Abstract
Background & Aims
Krüppel-like factor 5 (KLF5) is transcription factor that is expressed by dividing epithelial cells of the intestinal epithelium. KLF5 promotes proliferation in vitro and in vivo and is induced by mitogens and various stress stimuli. To study the role of KLF5 in intestinal epithelial homeostasis, we examined the phenotype of mice with conditional deletion of Klf5 in the gut.
Methods
Mice were generated with intestinal-specific deletion of Klf5 (Vil-Cre;Klf5fl/fl).
Morphological changes in the small intestine and colon were examined by immunohistochemistry, immunoblotting, and real-time PCR.
Results
Klf5 mutant mice were born at a normal Mendelian ratio but had high mortality compared to controls. Complete deletion of Klf5 from the intestinal mucosa resulted in neonatal lethality that corresponded with an absence of epithelial proliferation. Variegated intestinal-specific deletion of Klf5 in adult mice resulted in morphological changes that included a regenerative phenotype, impaired barrier function, and inflammation. Adult mutant mice exhibited defects in epithelial differentiation and migration. These changes were associated with reduced expression of Cdx 1, Cdx2, and Eph and ephrin signaling proteins. Concomitantly, Wnt signaling to β-catenin was reduced. Proliferation in regenerative crypts was associated with increased expression of the progenitor cell marker Sox9.
Conclusions
Deletion of Klf5 in the gut epithelium of mice demonstrated that KLF5 maintains epithelial proliferation, differentiation, and cell positioning along the crypt radial axis. Morphological changes that occur with deletion of Klf5 are associated with disruption of canonical Wnt signaling and increased expression of Sox9.
Keywords: intestinal homeostasis, gastrointestinal development, genetics, GI tract
The mammalian intestinal epithelium is a continuously renewing system in which several biological processes including stem cell self-renewal, proliferation, terminal differentiation, migration, and apoptosis are carefully choreographed in order to maintain tissue homeostasis. The epithelium of the small intestine is composed of a crypt and a villus compartment. Similarly, the colon consists of a crypt and a surface epithelium compartment. The bulk of the villus and surface epithelium of the small and large intestine, respectively, is composed of differentiated columnar epithelial cells that are divided into two main classes: absorptive and secretory. These cells are descendants of the multipotent stem cells residing in the base of the crypt and are no longer dividing. In contrast, crypt stem cells are capable of self-renewal and asymmetric division. They give rise to the proliferative progenitor cells, also residing in the crypt compartment, which then become fully differentiated daughter cells as they migrate along the crypt-villus axis of the intestine 1, 2.
Recent studies have illustrated the importance of several signaling pathways including the Wnt, Notch, Hedgehog, and bone morphogenetic protein pathways in controlling proliferation of intestinal stem cells and progenitor cells and their subsequent differentiation into different lineages of mature, differentiated epithelial cells 3-5. Among these, the Wnt pathway is the best characterized. Wnt genes, of which that are 19 in humans, encode secreted glycoproteins 6. Upon binding to cell surface receptors such as Frizzled (Fz) and low-density lipoprotein receptor-related protein (LRP), Wnt elicits a signaling cascade that cumulates in the formation of nuclear β-catenin/T cell factor (TCF) complexes, which then drive cell proliferation 7-9. It is well established that there is a Wnt gradient along the crypt-to-villus or crypt-to-surface epithelium axis of the small and large intestine, respectively, which provides important signals for proliferation of stem cells and progenitor cells in the crypt region 2, 3. Additionally, Wnt signaling participates in intestinal epithelial cell maturation through regulating expression of the caudal related homologue Cdx1, which, along with Cdx2, promotes development of a mature intestinal phenotype 10, 11. Wnt signaling also controls proper positioning of differentiated Paneth cells and enterocytes along the crypt-villus axis by reciprocally regulating expression of the EphB2/EphB3 receptors and their ligand ephrin B1 12-14.
The Krüppel-like factor 5 (KLF5) gene is one of over 20 mammalian genes which exhibit homology to the Drosophila Krüppel gene that is essential for embryogenesis 15-18. In the intestine, expression of KLF5 is enriched in the proliferating crypt epithelial cells 19, 20. Consistent with its cellular localization, KLF5 has been associated with, and in several instances, regulates, cellular proliferation in both physiological and pathophysiological conditions 21-25. Until recently, a definitive assessment of KLF5 function in the intestinal epithelium has not been possible, as homozygous deletion of the Klf5 gene is embryonic lethal 26. Here, we use the Cre-LoxP system to generate mice with conditional deletion of the Klf5 gene to investigate the physiological function of KLF5 in maintaining intestinal epithelial homeostasis.
Materials and Methods
Generation of mice with Intestinal-specific deletion of Klf5
C57BL/6 mice carrying Klf5 alleles flanked by loxP sites were described previously 27. C57BL/6 mice carrying the Cre recombinase gene under regulation of the villin promoter (Vil-Cre mice) 28 were obtained from Jackson Laboratories. Klf5fl/fl mice were crossed with Vil-Cre mice to generate Vil-Cre;Klf5fl/+ progeny. These mice were backcrossed to Klf5fl/fl mice to produce Vil-Cre;Klf5fl/fl offspring (designated Klf5Δ/S). The Animal Care Committee of Emory University approved all procedures performed, and handling of the mice was in accordance with the Guide for the Care and Use of Laboratory Animals, published by the United States Public Health Service.
