mice, was slightly but significantly elevated in TLR-deficient mice at 1 week post-infection and decreased thereafter (Fig. 4A). AST serum levels peaked at 2 weeks post-infection for both WT and TLR4 deficient mice. However, the levels of this enzyme decreased to normal levels in C57BL mice by 3 weeks post-infection. In contrast, the TLR4 deficient mice exhibited elevated AST through 4 weeks post-infection. AST levels were significantly higher in TLR4 deficient mice at week 4 post-infection compared to wild type mice (Fig. 4B). ALT levels also peaked at week 2 post-infection for both wild type and TLR4 deficient mice. However, ALT serum levels in wild type C57BL mice decreased to levels no different than control uninfected mice by 3 weeks post-infection. Conversely, the TLR4 deficient mice exhibited elevated levels of ALT through 4 weeks post-infection. ALT serum levels were significantly higher in TLR4 deficient mice at weeks 3 and 4 compared to wild-type C57BL mice (Fig. 4C). Bilirubin levels remained unchanged throughout the duration of the experimental protocol for both categories of mice (data not shown).
Figure 4.
Liver enzyme serum biochemistries. Serum was obtained from C. parvum infected wild type and TLR4 deficient C57BL mice. A. Serum alkaline phosphatase (Alk Phos) levels from wild type C57BL mice were not significantly elevated at any time point compared to uninfected controls (0 wks). In contrast, the serum alkaline phosphatase level of C. parvum infected TLR4 deficient mice was significantly elevated compared to matched uninfected controls at one week post-infection. B. Serum Aspartate transaminase levels peaked at 2 weeks post-infection for both wild type and TLR4 deficient C57BL mice, reaching significance only for the TLR4 deficient mice (*, p<0.05). AST levels diminish in wild type C57BL mice by 3 weeks post-infection, but remain significantly elevated in the TLR4 deficient mice compared to matched, uninfected controls at 3 and 4 weeks post-infection (*, p<0.05). In addition, the levels of AST were significantly elevated compared to infected wild type mice at 4 weeks post-infection (#, p<0.05). C. Serum Alanine transaminase levels again peaked at 2 weeks post-infection for both wild type and TLR4 deficient C57BL mice and were significantly elevated compared to matched, uninfected controls (p<0.05). ALT levels diminished to levels comparable to uninfected, matched controls by week three post-infection. In contrast, C. parvum infected TLR4 deficient mice exhibited elevated levels of ALT at weeks 3 and 4 post-infection compared to matched uninfected controls (*p<0.05) and wild type infected mice (#, p<0.05). Values are presented as mean +/− SE.
TLR4 deficient mice exhibit increased portal tract inflammation, hepatocellular necrosis, and cholangiocyte proliferation following C. parvum infection
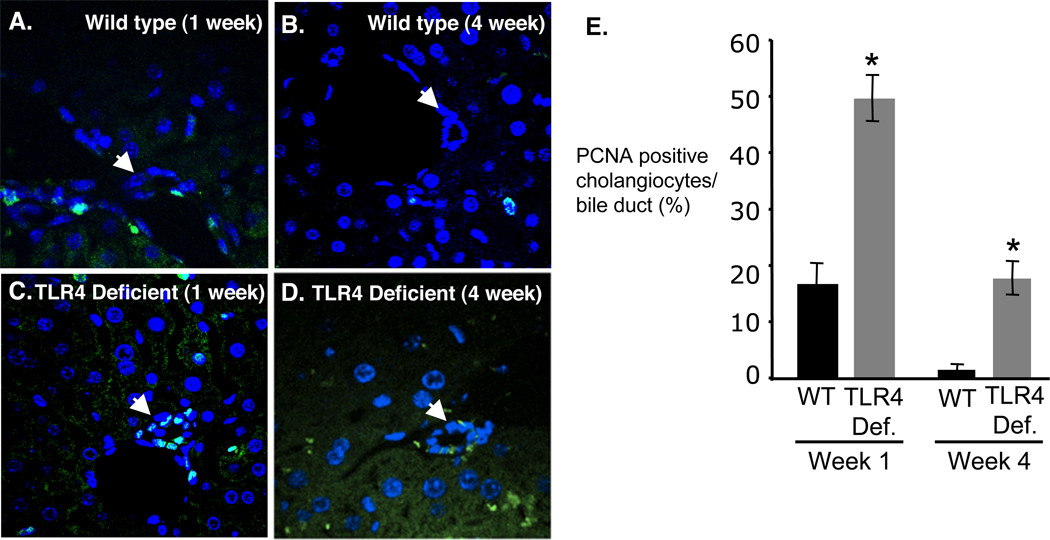
To see if the elevated IL6, AST, and ALT correlated with increased liver pathology, liver histology of H&E stained tissues was evaluated (Fig 5). Infected C57BL mouse livers exhibited minimal to moderate inflammation in the portal region, primarily polymorphonuclear leukocytes, at week 1 post-infection (Fig. 5A). Mild to moderate portal tract inflammation was also observed at 2–3 weeks post-infection, as was slight ductular proliferation (not shown). Inflammation levels were reduced in C57BL mice by 4 weeks