Abstract
In this review, we discuss the role of focal adhesion kinase (FAK), an intracellular tyrosine kinase, in endothelial cells in relation to neovascularization. Genetic and in vitro studies have identified critical factors, receptor systems, and their intracellular signaling components that regulate the neovasculogenic phenotypes of endothelial cells. Among these factors, FAK appears to regulate several aspects of endothelial cellular behavior, including migration, survival, cytoskeletal organization, as well as cell proliferation. Upon adhesion of endothelial cells to extracellular matrix (ECM) ligands, integrins cluster on the plane of plasma-membrane, while cytoplasmic domains of integrins interact with cytoskeletal proteins and signaling molecules including FAK. However, FAK not only serves as a critical component of integrin signaling, but is also a downstream element of the VEGF/VEGF-receptor and other ligand-receptor systems that regulate neovascularization. A complete understanding of FAK-mediated neovascularization, therefore, should address the molecular and cellular mechanisms that regulate the biology of FAK. Continued research on FAK may, therefore, yield novel therapies to improve treatment modalities for the pathological neovascularization associated with diseases.
Keywords: Angiogenesis, bFGF, FAK, neovascularization, Src, VEGF
Introduction
In mammals, the growth of blood vessels occurs mainly by vasculogenesis, angiogenesis, and lymphangiogenesis, together can be termed as neovascularization (Sabine 1917–1920; Ausprunk and Folkman, 1997; Risau, 1997; Hanahan and Folkman, 1996). Neovascularization is a fundamental biological process that plays a critical role during the development and growth of an organism, and it is required for wound healing and tissue repair in adults (Ausprunk and Folkman, 1997; Hanahan and Folkman, 1996; Hanahan and Weinberg, 2000; Veikkola et al., 2000; Carmeliet, 2003). Angiogenesis is defined as the branching and spreading out of capillaries that are formed from a preformed vessels, primarily through the migration, recruitment, interconnection and lumenization of endothelial cells (Lubarsky and Krasnow, 2003; Kamei M et al., 2006; Iruela-Arispe and Davis. 2009). Nevertheless, unwanted angiogenesis has been associated with the expansion of atherosclerotic lesions, diabetic retinopathy, psoriasis, and tumor progression (Wary, 2004; Kalluri et al.,, 2003; Carmeliet, 2003; Renault and Losordo, 2007; Adams and Alitalo, 2007). On the other hand, ischemic and cardiovascular diseases have been associated with insufficient angiogenesis (Renault and Losordo, 2007; Adams and Alitalo, 2007; Becker and D’Amato, 2007; Fukumura and Jain, 2007; Aird, 2008).
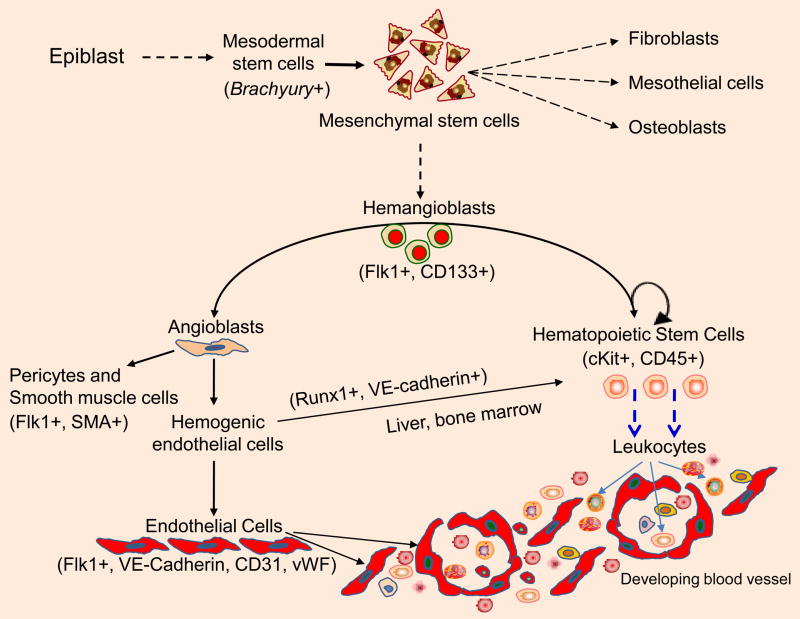
The vascular tree develops early in embryogenesis to nourish and provide oxygen to the developing organism (Risau, 1997; Watson and Cross, 2005; Robb and Elefanty, 1998; Hickey and Simon, 2006). In the mouse at embryonic day 7.5 (E7.5), the extraembryonic mesodermal cells of the yolk sac aggregate into clusters, representing the initial stage of blood island formation and hemoglobin accumulation (Sabine 1920; Robb and Elefanty, 1998; Hickey and Simon, 2006; Palis et al., 1995; Gerber et al., 2002; Rossant and Hirashima, 2003; Coultas et al., 2005; Hasegawa et al., 2007). Soon after this stage, blood islands differentiate into an external layer of endothelial cells and an inner core of blood cells (Sabine, 1920; Risau, 1997). Simultaneously, embryonic cells of the proximal lateral mesoderm assemble into pre-endocardial tubes that connect anteriorly to generate cardiac endocardium around the anterior intestinal portal (Palis et al., 1995; Gerber et al., 2002; Rossant and Hirashima, 2003; Coultas et al., 2005). Posteriorly, angioblasts organize into paired dorsal aortas, which later assemble in the midline to form a single tube. Correspondingly, mesodermal cells in the allantois generate cords that produce the umbilical blood vessels (Gerber et al., 2002; Rossant and Hirashima, 2003; Coultas et al., 2005; Hasegawa et al., 2007). The allantois develops rapidly inside the exocoelomic cavity and fuses with the chorionic extraembryonic mesoderm (Palis et al., 1995; Gerber et al., 2002; Rossant and Hirashima, 2003; Coultas et al., 2005; Hasegawa et al., 2007). This fusion results in a signal that initiates the differentiation of the chorionic vasculature, which indirectly connects to the maternal vascular system of the placenta. These early events are described as “vasculogenesis”, which signifies the de novo formation of blood vessels from angiogenic precursor cells. In the mouse, vasculogenesis is usually complete by E7.5 day. Later in embryogenesis, the vascular tree grows by the sprouting, cell division, migration and assembly of endothelial cells derived from pre-existing vessels through a process termed angiogenesis. At the cellular level, Brachyury acts as a switch to induce formation of the mesodermal lineage. Mesodermally derived mesenchymal stem cells give rise to hemangioblasts (Figure 1). Hemangioblasts are bipotential stem cells, which are characterized as CD133+/CD34+ and fetal liver kinase-1 (Flk1+) cells that can give rise to