Abstract
Thermal stability is important for the manufacture, distribution and administration of vaccines, especially in tropical developing countries, where particularly adverse field conditions exist. Current live-attenuated flavivirus vaccines exhibit relatively poor liquid stability in clinical settings, and clinicians are instructed to discard the yellow fever vaccine 1h after reconstitution. We have identified novel combinations of excipients that greatly enhance the thermal stability of live-attenuated DEN-2 PDK-53-based flavivirus vaccine candidates. Liquid formulations comprising a sugar, albumin and a pluronic polymer minimized the loss of flavivirus infectious titer to less than 0.5log(10)pfu after storage for at least 8h at 37°C, 7 days at room temperature or at least 11 weeks at 4°C. Additionally, these formulations prevented reduction of viral infectivity after two freeze-thaw cycles of virus. Formulated candidate vaccines were readily lyophilized and reconstituted with minimal loss of viral titers. In mice, the formulations were safe and did not hinder the ability of the vaccine virus to generate a potent, protective immune response. These formulations provided significantly greater liquid-phase stability than has been reported previously for other flavivirus vaccine formulations. The enhanced thermal stability provided by the formulations described here will facilitate the effective distribution of flavivirus vaccines worldwide.
Keywords: attenuated vaccine, dengue, flavivirus, thermal stability, recombinant viral vaccine
INTRODUCTION
Live-attenuated viruses (LAV) are widely used as vaccines, generating protective immune responses that can greatly reduce the incidence of infectious diseases in humans. LAV can mimic subclinical infection while expressing the full repertoire of viral immunogens and result in long-lived humoral and cell-mediated immunity. However, certain environmental factors can cripple the physical stability and affect the efficacy of LAV vaccines. For example, widespread distribution and use of the smallpox vaccine prior to World War II was limited because exposure of vaccine virus for only few days to elevated ambient temperatures resulted in complete inactivation [1].
Techniques such as lyophilization and use of various additives have been shown to promote the physical stability of live-attenuated vaccines[2]. Currently available flavivirus LAV vaccines, including yellow fever virus (YFV) 17D vaccine, are lyophilized in the presence of stabilizers. Nonetheless, these vaccines require storage and shipment at 2 – 8° C, a requirement that is difficult to achieve in the developing world and more remote regions of developed nations. Furthermore, upon reconstitution the YFV 17D vaccine rapidly loses potency. The yellow fever vaccine label recommends that the reconstituted vaccine should be stored on ice and disposed of after one hour. This limited liquid phase physical stability may result in accidental administration of suboptimal doses, prevents use of multi-dose vials for vaccination campaigns, and inhibits public health distribution in clinical settings where cold-chains are unavailable.
The attenuated dengue type 2 (DEN-2), strain PDK-53 has been used as a viral vector for the engineering of candidate dengue and West Nile vaccines [3–5]. Clinical trials conducted in the U.S. and Thailand have shown that DEN-2 PDK-53 is safe and immunogenic, eliciting both long-term humoral immunity [6–8] and cellular immunity [9, 10]. DEN-2 PDK-53-based chimeric viruses expressing the prM/E gene region of wild-type DEN-1, DEN-3, DEN-4, and West Nile viruses (WNV) have been constructed and shown to have attenuated phenotypes similar to the parental DEN-2 PDK-53 virus [3, 4]. To improve the thermal stability of these DEN-2 PDK-53-based chimeric attenuated vaccine viruses, we screened and identified combinations of excipients that significantly enhanced the thermal stability of these vaccines. When tested in various combinations, the presence of trehalose, F-127 (a polyoxyethylene-polyoxypropylene block copolymer), and albumin synergistically improved the thermal stability of these LAV vaccines.
MATERIALS AND METHODS
Attenuated flaviviruses
DEN-2 PDK-53 virus was generated by transfection of Vero cells by infectious DEN-2 PDK-53 cDNA clone, as described previously [4, 11]. Chimeric DEN-2/DEN-1, DEN-2/DEN-3, DEN-2/DEN-4, and DEN-2/West Nile viruses (all constructed in the attenuated DEN-2 PDK-53 genetic background) were also derived from their respective cDNA clones[4, 5]. Working seeds of these infectious clone-derived flaviviruses, as well as YFV 17D virus were grown in Vero cells and stored in the same serum-free Ex Cell media at −80°C (Sigma-Aldrich, St. Louis, MO) for this thermal stability study. Japanese encephalitis (JEV) SA 14-14-2 strain, was grown in MEM-2%FBS in PER.C6 cells. The YFV 17D vaccine was obtained from a vial of the vaccine (YF-VAX) manufactured by Sanofi Pasteur; JEV SA 14-14-2 virus was also prepared from the commercial vaccine.
Cell culture
Vero cells were grown in Dulbecco’s modified minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS, HyClone Laboratories, Logan, UT), sodium bicarbonate (3.7 g/L: GIBCO-BRL, Life Technologies, Gaithersburg, MD), and containing penicillin/streptomycin. Cultures were incubated in 5% CO2 at 37°C. Virus plaque titrations were performed under double agarose overlay in six well plates of confluent Vero cells as described previously [3]. A 100-μL inoculum of virus was adsorbed for 1.5 hours (hr) at 37°C, followed by the addition of agarose overlay medium containing 1% SeaKem LE agarose (FMC BioProducts, Rockland, ME) in nutrient medium. The second overlay containing neutral red stain was added 7 days (d) after infection, and plaques were counted 9–11 d post infection.
