Abstract
Background
Repeated exposure to drugs of abuse and stress increase dynorphin, a kappa opioid receptor (KOR) ligand, in the nucleus accumbens (NAc). Acute KOR activation produces dysphoria that may contribute to addictive behavior. How repeated KOR activation modulates reward circuitry is not understood.
Methods
We used intracranial self-stimulation (ICSS), a method that provides a behavioral index of reward sensitivity, to measure the effects of repeated administration of the KOR agonist salvinorin A (salvA; 2 mg/kg) on the reward-potentiating effects of cocaine (5.0 mg/kg). In separate rats, we measured the effects of salvA on activation of extracellular signal regulated kinase (ERK), cAMP response element binding protein (CREB), and c-Fos within the NAc.
Results
SalvA had biphasic effects on reward: an immediate effect was to decrease, whereas a delayed effect was to increase, the rewarding impact of ICSS. Repeated salvA produced a net decrease in the reward-potentiating effects of cocaine. In the NAc, both acute and repeated salvA administration increased phosphorylated ERK, whereas only acute salvA increased c-Fos and repeated salvA increased phosphorylated CREB. The KOR antagonist nor-Binaltorphimine (norBNI; 20 mg/kg) blocked the immediate and delayed effects of salvA and prolonged the duration of cocaine effects in ICSS.
Conclusions
Repeated salvA may trigger opponent processes such that “withdrawal” from the dysphoric effects of KOR activation is rewarding and decreases the net rewarding valence of cocaine. The temporal effects of salvA on ERK signaling suggest KOR-mediated engagement of distinct signaling pathways within the NAc that may contribute to biphasic effects on reward sensitivity.
Keywords: Intracranial self-stimulation, CREB, c-Fos, dynorphin, cocaine, opponent process
Introduction
Chronic administration of drugs of abuse and stress can lead to depressive-like states (1, 2), which are thought to promote addictive behaviors (3, 4). Converging evidence demonstrates that chronic drug exposure and stress increase activity of cAMP response element binding protein (CREB) (5–7), which in turn elevates synthesis of the neuropeptide dynorphin (8, 9)—an endogenous ligand at kappa opioid receptors (KORs; (10). Drug- and stress-induced KOR activation occurs within brain regions critical for mood regulation (11), and elevation in striatal dynorphin expression is coincident with the emergence of depressive-like states in drug-dependent humans and animals (12, 13). In the nucleus accumbens (NAc)—a critical substrate for motivated behavior—both CREB and KOR agonists elicit depressive-like states (7, 14–16) and decrease cocaine reward (17–19). Under some circumstances, KOR agonists decrease cocaine self-administration in rodents and non-human primates (20, 21), raising the possibility that KOR agonists might be used to treat psychostimulant intoxication (13) or other states characterized by hyperfunction of brain reward systems (19).
In contrast, there is emerging evidence that prolonged or prior KOR activation can potentiate the effects of cocaine. For example, repeated administration of KOR agonists can increase cocaine-induced locomotor activity and dopamine release (22, 23), and activation of KORs can increases reward-related effects of cocaine in a time-dependent manner (24–27). One explanation for these apparent inconsistencies is that KOR activation might trigger opponent processes that increase reward (23, 28). Alternatively, KOR-induced dysphoria may increase the valence (emotional value) of the reward-related effects of cocaine (29) by increasing the net change in hedonic state pre- to post-cocaine. One caveat is that KOR agonists decrease drug reward and drug seeking at times when KOR-mediated dysphoria and anhedonia are at their peak (19, 24). Thus, the interaction between KOR activation and drug reward is likely to involve a combination of opponent process and negative reinforcement mechanisms.
To determine the temporal effects of KOR activation on hedonic state and sensitivity to cocaine reward, we used intracranial self-stimulation (ICSS), an operant paradigm that measures reward function in real time: treatments that induce rewarding states in humans decrease the frequency of stimulation required to maintain responding (thresholds), whereas treatments with depressive effects increase the frequency of stimulation required to maintain responding (30). We hypothesized that repeated activation of KORs—as might occur during periods of chronic drug administration—would be dysphoric whereas a consequence of prior activation of KORs—as might occur after cessation of drug taking—would be increased reward. Furthermore, we hypothesized that if the rewarding valence of cocaine depends on relative changes in hedonic state (25), then a consequence of prior KOR activation would be a decrease in the net rewarding effects of cocaine. We treated rats repeatedly with the highly selective and short acting KOR agonist salvinorin A (salvA) (31) and assessed the effects on reward function over time using ICSS.
