Abstract
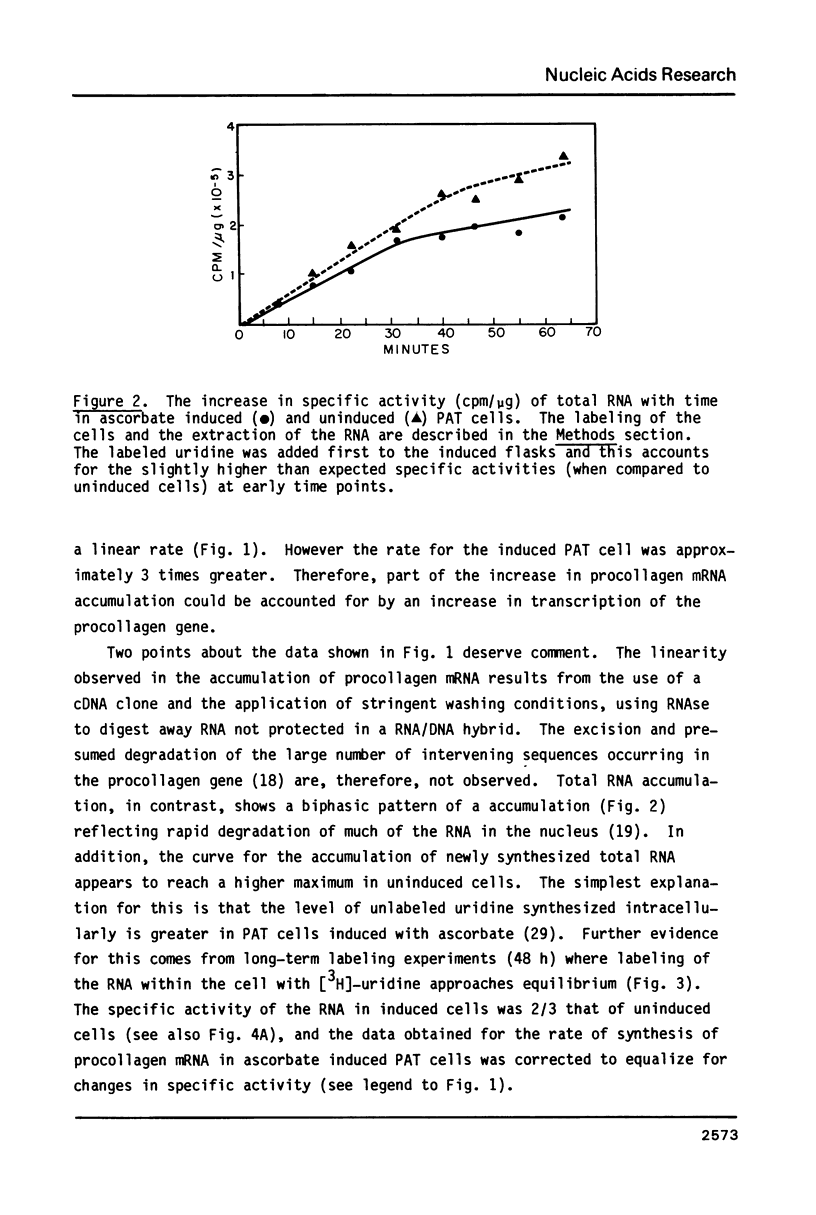
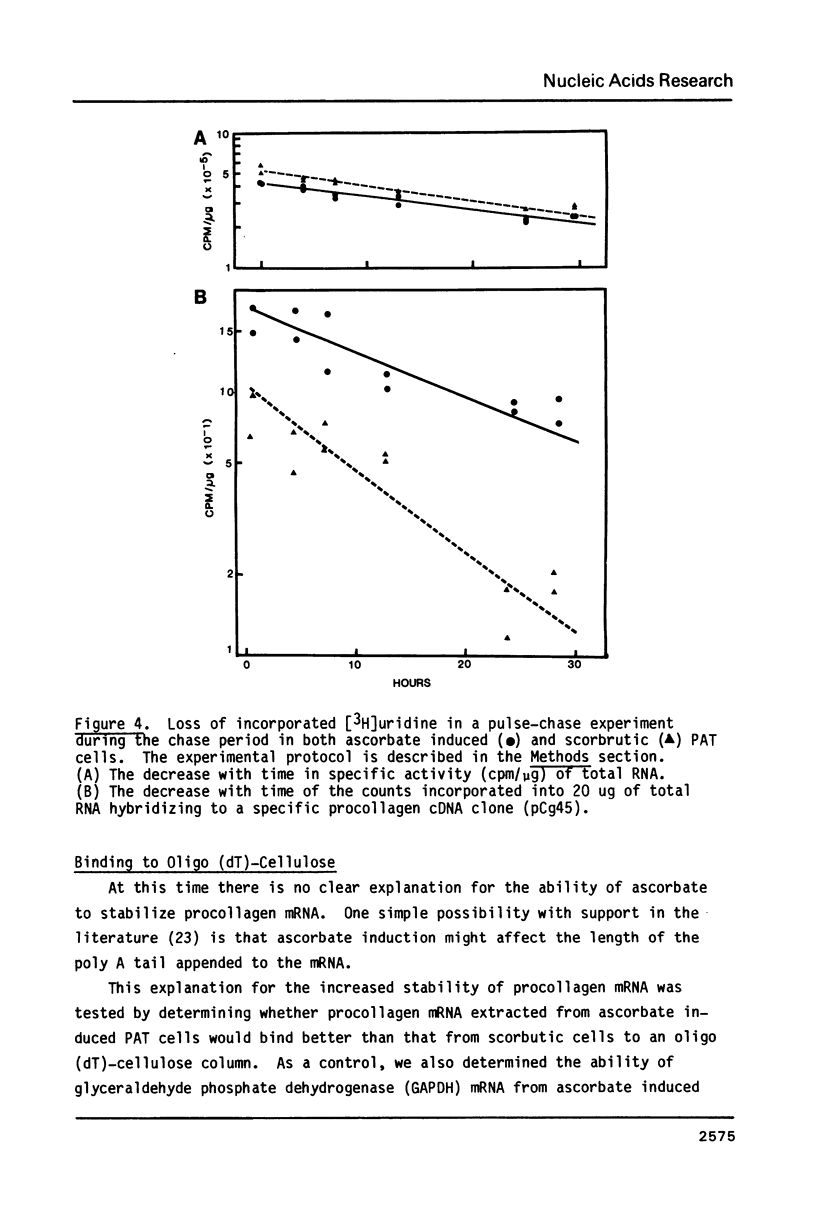
Procollagen alpha 2 (I) mRNA can be induced congruent to 6-fold in primary avian tendon (PAT) cells on addition of ascorbate to the culture medium. Previously, we have shown that the induction is linear after a 12 h lag and requires a total of 60-72 h to achieve maximum levels. We have now investigated in more detail the changes that have occurred in the metabolism of procollagen mRNA in fully induced cells to account for the observed induction. Ascorbate was found to triple the rate of procollagen gene transcription. In addition, there was a stabilization of the mRNA causing the half-life to increase from 10.5 h to 20 h. The increased stability of the procollagen mRNA, however, did not correlate with its ability to bind to oligo (dT)-cellulose. Since a 3-fold change in transcription rates and a 2-fold increase in half-life would account for the 6-fold overall increase in procollagen mRNA levels, we conclude that these are the primary alterations caused by ascorbate addition that give rise to the specific increase in procollagen mRNA.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. L., Wallace D. M., Siegal G. P., Hitchcock M. J., Birkenmeier C. S., Reber S. B. Preparation of interferon messenger RNAs with the use of ribonucleoside--vanadyl complexes. Methods Enzymol. 1981;79(Pt B):59–68. doi: 10.1016/s0076-6879(81)79013-x. [DOI] [PubMed] [Google Scholar]
- Dalgleish R., Trapnell B. C., Crystal R. G., Tolstoshev P. Copy number of a human type I alpha 2 collagen gene. J Biol Chem. 1982 Nov 25;257(22):13816–13822. [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Farmer S. R., Wan K. M., Ben-Ze'ev A., Penman S. Regulation of actin mRNA levels and translation responds to changes in cell configuration. Mol Cell Biol. 1983 Feb;3(2):182–189. doi: 10.1128/mcb.3.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Rosner C., Boedtker H. Procollagen complementary DNA, a probe for messenger RNA purification and the number of type I collagen genes. Biochemistry. 1978 Aug 8;17(16):3243–3249. doi: 10.1021/bi00609a011. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpold M. M., Wilson M. C., Darnell J. E., Jr Chinese hamster polyadenylated messenger ribonucleic acid: relationship to non-polyadenylated sequences and relative conservation during messenger ribonucleic acid processing. Mol Cell Biol. 1981 Feb;1(2):188–198. doi: 10.1128/mcb.1.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi-Scherrer M. T., Maundrell K., Civelli O., Scherrer K. Transcriptional and post-transcriptional regulation in duck erythroblasts. Dev Biol. 1982 Sep;93(1):126–138. doi: 10.1016/0012-1606(82)90246-9. [DOI] [PubMed] [Google Scholar]
- Jungmann R. A., Kelley D. C., Miles M. F., Milkowski D. M. Cyclic AMP regulation of lactate dehydrogenase. Isoproterenol and N6,O2-dibutyryl cyclic amp increase the rate of transcription and change the stability of lactate dehydrogenase a subunit messenger RNA in rat C6 glioma cells. J Biol Chem. 1983 Apr 25;258(8):5312–5318. [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Frischauf A. M., Hanahan D., Wozney J., Fuller F., Crkvenjakov R., Boedtker H., Doty P. Construction and characterization of a 2.5-kilobase procollagen clone. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5417–5421. doi: 10.1073/pnas.75.11.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Ceccarelli A., Lodish H. F. Cyclic AMP stabilizes a class of developmentally regulated Dictyostelium discoideum mRNAs. Nature. 1983 Feb 17;301(5901):616–618. doi: 10.1038/301616a0. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Milner R. J., Brow M. D., Cleveland D. W., Shinnick T. M., Sutcliffe J. G. Glyceraldehyde 3-phosphate dehydrogenase protein and mRNA are both differentially expressed in adult chickens but not chick embryos. Nucleic Acids Res. 1983 May 25;11(10):3301–3315. doi: 10.1093/nar/11.10.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profous-Juchelka H. R., Reuben R. C., Marks P. A., Rifkind R. A. Transcriptional and post-transcriptional regulation of globin gene accumulation in murine erythroleukemia cells. Mol Cell Biol. 1983 Feb;3(2):229–232. doi: 10.1128/mcb.3.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe L. B., Schwarz R. I. Role of procollagen mRNA levels in controlling the rate of procollagen synthesis. Mol Cell Biol. 1983 Feb;3(2):241–249. doi: 10.1128/mcb.3.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmeyer S., Gallis B., Bornstein P. Coordinate transcriptional regulation of type I procollagen genes by Rous sarcoma virus. J Biol Chem. 1981 May 25;256(10):5022–5028. [PubMed] [Google Scholar]