Excipients and liquid stability studies
Several substances were tested as stabilizing vehicles for live-attenuated flavivirus vaccine. These excipients included Pluronic® block copolymers F127 (Sigma-Aldrich), F68 (BASF), P85 (BASF) and P123 (BASF), human serum albumin (HSA), bovine serum albumin (Sigma-Aldrich) the linear polysaccharide chitosan (Sigma-Aldrich), trehalose (Sigma-Aldrich), sucrose (Sigma-Aldrich), lactose (MP-BioMedicals, Solon, OH), Tween-20 and Tween-80 (Sigma-Aldrich), and DEAE-β-cyclodextran 0.5–5% (Carbomer, San Diego, CA). Both rice-derived (Cellastim®, InVitria, Fort Collins, CO) and yeast-derived (New Century Pharmaceuticals, Huntsville, AL) recombinant HSA (rHSA), as well as native HSA (Grifols USA, Los Angeles, CA) were tested. Calcium chloride and magnesium sulfate were also included in some formulations. Stock solutions of each excipient were prepared in PBS and the pH adjusted to 7.2, except for chitosan where the pH of the stock solution was adjusted to approximately 6.8. Single excipients or combinations of excipients were diluted further in PBS to obtain the final working concentrations (w/v). Vaccine stability was examined by incubating 104 plaque forming units (pfu) of virus in a total volume of 0.5mL of PBS or working excipient solution in 1.7mL tubes at 4°C, −80°C, room temperature (~ 25°C) or 37°C. Following incubation, the remaining viral infectious titer was determined by plaque titration assay in Vero cells as described above. For each stability timepoint, a 10-fold dilution series was generated (beginning with a 1:10 dilution). 100–200μL of each diluted sample was adsorbed on Vero cell monolayers, providing an assay detection limit of 50 or 100 pfu or 0.25–0.5% of the initial input virus. For the freeze-thaw experiment, 104 pfu of DEN-2 PDK-53 vaccine candidate virus was suspended in 0.5 mL of each composition, stored at −80°C for 24 hrs and thawed gradually at RT for 60 minutes. This was immediately followed by a second freeze-thaw cycle where the vials were frozen at −80°C for 1 hr and thawed at RT 60 minutes. Viral infectivity was then assessed by plaque titration assay. Samples were tested in triplicate in each stability experiment, and each experiment was repeated at least twice. Experimental results shown in the figures and tables are mean values of triplicate samples from a representative stability experiment.
Lyophilization
Formulated vaccines were placed in vaccine vials and subjected to lyophilization procedures using a VirTis freeze-dryer with controlled lyophilization cycles. Dried vaccines were stoppered under vacuum, stored at 37°C, room temperature, or 4°C for the indicated length of time, and then reconstituted to the original liquid volume by addition of sterile water. Viral infectivity of the reconstituted vaccine was assessed by plaque titration on Vero cells.
Immunogenicity and protection in mice
Neutralizing antibody responses were tested in female NIH Swiss mice (Harlan Sprague Dawley, Indianapolis, Indiana). Groups of 8 or 9 mice were immunized by subcutaneous (sc) injection with 105 pfu of DEN-2/WN candidate vaccines in four different excipient solutions or PBS, on day 0 (d0), and then boosted with the same vaccine on d29. Mice were challenged intraperitoneally (ip) with a lethal dose (103 pfu) of the pathogenic, wild-type West Nile strain NY99, on d45. Four groups of control mice (n=8) each received one of the four excipient solutions. Sera were collected prior to immunization on d0, prior to boost on d28, prior to challenge on d44, and post-challenge on d75. Mice were monitored every day for signs of illness after primary and secondary immunizations. Signs of morbidity including weight loss, rough fur, lethargy, erratic movement, and paralysis were considered as non-survival endpoints requiring euthanasia. Mice were further monitored for 28 days after challenge with wild-type WNV virus. The animal study protocol was reviewed and approved by the Institutional Animal Care and Use Committee at the Division of Vector Borne Diseases, CDC.
Neutralization assays
West Nile neutralizing antibody titers in the mouse sera were determined by plaque reduction neutralization test (PRNT). The PRNT was performed in six-well plates of Vero cells as described previously [3]. The mouse sera were heat inactivated (56°C for 30 min), and the tests were performed without addition of exogenous complement. Back titrations of the input WNV NY99, a clinical isolate from the CDC collection, were included in each assay. The neutralizing antibody titer was identified as the highest serum dilution (serial two-fold dilution series) that reduced the number of input virus plaques in the test by at least 50% (PRNT50).
RESULTS
Initial screen of pharmaceutically accepted excipients
The DEN-2 PDK-53 and chimeric DEN-2/WN vaccines were used as representatives of live-attenuated flaviviruses to determine the effect of various excipients on viral stability at different temperatures. Liquid preparations of viruses were formulated with a variety of excipients including trehalose (5–30%), sucrose (5–20%), lactose (2–15%), chitosan (0.05–0.5%), proteins (human serum albumin, FBS, BSA), surfactants (Tween-20, Tween-80 0.001–2%, DEAE-β-cyclodextran (0.5–5%) ), and Pluronic® block copolymers (F127, F68, P123, P85) in phosphate-buffered saline. Following incubation of 104 pfu of DEN-2 PDK-53 virus in PBS alone for ~21 hrs at 37°C, infectious virus was undetectable (< 0.25–0.5% of the input virus remaining) (Table 1). Mixture with individual excipients such as 15% trehalose, 2% F127, 1% rHSA (rice), or 0.05% chitosan imparted minimal but reproducible stabilization of 1.0 – 5.5% of the input virus, under these conditions (Table 1). Other excipients tested had no reproducible effect on virus stability (data not shown). Results of comparing certain pairs of excipients (15% trehalose + 2% F127, 15% trehalose + 1% rHSA, 2% F127 + 0.05% chitosan, 2% F127 + 1% rHSA) clearly indicated the significant stabilizing effect of rHSA, which resulted in 18.0 and 20.3% viral survival in two of these formulations (Table 1). The two excipient pairs lacking rHSA resulted in only 1.2 and 3.0% viral survival values, which were significantly lower than that afforded by either rHSA-containing pair (p< 8.4E-7, Student’s t-test). Interestingly, certain combinations of excipients containing F127, trehalose and rHSA (hereafter referred to as FTA), imparted the greatest stability levels of 32.7 – 45.3% viral survival after ~21 hrs at 37°, versus formulations containing one or two excipients (p<8.9E-5, Table 1). Unexpectedly, the combined stabilizing effect of three excipients (trehalose, F127, and HSA) was greater than the sum of those observed with each individual component, suggesting synergy between the components. The addition of chitosan (40.0% survival) or increasing the rHSA concentration from 1% to 2% (45.3%) resulted in modest enhancement of viral survival afforded by the 15%T + 2%F + 1%A formulation (32.7%) (Table 1).