post-infection and histology appeared normal (Fig. 5B), which is consistent with the observed cytokine and liver enzyme levels observed in these mice. In contrast, H&E stained liver sections from TLR4-deficient mice exhibited moderate to severe inflammation with multifocal necrosis and ductular proliferation from weeks 1 to 4 post-infection (Fig. 5C,D). These observations were in accordance with the high levels of AST, and ALT in TLR4 deficient mice at week 4 post-infection indicating hepatocellular liver injury. Given that IL-6 is known to induce cholangiocyte proliferation in response to lipopolysaccharide (Park et al., 1999), and was elevated throughout the course of the experiment in TLR4 deficient mice, we examined cholangiocyte proliferation using PCNA staining. Liver sections of C57BL mice, stained for PCNA, demonstrated a minimally increased level of cholangiocyte proliferation at 1 week post-infection (Fig. 6A) and minimal proliferation at week four post-infection (Fig. 6B). In contrast, TLR4-deficient mice exhibited persistent proliferation from 1 week through 4 weeks post-infection, consistent with the observed elevated expression of IL-6 (Fig. 6C,D). The level of cholangiocyte proliferation was quantified by counting the number of PCNA positive cells per bile duct from a minimum of 10 ducts and 3 mice for each group (30 ducts minimum). TLR4 deficient mice exhibited increased PCNA staining compared to wild-type controls at both 1 week and 4 weeks post-infection (Fig. 6E).
Figure 5.
Hematoxylin and Eosin staining of C. parvum infected wild type and TLR4 deficient C57BL mouse livers. Top panels show typical staining of wild type mouse livers from week one to four post-infection. A. At week 1 post infection the livers typically exhibit minimal to moderate inflammation, with the infiltration of polymorphonucleocytes. Inflammatory cell infiltration remains mild to moderate through weeks 2 and 3 with some cholangitis and grade 2 ductular proliferation. B. By week 4 post-infection inflammation is reduced to minimal with normal histology. C. In contrast, H&E staining of TLR4 deficient mouse livers at 1 week post-infection demonstrates moderate to severe inflammation with multifocal necrosis and grade 3 ductular proliferation. By week two post-infection mild to moderate inflammation was observed in the portal tracts of TLR4 deficient mice. D. By week four post-infection, severe portal tract inflammation with hepatocellular necrosis and grade 2–3 ductular proliferation was observed.
Figure 6.
Cholangiocyte proliferation was assessed by PCNA staining of C. parvum infected wild type (top panels) and TLR4 deficient (bottom panels) mice. A. Wild type C57BL mice exhibited minimal ductular proliferation at week 1. B. By week four post-infection the levels of proliferation decreased to undetectable levels in wild-type mice. C. parvum infected TLR4 deficient mouse livers exhibited increased levels of cholangiocyte proliferation at I week post-infection (C) through week four post-infection (D). E. The level of cholangiocyte proliferation was quantified by counting the number of PCNA positive cells per bile duct from a minimum of 10 ducts and 3 mice for each group (30 ducts minimum). TLR4 deficient mice exhibited increased PCNA staining compared to wild-type controls at both 1 week and 4 weeks post-infection. *, p<0.01 compared to wild type at same time point.