hematopoietic cells (HSCs) and angioblasts. The vascular tree grows by sprouting endothelial cells derived from angioblasts. Concurrent with VEGFR activation, cell-cell and cell-matrix interactions provide positional information related to when endothelial cells will elongate, interconnect, migrate, divide, and assemble into tube-like structures (Risau, W., 1997; Rossant and Hirashima, 2003; Coultas et al., 2005; Hasegawa et al., 2007). More recently, a subset of VE-cadherin+ and Runx-1+ endothelial cells, derived from dorsal aorta has been termed as “hemogenic endothelial cells”. Hemogenic endothelial cells originating from dorsal aorta between E8.5 to E11.5 days can give rise to hematopoietic cells that directly home into liver (Chen MJ et al. 2009; Swiers G, et al. 2010). Nevertheless, productive neovascularization requires not only endothelial cells, but also hematopoietic cells and the recruitment of pericytes and smooth muscle cells.
Fig. 1.
Angiogenesis. Mesenchymal stem cells of mesodermal origin give rise to hemangioblasts are derived from mesoderm. Transcription factor Brachyury is required for mesoderm formation and differentiation. Hemangioblasts can give rise to hematopoietic cells and angioblasts. Whereas a subset of VE-cadherin+ and Runx-1+ endothelial cells, called “hemogenic endothelial cells” can also give rise to hematopoietic stem cells that have the ability to home into the liver. Angiogenesis requires activities of endothelial cells, as well as accessory cells including hematopoietic, pericytes and smooth muscle cells.
In vitro transgenic and knockout studies have provided evidence that vascular endothelial growth factor (VEGF) and the VEGF receptor-2 system (also known as fetal liver kinase (Flk-1) and kinase domain receptor (KDR)) play fundamental roles in the formation of blood vessels (Shima et al., 1996; Shalaby et al., 1997; Park et al., 2004). Flk-1 is one of earliest markers of endothelial cells and is first detected during mouse development in the extra-embryonic mesoderm of the yolk sac, where the blood islands form shortly after gastrulation, around day 7 postcoitum (Shima et al., 1996; Shalaby et al., 1997; Park et al., 2004; Lugus et al., 2009; Hellström et al., 2009). In the extraembryonic mesoderm, flk-1 appears shortly after gastrulation, followed sequentially by tie-2 and tie-1 over the next 24 hours. In addition, flk-1 is initially observed in the extraembryonic mesoderm prior to its expression in the embryonic mesoderm (Shima et al., 1996; Shalaby et al., 1997; Park et al., 2004). Activation of the VEGFR-2/Flk1 receptor promotes endothelial elongation, motility and proliferation. Specifically, the activation of endothelial cells by VEGF induces expression of Delta like ligand-4 (Dll4) by one of the endothelial cells, which is referred to as the “tip cell”. Tip cells display a highly motile phenotype. Cell surface associated Dll4 expressed by the tip cell then ligate Notch receptor on the adjacent endothelial cell to induce intracellular signaling to activate the Hes and Hey transcriptional target genes (Siekmann and Lawson, 2007; Hoffmann and Iruela-Arispe, 2007; Nakatsu et al., 2003; Benedito et al., 2009; Kume, 2009; Jakobsson et al., 2010). Activation of Hes and Hey promote cell proliferation and these subset of proliferating cells are termed as the “stalk cells” (Siekmann and Lawson, 2007; Hoffmann and Iruela-Arispe, 2007; Nakatsu et al., 2003; Benedito et al., 2009; Kume, 2009; Jakobsson et al., 2010). Proliferative cells at some point receive a cell cycle “arrest” signal which induces morphogenic differentiation, lumenization and tube formation (Hoffmann and Iruela-Arispe, 2007; Nakatsu et al., 2003; Benedito et al., 2009; Kume, 2009; Iruela-Arispe and Davis, 2009; Jakobsson et al., 2010).
All of these processes may involve FAK signaling, either directly or indirectly, in the context of key molecules and pathways controlling cell migration, VEGF production, cell proliferation and the formation of new vessels. Below, we review and discuss the structure, expression, and FAK regulation of vasculogenesis and angiogenesis in developmental settings and under pathological conditions.
Regulation of FAK expression
It became clear that upon transformation of fibroblast cells with v-Src, an intracellular tyrosine kinase, v-Src phosphorylated several intracellular proteins in these cells. In the early 1990s, several laboratories generated monoclonal antibodies using proteins derived from v-Src-transformed cells. By screening a chicken embryo cDNA expression library with such an antibody raised by J.T. Parsons’ group, an intracellular tyrosine kinase protein, designated p125, was cloned (Schaller et al., 1992; Schaller et al., 1993; Hildebrand et al., 1993; Schlaepfer et al., 1994). An immunofluorescent labeling experiment using chicken embryo fibroblast cells plated on fibronectin showed colocalization of the p125 protein with the focal adhesion protein tensin (Schaller et al., 1992; Schaller et al., 1993; Hildebrand et al., 1993; Schlaepfer et al., 1994). Accordingly, this protein was designated pp125 focal adhesion kinase (FAK) (Schaller et al., 1992; Schaller et al., 1993; Hildebrand et al., 1993; Schlaepfer et al., 1994).