The intracellular mechanisms through which KORs modulate motivated behavior are not fully understood. Whether through direct actions on NAc neurons or indirectly through modulation of inputs (32–35), KORs activate a variety of signal transduction pathways including ERK1/2—a member of the mitogen-activated protein kinase (MAPK) family (36). ERK substrates include the transcription factors CREB and c-Fos, with c-Fos acting as a “sensor” for ERK activation dynamics (37). Pharmacological inhibition of ERK modulates reward-related effects of cocaine (38, 39), suggesting that ERK activation in the NAc contributes to reward function. Therefore, we examined the temporal effects of salvA on ERK, CREB and c-Fos in the NAc and dorsal striatum.
Methods and Materials
Animals
A total of 121 male Sprague-Dawley rats (Charles River Laboratories) weighing approximately 350–400 g at the time of testing were used for these studies. Rats were individually housed with a 12h/12h light/dark cycle and all experiments were conducted during the light phase. Rats were maintained on ad libitum food and water. All rats were treated according to the guidelines recommended by the Institutional Animal Care and Use Committee of McLean Hospital and in strict accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (1996).
Drugs
SalvA was provided by Dr. Cécile Béguin (McLean Hospital, Belmont, MA). The drug was extracted and purified according to established methods (40). SalvA was dissolved in a vehicle of 75% dimethyl sulfoxide (DMSO) plus 25% distilled water (vehicle), and administered intraperitoneally (i.p.) at a dose of 2.0 mg/kg/d in a volume of 1 ml/kg. This regimen is based on prior work showing that repeated KOR activation potentiates the locomotor-stimulant effects of cocaine (22), and that salvA (2.0 mg/kg) is maximally effective at increasing ICSS thresholds without decreasing rates of responding (41). Nor-binaltorphimine (norBNI; NIDA drug supply) was dissolved in distilled water at a concentration of 10 mg/ml and administered i.p. in a volume of 2 ml/kg for a final dose of 20 mg/kg. Given the delayed onset and prolonged activity of KOR-specific antagonism (42), norBNI was administered only once, 24 hr prior to salvA treatment.
Intracranial Self-Stimulation Procedures
Fifty-nine rats were used for ICSS. For details on ICSS procedures, refer to Supplement 1 and references (30, 35). Rats were trained until mean ICSS thresholds remained stable (± 10% for 4 consecutive days). The average of these pre-testing thresholds were designated as “original baseline” parameters.
For drug testing, three rate-frequency functions (3 × 15 min) were determined for each rat immediately before each day’s drug injection. The ICSS thresholds and maximum rates were averaged and were designated as “daily baseline” parameters. Each rat then received an IP injection of drug or vehicle and 8 more 15-min rate frequency functions were obtained (2 hr). Each rat was treated with either vehicle (water) or norBNI (20 mg/kg) on d0, followed by vehicle (75% DMSO) or salvA (2.0 mg/kg) on days 1–5 and 8, followed by vehicle (0.9% saline) or cocaine (5.0 mg/kg) on d9. Drug treatments were administered 24 hr apart, and on days 6 and 7 the rats remained untreated in their home cages.
Western blot analysis
Fifty-two rats were used to examine the effects of acute or repeated salvA on ERK and CREB activity. For acute salvA, rats were treated with either vehicle (75% DMSO) or salvA (2.0 mg/kg) and sacrificed 15 min later by decapitation. For repeated salvA, rats were treated with either vehicle (75% DMSO) or salvA (2.0 mg/kg) on days 1–5 and 8. On day 9, 24 hr after the last salvA injection, rats were sacrificed by decapitation. The procedures used for tissue dissection, protein isolation, SDS polyacrylamide electrophoresis, Western blotting and antibody detection are described in Supplement 1 and (43).
To ensure the accuracy of tissue punches, 40 μM coronal sections were taken from each dissected region and stained with cresyl violet for histological examination.