In vivo Permeability Assay
Gut permeability assays were performed to determine barrier function in control and mutant mice at 6 weeks of age. Mice were administered FITC dextran by gavage (60 mg/100 g body weight, FD-4, M 4000, Sigma Aldrich, St. Louis, MO) to determine the amount of dextran crossing the gut epithelial barrier and entering the bloodstream. Blood serum was collected 4 hours post-treatment. Levels of FITC dextran in the blood serum were measured by fluorescence intensity of the samples (excitation, 492, nm; emission, 525 nm; Cytofluor 2300). Results were normalized to the amount of serum protein assayed.
Myeloperoxidase (MPO) Activity
Neutrophil infiltration into tissue was quantified by measuring MPO enzyme activity (a marker for neutrophils). Briefly, colon tissues were washed to remove luminal contents and homogenized with a Tissuemizer (Fisher Scientific, Hampton, NH) in 0.5% hexadecyltrimethyl ammonium bromide (Sigma Aldrich) in 50 mM PBS, pH 6.0. Samples were subjected to three rounds of freeze/thaw and homogenized in the Bullet Blender with .1 mm zirconium silicate beads (Next Advance, Averill Park, NY). MPO activity was assayed from cleared supernatants by adding 1 mg/ml of dianisidine dihydrochloride (Sigma Aldrich) and 5 × 10−4% H2O2 (Sigma Aldrich) and measuring the change in optical density at 450 nm. Activity was normalized to total protein assayed.
Immunohistochemistry
Tissue isolated from the colon and small intestine (duodenum, jejunum, ileum) of 8-week-old mice were extracted, cut longitudinally and formed into Swiss rolls. Samples were prepared and subjected to immunohistochemical staining as described previously 29. Immunofluorescence staining was performed by blocking with 3% BSA/PBS/Tween20 solution, incubating with primary antibody overnight at 4°C and incubation with Alexa fluor 488 or Alexa fluor 568 secondary antibodies (Invitrogen, Carlsbad, CA). Images were taken using Zeiss Axioskop2 plus microscope (Carl Zeiss Microimaging, Inc.). Areas of Klf5 deletion were verified in stained sections by co-staining with Klf5 antibody or by comparing with serial sections stained for Klf5.
Alcian blue/Periodic acid Schiff (PAS) staining
Alcian blue/PAS staining of paraffin-embedded sections to identify goblet cells was carried out using the Alcian Blue and PAS Staining Kit (Biocare Medical, Concord, CA) per manufacturer's instructions.
Western Blot Analysis
Protein lysates were prepared from distal ileum or proximal colon where deletion of Klf5 was most complete. Epithelial tissues were scraped into cell extraction buffer (Invitrogen) supplemented with Complete Protease Inhibitor Cocktail tablets (Roche, Indianapolis, IN). Samples were homogenized briefly with a Tissuemizer (Fisher Scientific) and further homogenized with 0.1 mm zirconium silicate beads in the Bullet Blender (Next Advance). Lysates were prepared in a 1:1 ratio with 2X Laemmli Buffer, subjected to polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. Membranes were blocked for 30 minutes in 5% non-fat dried milk in Tween/Tris-buffered salt solution (20mmol/L Tris-HCl, 3mmol/L KCl, 137 mmol/L NaCl, 0.1% Tween20, pH 7.5) and hybridized overnight at 4°C with primary antibodies.
Quantitative Real-time Polymerase Chain Reaction (qRT-PCR) Analysis
Total RNA from scraped colonic musosa of 3 control and 3 Klf5 mutant mice was prepared using Trizol reagent (Invitrogen) followed by DNase treatment and RNA cleanup with RNeasy columns (Qiagen, Valencia, CA). First strand synthesis of cDNA was performed with the RT2 First Strand Kit per manufacturers' instructions (SA Biosciences, Frederick, MD). For analysis of Wnt pathway genes, PCR reactions were set up using the Wnt Signaling Pathway PCR Array plates (SA Biosciences) and analyzed with the manufacturer's software. Reactions were run on the Eppendorf Realplex PCR machine. Information on primers is included in Supplementary Methods.
Antibodies
See Supplementary Methods for a list of antibodies and dilutions used for Western blotting and immunohistochemistry.
Statistics
Numerical values in the figures are expressed as the mean ± S.E. Sample sizes are indicated in the figure legends. P values comparing the data for two groups of mice were calculated using a 2-tailed t test. Differences were considered to be significant if the P value was less than 0.05.