Most primary anchorage-dependent cultured cells of mesenchymal origin express high levels of FAK. For example, osteoblasts, endothelial cells, chondrocytes, smooth muscle cells, and fibroblast cells express large amounts of FAK. Although neoplastic cells are anchorage-independent cells, FAK expression is not lost in these cells. However, most immune cells express PYK2, while FRNK is expressed at a low levels in most cells types. Because specific antibodies to FAK, PYK2, and FRNK are commercially available, the expression of these molecules has been studied and reported by dozens of laboratories.
Using RT-PCR and immunostaining analyses, it has been demonstrated that cultured endothelial cells express high levels of FAK. This result suggests that endothelial cells may be particularly important to integrin signaling and, possibly, to endothelial cell adhesion and migration regulation. FAK signaling regulates fundamental aspects of neovascularization (Orr and Murphy-Ullrich, 2004; Vadali et al., 2007; Zhao and Guan, 2009). The widely studied and well-understood FAK signaling pathway is mediated by integrins. Because integrins mediate both cell-cell and cell-matrix interactions, integrin-mediated cell adhesion events are considered a key element of angiogenesis. Although FAK mediates integrin signaling, FAK is also a critical component of the VEGF/VEGFR2 and Ang/Tie-2 receptor systems (Orr and Murphy-Ullrich, 2004; Vadali et al., 2007; Zhao and Guan, 2009). Thus, FAK is not only involved in endothelial cell migration and survival, it may also be involved in vessel stabilization and maturation by acting as a downstream signaling molecule for the VEGF/VEGFR2 and Ang/Tie-2 receptor systems.
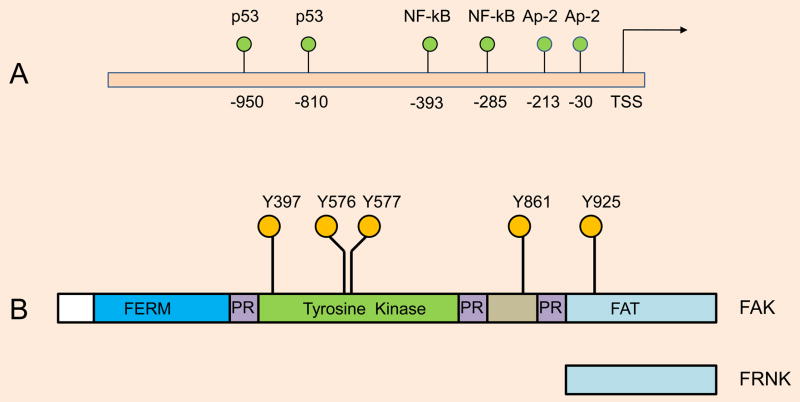
What regulates FAK expression? FAK expression is ubiquitous, suggesting that the promoter of FAK promoter is likely regulated by multiple transcription factors, including the AP-2, NF-κB and p53 proteins (Golubovskaya, V., et al., 2004) (Figure 2A). There are at least 20 potential KLF4/KLF2 binding sites (CACCC) in the human FAK promoter spread across 3.2 kb upstream of transcription start site (TSS). Unlike the VEGFR2/FLK1 gene promoter, which contains an endothelial-specific promoter, the FAK gene promoter does not harbor endothelial-specific DNA sequences. Additionally, amplification or mutation of the FAK gene in tumors or vascular lesions has not been reported. There are also no known CpG islands in the FAK promoter. Whether the FAK promoter/enhancer region is epigenetically regulated is also unknown. Thus, it will be interesting to find answers to these questions, including whether epigenetic modification of the FAK promoter/enhancer has any role in angiogenesis.
Fig. 2.
A. Human FAK promoter. transcription start site (TSS), Known binding sites for AP-2, p53, and NF-kB are as shown. Although, there are at least 20 potential (CACCC) KLF2/KLF4 binding sites in the human FAK promoter, however these binding sites have not been experimentally proven. FAK promoter also lack CpG islands. B. Structure of FAK. The indicated tyrosine phosphorylation sites (Y397, Y576, Y577, Y861, and Y925) act as recruitment and docking sites for signaling and adaptor proteins. For example, autophosphorylation of FAK at Y397 recruits the Src-family protein tyrosine kinase (PTK) via its SH2 domain, while Y925 phosphorylation provides docking sites for the Grb2 adaptor protein via its SH2 domain. The N-terminal four-point-one, ezrin, radixin, and moesin-homology (FERM) segment serves as an integrin and growth factor receptor activation domain, while the C-terminal focal adhesion-targeting (FAT) domain targets localization of FAK to focal adhesions by interacting with paxillin and talin. The FAT domain can induce Rho GTPase activation through an association with p190RhoGEF. Proline-rich (PR)-1, -2, and -3 represent proline-rich regions. The FAK-related non-kinase molecule FRNK is a short form of FAK that acts as a dominant-negative form.