Immunohistochemistry (IHC)
To determine the molecular phenotype of NAc neurons expressing c-Fos in response to acute salvA, rats were sacrificed 2 hr after a single vehicle or salvA (2.0 mg/kg) injection (N=5/group), and double label IHC for c-Fos and prodynorphin was performed in serial reactions, following a standard IHC protocol (see Supplement 1 and reference (22)). For each, sections were incubated overnight in the primary antiserum, and the sections were then incubated in biotinylated secondary serum and in streptavidin. The first antigen (c-Fos) was detected with nickel-enhanced DAB (purple-gray reaction product) and the second (prodynorphin) with cacodylate-buffered DAB (brown reaction product). Between the two procedures, the sections were washed overnight in PBS-T, followed by blocking steps in H2O2, avidin, and biotin. Primary antibodies were: polyclonal antibody made in rabbit directed against c-Fos (PC38T, Calbiochem, La Jolla, CA), diluted 1:10,000, and an antibody made in guinea pig directed against prodynorphin (AB5519, Chemicon), diluted 1:4,000.
To mark striatal projection neurons, we immunostained for dynorphin, a phenotypic marker of direct pathway projection neurons (44). To estimate the relative levels of c-Fos induction in dynorphin-expressing neurons, we chose doubly immunostained sections in which c-Fos immunoreactivity in the NAc was most intense and manually counted neurons that expressed c-Fos alone without dynorphin and neurons that expressed both c-Fos and dynorphin, as in (45).
Statistics
The time course of drug effects on ICSS thresholds and maximum rates were analyzed using two-way (treatment x time) ANOVAs with repeated measures on time. The effect of cocaine on the change in absolute ICSS thresholds (Hz) following repeated salvA was measured with a one-way ANOVA. The effects of salvA on ERK and CREB activation were analyzed using separate 3-way (treatment × brain region × protein) ANOVAs with repeated measures on brain region and protein (ERK, P-ERK and CREB, P-CREB). The effect of acute salvA on co-localization of c-Fos and prodynorphin were analyzed with a two-way ANOVA (treatment × antibody) with repeated measures on antibody. All significant effects and interactions were analyzed further using Bonferroni/Dunn’s correction tests.
Results
An immediate response to repeated salvA is a decrease in the rewarding effects of ICSS
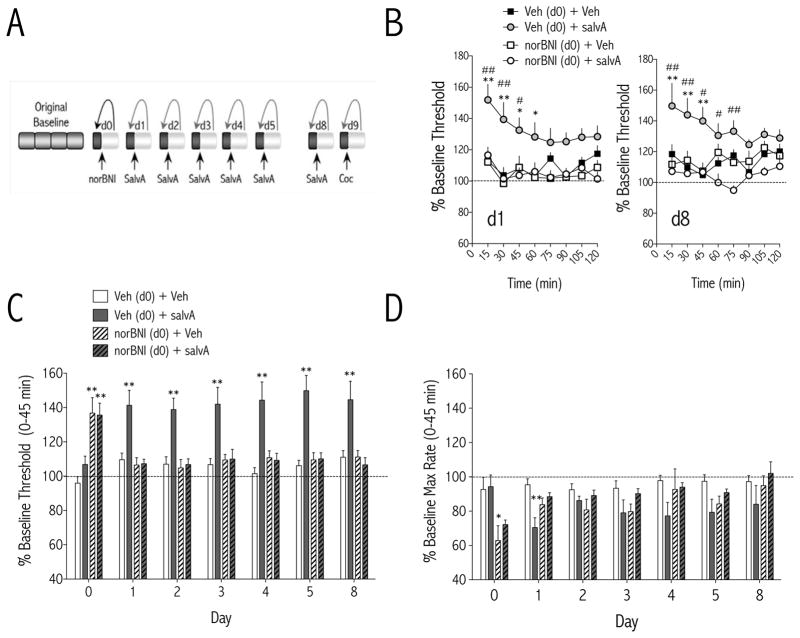
The effect of daily salvA treatment on ICSS thresholds was compared to each day’s pre-salvA thresholds in order to capture the real-time impact of repeated KOR activation on reward function relative to daily baseline hedonic state (Figure 1A). The effects of KOR ligands (salvA and norBNI) on ICSS thresholds on d1 (Figure 1B) depended on treatment [F(3,37) = 8.24, p<0.01] and time [F(7,37) = 3.21, p<0.01], whereas the effects of KOR ligands on ICSS thresholds on d8 (Figure 1B) depended on an interaction between treatment and time [F(21,36)<0.05]. When data are expressed as single means for the first 45 min of the test session (when effects are maximal: see Figure 1B), the effects of KOR ligands on ICSS thresholds depended on an interaction between treatment and day [F(18,228) = 5.7, p<0.01; Figure 1C]. SalvA also had effects on response rates that depended on an interaction between treatment and day [F(18,228) = 2.7, p<0.01; Figure 1D]. When data are expressed as single means for the first 45 min of the test session, salvA significantly decreased maximum rates of responding only on d1 (p<0.01), suggesting tolerance to the rate-decreasing effects of salvA. The long-acting KOR antagonist norBNI (20 mg/kg, i.p.) had immediate, threshold-increasing effects (p<0.01), likely due to non-specific blockade of all opioid receptors at this time point (46), but blocked the threshold-increasing effects of salvA on subsequent days (1–8).