Table 1.
Effects of formulations on the stability of DEN-2 PDK-53 virus at 37°C.
| Formulation1 | % viral titer Remaining2,3 | P value4 Compared to FTA (15%T+2%F+1%A) |
|---|---|---|
| PBS | <0.5 ± 0.0 | 1.2E-07 |
| 15% Trehalose (T) | 5.1 ± 0.2 | 1.1E-06 |
| 2% F127 (F) | 2.4 ± 0.1 | 8.4E-07 |
| 1%rHSA (recombinant-rice) (A) | 5.5 ± 0.2 | 7.5E-07 |
| 0.05% Chitosan (C) | 1.0 ± 0.3 | 6.8E-07 |
| 15%T+2%F | 3.0 ± 1.7 | 8.0E-06 |
| 15%T+1%A | 18.0 ± 1.7 | 0.00013 |
| 2%F+0.05%C | 1.2 ± 0.3 | 8.9E-5 |
| 2%F+1%A | 20.3 ± 3.2 | 0.0016 |
| 15%T+2%F+1%A (FTA) | 32.7 ± 3.1 | NA |
| 15%T+2%F+1%A+0.05%C | 40.0 ± 3.5 | 0.0127 |
| 15%T+2%F+ 2%A (FTA) | 45.3 ± 2.3 | 0.0005 |
Approximately 104 pfu of virus was incubated in 500 μL of the indicated formulation.
Percent viral titer remaining after incubation at 37°C for ~21 hours.
Average ± SD of triplicate samples, data representative of an experiment performed three times.
p-value determined by Student’s t-test.
Kinetics of flavivirus inactivation at 37°C
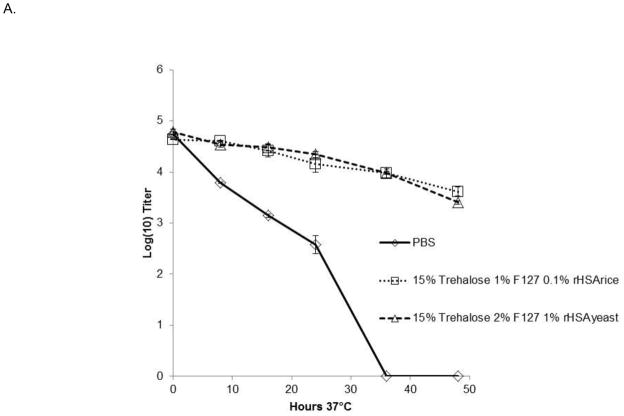
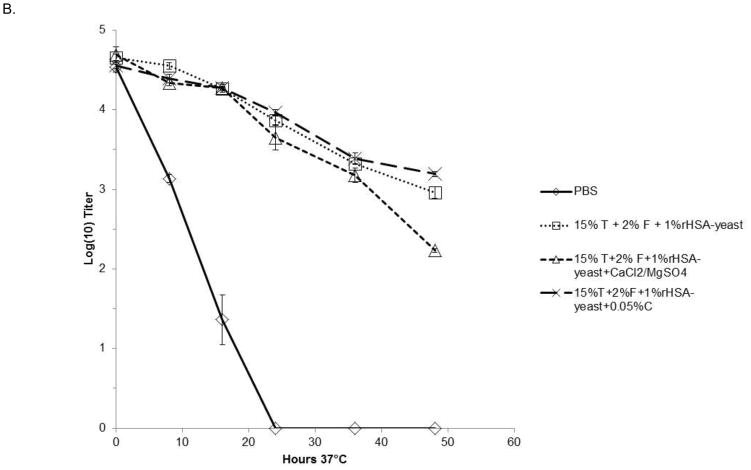
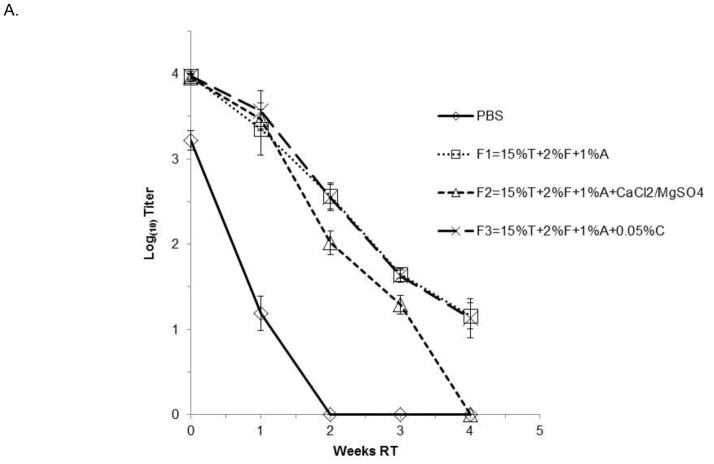
The kinetics of inactivation of both DEN-2 PDK-53 and DEN-2/WN candidate vaccine viruses were further investigated by assessing viral stability in select formulations at 37°C for 48 hrs. Aliquots were collected at indicated time points and titrated on Vero cell monolayers. As shown in Fig. 1A, formulations containing 15% trehalose, 1 or 2% F127 and 0.1% rice-derived rHSA or 1.0% yeast-derived rHSA, resulted in less than 1 log10 pfu titer loss over 36 hours for the DEN-2 PDK-53 virus. The different concentrations of F127 and rHSA showed equivalent improvements to viral stability, compared to PBS alone in which virus was undetectable after 36 hrs at 37°C (Fig. 1A). Additionally, we tested DEN-2/WN virus in formulations containing 0.9mM Ca2+ and 0.5mM Mg2+, or 0.05% chitosan, as well as 15% trehalose, 2% F127 and 1% yeast-derived rHSA (Fig. 1B). All three formulations resulted in the loss of less than 1 log10 pfu of DEN-2/WNV during the first 24 hours of incubation at 37°C, versus PBS alone in which the virus was undetectable after 24 hours. The addition of divalent cations had minimal, or perhaps a slightly negative impact, on long term liquid phase viral stability in the context of formulations containing trehalose, F127 and rHSA. The addition of 0.05% chitosan provided similar stabilizing effects to formulations containing a combination of only trehalose, F127 and rHSA (Fig. 1B).