FAK signaling in cell migration and angiogenesis
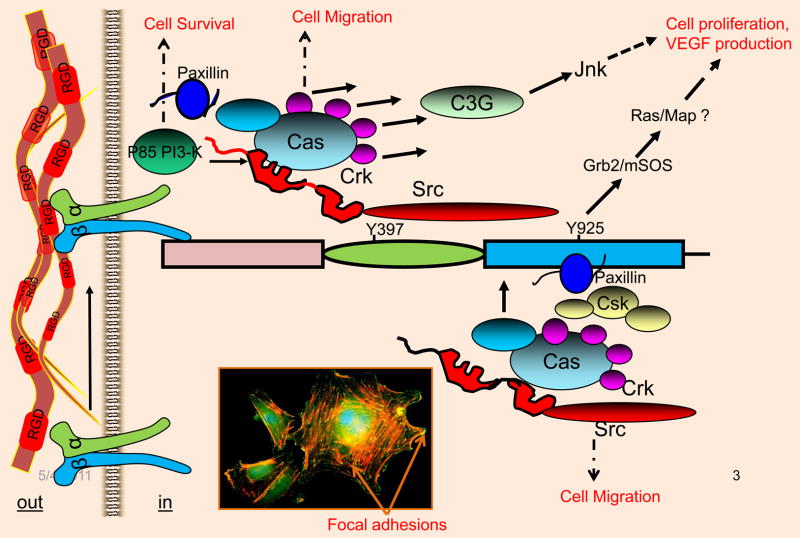
Structurally, FAK contains a N-terminal FERM domain, a central tyrosine kinase domain, proline-rich (PR) regions, and a focal adhesion-targeting (FAT) domain at the C-terminus (Figure 2B). Schaller et al provided evidence on how FAK mediates integrin signaling (Schaller et al., 1994). There are two major tyrosine phosphorylation sites, Y397 and Y925, that are central to FAK functioning (Schaller et al., 1993; Schaller et al., 1994) and serve to recruit key signaling and adaptor molecules. For example, Y397 serves as an autophosphorylation site, which then recruits c-Src via its SH2 domain. When the SH2 domain of Src is bound to the autophosphorylated Y397 site, Src tyrosine kinase unfolds to transphosphorylate Y925 at the C-terminus, which then recruits the Grb-2/mSOS complex to activate the Ras/MAP kinase pathway in response to integrin ligation (Schaller et al., 1993, Schlaepfer et al., 1997). The recruitment of Src at the Y397 autophosphorylation site results in the transphosphorylation of FAK molecules at Y407, Y576, and Y577, which subsequently increases the tyrosine kinase activity of FAK (Figure 3). The FERM domain can serve as an integrin, or EGFR, or PDGFR, or VEGFR2 activating domain. The N-terminal proline-rich (PR1) segment mediates interactions with the SH3-domain-containing proteins, including c-Src, while the PR2 and PR3 segments in the C-terminus promote interaction with the adaptor protein p130Cas, which is a RhoA-specific GTPase-activating protein known as Graf (GTPase regulator associated with FAK), and ASAP1 (ARF-GAP containing SH3, ANK repeats, and PH domain) (Schlaepfer et al., 1997; Cary and Guan, 1999; Schaller, 2001; Parsons, 2003). The observation that a FAK mutant lacking the FAT domain does not localize to focal adhesion sites indicated that the FAT domain is critical to its localization to focal adhesion sites in cultured cells (Schaller, 2001). The ability of the FAT domain of FAK to interact with the cytoskeletal proteins paxillin and talin suggested the means through which FAK might regulate cellular behavior. Because focal adhesion sites are highly enriched with numerous tyrosine-phosphorylated phosphoproteins, and FAK contained several tyrosine phosphorylation sites, it was hypothesized that many signaling and cytoskeletal proteins could directly or indirectly interact with FAK (Schlaepfer et al., 1997; Cary and Guan, 1999; Schaller, 2001; Parsons, 2003). Accordingly, PYK2, which is a structural homolog of FAK, binds similar cytosolic proteins analogous to those that are bound by FAK, while the non-catalytic C-terminal protein-binding domain of FAK is expressed as a distinct protein designated FAK-related nonkinase (FRNK). FRNK acts as a dominant-negative form of FAK; for example, in smooth muscle cells, FRNK can inhibit FAK-mediated migration (Taylor et al., 2001). Thus, activation of the Src-FAK protein complex regulates several signaling pathways that directly or indirectly alter cellular phenotypes, including cytoskeletal organization, cell migration, survival and proliferation.
Fig. 3.
Interaction of FAK with signaling and cytoskeletal proteins. In response to integrin ligation, FAK is autophosphorylated at the Y397 residue. Phosphorylated Y397 recruits Src via its SH2 domain. Recruited Src tyrosine kinase then transphosphorylates the Y929 residue, which is the binding site for the Grb2/mSOS complex. The N-terminal FERM domain can also be activated by growth factor receptor systems, including the VEGF/VEGFR2 system. This event can further induce tyrosine phosphorylation of other sites, which creates additional docking sites for signaling and cytoskeletal proteins, including p130Cas, paxillin, PI-3-kinase and Crk. Inset: Endothelial cells plated onto fibronectin coated glass slide was immunostained with anti-FAK antibody (green) and TRITC-Phalloidin (red); yellow structures indicated by arrows show colocalization at Focal adhesion sites.
Most of the endothelial cell genes including FAK have been deleted in mice using homologous recombination. A common feature of these knockouts is embryonic lethality as a result of abnormal endothelial development. Their corresponding phenotypes, however, are distinctive. For example, conventional deletion of Fak is incompatible with survival because these embryos die in utero owing to defects in cell migration, which are secondary to reduced focal adhesion turnover (Ilic et al., 1995b).
In adults, endothelial cells usually remain in a dormant state (G0-phase of the cell cycle) within the vascular walls, which are subject to microenvironmental regulation. Only a subset of endothelial cells, however, respond to angiogenic stimuli producing tip cells, which are referred to as sprouting endothelial tip cells. The selection process essentially limits the ability of endothelial cells to form angiogenic sprouts in a controlled manner and to perfuse tissue when required. To form endothelial interconnections, tip cells must reduce endothelial cell migratory behavior. This reduction is necessary to establish cell-ECM and cell-cell interactions. These cell adhesion events enhance the generation of blood-transporting vessels by these endothelial cells. Thus, during angiogenesis, endothelial cells receive biochemical cues from soluble and extracellular matrix proteins to elongate, migrate, proliferate, and resist anoikis. A reciprocal relationship is initiated between endothelial cells and the extracellular matrix, favoring angiogenesis. Stromal changes include modifications of the extracellular matrix composition, activation of integrin and VE-cadherin signaling, pericyte or smooth muscle cell recruitment, and involvement of various accessory cells of the immune system. However, angiogenesis involves signaling from not only cell-cell and cell-matrix interactions but also soluble growth factors and cytokines.