Figure 1.
Immediate effects of repeated salvinorin A (salvA) on intracranial self-stimulation. (A) Experimental design: Each test day, baseline stimulation thresholds were determined for 60 min prior to injection of drug (norBNI; salvA; or Coc, cocaine) or vehicle (water; 75% DMSO; 0.9% saline, respectively). Post-drug stimulation thresholds were determined immediately after drug injection for 120 min and compared to pre-drug baseline thresholds to obtain a daily % change from baseline thresholds. Data are expressed as mean (±SEM) % change from daily baseline thresholds. (B) Time course of the immediate effects of salvA (2.0 mg/kg, i.p.) on ICSS thresholds on d1 and d8. Pretreatment with norBNI (20 mg/kg, i.p.) on d0 did not affect ICSS thresholds on its own, but blocked the threshold elevating effects of salvA. (C) On day 0, norBNI (20 mg/kg, i.p.) significantly increased thresholds. On days 1–5 and 8, SalvA (2.0 mg/kg/d) significantly increased thresholds in rats pre-treated with vehicle (water) on d0 but not in rats pre-treated with norBNI on d0. (D) On day 0, norBNI (20 mg/kg, i.p.) significantly decreased maximum rates of responding. On day 1, SalvA (2.0 mg/kg/d) significantly decreased maximum rates of responding in rats pre-treated with vehicle (water) on d0 but not in rats pre-treated with norBNI on d0. *p<0.05; **p<0.01 compared with control [0 hr post-veh (H2O d0)], Bonferroni/Dunn’s correction. N=7–13 rats/group.
A delayed response to repeated salvA is an increase in the rewarding effects of ICSS
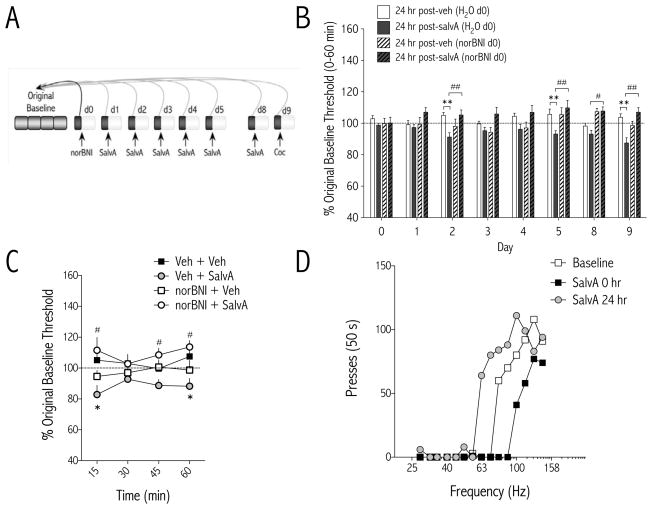
The one-hour daily baseline threshold sessions obtained 24 hr after the prior days’ salvA treatment were compared to the original baseline thresholds established during training (Figure 2A). We found that the delayed (24 hr) effects of salvA depended on an interaction between treatment and day [F(21,266) = 2.08, p<0.01; Figure 2B]. When data are expressed as single means for the 60 min daily baseline session, ICSS thresholds were significantly decreased compared to vehicle (p<0.01) on days 2, 5, and 9, 24-hr post-salvA. These delayed effects of salvA on reward function were normalized by norBNI pretreatment. We analyzed time course data from tests conducted 24 hr after the last salvA treatment (i.e. d9). The delayed effects of salvA on reward function depended on treatment [F(3,36) = 8.14, p<0.01; Figure 2C]. There were no delayed effects of salvA or norBNI on maximum rates of responding compared to vehicle [F(3,36) = 0.72, ns; data not shown]. Raw data from a representative rat illustrate how salvA causes an immediate rightward, and a delayed leftward, shift in the function that relates response rates to frequency of stimulation (Figure 2D).