Figure 1. Kinetics of flavivirus inactivation at 37°C.
Triplicate samples of virus were incubated with the FTA formulations for 48 hours at 37°C. Graphs depict mean values ± s.d. (A). Kinetics of DEN-2 PDK-53-V viral inactivation at 37°C in FTA containing rice or yeast HSA. (B). Kinetics of DEN-2/WN viral inactivation at 37°C in FTA contain trehalose (T), F127 (F) and rHSA (derived from rice or yeast) in PBS. Two formulations (in B) also contained 0.0009M CaCl2 and 0.0005M MgSO4, or 0.05% chitosan (C). Titers shown at zero represent undetectable titers, with the detection limit being 1.7 log10pfu.
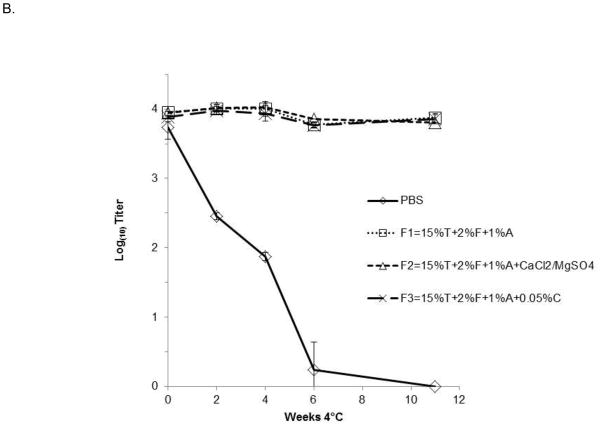
Kinetics of flavivirus inactivation at various temperatures
To further investigate the effectiveness of combination formulations containing F127, trehalose, and rHSA (FTA), as well as FTA containing chitosan or divalent cations, we tested the stability of DEN-2/WNV in liquid compositions stored for various lengths of time at room temperature (RT,~25°C, Fig. 2A), 4°C (Fig. 2B), or −80°C (data not shown). Approximately 104 pfu of virus were incubated at each temperature. Figure 2 demonstrates that formulations containing FTA significantly improved the thermal stability of the virus during storage at RT and 4°C. At RT, loss of DEN-2/WN viral activity was less than 1 log10 pfu over 7 days (Fig. 2A), as compared to 2.0 log10 pfu of viral titer loss when incubated in PBS alone. Significantly, virus formulated in FTA was very stable for long periods of time at 4°C. Titer reduction of DEN-2/WNV was negligible during the 11 weeks of testing at 4°C (Fig. 2B). Again, addition of calcium and magnesium, or chitosan, to the FTA formulation did not improve viral stability (Fig. 2A, B). Observation of minimal DEN-2 PDK-53 viral titer loss at 4°C and −80°C for a period of eight weeks (data not shown) suggested that the thermal stability of these candidate flavivirus vaccines in a liquid FTA formulation is comparable in a standard refrigerator or in a deep freezer. In other testing, we demonstrated that the source of HSA did not influence the stabilizing capability of this component of FTA. FTA stabilization of viral titer was equivalent in formulations containing rHSA from rice, rHSA from yeast, and native HSA purified from humans (data not shown).
Figure 2. Kinetics of flavivirus virus inactivation at various temperatures.
DEN-2/WN virus was mixed with the indicated FTA formulations and stored for (A) 4 weeks at room temperature (~25°C), and (B) for 11 weeks at 4°C. Triplicate aliquots were taken at the indicated time points. Graphs depict mean values ± s.d. FTA formulations contain 15% trehalose (T), 2% F127 (F), 1% rHSA-yeast (1%A) in PBS. Two of the formulations also contained either 0.0009M CaCl2 and 0.0005M MgSO4, or 0.05% chitosan (C). Titers shown at zero represent undetectable titers, with the detection limit being 1.7 log10pfu.
Stability of flaviviruses after freeze-thaw
During storage and shipping, vaccines are often inadvertently subjected to potentially detrimental cycles of freezing and thawing. This ultimately leads to expensive wastage, as these vaccines would be discarded according to cGCP guidelines. Developing a formulation which would maintain vaccine viability upon freeze thaw would be advantageous for vaccine manufacturing and commercial distrubition. We therefore examined the ability of FTA and its component excipients to preserve viral activity after two freeze-thaw cycles (Table 2). Single-component formulations containing F127 (21.0% viral titer remaining) or trehalose (27%), but not rHSA alone (1.2%); dual-component formulations with trehalose/F127 (53.2%), trehalose/HSA (86.0%), and F127/HSA (67.7%); and fully formulated FTA showed incremental improvements to viral stability upon freeze thaw, versus PBS alone (1.2%) (Table 2). FTA, the most protective formulation tested, preserved 100% of DEN-2 PDK-53 viral activity through two freeze-thaw cycles, which was similar to the 96.8% viability of the virus stored in 20% fetal bovine serum (Table 2). The results of this experiment suggested that FTA is an effective cryoprotectant for flaviviral seeds and vaccines, and may facilitate viral preservation to freeze-thaw cycles during manufacture, early clinical testing and during vaccine distribution.