How does FAK regulate angiogenesis? Conventional deletion of the Fak gene in mouse embryos resulted in a general defect in mesoderm development, and cells from these embryos exhibited reduced mobility in vitro; significantly, these embryos did not survive beyond day E8.5 to 9.0 (Ilic et al., 1995b). In Fak−/− cells, the number of focal adhesions was increased, suggesting that FAK regulates focal adhesion turnover (assembly and disassembly) during cell migration (Ilic et al., 1995b; Ilic et al., 1998; Ilic et al., 2003). Subsequent studies have shown that FAK not only mediates cell migration but also survival signals from the extracellular matrix protein fibronectin by suppressing p53-mediated apoptosis. If FAK or the correct type of ECM is absent, cells undergo apoptosis through a p53-dependent pathway activated by protein kinase C (Ilic et al., 1995b; Ilic et al., 1998; Ilic et al., 2003), suggesting that FAK has the ability to regulate cell survival (Ilic et al., 1995b; Ilic et al., 1998; Ilic et al., 2003).
To investigate the biology of FAK in angiogenesis, Ilic et al. used FAK+/+ and FAK−/− embryoid bodies, FAK+/+ and FAK−/− endothelial cells, and HUVECs expressing antisense FAK, a dominant-negative fragment of FAK, or wild-type FAK to examine the role of FAK in Matrigel tube formation assays (Ilic et al., 2003). Their results showed that FAK-deficient endothelial cells fail to organize into functional vascular networks. Additionally, in an avian chorioallantoic membrane (CAM) assay, FGF- or VEGF-induced angiogenesis was blocked by FRNK overexpression (Orr and Murphy-Ullrich, 2004; Vadali et al., 2007; Zhao and Guan, 2009; Ilic et al., 1995b; Ilic et al., 1998; Ilic et al., 2003). Conversely, FAK overexpression induced increased angiogenesis in a transgenic mouse model in both hind limb ischemia and wound-induced angiogenesis model (Orr and Murphy-Ullrich, 2004; Vadali et al., 2007; Zhao and Guan, 2009; Ilic et al., 1995b; Ilic et al., 1998; Ilic et al., 2003).
The regulatory role of FAK on angiogenesis has been reinforced by the finding that EC-specific deletion of Fak using a Cre-loxP system led to defective angiogenesis in embryos, yolk sacs, and placentas and to a late embryonic lethal phenotype. Staining of the mutant embryos showed disorganized, detached, and apoptotic endothelial cells. ECs isolated from floxed Fak−/− mice exhibited reduced tube formation, cell survival, proliferation, and migration in vitro. These results indicate a key role of FAK in angiogenesis and vascular development due to its ability to regulate multiple EC activities (Shen et al., 2005). In another study, Fak was deleted in endothelial cells (ECs) in Tie2-Cre mice, in these mice, conditional Fak deletion did not affect vasculogenesis, but mutants exhibited hemorrhagic vessels and lethality between days E10.5 and 11.5. In these mutant embryos, time-lapse microscopy of EC behavior during vascular formation revealed increased cell retraction and death, leading to reduced vessel growth and regression. Accordingly, ECs isolated from mutant embryos displayed unusual lamellipodial extensions and altered cytoskeletal organization. However, the results of this study also suggested that FAK regulated endothelial survival and morphology (Braren et al., 2006). Fak−/− endothelial cells showed increased apoptosis in vivo, indicating FAK regulation of cell survival (Shen et al., 2005; Braren et al., 2006). A more recent study has suggested that endothelial cell migration during murine yolk sac vascular remodeling arises via Rac1 and FAK activation in vivo (Enciso et al., 2010). Endothelial cell migration in large part is mediated by integrin-ECM interactions, that require activation of Src and or FAK, including the action of small GTPases of the Rho family and their modulators such as ASAP1 and Graf. Signaling networks regulated by these proteins generate the motility force for both mesenchymal and endothelial cells.
During embryonic development endothelial cells can go through normal apoptosis. This process known as “vascular pruning,” is especially important to control the architecture of the new vessels. However, during proliferation (e.g., mitosis) and migration, endothelial cells partially detach from the ECM substrate. How do endothelial cells resist apoptosis during these events? The SH3 domain of Src is known to interact with the p85 subunit of PI-3 kinase. NIH3T3 cells that were kept in suspension also showed increased Src activity (Lock P, et al. 1998). In response to integrin ligation, FAK recruits phosphatidylinositol 3-kinases (PI3-K) indirectly through Src tyrosine kinase. The activation of PI3-K results in the synthesis of the second messenger PtdIns(3,4,5)P3 (PIP3) from PtdIns(4,5)P2 (PIP2). This event generates activation of various downstream signals, including the serine/threonine kinase Akt, which regulates cell survival (Vivanco and Sawyers, 2002). Integrin ligation (α6β4) results in the activation of PI3-K, this event is also shown to promote cell elongation and invasion (Shaw et al, 1997). Autophosphorylation of FAK-Y397 following integrin-ligation and or growth factor receptor activation results in the recruitment of PI3-K, thereby promoting PI3-K activation. Thus, endothelial cell FAK can directly or indirectly recruit and activate PI3-K generating a signal to Akt for survival. Survival signaling is critical not only for differentiation of endothelial cells, but also for resistance from anoikis during migration and proliferation. Thus, FAK mediated PI3-K activation appears to regulate angiogenesis at several cellular levels. However, these mechanisms are far from clear. Therefore, it would be interesting to monitor FAK and PI-3 kinase activities, their localization(s), intrinsic to dormant versus activated endothelial cells, to better understand and target this pathway in angiogenesis.