Results
To examine the consequences of intestinal-specific deletion of the Klf5 gene, we generated Vil-Cre;Klf5fl/fl mice (designated Klf5Δ/S for “intestinal-specific deletion”) by crossing mice carrying floxed alleles of the Klf5 gene (Klf5fl/fl) with mice expressing the Cre recombinase under the control of a 12.4-kb regulatory region of the murine villin gene, followed by backcrossing to obtain the mutant mice. Klf5Δ/S mice were born at a normal Mendelian ratio, but approximately two-thirds of these mice died within two days after birth. The remainder survived up to 8 weeks before dying or being sacrificed due to significant weight loss and rectal prolapse. Deletion of Klf5 in intestinal tissues was confirmed by immunohistochemical staining of Klf5. In mutant mice that died shortly after birth, Klf5 was deleted completely in the small intestine and colon (Figure 1A, panels a-d). Intestinal villi were sparse in newborn mutant mice compared to control mice, with the villus tips having a bulbous rather than tapered shape. In the colon, tissue architecture was distorted, with no identifiable crypt structures. To examine the effect of intestinal-specific deletion of Klf5 on epithelial proliferation in the neonatal gut, tissues from control and Klf5Δ/S mice were stained for expression of the proliferation marker, Ki67 (Figure 1A, panels e-h). Whereas proliferating cells were abundant in the crypts of control mice, they were virtually absent in corresponding areas in the mutant mice in both the small and large intestine.
Figure 1.

Deletion of Klf5 from the intestinal epithelium of moribund newborn mice. Immunohistochemical staining of Klf5 (A) and Ki67 (B) was performed on tissues from the small intestine and colon of control (Klf5fl/fl) and Klf5Δ/S (Vil-Cre;Klf5fl/fl) newborn mice one day after birth.
In examining Klf5 expression in surviving mice at 8 weeks of age, small and large intestines from Klf5fl/fl control mice and Klf5 mutant mice were prepared as Swiss rolls and stained for Klf5 (Figure2A). Adult control mice showed Klf5 localized to epithelial cells in proliferating regions of intestinal crypts. In contrast, adult Klf5Δ/S mice exhibited patchy expression of Klf5 in segments of the small and large intestine, indicating incomplete deletion of the Klf5 gene from intestinal stem cells. Klf5 was deleted in approximately 50% of small intestinal tissues and in 80% of colon tissues (Figure 2B).
Figure 2.

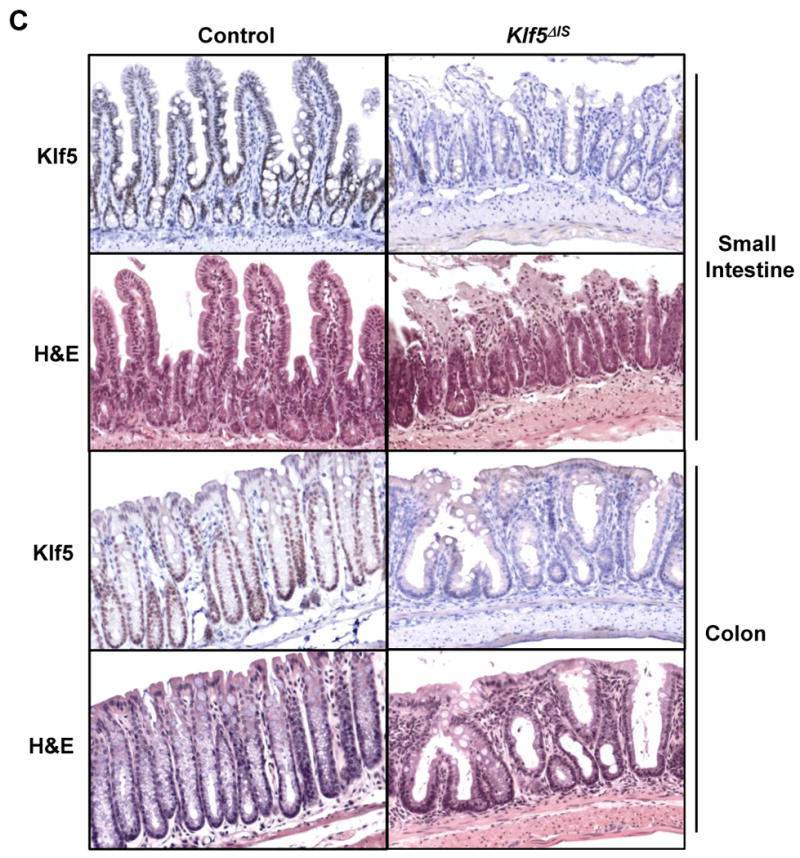
Variegated deletion of Klf5 and evidence of a regenerative phenotype in Klf5 mutant mice. (A) Immunohistochemical staining of Klf5 in Swiss rolls from the small intestine and colon of mice at 8 weeks of age. (Red arrows: Klf5-positive crypts; black arrows: Klf5-negative crypts). (B) Average percent deletion of Klf5 in Swiss rolls of duodenum, jejunum, ileum and colon stained for Klf5 protein, n=5. (C) Histolology in areas of Klf5 deletion. Serial sections from the small intestine and colon of adult control and Klf5Δ/S mice were stained with Klf5 antibody or H&E.