Table 2.
Effects of formulations on freeze-thawing of DEN-2 PDK-53 virus.
| Formulation1 | % viral titer remaining2,3 |
|---|---|
| PBS | 1.2 ± 0.02 |
| 2% F127 | 21.0 ± 0.02 |
| 1% rHSAyeast | 1.2 ± 0.01 |
| 15% Trehalose | 27.4± 0.03 |
| Trehalose/F127 | 53.2 ± 0.08* |
| Trehalose/rHSAyeast | 86.0 ± 0.09 |
| F127/rHSAyeast | 67.7 ± 0.21* |
| FTAyeast | 100 ± 0.10 |
| DMEM 20% FBS | 96.8 ± 0.16 |
Approximately 104 pfu of virus was incubated in 500 μL of the indicated formulation.
Percent viral titer remaining after two freeze-thaw cycles at −80°C and room temperature.
Average ± SD of triplicate samples.
Indicates a P value of <0.25 compared to FTA by Student’s t-test.
Stability of lyophilized vaccine virus with FTA
DEN-2 PDK-53 virus formulated with 2% F127, 15% trehalose, and either 1% (FTA-1%A) or 0.1% (FTA-0.1%A) yeast-derived rHSA was lyophilized. The rehydrated samples retained full input viral titer following incubation of lyophilized vial for 5d at 4°C followed by reconstitution (data not shown), demonstrating initial effective preservation of a dehydrated viral vaccine utilizing compositions containing FTA. More importantly, lyophilized virus incubated for 14d at 37°C showed decreases of only 1.1 – 1.2 log10 pfu of viral titer (Table 3). These results suggested that, at high temperatures, lyophilized FTA-formulated virus was more stable than FTA-formulated virus in liquid form. The lyophilized FTA-formulated virus was stable for long term storage at room temperature as indicated by the 0.59–0.764 log10 pfu loss in viral titer after 195d (Table 3). FTA compositions containing 1 and 0.1% rHSA were equally effective (Table 3).
Table 3.
Effects of formulations on lyophilizing DEN-2 PDK-53 virus.
| Temperature/Time | Log10loss in viral titer±SD1 | Formulation2 |
|---|---|---|
| 37°C 14 days | 1.1 ± 0.53 | FTA-1%A |
| 1.1 ± 0.21 | FTA-0.1%A | |
| Room Temp. 195 days | 0.59 ± 0.04 | FTA-1%A |
| 0.76 ± 0.04 | FTA-0.1%A |
Approximately 104 pfu of DEN-2 PDK-53 virus was diluted in 500 μL of the indicated formulation prior to lyophilization, and then incubated at the indicated temperature/time combinations.
Formulations comprised 15% trehalose, 2% F127, and either 1%, or 0.1% rHSA (yeast).
Stability of various chimeric flaviviruses
To determine whether FTA was also an effective formulation for liquid phase stabilization of other flavivirus vaccine strains, we compared the stability of DEN-2 PDK-53 candidate vaccine virus to the Sanofi Pasteur YFV 17-D (YF-VAX) and the JEV SA 14-14-2 live-attenuated vaccine viruses. Approximately 104 pfu of virus was incubated in 0.5mL of PBS, FTA containing rHSA (rice) or native HSA, or a stabilizing formulation proposed for future manufacture of the YFV 17D vaccine (2% sorbitol, 4% lactose, 1mM CaCl2, 0.5mM MgSO4, 10mM histidine, and 10mM alanine in PBS) [12] for ~21 hours at 37°C (Table 4). The DEN-2 PDK53 and JEV SA 14-14-2 viruses displayed 55.2 – 62.6% and 55.7% stability after incubation in FTA, versus 5.3% and 16.0% survival in PBS alone, respectively (Table 4). In contrast, the YFV 17D virus displayed equivalent stabilities after incubation in FTA (44.1%) or PBS (41.7%). Interestingly, the formulation containing sorbitol and lactose afforded the DEN-2 PDK-53 virus no stability advantage versus PBS alone (0.7% versus 5.3% virus remaining, respectively). Additionally, we tested the liquid stability of chimeric flaviviruses DEN-2/DEN-1, DEN-2/DEN-3, and DEN-2/DEN-4 viruses [4]. Each of these DEN-2 PDK-53-based chimeras in FTA displayed the same level of stability as the DEN-2 PDK-53 virus at room temperature (48 hrs), 4°C (48 hrs), and 37°C (~21 hrs) (data not shown). In addition to the earlier data presented for DEN-2 PDK-53 virus and the DEN-2/WNV chimera, these results suggested that the FTA composition may be useful for liquid phase stabilization of diverse members of the family of Flaviviridae.
Table 4.
Effects of formulations on the stability of three attenuated flaviviruses at 37°C.
| virus | formulation | % remaining after ~21 hours 37°C |
|---|---|---|
| DEN-2 PDK-53 | FTA1 0.1% recombinant | 62.6 ± 11.2 |
| DEN-2 PDK-53 | FTA1 0.1% native | 55.2 ± 5.3 |
| DEN-2 PDK-53 | Sorbitol/Lactose2 | 0.7 ± 0.7 |
| DEN-2 PDK-53 | PBS | 5.3 ± 1.5 |
| Yellow Fever 17D | FTA1 0.1% native | 44.1 ± 4.5 |
| Yellow Fever 17D | PBS | 41.7 ± 5.6 |
| JE SA 14-14-2 | FTA1 0.1% native | 55.7 ± 22.7 |
| JE SA 14-14-2 | PBS | 16.0 ± 7.7 |
FTA (with 15% Trehalose, 1–2% F127, 0.1% recombinant rice-derived human serum albumin or 0.1% purified human native serum albumin).