Role of FAK in endothelial cell proliferation and differentiation
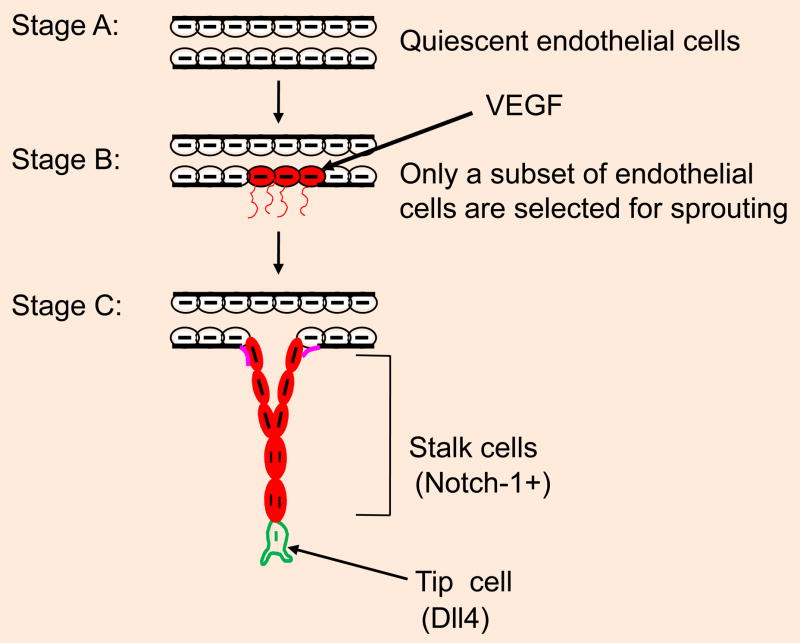
Endothelial cell proliferation is one of the defining hallmarks of angiogenesis. However, mature endothelial cells have also been known to exhibit limited turnover, i.e., these cells have a finite proliferative capacity. Although primary endothelial cells proliferate relatively well in vitro, the proliferation of endothelial cells in vivo is difficult to detect. In particular, the detection of endothelial cell proliferation in developing embryos is technically challenging. In contrast, it is relatively easy to detect endothelial cell proliferation in tumor angiogenesis and Matrigel plug assays. Nevertheless, studies utilizing tumor angiogenesis or developmental models have shown that only a small percentage of endothelial cells are proliferative (Figure 4). Proliferative endothelial cells have been redefined as stalk cells that are Notch-1+, while the tip cells are Dll4+ (Benedito et al., 2009; Phng et al., 2009; Eilken and Adams, 2010; Jakobsson et al., 2010). Integrin-mediated endothelial cell cycle progression through G1-phase is mediated by the adapter protein Fyn and the Shc protein complex, as this complex activated the Ras/Map kinase pathway via a caveolin-1-dependent pathway but not FAK signaling (Wary et al., 1996; Wary et al., 1998). Accordingly, conventional deletion of Shc has been shown to result in profound endothelial cell defects, including reduced endothelial cell proliferation, migration, and angiogenesis (Lai and Pawson, 2000). In addition, in vitro studies have shown that FAK may mediate cell proliferation in cultured cells (Schlaepfer et al., 1994; Schlaepfer et al., 1997; Cary and Guan, 1999; Schaller, 2001; Parsons, 2003; Taylor et al., 2001). To determine the role of FAK in endothelial cell proliferation more directly, two laboratories deleted Fak in endothelial cells (Shen et al., 2005; Braren et al., 2006). One of these groups observed reduced proliferation of endothelial cells in Fak−/− cells in vivo (Shen et al., 2005), though the other group did not observe proliferative defects in these cells, either in vivo or under ex vivo experimental conditions (Braren et al., 2006). In cultured lung endothelial cells, a role for FAK has also been established following ligation of αvβ3 integrin to type IV collagen in Nitric oxide (NO) induced angiogenesis (Wang and Su, 2011).
Fig. 4.
A model of tube formation. Stage A represents the quiescent endothelium. In stages B and C, only a subset of endothelial cells are activated in response to VEGF/VEGFR2 ligation, and this process is characterized by the dissolution of the basement membrane, crawling of endothelial cells along the underlying matrix, and proliferation of these cells (Notch-1+ cells). Stage C depicts endothelial cells synthesizing new basement membrane. Failing to synthesize and organize a functional matrix results in the apoptosis of the endothelial cells, which is a mechanism through which the extent of capillary formation can be minimized or unwanted angiogenesis can be prevented. Tip cells (Dll4+) regulate the extent of endothelial sprouting and stalk cell functioning.
Fak−/− mouse ES cells injected subcutaneously into immunocompromised nude mice gave rise to derivatives of all three germ layers, suggesting that FAK may not be required for endothelial cell differentiation (Ilic et al., 1995a). These data suggest that the differentiation of endothelial cells is not affected by Fak deletion, but the absence of FAK likely affects cell elongation and the interconnections of endothelial cells.
Thus, FAK appears to regulate not only cell migration, focal adhesion disassembly, and cytoskeletal rearrangements, but also endothelial cell proliferation in vivo and endothelial cell permeability (Benedito et al., 2009; Phng et al., 2009; Eilken and Adams, 2010; Jakobsson et al., 2010). These conclusions remain mostly suggestive regarding how FAK may regulate cell proliferation, because only a few cells have been selected to proliferate in vivo. Therefore, it could be worthwhile to reexamine the role of FAK in endothelial cell proliferation in vivo using cutting-edge technology and to determine if there is a link between the formation of stalk cells (proliferative endothelial cell phenotype) and FAK activation following VEGF/VEGFR-2 (FLK1) signaling.