DISCUSSION
The results of our study provide the first direct evidence that TLR4 is required for efficient elimination of C. parvum from the biliary tree, and lack of this receptor results in increased hepatic injury following infection. Using an in vivo model of biliary cryptosporidiosis we demonstrate that: (i) TLR4 deficient C57BL mice are less efficient at clearing the parasite from the biliary tree; (ii) TLR4 deficient mice show an altered proinflammatory cytokine response, particularly with respect to the proinflammatory cytokine, IL-6; and, (iii) TLR4 deficient mice exhibit a more severe liver pathology following infection with C. parvum. Our findings demonstrate a role for TLR4 in the elimination of C. parvum as well as an unexpected protective role for TLR4 in regulating proinflammatory cytokine expression.
TLRs are an evolutionarily conserved family of cell surface pattern recognition proteins that play a key role in host immunity through detection of pathogens and the initiation of the innate immune response (Takeda et al., 2003; Akira and Takeda 2004; Modlin and Cheng 2004). Epithelial cells express TLRs and activation of TLRs triggers an array of epithelial defense responses. Using both cultured human cholangiocytes and normal human liver, we previously demonstrated that all ten known TLRs are expressed in cholangiocytes (Chen et al., 2005). Furthermore, we demonstrated that C. parvum infection induced an increase in TLR4 protein expression in cholangiocytes, and that this molecule accumulates at sites of parasite-host cell interaction (Chen et al., 2005; Chen et al., 2007). Having shown that TLR4 is involved in the infection dynamics of C. parvum in cholangiocytes in vitro, we were able to show, through our current study, that mice lacking TLR4 were less efficient at clearing the infection from the biliary tract as evident by increased shedding of oocysts in the stool and an increased persistence of parasite found in bile and on the apical membrane of the intrahepatic biliary epithelium. Previous research has shown that, in the absence of MyD88, an adaptor protein involved in most TLR signaling pathways, mice infected with C. parvum had a greater parasite burden in the intestinal tract compared to wild type mice (Rogers et al., 2006). Additionally, MyD88 deficiency coupled with IFNγ inactivation resulted in an increase in intestinal parasite burden and inflammation (Rogers et al., 2006). In the current study, we demonstrate a functional role for TLR4 in mounting an efficient inflammatory response against biliary cryptosporidiosis in mice resulting in parasite clearance and limiting tissue damage. Indeed, we propose that the inability to activate TLR4 is associated with the overexpression of the proinflammatory cytokine IL-6, which may promote liver pathology.
CD4+T cells and INFγ play a dominant role during the immunological response to murine C. parvum infection. Indeed, clearance of C. parvum infection in mice requires CD4+ T-cell production of IFNγ (Ungar et al., 1991; Wyatt et al., 1997; Theodos 1998; McDonald et al., 2000; Gomez Morales et al., 2004). The role of the proinflammatory cytokine TNFα during C. parvum infection is less clear. This cytokine is increased in the intestine following infection of mice (Lacroix et al., 2001; Ehigiator et al., 2005) and administration of TNFα to C57BL6 IFN-knockout mice, which typically die as a result of fulminant infection, reduced oocyst shedding (Lacroix et al., 2001). It is proposed that murine INFγ is essential in the initial response to the parasite, while TNFα may play a protective role and contribute to inhibition of parasite development (Lean et al., 2006). We demonstrated that hepatic protein expression of IFNγ and TNFα modestly increased over uninfected control levels in C57BL mice. In contrast, we demonstrated that TLR4 deficient mice did not mount a significant IFNγ response throughout the duration of the experiment. Furthermore, our results show elevated levels TNFα at weeks 3 and 4 post-infection in the TLR4-deficient mice compared to uninfected controls. Interestingly, TLR4 deficient mice exhibited increased expression of the proinflammatory cytokine IL-6 throughout the duration of the experiment. Moreover, we also demonstrated increased IL-6 expression in TLR4-DN expressing cultured cholangiocytes (H69 cells) compared to untransfected H69 cells in vitro indicating that this receptor may function to regulate IL-6 expression in these cells. TNFα and IL-6 are critically involved in many hepatobiliary diseases. It has been proposed that IL-6, which is expressed by multiple cell types including epithelial cells, can control the balance between T helper (Th) 1/Th2 differentiation. In this early model of Th1/Th2 differentiation, IL-6 works by both suppressing Th1 differentiation by inducing the expression of suppressor of cytokine signaling 1 (SOCS1), hence diminishing IFNγ expression (Diehl et al., 2000), and promoting Th2 differentiation through the upregulation IL4 expression (Rincon et al., 1997). More recently, IL-6 with concurrent TGFβ expression has been implicated in the promotion of Th17 differentiation of CD4+ T cells. Interestingly, Th17 proinflammatory responses have been implicated in several hepatobiliary diseases (Harada et al., 2009; Ye et al., 2010). While we did not directly address T cell differentiation, TLR4 deficient mice, which exhibited persistently elevated IL-6 production, did not express significant levels of IFNγ. This observation suggests that TLR4 may not only be involved in the eradication of C. parvum from the biliary tree, but lack of this receptor may promote proinflammatory T cell differentiation pathways in response to C. parvum (Schnare et al., 2001).