We next examined morphology of the small and large intestines in control and surviving Klf5 mutant mice. Klf5 expression in areas of representative histology was confirmed by staining serial sections with Klf5 antibody (Figure 2C). Hematoxylin and eosin (H&E) staining indicated that normal crypt architecture was disrupted throughout the gut of the mutant mice. In the small bowel, villi were shortened and crypts were elongated, with some areas exhibiting erosion at the villus tips. In the colon, crypts were severely distorted, with dilated glands and reduced numbers of goblet cells. Somewhat surprisingly, mutant mice displayed active proliferation, with increased crypt lengths and crypt densities in the small intestine and increased crypt fission and proliferation in the colon (Supplementary Figure 1). These results were in contrast to those in newborn mice which showed absence of epithelial proliferation. Altered morphologies in the adult Klf5Δ/S mice are consistent with a regenerative phenotype.
The average lifespan of Klf5Δ/S mice with variegated Klf5 expression was approximately 8 weeks, with the mice succumbing to severe wasting or being sacrificed due to rectal prolapse (Figure 3 A and B). Histological analysis of mutant mice with rectal prolapse revealed cryptitis, with infiltration of neutrophils into the epithelium and lamina propria of the small and large intestines, disruption of crypt architecture in the small intestine, and the presence of neutrophil exudate in the glands of the colon (Figure 3C). Neutrophil infiltration was also examined in Klf5 mutant mice by myeloperoxidase (MPO) activity, a lysosomal enzyme abundant in neutrophils (Figure 3D). MPO activity was significantly higher in Klf5 mutant mice than in control mice at 6 weeks of age, prior to the development of rectal prolapse. To determine whether inflammation was associated with epithelial barrier defects, barrier function was measured in control and mutant mice at 6 weeks of age by the FITC dextran method (Figure 3E). Results indicated a significantly increased permeability across the intestinal epithelium of Klf5Δ/S mice, corresponding to increased MPO activity.
Figure 3.

Wasting and enterocolitis in Klf5Δ/S mice. (A) Comparison of size difference of control and Klf5Δ/S littermates at 8 weeks of age. (B) Rectal prolapse is frequently observed in Klf5Δ/S mice by approximately 8 weeks of age. (C) H&E staining indicates infiltration of immune cells and disruption of normal crypt architecture in the small intestine and colon of Klf5Δ/S mice with rectal prolapse. (D) Measurement of neutrophil infiltration by myeloperoxidase assay using colon tissue from control and Klf5Δ/S mice at 6 weeks of age (*P < 0.001, n=7). (E) Measurement of gut permeability of mice at 6 weeks of age with the FITC dextran permeability assay (**P = 0.05, n=7).
Accompanying the perturbed crypt architecture and barrier defects in Klf5 mutant mice were alterations in intestinal epithelial differentiation, with aberrant localization of differentiated cells. In control mice, immunohistochemical staining for carbonic anhydrase-I (CA-I), a colonic marker for epithelial differentiation 30, showed CA-1 restricted to epithelial cells in the upper third of the colonic crypt (Figure 4A). However, in Klf5 mutant mice, CA-I expression was seen throughout the epithelial mucosa. Disruption of normal patterns of terminal differentiation was associated with reduced mRNA expression of key regulators of differentiation in the small and large intestine, Cdx1 and Cdx2 10 (Figure 4B).
Figure 4.

Loss of a restricted differentiation zone and changes in epithelial cell lineage with deletion of Klf5. Paraffin-embedded colon sections from adult control and Klf5 mutant mice were stained with various epithelial markers to identify changes in differentiation. (A) Immunohistochemical staining with the colonic differentiation marker, carbonic anhydrase-I. (B) Quantitative real time polymerase chain reaction (qRT-PCR) to measure relative mRNA expression of Cdx1 and Cdx2 in colon tissues from control and mutant mice (*P < 0.001, **P = 0.004, n=3). (C) Alcian blue/Periodic acid-Schiff (PAS) staining to identify goblet cells and immunohistochemical staining of chromogranin A as a marker for enteroendocrine cells.
Epithelial cell lineages also appeared to be altered in the Klf5Δ/S mice. Numbers of goblet cells were reduced in the colon of mutant mice as determined by Alcian blue/Periodic acid-Schiff staining (Figure 4C). A reduction in goblet cell number was accompanied by a reciprocal increase in the number of enteroendocrine cells, as indicated by chromogranin A staining (Figure 4D). In the small intestine, no significant changes in epithelial cell lineage decisions were noted in mutant mice compared to control mice. Numbers of goblet cells and enteroendocrine cells as a percentage of total epithelial cells were comparable between the two groups (data not shown).