FAK in tumor angiogenesis
Tumor angiogenesis usually indicates increased tumor growth and metastatic phenotypes. Tumor angiogenesis requires both cell adhesion events and VEGF action. The observations that FAK promotes cell migration and proliferation suggested that FAK could be necessary for tumor angiogenesis. Although the overexpression of FAK in tumor cells has been documented in the literature, constitutive overexpression as a result of gene amplification or somatic mutation of FAK has not been observed or linked to any vascular defects. The ability of Flt-1 kinase to regulate tubulogenic activity was dependent on FAK (Maru Y., et al. 2001). Increased expression of FAK in tumor angiogenic vessels has been reported (Haskell et al., 2003). However, interference with FAK activity in 4T1 breast carcinoma cells resulted in reduced VEGF expression, thereby causing small avascular tumors in mice (Mitra et al., 2006). This effect was attributed to the ability of FAK-Y927 to recruit GRB2 and stimulate the Ras-MAPK pathway. Thus, overexpression of FAK in breast tumor cells is likely to enhance VEGF production and aid in the recruitment and angiogenesis of tumors in vivo (Mitra et al., 2006), indicating that the role of FAK in tumor angiogenesis is likely indirect. Other authors have reported that deletion of Fak impaired VEGF-mediated tumor angiogenesis in vivo and in vitro (Tavora et al., 2010 Thus, the function of FAK in tumor angiogenesis is only beginning to be investigated.
Conclusions
Our discussion indicates that FAK is a multifunctional protein in that it regulates non-genomic responses, such as cell migration, cytoskeletal organization, and cell spreading, and genomic responses, including the cell cycle and proliferation, via different mechanisms. The role of FAK in the regulation of cell migration has been studied in vitro and in vivo. Because of the embryonic lethality associated with Fak-knockout mice, the role of FAK in vasculogenesis and angiogenesis is still not completely understood; thus, further investigation will be needed to define and re-define the functions of FAK and at which steps of angiogenesis FAK may act. A clearer understanding of how FAK gene expression is regulated in sprouting endothelial versus quiescent endothelial cells may aid in the development of small molecules targeting FAK at the level of gene transcription. For example, a molecule that inhibits FAK kinase activity may block cell migration, thereby blocking angiogenesis. Accordingly, the development of small molecules targeting the Src-FAK complex could also be useful in improving treatment modalities for diseases, including tumor angiogenesis, atherosclerosis, diabetic retinopathy and psoriasis.
Research highlights.
Focal adhesion kinase (FAK) is a 125kD polypeptide, an intracellular tyrosine kinase that primarily localizes to focal adhesion structures.
Understanding the biology of FAK in the regulation of angiogenesis is a significant topic.
FAK appears to regulate multiple aspects of angiogenesis.
Here we review current literature as they relate to pathophysiology of angiogenesis.
Acknowledgments
We would like to apologize for not being able to accommodate many references of our colleagues who have contributed to the understanding of FAK function.
Sources of Funding
The authors were supported by the National Institutes of Health (R01HL079356; HL079356-03S1), American Heart Association (GRNT4520014) grants and the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS) Award Number UL1RR029879 from the National Center for Research Resources to K.K.W. E.E.K. was supported by T32GM070388 and T32HL072742 NIH training grants.
Footnotes
Competing interests statement.
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- Aird WC. Endothelium in health and disease. Pharmacol Rep. 2008;60:139–143. [PubMed] [Google Scholar]
- Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1997;14:53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009;16:222–231. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CM, D’Amato RJ. Angiogenesis and antiangiogenic therapy in endometriosis. Microvasc Res. 2007;74:121–130. doi: 10.1016/j.mvr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Benedito R, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Braren R, et al. Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J Cell Biol. 2006;172:151–162. doi: 10.1083/jcb.200506184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Cary LA, Guan JL. Focal adhesion kinase in integrin-mediated signaling. Front Biosci. 1999;4:D102–113. doi: 10.2741/cary. [DOI] [PubMed] [Google Scholar]
- Chen MJ, et al. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, et al. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Enciso JM, et al. Endothelial cell migration during murine yolk sac vascular remodeling occurs by means of a Rac1 and FAK activation pathway in vivo. Dev Dyn. 2010;239:2570–2583. doi: 10.1002/dvdy.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber HP, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- Golubovskaya V, Kaur A, Cance W, et al. Cloning and characterization of the promoter region of human focal adhesion kinase gene: nuclear factor kappa B and p53 binding sites. Biochim Biophys Acta. 2004;1678:111–125. doi: 10.1016/j.bbaexp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, et al. The embryonic human choriocapillaris develops by hemo-vasculogenesis. Dev Dyn. 2007;236:2089–2100. doi: 10.1002/dvdy.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell H, et al. Focal adhesion kinase is expressed in the angiogenic blood vessels of malignant astrocytic tumors in vivo and promotes capillary tube formation of brain microvascular endothelial cells. Clin Cancer Res. 2003;9:2157–2165. [PubMed] [Google Scholar]
- Hellström M, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;15:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- Hickey MM, Simon MC. Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. Curr Top Dev Biol. 2006;76:217–257. doi: 10.1016/S0070-2153(06)76007-0. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, et al. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J Cell Biol. 1993;123:993–1005. doi: 10.1083/jcb.123.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JJ, Iruela-Arispe M. Notch expression patterns in the retina: An eye on receptor-ligand distribution during angiogenesis. Gene Expr Patterns. 2007;7:461–470. doi: 10.1016/j.modgep.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D, et al. Focal adhesion kinase is not essential for in vitro and in vivo differentiation of ES cells. Biochem Biophys Res Commun. 1995a;209:300–330. doi: 10.1006/bbrc.1995.1503. [DOI] [PubMed] [Google Scholar]