Whereas IL-6 secretion is involved in many aspects of an inflammatory and host defense response, excess production of this cytokine can be involved in sustaining inflammation and cell proliferation. More specifically, IL-6 has been implicated in many chronic inflammatory diseases of the liver and may be involved in the development of cholangiocarcinoma (Okada et al., 1994; Meng et al., 2006; Wehbe et al., 2006). From our study, infection of TLR4-deficient mice, which correlates with elevated IL-6 expression, is associated with severe liver pathology. Evidence of this is shown by significantly elevated levels of ALT and AST, known indicators of hepatocellular injury, through 4 weeks post-infection in the TLR4-deficient mice. Histology and PCNA staining of liver samples taken from wild type and TLR4 deficient mice provide further evidence of the hepatobiliary pathology. H&E staining of C. parvum infected liver tissue from TLR4 deficient mice showed moderate to severe portal tract inflammation with hepatocellular necrosis, and PCNA staining demonstrated proliferation of biliary epithelial cells in these same mice, an indicator of cholestatic disease. Taken together, we show that a deficiency of TLR4 results in altered cytokine production and chronic inflammation.
While our study demonstrates an essential role of TLR4 in the efficient eradication of biliary cryptosporidiosis in vivo, and thus the maintenance of a protective, but not destructive, immune response, it does not address the mechanisms of how TLR4 activation clears the infection. We demonstrate that TLR4 is required for an efficient IFNγ response, yet it is likely that other components of the host innate immune response are critical for the eradication of C. parvum and dependent on TLR4 signaling. For instance, cholangiocyte expression of human beta defensin-2 (HBD2), an antimicrobial peptide, is TLR-dependent, and C. parvum infection of cultured H69 cells causes increased expression of HBD2 (Chen et al., 2005). Therefore, it is probable if not likely that the mouse homologue of hBD2 (mBD3) contributes to the eradication of biliary C. parvum in our mouse model, and merits further investigation.
In summary, using a mouse model of C. parvum biliary infection, we have identified a functional role for TLR4 in the eradication of the parasite and the regulation of an effective cytokine response to infection. It would be of interest to extend these studies to identify the mechanism and consequences of TLR4—dependent regulation of the proinflammatory cytokine IL-6, and determine whether aberrant IL-6 expression alters CD4+ T-cell differentiation and ultimately promotes liver pathology. Furthermore, an investigation of the precise mechanism through which TLR4 promotes parasite eradication from the biliary tree is merited.
Acknowledgements
We thank Deb Hintz for secretarial assistance. This work was supported by the National Institutes of Health Grants DK76922 (S.P.O.), DK57993 (N.F.L.), and the Optical Microscopy Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK084567).