Figure 2.
Delayed effects of repeated salvinorin A (salvA) on intracranial self-stimulation. (A) Experimental design: Each test day, stimulation thresholds were determined for 60 min 24 hr after the previous days’ treatment and immediately prior to administration of the current days’ treatment and compared to original baseline thresholds determined during stability training. Data are expressed as mean (±SEM) % change from original baseline thresholds. (B) On day 0, prior to any drug treatments, stimulation thresholds were unchanged from original baseline. On day 1, 24 hr after norBNI but prior to any salvA treatment, thresholds were unchanged. Starting on d2, stimulation thresholds were lower in salvA- treated compared to vehicle-treated rats. Pretreatment with norBNI (20 mg/kg, i.p.) on d0 blocked the delayed effects of salvA. *p<0.05; **p<0.01 compared with each days’ vehicle control group [24 hr post-veh (H2O d0)]; Bonferroni/Dunn’s correction. N=7–13 rats/group. (C) Time course of delayed effects of salvA on ICSS thresholds on d9, 24 hr after salvA injection. SalvA treatment [24 hr post-salvA (H2O d0)] significantly decreased stimulation thresholds compared to controls [24 hr post-veh (H2O d0)], *p<0.05, and pretreatment with norBNI [24 hr post-salvA (norBNI d0)] blocked this effect #p<0.05; Bonferroni/Dunn’s correction. (D) SalvA (2.0 mg/kg) caused immediate parallel rightward, and delayed parallel leftward, shifts in rate-frequency functions. Data are from a single representative rat; for clarity, only the first of 4 rate-frequency determinations is presented.
Given a report that very low doses of salvA (0.1 – 40 μg/kg) are rewarding (47), we administered salvA (30 μg/kg and 500 μg/kg) to a separate cohort of rats using the same conditions as Braida et al. (2008) and found no effect on ICSS thresholds [F(1,10) = 0.24, ns; Figure S1 in Supplement 1].
A delayed effect of repeated salvA is a decrease in the rewarding valence of cocaine
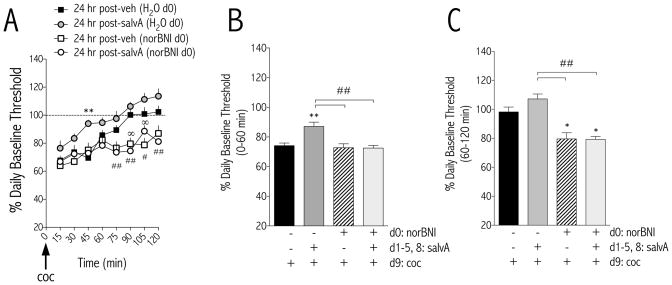
A cocaine challenge was administered to rats 24 hr after the last salvA treatment. Data are presented as percent change (±SEM) from d9 pre-cocaine baseline thresholds (i.e. “daily baseline”). Time course analysis revealed an interaction between treatment condition and time [F(21,266) = 2.30, p<0.01; Figure 3A]: the ability of cocaine to decrease ICSS thresholds from baseline was blunted in rats with prior, repeated salvA experience. Pretreatment with norBNI normalized the effects of salvA on the reward-related effects of cocaine and prolonged the duration of cocaine-potentiated reward function—regardless of salvA treatment. When data are expressed as single means for the first (0–60 min) and second (60–120 min) hours of the test session, pretreatments affected the threshold-reducing effects of cocaine in both the first [F(3,37) = 9.07, p<0.01; Figure 3B] and second [F(3,37) = 12.29, p<0.01; Figure 3C] hours after a cocaine challenge.