2% Sorbitol+4%Lactose+1mM CaCl2/0.5 mM MgSO4+10mM histidine+10mM alanine.
Safety and efficacy of formulated chimeric DEN-2/WNV vaccine in mice
The effect of excipients on the safety and efficacy of live attenuated DEN-2/WNV vaccine was determined in mice. Outbred mouse models have been well established for West Nile virus but not for dengue virus infection. Mice were immunized with 105 pfu of DEN-2/WNV diluted in five different excipient combinations: (Group 1) 15% trehalose, 2% F127, 1% rHSAyeast; (Group 2) 15% trehalose, 2% F127, 1% rHSAyeast, 1mM CaCl2/0.5mM MgSO4; (Group 3) 15% trehalose, 2% F127, 1% rHSAyeast, 0.5% chitosan; (Group 4) 22.5% trehalose, 3% F127, 1.5% rHSAyeast; and (Group 5) PBS alone (Table 5). Control groups (n=8) received the excipient solutions used for the DEN-2/WN virus formulation. (Table 5). All excipients were well tolerated as there was no evidence of illness following primary or secondary immunizations (Table 5). The majority of the animals receiving the DEN-2/WNV vaccine seroconverted after the first dose regardless of formulation, and all of the vaccinated animals seroconverted after the booster administration (Table 5). Geometric mean PRNT titers (GMT) showed few differences among the treatment groups. Low reciprocal GMT of 10 – 30 were evident after primary immunization. Reciprocal GMT increased to 109 – 226 after the boost, and then showed a dramatic anamnestic response following challenge with the WNV NY99 virus (Table 5). With the exception of a single mouse in Group 4, DEN-2/WNV formulations fully protected against lethal WNV NY99 viral challenge. One mouse in Group 4 became ill on day 13 after WNV challenge, and was euthanized. This mouse seroconverted with 1:20 WN viral-neutralizing antibody before challenge. We are not certain whether the morbidity in this mouse was caused by the WNV viral challenge or by a different, unexpected cause, since all other mice with ≥ 1:10 neutralizing antibody against WNV survived without any sign of illness. Only 21.9% of the pooled controls animal group survived; those that did survive showed evidence of potent neutralizing antibody responses after challenge. It is common for some naïve, older mice to survive challenge with WN NY99 virus [5].
Table 5.
Effects of formulations on the immunogenic and protective efficacies of DEN-2/WN virus in mice.
| Group5 | N | Post-primary (d28) | Post-boost (d44) | Post-Challenge (d75) | Survival | Survive% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GMT1 | SD2 | SC3% | GMT1 | SD2 | SC% | GMT4 | SD4 | SC4% | ||||
| 1 | 8 | 1.48 | 0.68 | 87.5% | 2.09 | 0.45 | 100.0% | 2.88 | 0.52 | 100.0% | 8/8 | 100.0% |
| 2 | 8 | 1 | 0.85 | 62.5% | 2.35 | 0.62 | 100.0% | 2.92 | 0.52 | 100.0% | 8/8 | 100.0% |
| 3 | 8 | 1.6 | 0.45 | 100.0% | 2.09 | 0.62 | 100.0% | 3.26 | 0.3 | 100.0% | 8/8 | 100.0% |
| 4 | 9 | 1 | 0.8 | 66.7% | 2.14 | 0.72 | 100.0% | 3.22 | 0.4 | 100.0% | 8/9 | 88.9% |
| 5 | 9 | 1 | 1 | 66.7% | 2.04 | 0.74 | 100.0% | 3.24 | 0.38 | 100.0% | 9/9 | 100.0% |
| Control | 32 | 0 | NA | NA | 0 | NA | NA | 3.11 | 0.32 | 100.0% | 7/32 | 21.9% |
GMT = geometric mean PRNT titer in Log10 value; titers under detection limit (<10) arbitrarily assigned a value of 1 (=0Log10) for calculation.
SD = standard deviation of GMT in Log10 value.
% SC = percentage of animals that sero-converted with detectable PRNT titers (>10).
Results represent surviving animals post challenge.
FTA formulation of groups: 1) 15% Trehalose, 2% F127, 1% rHSA yeast; 2)15% Trehalose, 2% F127, 1% rHSA, 1mM CaCl2/0.5mM MgSO4; 3) 15% Trehalose, 2% F127, 1% rHSA, 0.5% chitosan; 4) 22.5% Trehalose, 3% F127, 1.5% rHSA; 5) PBS.
DISCUSSION
Previous studies have assessed the stability of flavivirus vaccines using the existing yellow fever and Japanese encephalitis live-attenuated viruses. The unstabilized lyophilized YF 17D vaccine loses 1.5–2.5 log10/dose (up to 99.7% ) after14 days at 37°C [12]. A combination of lactose, sorbitol, the divalent cations calcium and magnesium, and at least one amino acid significantly added to the stability of the lyophilized yellow fever vaccine [12], reducing the vaccine titer loss to less than 1 log after incubation at 37° C for 14 d (U.S. Patent No. 4,500,512). However, this formulation was not effective at preserving DEN-2 PDK-53- candidate vaccine viral titer for 21 hrs at 37°C in the present study. Another study determined that formulations consisting of 10% sucrose alone, 2% sorbitol with 4% inositol, or 10% sucrose with 5% lactalbumin, 0.1g/L CaCl2 and 0.076 g/L MgSO4 provided the best stability for YFV 17D [13]. However, in all cases after reconstitution, yellow fever vaccine is still very unstable, rapidly losing titer after 2 hrs at 37°C, and must be discarded after only about one hour at room temperature [12, 13]. This instability leads to vaccine wastage and the potential administration of an ineffective vaccine dose under field conditions. Another live-attenuated flavivirus vaccine against Japanese encephalitis, JEV SA 14-14-2, has been widely distributed in China [14]. One stabilizing formulation that included 1% gelatin and 5% sorbitol afforded stabilization of the lyophilized vaccine for at least 1.5 yrs at 2–8° C, 4 months at RT, and 10 ds at 37°C. However, the reconstituted liquid vaccine is very labile and its infectious titer decreased significantly after 2 hours at RT [15]. Although the lyophilized measles mumps and rubella vaccine is stable for months at various temperatures, the reconstituted vaccine loses 2/3 of its infectivity after seven hours at 37°C[16].