Paneth cell numbers and distribution in the small intestine of mutant and control mice were examined by staining for the Paneth cell marker, lysozyme 31. While numbers of Paneth cells per crypt were similar in control and mutant mice, the localization of Paneth cells within the crypts was altered in Klf5 mutant mice. In control mice, Paneth cells were anchored at the very base of the crypts (Figure 5A, panel a). However, in mutant mice, some of the lysozyme-positive cells were no longer retained at the crypt base and could be found at higher positions in the crypts (Figure 5A, panels b,c). Mispositioning of cells was also observed for actively proliferating epithelial cells in Klf5 mutant mice. Whereas cells stained positive for the proliferation marker, Ki67, were localized to the base of the crypts in the small and large intestines of control mice (Figure 5B, panels d,g), Ki67-positive proliferating cells were intermingled with non-proliferating cells throughout the intestinal crypts of mutant mice (Figure 5B, panels e, f, h and i). These results suggest that one of the physiological functions of KLF5 is to control cell migration and position along the crypt/villus axis.
Figure 5.

Impaired migration of epithelial cells in Klf5 mutant mice. Tissues from adult control and Klf5Δ/S mice were examined for alterations in epithelial cell migration. (A) Immunohistochemical staining of the small intestine with lysozyme as a Paneth cell-specific marker. Right panel shows a higher magnification of Klf5Δ/S staining. The red arrowhead indicates a Paneth cell that has migrated from the crypt base. (B) Immunohistochemical staining with the proliferation marker, Ki67, in the small intestine and colon. Red dotted lines indicate the normal boundaries between proliferative and differentiated compartments. Right panels are higher magnification images of adjacent panels. Green arrowheads indicate Ki67-positive proliferating cells. Red arrowheads indicate Ki67-negative non-cycling cells.
The scattered distribution of Paneth cells and Ki67-positive cells in the intestines of Klf5 mutant mice is reminiscent of the phenotype observed in mice deficient for the EphB2/EphB3 receptors 12,13. These receptors are normally expressed in the intestinal crypts. Together with their ligand, ephrin B1 which is expressed in the villi, EphB2/EphB3 regulate positioning of cells in the intestinal epithelium 12, 14. We therefore performed immunohistochemical staining and Western blot analysis for EphB2, EpB3 and ephrin B1 in intestinal tissues from Klf5 mutant and control mice. Deletion of Klf5 in regions of immunohistochemical staining was verified by co-staining for Klf5 or staining serial sections for Klf5 (Supplementary Figure 2). In examining expression of EphB2 and EphB3 in the colon, both receptors were confined to cells at the base of the crypts in control mice (Figure 6A). In the mutant mice, however, EphB2 exhibited a more diffuse staining throughout the crypt, with expression levels only slightly reduced compared to those in control mice (Figure 6A and B). In contrast, EphB3 expression was not detectable in the colon of mutant mice by Western blot or immunohistochemistry. Likewise, ephrin B1, which is normally expressed in cells in the upper region of the colonic epithelium, was undetectable in mutant mice. Similar although less dramatic changes in EphB3 and ephrin B1 expression were seen in the small intestine (Supplementary Figure 3). Thus, KLF5 regulates cell positioning in the gut epithelium by controlling expression of EphB2/EphB3 receptors and the ephrin B1 ligand.
Figure 6.

Disrupted expression of Eph/ephrin proteins in Klf5ΔIS mice. (A) Immunofluorescence staining of EphB2, EphB3 and ephrin B1 in colon tissues from control and Klf5 mutant mice. White arrows indicate expected localization of the Eph/ephrin proteins. (B) Western blot analysis of Eph/ephrin levels in colon lysates from control and Klf5 mutant mice, n=3.
KLF5 has been reported to promote β-catenin activity through its effects on β-catenin nuclear localization 29. Since differentiation and cell positioning are regulated by Wnt/ β-catenin signaling in the intestine, we investigated whether deletion of Klf5 affects Wnt pathway activation. Immunohistochemical staining of β-catenin revealed reduced nuclear localization at the base of colonic crypts in Klf5 mutant mice compared to controls (Figure 7A). Klf5 expression in regions stained for β-catenin was verified by immunohistochemical staining of Klf5 in serial sections (Supplementary Figure 4.) Levels of β-catenin in nuclear lysates from mucosal scrapings were also reduced in Klf5Δ/S mice (Figure 7B). Using a quantitative PCR array for analysis of Wnt pathway genes, numerous Wnt-related genes were found to be downregulated in colonic tissues of Klf5Δ/S mice (Figure7C). Genes with reduced expression included direct targets of Wnt signaling (Ccnd1, Fgf4, Jun, Sox17) as well as genes encoding receptors and ligands for Wnt signaling (Dvl2, Fzd4, Fzd7, Lrp5, Wnt2, Wnt4) and proteins involved in Wnt activation (Lef1 and Pygo1). Thus, deletion of Klf5 results in reduced activation of β-catenin and inhibition of Wnt signaling in the intestine.
Figure 7.

Impaired Wnt signaling and induction of Sox9 in colonic tissues of Klf5ΔIS mice. (A) Colonic tissues of control and mutant mice were stained by immunohistochemistry to examine localization of β-catenin. Red arrows indicate the presence or absence of nuclear localization of β-catenin in control and mutant mice, respectively. (B) Nuclear lysates prepared from colons of 3 control and 3 mutant mice were pooled and subjected to Western blotting to determine levels of nuclear-localized β-catenin, n=3. Tata-binding protein (TBP) is shown as a nuclear protein loading control. (C) Wnt pathway PCR array analysis was conducted on RNA samples from control and Klf5Δ/S mice to identify differentially expressed mRNAs. RNA samples were pooled for analysis, n=3. Fold changes in parentheses indicate a fold decrease.