Figure 3.
Delayed effects of repeated salvinorin A (salvA) on the reward-potentiating effects of cocaine. (A) Time course of effects of a cocaine (Coc, 5.0 mg/kg, i.p.) challenge (d9) on intracranial self-stimulation thresholds 24 hr after repeated salvA (2.0 mg/kg) treatment. Data are expressed as mean (±SEM) % change from pre-cocaine baseline thresholds. Repeated salvA pretreatment significantly blunted the cocaine-induced % decrease from baseline thresholds compared to rats pretreated with repeated vehicle. Pre-treatment with norBNI (20 mg/kg) on d0 normalized the delayed effects of salvA on the magnitude of cocaine effects and prolonged the time that stimulation thresholds were decreased after cocaine compared to vehicle controls. (B) In the first 60 min after cocaine (5.0 mg/kg), the % change from baseline thresholds was significantly blunted in rats previously treated with repeated salvA (2.0 mg/kg/d) compared to rats treated with repeated vehicle. Pretreatment with norBNI (20 mg/kg) on d0 blocked the effects of salvA on cocaine-mediated decreases in thresholds. (C) In the second 60 min of the test session (60 – 120 min) after cocaine (5.0 mg/kg), there was no difference in cocaine-mediated decreases in thresholds between rats previously treated with salvA (2.0 mg/kg/d) or vehicle. Cocaine-mediated decreases in thresholds were significantly decreased in rats pretreated with norBNI on d0 compared to rats that received vehicle on d0. *p<0.05; **p<0.01 compared with control [24 hr post-veh (H2O d0)]; ##p<0.01 compared to salvA pretreated rats [24 hr post-salvA (H2O d0)], Bonferroni/Dunn’s correction. N=7–13 rats/group.
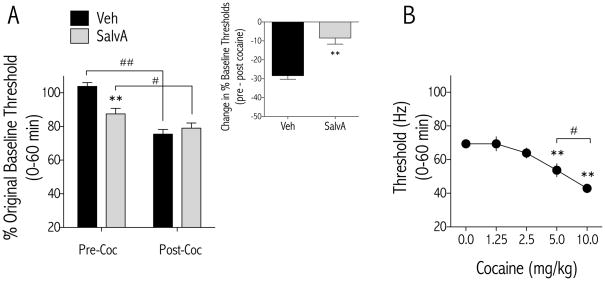
The ability of prior, repeated salvA treatment to reduce the reward-related effects of cocaine might be due to a decreased ability of cocaine to lower absolute stimulation thresholds or to a decrease in the net change in thresholds from pre- to post-cocaine. To distinguish between these possibilities, pre- and post-cocaine stimulation thresholds obtained on d9 were compared to original baseline thresholds obtained during training. The percent change in thresholds from original baseline depended on an interaction between salvA treatment and test condition [F(1,24) = 28.40, p<0.01; Figure 4A]. Pre-cocaine thresholds were significantly decreased in rats treated repeatedly with salvA compared to those in rats treated with vehicle (p<0.01). Irrespective of salvA treatment, a cocaine challenge significantly decreased thresholds compared to original baseline. However, the net change in thresholds was significantly less in rats treated repeatedly with salvA (Figure 4A inset). To ensure that the dose of cocaine (5.0 mg/kg) used in this study did not maximally reduce ICSS thresholds, a separate cohort of rats was treated with a range of cocaine doses (0.0 – 10.0 mg/kg) and absolute thresholds (Hz) determined. The effects of cocaine on ICSS thresholds were dose dependent [F(4,54) = 21.21, p<0.01; Figure 4B], with the 10 mg/kg dose significantly decreasing thresholds compared to the 5.0 mg/kg dose.
Figure 4.