Footnotes
Footnote: Medical Microbiology & Immunology. Creighton School of Medicine. Omaha, NE 68178
REFERENCES
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Chen XM, Keithly JS, et al. Cryptosporidiosis. N Engl J Med. 2002;346(22):1723–1731. doi: 10.1056/NEJMra013170. [DOI] [PubMed] [Google Scholar]
- Chen XM, Levine SA, et al. Cryptosporidium parvum activates nuclear factor kappaB in biliary epithelia preventing epithelial cell apoptosis. Gastroenterology. 2001;120(7):1774–1783. doi: 10.1053/gast.2001.24850. [DOI] [PubMed] [Google Scholar]
- Chen XM, O'Hara SP, et al. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. J Immunol. 2005;175(11):7447–7456. doi: 10.4049/jimmunol.175.11.7447. [DOI] [PubMed] [Google Scholar]
- Chen XM, Splinter PL, et al. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J Biol Chem. 2007;282(39):28929–28938. doi: 10.1074/jbc.M702633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank SM, Southgate J, et al. Expression and cytokine regulation of immune recognition elements by normal human biliary epithelial and established liver cell lines in vitro. J Hepatol. 1998;29(4):550–558. doi: 10.1016/s0168-8278(98)80149-9. [DOI] [PubMed] [Google Scholar]
- Demetris AJ, Lunz JG, 3rd, et al. Biliary wound healing, ductular reactions, and IL-6/gp130 signaling in the development of liver disease. World J Gastroenterol. 2006;12(22):3512–3522. doi: 10.3748/wjg.v12.i22.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl S, Anguita J, et al. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13(6):805–815. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- Ehigiator HN, Romagnoli P, et al. Mucosal cytokine and antigen-specific responses to Cryptosporidium parvum in IL-12p40 KO mice. Parasite Immunol. 2005;27(1–2):17–28. doi: 10.1111/j.1365-3024.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- Gomez Morales MA, Mele R, et al. Cryptosporidium parvum-specific CD4 Th1 cells from sensitized donors responding to both fractionated and recombinant antigenic proteins. Infect Immun. 2004;72(3):1306–1310. doi: 10.1128/IAI.72.3.1306-1310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubman SA, Perrone RD, et al. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol. 1994;266(6 Pt 1):G1060–G1070. doi: 10.1152/ajpgi.1994.266.6.G1060. [DOI] [PubMed] [Google Scholar]
- Harada K, Ohba K, et al. Peptide antibiotic human beta-defensin-1 and -2 contribute to antimicrobial defense of the intrahepatic biliary tree. Hepatology. 2004;40(4):925–932. doi: 10.1002/hep.20379. [DOI] [PubMed] [Google Scholar]
- Harada K, Shimoda S, et al. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin Exp Immunol. 2009;157(2):261–270. doi: 10.1111/j.1365-2249.2009.03947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix S, Mancassola R, et al. Cryptosporidium parvum-specific mucosal immune response in C57BL/6 neonatal and gamma interferon-deficient mice: role of tumor necrosis factor alpha in protection. Infect Immun. 2001;69(3):1635–1642. doi: 10.1128/IAI.69.3.1635-1642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent F, Eckmann L, et al. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect Immun. 1997;65(12):5067–5073. doi: 10.1128/iai.65.12.5067-5073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent F, Kagnoff MF, et al. Human intestinal epithelial cells respond to Cryptosporidium parvum infection with increased prostaglandin H synthase 2 expression and prostaglandin E2 and F2alpha production. Infect Immun. 1998;66(4):1787–1790. doi: 10.1128/iai.66.4.1787-1790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaridis KN, Strazzabosco M, et al. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127(5):1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Lean IS, Lacroix-Lamande S, et al. Role of tumor necrosis factor alpha in development of immunity against Cryptosporidium parvum infection. Infect Immun. 2006;74(7):4379–4382. doi: 10.1128/IAI.00195-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald V, Smith R, et al. Host immune responses against Cryptosporidium. Contrib Microbiol. 2000;6:75–91. doi: 10.1159/000060371. [DOI] [PubMed] [Google Scholar]
- Meng F, Yamagiwa Y, et al. Over-expression of interleukin-6 enhances cell survival and transformed cell growth in human malignant cholangiocytes. J Hepatol. 2006;44(6):1055–1065. doi: 10.1016/j.jhep.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin RL, Cheng G. From plankton to pathogen recognition. Nat Med. 2004;10(11):1173–1174. doi: 10.1038/nm1104-1173. [DOI] [PubMed] [Google Scholar]