To identify mechanisms promoting colonic epithelial proliferation in regions with no Klf5 expression and reduced Wnt signaling, several candidate pathways and molecules were examined. Canonical TCF/β-catenin targets, cyclin D1 and c-Myc, were reduced or unchanged, respectively, in areas of Klf5 deletion (Supplementary Figure 5). Notch signaling was likewise modestly reduced in colonic tissues of Klf5 mutant mice (Supplementary Figure 6). However, expression of Sry (sex determining region of Y)-box 9 (Sox9), a transcription factor that participates in decisions of self-renewal and differentiation 32, 33, was increased approximately four-fold in colonic tissues of Klf5 mutant mice (Figure 7D and E). Staining of Sox9 in Klf5 mutant mice revealed high levels throughout the regenerative glands. Thus, Sox9 may mediate aspects of the regenerative phenotype in intestinal tissues of Klf5Δ/S mice.
Discussion
The results presented here demonstrate that Klf5 plays a critical role in homeostasis of the intestinal epithelium, with loss of Klf5 affecting mucosal crypt architecture and barrier function. Deletion of Klf5 from the gut epithelium culminates in a regenerative phenotype that is characteristic of wound repair. This phenotype is likely a compensatory response to changes in the mucosal epithelium that are associated with reduced Wnt signaling. Interestingly, changes in tissue architecture with deletion of Klf5 are more severe in the colon than in the small intestine. This may be due to a more complete deletion of Klf5 in colonic tissues (Figure 2B) or to Klf5 playing a more significant role in maintenance of epithelial homeostasis in the colon.
Our results indicate that Klf5 is not essential to maintain intestinal epithelial proliferation in adult mice, in contrast to its requirement for proliferation in newborn mice. This finding may be attributable to regenerative processes in adult intestinal tissues that are independent of canonical Wnt signaling. In adult Klf5 mutant mice, epithelial cell proliferation was observed in regenerative crypts where KLF5 is deleted and Wnt signaling is reduced. Concomitant with these changes was increased expression of the progenitor cell marker, Sox9. Sox9 is linked to progenitor status in adult liver, exocrine pancreas and intestine, and is proposed to maintain the precursor cell population during physiological cell replacement and/or regeneration after injury 34. Thus, Sox9 may promote proliferation in Klf5 mutant mice by maintaining epithelial cells in a progenitor state.
In regard to changes in tissue morphology, we show a direct effect of KLF5 deletion on epithelial differentiation within the gut epithelium. Defects in terminal differentiation along the crypt-luminal axis are apparent in the colon of Klf5 mutant mice stained with the differentiation marker, carbonic anhydrase-I. Normal expression of CA-I is limited to colonocytes in the upper one third of the crypt 30; however, in KLF5Δ/S mice, CA-I-expressing cells are scattered throughout the crypt, indicating that terminal differentiation is no longer restricted to the luminal surface. A similar effect is seen with the differentiation marker alkaline phosphatase in mice with transgenic intestinal-specific expression of the Wnt inhibitor Dkk1 35. Perturbations in terminal differentiation in Klf5 mutant mice were associated with reduced expression of the homeobox transcription factors, Cdx1 and Cdx 2. These factors are reported to stimulate differentiation and inhibit proliferation of intestinal epithelial cells 10, 11. Cdx1 is a transcriptional target of TCF4/β-catenin 36, while Cdx2 is repressed by Sox9 37. In accordance with reports on regulation of Cdx1 and Cdx2, reduced expression of these factors in Klf5 mutant mice correlates with impaired Wnt signaling and increased Sox9 expression. In examining epithelial lineage decisions, Klf5Δ/S mice exhibit changes in the prevalence of secretory cells, with fewer goblet cells and increased numbers of enteroendocrine cells in the colon compared to controls. These effects are similar to those seen with deletion of the transcriptional repressor, Gfi1, which drives the selection of goblet and Paneth cells over enteroendorcrine progenitors 38.
Defects in epithelial migration in Klf5 mutant mice are reminiscent of mice with disrupted Eph/ephrin signaling, correlating with altered expression of Eph/ephrin proteins in the mutant mice. In the intestine of EphB3-null mice, localization of Paneth cells is no longer restricted to the crypt base; rather, Paneth cells migrate upwards and are found dispersed throughout the epithelium 12. Mislocalization of Paneth cells is also observed in mice with intestinal specific deletion of ephrin B1 39. Other architectural changes in Klf5Δ/S mice are similar to those reported in EphB2-/-;EphB3-/- double knockout mice including intermingling of proliferative and differentiated cells 12. Future studies will examine whether genes encoding EphB3 or ephrinB1 are direct transcriptional targets of KLF5.