- Ilic D, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995b;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Ilic D, et al. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol. 1998;143:547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D, et al. Focal adhesion kinase is required for blood vessel morphogenesis. Circ Res. 2003;92:300–307. doi: 10.1161/01.res.0000055016.36679.23. [DOI] [PubMed] [Google Scholar]
- Jakobsson L, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- Kamei M, et al. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- Kalluri R, et al. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- Kume T. Novel insights into the differential functions of Notch ligands in vascular formation. J Angiogenes Res. 2009;1:8. doi: 10.1186/2040-2384-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KM, Pawson T. The ShcA phosphotyrosine docking protein sensitizes cardiovascular signaling in the mouse embryo. Genes Dev. 2000;14:1132–1145. [PMC free article] [PubMed] [Google Scholar]
- Lock P, et al. A new method for isolating tyrosine kinase substrates used to identify fish, an SH3 and PX domain-containing protein, and Src substrate. EMBO J. 1998;17:4346–4357. doi: 10.1093/emboj/17.15.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubarsky P, Krasnow MA. Tube morphogenesis. Making and shaping biological tubes. Cell. 2003;112:19–28. [Google Scholar]
- Lugus JJ, et al. Both primitive and definitive blood cells are derived from Flk-1+ mesoderm. Blood. 2009;113:563–566. doi: 10.1182/blood-2008-06-162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maru Y, et al. The tubulogenic activity associated with an activated form of Flt-1 kinase is dependent on focal adhesion kinase. Biochim Biophys Acta. 2001;1540:147–153. doi: 10.1016/s0167-4889(01)00127-6. [DOI] [PubMed] [Google Scholar]
- Mitra SK, et al. Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene. 2006;25:5969–5984. doi: 10.1038/sj.onc.1209588. [DOI] [PubMed] [Google Scholar]
- Nakatsu MN, et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvasc Res. 2003;66:102–112. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- Orr AW, Murphy-Ullrich JE. Regulation of endothelial cell function BY FAK and PYK2. Front Biosci. 2004;9:1254–1266. doi: 10.2741/1239. [DOI] [PubMed] [Google Scholar]
- Palis J, et al. Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood. 1995;86:156–163. [PubMed] [Google Scholar]
- Park C, et al. A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development. 2004;131:2749–2762. doi: 10.1242/dev.01130. [DOI] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Phng LK, et al. Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. Dev Cell. 2009;16:70–82. doi: 10.1016/j.devcel.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault MA, Losordo DW. Therapeutic myocardial angiogenesis. Microvasc Res. 2007;74:159–171. doi: 10.1016/j.mvr.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Robb L, Elefanty AG. The hemangioblastan elusive cell captured in culture. BioEssays. 1998;20:611–614. doi: 10.1002/(SICI)1521-1878(199808)20:8<611::AID-BIES3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Rossant J, Hirashima M. Vascular development and patterning: making the right choices. Curr Opin Genet Dev. 2003;13:408–412. doi: 10.1016/s0959-437x(03)00080-7. [DOI] [PubMed] [Google Scholar]
- Sabin FR. Origin and development of the primitive vessels of the chick and of the pig. Contrib Embryol Carnegie Inst Washington. 1917;6:61–124. [Google Scholar]
- Sabine FR. Studies on the origin of the blood vessels and of red blood corpuscles as seen in the living blastoderm of chick during the second day of incubation. Contr Embryol. 1920;9:215–262. [Google Scholar]
- Schaller MD, et al. PPI 25FAK, a structurally unique protein kinase associated with focal adhesions. Proc Natl A cad Sci USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, et al. Autonomous expression of a noncatalytic domain of the focal adhesion-associated protein tyrosine kinase pp125FAK. Mol Cell Biol. 1993;13:785–791. doi: 10.1128/mcb.13.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, et al. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. BBA. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, et al. Integrin-mediated signal transduction linked to ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, et al. Fibronectin-stimulated signaling from a focal adhesion kinase–c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997;17:1702–1710. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby F, et al. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Shaw LM, et al. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Shen TL, et al. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol. 2005;169:941–952. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima DT, et al. The mouse gene for vascular endothelial growth factor. Genomic structure, definition of the transcriptional unit, and characterization of transcriptional and post-transcriptional regulatory sequences. J Biol Chem. 1996;271:3877–3883. doi: 10.1074/jbc.271.7.3877. [DOI] [PubMed] [Google Scholar]
- Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;15:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- Swiers G, et al. Hematopoietic stem cell emergence in the conceptus and the role of Runx1. Int J Dev Biol. 2010;54:1151–1163. doi: 10.1387/ijdb.103106gs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavora B, et al. Endothelial FAK is required for tumour angiogenesis. EMBO Mol Med. 2010;2:516–528. doi: 10.1002/emmm.201000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, et al. Selective expression of an endogenous inhibitor of FAK regulates proliferation and migration of vascular smooth muscle cells. Mol Cell Biol. 2001;21:1565–1572. doi: 10.1128/MCB.21.5.1565-1572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadali K, et al. Focal adhesion kinase: an essential kinase in the regulation of cardiovascular functions. IUBMB Life. 2007;59:709–716. doi: 10.1080/15216540701694245. [DOI] [PubMed] [Google Scholar]
- Veikkola T, et al. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203–212. [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Wary KK, et al. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Wary KK, et al. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- Wary KK. Molecular targets for anti-angiogenic therapy. Curr Opin Mol Ther. 2004;6:54–70. [PubMed] [Google Scholar]
- Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 2005;20:180–193. doi: 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]
- Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009;28:35–49. doi: 10.1007/s10555-008-9165-4. [DOI] [PubMed] [Google Scholar]