We have demonstrated improved stability of our live-attenuated flavivirus vaccine candidates with the combination of rHSA, trehalose, and the polyoxyethylene-polyoxypropylene (EO-PO) block copolymer F127. The synergistic effect of these excipients suggests that interactions between these three components and/or between the components and the viral particles contribute to their stabilizing mechanism. The stabilizing role of the albumin in the FTA formulation is not simply as a general carrier protein; other proteins such as gelatin and lactoferrin failed to improve virus stability in our testing (data not shown). We have tested a variety of sources of HSA including recombinant yeast, recombinant rice, and native, human derived proteins, and all provided similar levels of stability for FTA-formulated virus. Use of rHSA would be advantageous, as this would mitigate potential hazards associated with use of human-derived products.
Surfactants have been incorporated into vaccine formulations to prevent material loss to surfaces such as glass vials [17]. However, commonly used pharmaceutical surfactants such as Polysorbate 20 (Tween 20) were not effective in stabilizing DEN-2 PDK-53, relative to formulations containing a pluronic copolymer (data not shown). Our studies suggested better stabilizing efficiencies with formulations containing distinct, high molecular weight pluronic copolymer surfactants. The pluronic block copolymers have been investigated as potential delivery agents for a variety of hydrophobic drugs, proteins, DNA, or inactivated vaccines [18, 19]. F127 has also been tested as a carrier for a lentiviral vector to cells of the central nervous system [20]. Interestingly, non-ionic block copolymers have also been rationally designed to act as adjuvants, and the type of immune response varies by both size and polyolyethylene content [21]. However, in our studies the FTA formulation does not significantly enhance the immunogenicity of the DEN-2/WN vaccine.
The surfactants studied here display some unusual biochemical properties. Pluronic F127 is a non-ionic polyoxyethylene-poloxypropylene block copolymer, consisting of hydrophilic ethylene oxide and hydrophobic propylene oxide blocks. F127 (at concentrations >10%) undergoes a process known as reverse thermogelation, as it undergoes a phase transition from liquid to a gel upon reaching physiological temperatures. Additionally, when these block copolymers reach above the critical concentration they assemble into micelles in aqueous solutions, which is also dependent on temperature [22]. The critical micelle concentration is 500μM at 20°C, and 2μM at 37°C, respectively [22]. Under the conditions evaluated here, the FTA formulations have inadequate concentrations to promote reverse thermogelation, but the 1–2% (approximately 800–1600 μM) F127 concentrations tested here permit micelle formation at room temperature and 37°C. The unique biophysical properties of F127 also promote the association of F127 with membranes, and these attributes may result in intercalation of F127 within the membrane of the enveloped flavivirus, promoting its stability.
The studies presented here have successfully identified a combination of pharmaceutically accepted excipients (FTA) which significantly enhances the liquid and freeze-dried thermal stability of candidate DEN-2 PDK-53-based flaviviral vaccines. The excipient components comprising FTA are currently present in approved vaccines, so regulatory issues associated with these components for a live-attenuated vaccine are minimal. We showed that a formulation consisting of 15% trehalose, 1–2% F127 and 0.1–2% human serum albumin synergistically improved the thermal stability of these candidate vaccine viruses. This FTA formulation permitted stable storage of our liquid vaccine for at least 8 hours at 37°C, for 1 week at 25°C, and for at least 11 weeks at 4°C, all with less than 0.5 log titer loss. The formulated vaccine also maintained full viral activity after two freeze/thaw cycles. In addition, FTA formulations were safe in mice and did not affect the immunogenicity of the candidate DEN-2/WN vaccine. Although FTA formulations did not perform better than existing formulations used for stabilization of the liquid yellow fever vaccines, they were effective at stabilizing the DEN-2 PDK-53 and JEV SA 14-14-2 viruses in liquid-phase. Interestingly, YFV17D virus in PBS control was quite stable at 37°C for 21 hours in our experiment, and FTA did not significantly improve the stability (Table 4). FTA did not show a stabilizing effect for the liquid YFV 17D vaccine, but did constitute a highly effective stabilizer for DEN-2 PDK-53 virus and its associated DEN-1, DEN-3, DEN-4 and WN chimeras, as well as the JEV SA 14-14-2. In reviewing the phylogeny of the genus Flavivirus, we hypothesize that the formulation is effective for the dengue virus clade and the Japanese encephalitis virus clade (of which West Nile is a member) but not the yellow fever clade of mosquito-borne flaviviruses [23].
We have shown that FTA formulations enhanced thermal stability of the tested live-attenuated vaccine candidates. Based on these data, the formulations appeared to be safe and had no adverse effect on the immunogenic and protective efficacy of the DEN-2/WNV vaccine. In other mouse experiments, FTA did not appear to afford any dose-sparing effect for the candidate DEN-2/WN vaccine (data not shown). Further development of this novel combination of excipients will facilitate distribution of live-attenuated flavivirus vaccines to populations living in areas where maintaining the “cold chain” is difficult. Currently, Inviragen’s DEN-2-PDK-53-based tetravalent dengue vaccine (DENVax), formulated in FTA containing native HSA, is being evaluated for safety and efficacy in Phase 1 clinical trials in healthy adults.