Reduced expression of Eph/ephrin proteins and Cdx1 in Klf5Δ/S mice indicates that Wnt signaling is inhibited in the mutant mice. Absence of epithelial proliferation in the intestines of Klf5Δ/S newborn mice (Figure 1B) phenocopies genetic models for disruption of Wnt signaling that include deletion of Tcf4 and transgenic expression of the Wnt inhibitor Dkk-1 35, 40-42. In these models, the neonatal epithelium was composed entirely of differentiated, non-dividing villus cells, resulting in loss of intestinal stem cell compartments and neonatal lethality. In surviving Klf5Δ/S mice, we observe downregulation of numerous Wnt target genes, corresponding to reduced expression of active β-catenin at the base of the crypts (Figure 7). Previous evidence from our laboratory demonstrates that changes in expression of Klf5 have a significant impact on β-catenin localization and activity 29. Haploinsufficiency of Klf5 in the ApcMin/+ mouse rescues the tumor-initiating effects of the ApcMin mutation by inhibiting β-catenin nuclear accumulation. Furthermore, downregulation of KLF5 in colon cancer cell lines inhibits nuclear localization of β-catenin and reduces expression of TCF/ β-catenin target genes 29. Inhibition of tumor formation by heterozygous deletion of Klf5 is also reported in mice with combined KRas and ApcMin mutations, indicating a critical role for KLF5 in proliferative and transforming events associated with intestinal tumorigenesis 43.
Homeostasis of the intestinal epithelium is dependent on tight regulation of pathways that regulate cell proliferation, differentiation and migration. An unexpected finding of our work is the necessity of intestinal-specific expression of KLF5 for viability. By targeting expression of Klf5, we have identified a novel effector of Wnt signaling that is critical for maintenance of proper crypt organization and structure.
Supplementary Material
Acknowledgments
Grant Support: This work was supported in part by grants from the National Institutes of Health (DK052230, DK055679, DK059888, DK0644399, DK076742, DK089131, CA084197, and CA130308).
Abbreviations
- Fz
Frizzled
- LRP
lipoprotein receptor-related protein
- KLF5
Krüppel-like factor 5
- MPO
myeloperoxidase
- PAS
Periodic acid-Schiff
- Sox9
Sry (sex determining region of Y)-box 9
- TCF
T cell factor
Footnotes
Disclosures: The authors have no potential conflicts of interest to disclose.
Authors' Contributions:
BBM designed and performed experiments, analyzed data and drafted the manuscript
SSK conducted mouse experiments and immunohistochemical staining
KY conducted immunohistochemical staining and quantitative RT-PCR experiments
AMG provided technical expertise for tissue staining
NT developed the mouse model used in experiments
MI developed the mouse model used in experiments
AN provided histological analysis, advised on study design
RN provided the mouse model for these studies and contributed to study design
VWY advised on experimental design, analyzed data and edited the manuscript
All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–9. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 2.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 3.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–59. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 4.Fre S, Pallavi SK, Huyghe M, Lae M, Janssen KP, Robine S, Artavanis-Tsakonas S, Louvard D. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci U S A. 2009;106:6309–14. doi: 10.1073/pnas.0900427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishizuya-Oka A, Hasebe T. Sonic hedgehog and bone morphogenetic protein-4 signaling pathway involved in epithelial cell renewal along the radial axis of the intestine. Digestion. 2008;77 1:42–7. doi: 10.1159/000111487. [DOI] [PubMed] [Google Scholar]
- 6.Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–38. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 8.Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–30. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 9.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–5. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 10.Guo RJ, Suh ER, Lynch JP. The role of Cdx proteins in intestinal development and cancer. Cancer Biol Ther. 2004;3:593–601. doi: 10.4161/cbt.3.7.913. [DOI] [PubMed] [Google Scholar]
- 11.Crissey MA, Guo RJ, Funakoshi S, Kong J, Liu J, Lynch JP. Cdx2 levels modulate intestinal epithelium maturity and Paneth cell development. Gastroenterology. 2011;140:517–528 e8. doi: 10.1053/j.gastro.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–63. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 13.Holmberg J, Genander M, Halford MM, Anneren C, Sondell M, Chumley MJ, Silvany RE, Henkemeyer M, Frisen J. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125:1151–63. doi: 10.1016/j.cell.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 14.Merlos-Suarez A, Batlle E. Eph-ephrin signalling in adult tissues and cancer. Curr Opin Cell Biol. 2008;20:194–200. doi: 10.1016/j.ceb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–60. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 16.Dang DT, Pevsner J, Yang VW. The biology of the mammalian Kruppel-like family of transcription factors. Int J Biochem Cell Biol. 2000;32:1103–21. doi: 10.1016/s1357-2725(00)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaczynski J, Cook T, Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4:206. doi: 10.1186/gb-2003-4-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson R, Fleetwood J, Eaton S, Crossley M, Bao S. Kruppel-like transcription factors: a functional family. Int J Biochem Cell Biol. 2008;40:1996–2001. doi: 10.1016/j.biocel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Conkright MD, Wani MA, Anderson KP, Lingrel JB. A gene encoding an intestinal-enriched member of the Kruppel-like factor family expressed in intestinal epithelial cells. Nucleic Acids Res. 1999;27:1263–70. doi: 10.1093/nar/27.