- Morita M, Watanabe Y, et al. Inflammatory cytokines up-regulate intercellular adhesion molecule-1 expression on primary cultured mouse hepatocytes and T-lymphocyte adhesion. Hepatology. 1994;19(2):426–431. [PubMed] [Google Scholar]
- O'Hara SP, Small AJ, et al. HIV-1 Tat protein suppresses cholangiocyte toll-like receptor 4 expression and defense against Cryptosporidium parvum. J Infect Dis. 2009;199(8):1195–1204. doi: 10.1086/597387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara SP, Yu JR, et al. A novel Cryptosporidium parvum antigen, CP2, preferentially associates with membranous structures. Parasitol Res. 2004;92(4):317–327. doi: 10.1007/s00436-003-1057-5. [DOI] [PubMed] [Google Scholar]
- Okada K, Shimizu Y, et al. Interleukin-6 functions as an autocrine growth factor in a cholangiocarcinoma cell line. J Gastroenterol Hepatol. 1994;9(5):462–467. doi: 10.1111/j.1440-1746.1994.tb01275.x. [DOI] [PubMed] [Google Scholar]
- Park J, Gores GJ, et al. Lipopolysaccharide induces cholangiocyte proliferation via an interleukin-6-mediated activation of p44/p42 mitogen-activated protein kinase. Hepatology. 1999;29(4):1037–1043. doi: 10.1002/hep.510290423. [DOI] [PubMed] [Google Scholar]
- Rincon M, Anguita J, et al. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185(3):461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers KA, Rogers AB, et al. MyD88-dependent pathways mediate resistance to Cryptosporidium parvum infection in mice. Infect Immun. 2006;74(1):549–556. doi: 10.1128/IAI.74.1.549-556.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnare M, Barton GM, et al. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2(10):947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- Scholz M, Cinatl J, et al. Expression of human leukocyte antigens class I and class II on cultured biliary epithelial cells after cytomegalovirus infection. Tissue Antigens. 1997;49(6):640–643. doi: 10.1111/j.1399-0039.1997.tb02813.x. [DOI] [PubMed] [Google Scholar]
- Selmi C, Mackay IR, et al. The immunological milieu of the liver. Semin Liver Dis. 2007;27(2):129–139. doi: 10.1055/s-2007-979466. [DOI] [PubMed] [Google Scholar]
- Seydel KB, Zhang T, et al. Cryptosporidium parvum infection of human intestinal xenografts in SCID mice induces production of human tumor necrosis factor alpha and interleukin-8. Infect Immun. 1998;66(5):2379–2382. doi: 10.1128/iai.66.5.2379-2382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JY, Costerton JW, et al. Defense system in the biliary tract against bacterial infection. Dig Dis Sci. 1992;37(5):689–696. doi: 10.1007/BF01296423. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, et al. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Theodos CM. Innate and cell-mediated immune responses to Cryptosporidium parvum. Adv Parasitol. 1998;40:87–119. doi: 10.1016/s0065-308x(08)60118-9. [DOI] [PubMed] [Google Scholar]
- Theodos CM, Sullivan KL, et al. Profiles of healing and nonhealing Cryptosporidium parvum infection in C57BL/6 mice with functional B and T lymphocytes: the extent of gamma interferon modulation determines the outcome of infection. Infect Immun. 1997;65(11):4761–4769. doi: 10.1128/iai.65.11.4761-4769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar BL, Kao TC, et al. Cryptosporidium infection in an adult mouse model. Independent roles for IFN-gamma and CD4+ T lymphocytes in protective immunity. J Immunol. 1991;147(3):1014–1022. [PubMed] [Google Scholar]
- Verdon R, Polianski J, et al. Cryptosporidium parvum biliary tract infection in adult immunocompetent and immunosuppressed mice. J Med Microbiol. 1998;47(1):71–77. doi: 10.1099/00222615-47-1-71. [DOI] [PubMed] [Google Scholar]
- Wehbe H, Henson R, et al. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res. 2006;66(21):10517–10524. doi: 10.1158/0008-5472.CAN-06-2130. [DOI] [PubMed] [Google Scholar]
- Wyatt CR, Brackett EJ, et al. Activation of intestinal intraepithelial T lymphocytes in calves infected with Cryptosporidium parvum. Infect Immun. 1997;65(1):185–190. doi: 10.1128/iai.65.1.185-190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Xie X, et al. Involvement of Th17 and Th1 effector responses in patients with Hepatitis B. J Clin Immunol. 2010;30(4):546–555. doi: 10.1007/s10875-010-9416-3. [DOI] [PubMed] [Google Scholar]