Acknowledgments
We would like to thank Kaitlyn Mulhern for her contribution to this study.
Funding. This work was supported by NIH grant # 1 U01 AI070443-01 and a Rocky Mountain Regional Centers for Excellence grant.
List of Abbreviations Used
- FTA
DEN, WN
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collier LH. The development of a stable smallpox vaccine. J Hyg. 1955;53:76–101. doi: 10.1017/s002217240000053x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandau DT, et al. Thermal stability of vaccines. J Pharm Sci. 2003;92(2):218–31. doi: 10.1002/jps.10296. [DOI] [PubMed] [Google Scholar]
- 3.Huang CY, et al. Chimeric dengue type 2 (vaccine strain PDK-53)/dengue type 1 virus as a potential candidate dengue type 1 virus vaccine. J Virol. 2000;74(7):3020–8. doi: 10.1128/jvi.74.7.3020-3028.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang CY, et al. Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. J Virol. 2003;77(21):11436–47. doi: 10.1128/JVI.77.21.11436-11447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang CY, et al. Chimeric dengue 2 PDK-53/West Nile NY99 viruses retain the phenotypic attenuation markers of the candidate PDK-53 vaccine virus and protect mice against lethal challenge with West Nile virus. J Virol. 2005;79(12):7300–10. doi: 10.1128/JVI.79.12.7300-7310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhamarapravati N, et al. Immunization with a live attenuated dengue-2-virus candidate vaccine (16681-PDK 53): clinical, immunological and biological responses in adult volunteers. Bull World Health Organ. 1987;65(2):189–95. [PMC free article] [PubMed] [Google Scholar]
- 7.Kanesa-thasan N, et al. Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers. Vaccine. 2001;19(23–24):3179–88. doi: 10.1016/s0264-410x(01)00020-2. [DOI] [PubMed] [Google Scholar]
- 8.Vaughn DW, et al. Testing of a dengue 2 live-attenuated vaccine (strain 16681 PDK 53) in ten American volunteers. Vaccine. 1996;14(4):329–36. doi: 10.1016/0264-410x(95)00167-y. [DOI] [PubMed] [Google Scholar]
- 9.Dharakul T, et al. Dengue virus-specific memory T cell responses in human volunteers receiving a live attenuated dengue virus type 2 candidate vaccine. J Infect Dis. 1994;170(1):27–33. doi: 10.1093/infdis/170.1.27. [DOI] [PubMed] [Google Scholar]
- 10.Rothman AL, et al. Induction of T lymphocyte responses to dengue virus by a candidate tetravalent live attenuated dengue virus vaccine. Vaccine. 2001;19(32):4694–9. doi: 10.1016/s0264-410x(01)00236-5. [DOI] [PubMed] [Google Scholar]
- 11.Kinney RM, et al. Construction of infectious cDNA clones for dengue 2 virus: strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology. 1997;230(2):300–8. doi: 10.1006/viro.1997.8500. [DOI] [PubMed] [Google Scholar]
- 12.Monath TP. Stability of yellow fever vaccine. Dev Biol Stand. 1996;87:219–25. [PubMed] [Google Scholar]
- 13.Adebayo AA, et al. Stability of 17D yellow fever virus vaccine using different stabilizers. Biologicals. 1998;26(4):309–16. doi: 10.1006/biol.1998.0157. [DOI] [PubMed] [Google Scholar]
- 14.Bista MB, et al. Efficacy of single-dose SA 14-14-2 vaccine against Japanese encephalitis: a case control study. Lancet. 2001;358(9284):791–5. doi: 10.1016/s0140-6736(01)05967-0. [DOI] [PubMed] [Google Scholar]
- 15.Wang SG, YH, Den YY, et al. Studies on the production of SA14-2 Japanese encephalitis live vaccine. Chin J Virol. 1990;6:38–43. [Google Scholar]
- 16.McAleer WJ, et al. Stability on storage at various temperatures of live measles, mumps and rubella virus vaccines in new stabilizer. Journal of biological standardization. 1980;8(4):281–7. doi: 10.1016/s0092-1157(80)80005-9. [DOI] [PubMed] [Google Scholar]
- 17.Burke CJ, Hsu TA, Volkin DB. Formulation, stability, and delivery of live attenuated vaccines for human use. Crit Rev Ther Drug Carrier Syst. 1999;16(1):1–83. [PubMed] [Google Scholar]
- 18.Lemieux P, et al. A combination of poloxamers increases gene expression of plasmid DNA in skeletal muscle. Gene Ther. 2000;7(11):986–91. doi: 10.1038/sj.gt.3301189. [DOI] [PubMed] [Google Scholar]
- 19.Todd CW, et al. Systematic development of a block copolymer adjuvant for trivalent influenza virus vaccine. Dev Biol Stand. 1998;92:341–51. [PubMed] [Google Scholar]
- 20.Strappe PM, et al. Delivery of a lentiviral vector in a Pluronic F127 gel to cells of the central nervous system. Eur J Pharm Biopharm. 2005;61(3):126–33. doi: 10.1016/j.ejpb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Newman MJ, Todd CW, Balusubramanian M. Design and development of adjuvant-active nonionic block copolymers. J Pharm Sci. 1998;87(11):1357–62. doi: 10.1021/js980072c. [DOI] [PubMed] [Google Scholar]
- 22.Lee K, Shin SC, Oh I. Fluorescence spectroscopy studies on micellization of poloxamer 407 solution. Arch Pharm Res. 2003;26(8):653–8. doi: 10.1007/BF02976716. [DOI] [PubMed] [Google Scholar]
- 23.Kuno G, et al. Phylogeny of the genus Flavivirus. Journal of virology. 1998;72(1):73–83. doi: 10.1128/jvi.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]