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McConnell BB, Ghaleb AM, Nandan MO, Yang VW. The diverse functions of Kruppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays. 2007;29:549–57. doi: 10.1002/bies.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun R, Chen X, Yang VW. Intestinal-enriched Kruppel-like factor (Kruppel-like factor 5) is a positive regulator of cellular proliferation. J Biol Chem. 2001;276:6897–900. doi: 10.1074/jbc.C000870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chanchevalap S, Nandan MO, Merlin D, Yang VW. All-trans retinoic acid inhibits proliferation of intestinal epithelial cells by inhibiting expression of the gene encoding Kruppel-like factor 5. FEBS Lett. 2004;578:99–105. doi: 10.1016/j.febslet.2004.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nandan MO, Chanchevalap S, Dalton WB, Yang VW. Kruppel-like factor 5 promotes mitosis by activating the cyclin B1/Cdc2 complex during oncogenic Ras-mediated transformation. FEBS Lett. 2005;579:4757–62. doi: 10.1016/j.febslet.2005.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McConnell BB, Klapproth JM, Sasaki M, Nandan MO, Yang VW. Kruppel-like factor 5 mediates transmissible murine colonic hyperplasia caused by Citrobacter rodentium infection. Gastroenterology. 2008;134:1007–16. doi: 10.1053/j.gastro.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nandan MO, McConnell BB, Ghaleb AM, Bialkowska AB, Sheng H, Shao J, Babbin BA, Robine S, Yang VW. Kruppel-like factor 5 mediates cellular transformation during oncogenic KRAS-induced intestinal tumorigenesis. Gastroenterology. 2008;134:120–30. doi: 10.1053/j.gastro.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shindo T, Manabe I, Fukushima Y, Tobe K, Aizawa K, Miyamoto S, Kawai-Kowase K, Moriyama N, Imai Y, Kawakami H, Nishimatsu H, Ishikawa T, Suzuki T, Morita H, Maemura K, Sata M, Hirata Y, Komukai M, Kagechika H, Kadowaki T, Kurabayashi M, Nagai R. Kruppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat Med. 2002;8:856–63. doi: 10.1038/nm738. [DOI] [PubMed] [Google Scholar]
- 27.Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P, Conway SJ, Nagai R. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120:254–65. doi: 10.1172/JCI40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–83. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 29.McConnell BB, Bialkowska AB, Nandan MO, Ghaleb AM, Gordon FJ, Yang VW. Haploinsufficiency of Kruppel-like factor 5 rescues the tumor-initiating effect of the Apc(Min) mutation in the intestine. Cancer Res. 2009;69:4125–33. doi: 10.1158/0008-5472.CAN-08-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bekku S, Mochizuki H, Takayama E, Shinomiya N, Fukamachi H, Ichinose M, Tadakuma T, Yamamoto T. Carbonic anhydrase I and II as a differentiation marker of human and rat colonic enterocytes. Res Exp Med (Berl) 1998;198:175–85. doi: 10.1007/s004330050101. [DOI] [PubMed] [Google Scholar]
- 31.Bry L, Falk P, Huttner K, Ouellette A, Midtvedt T, Gordon JI. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc Natl Acad Sci U S A. 1994;91:10335–9. doi: 10.1073/pnas.91.22.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande F, Blache P, Jay P. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635–48. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Formeister EJ, Sionas AL, Lorance DK, Barkley CL, Lee GH, Magness ST. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1108–18. doi: 10.1152/ajpgi.00004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 35.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–13. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lickert H, Domon C, Huls G, Wehrle C, Duluc I, Clevers H, Meyer BI, Freund JN, Kemler R. Wnt/(beta)-catenin signaling regulates the expression of the homeobox gene Cdx1 in embryonic intestine. Development. 2000;127:3805–13. doi: 10.1242/dev.127.17.3805. [DOI] [PubMed] [Google Scholar]
- 37.Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund JN, Clevers H, Jay P. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. 2004;166:37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shroyer NF, Wallis D, Venken KJ, Bellen HJ, Zoghbi HY. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 2005;19:2412–7. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cortina C, Palomo-Ponce S, Iglesias M, Fernandez-Masip JL, Vivancos A, Whissell G, Huma M, Peiro N, Gallego L, Jonkheer S, Davy A, Lloreta J, Sancho E, Batlle E. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet. 2007;39:1376–83. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]
- 40.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–83. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 41.Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ, Winton DJ. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology. 2004;126:1236–46. doi: 10.1053/j.gastro.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 42.Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A. 2004;101:266–71. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nandan MO, Ghaleb AM, McConnell BB, Patel NV, Robine S, Yang VW. Kruppel-like factor 5 is a crucial mediator of intestinal tumorigenesis in mice harboring combined ApcMin and KRASV12 mutations. Mol Cancer. 2010;9:63. doi: 10.1186/1476-4598-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