- Schwarz R. I., Bissell M. J. Dependence of the differentiated state on the cellular environment: modulation of collagen synthesis in tendon cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4453–4457. doi: 10.1073/pnas.74.10.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. I., Farson D. A., Bissell M. J. Requirements for maintaining the embryonic state of avian tendon cells in culture. In Vitro. 1979 Dec;15(12):941–948. doi: 10.1007/BF02619153. [DOI] [PubMed] [Google Scholar]
- Schwarz R. I., Mandell R. B., Bissell M. J. Ascorbate induction of collagen synthesis as a means for elucidating a mechanism of quantitative control of tissue-specific function. Mol Cell Biol. 1981 Sep;1(9):843–853. doi: 10.1128/mcb.1.9.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima S., Pinnell S. R. Regulation of collagen synthesis by ascorbic acid. Ascorbic acid increases type I procollagen mRNA. Biochem Biophys Res Commun. 1982 May 31;106(2):632–637. doi: 10.1016/0006-291x(82)91157-3. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstoshev P., Haber R., Trapnell B. C., Crystal R. G. Procollagen messenger RNA levels and activity and collagen synthesis during the fetal development of sheep lung, tendon, and skin. J Biol Chem. 1981 Sep 25;256(18):9672–9679. [PubMed] [Google Scholar]
- Volloch V., Housman D. Stability of globin mRNA in terminally differentiating murine erythroleukemia cells. Cell. 1981 Feb;23(2):509–514. doi: 10.1016/0092-8674(81)90146-x. [DOI] [PubMed] [Google Scholar]
- Wozney J., Hanahan D., Tate V., Boedtker H., Doty P. Structure of the pro alpha 2 (I) collagen gene. Nature. 1981 Nov 12;294(5837):129–135. doi: 10.1038/294129a0. [DOI] [PubMed] [Google Scholar]
- Zeevi M., Nevins J. R., Darnell J. E., Jr Newly formed mRNA lacking polyadenylic acid enters the cytoplasm and the polyribosomes but has a shorter half-life in the absence of polyadenylic acid. Mol Cell Biol. 1982 May;2(5):517–525. doi: 10.1128/mcb.2.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]