Effects of prior, repeated salvA and acute cocaine on stimulation thresholds. (A) Compared to original baseline thresholds obtained during ICSS training, stimulation thresholds were significantly lower in salvA-pretreated rats compared to Veh-pretreated rats on d9, 24 hr after the last drug injection (Pre-Coc). Cocaine significantly reduced thresholds in rats pretreated with vehicle and salvA, and there was no difference in thresholds between Veh and salvA groups after cocaine. The change (delta) in thresholds of veh and salvA treated rats from pre-cocaine to post-cocaine are significantly different (Inset). (B) In a separate group of rats, cocaine dose-dependently decreased the minimum stimulation (Hz) required to sustain responding for brain stimulation reward (Theta 0). **p<0.01 compared with vehicle of corresponding treatment group; #p<0.05, ##p<0.01 comparing groups under bars. Bonferroni/Dunn’s correction, N=11–13 rats/group.
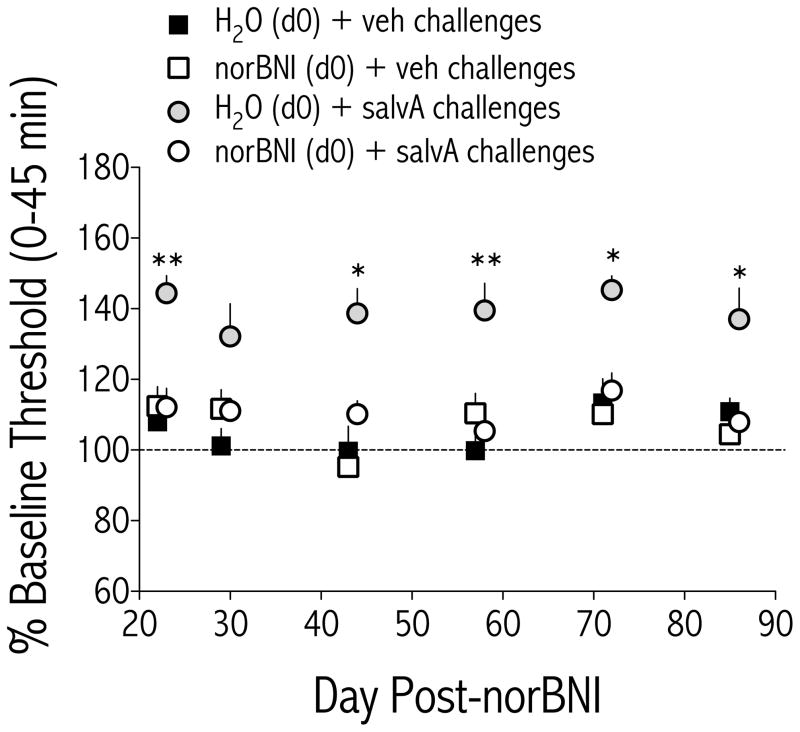
Currently available selective KOR antagonists have an exceptionally long duration of action (46, 48), but time course data has not yet been reported for effects on ICSS behavior. We performed a time course experiment in a subset of rats that had participated in the repeated salvA experiment and found that the effects on ICSS thresholds depended on treatment [F(3,24) = 25.3, p<0.01; Figure 5]. A single norBNI treatment (20 mg/kg, IP) blocked salvA-mediated increases in ICSS thresholds for at least 86 days.
Figure 5.
The KOR antagonist norBNI blocked salvA-induced increases in stimulation thresholds for at least 86 days. Rats that had been treated with either vehicle (H2O) or norBNI (20 mg/kg) on d0 were challenged with either vehicle (75% DMSO) or salvA (2.0 mg/kg) on the indicated days. Data are presented as mean (±SEM) % change from daily pre-drug baseline thresholds. ICSS thresholds depended on treatment [F(3,24) = 25.3, p<0.01]. Main effects tests revealed that, in rats pretreated with vehicle, salvA significantly increased ICSS thresholds compared to vehicle [F(1,8) = 39.8, p<0.01], whereas in rats pretreated with norBNI, salvA had no effect on ICSS thresholds compared to vehicle [F(1,16) = .68, ns]. *p<0.05; **p<0.01 comparing H2O (d0) + salvA challenges to norBNI (d0) + salvA challenges. Bonferroni/Dunn’s correction, N=5–9 rats/group.
Immediate and delayed effects of salvA include increased ERK activity
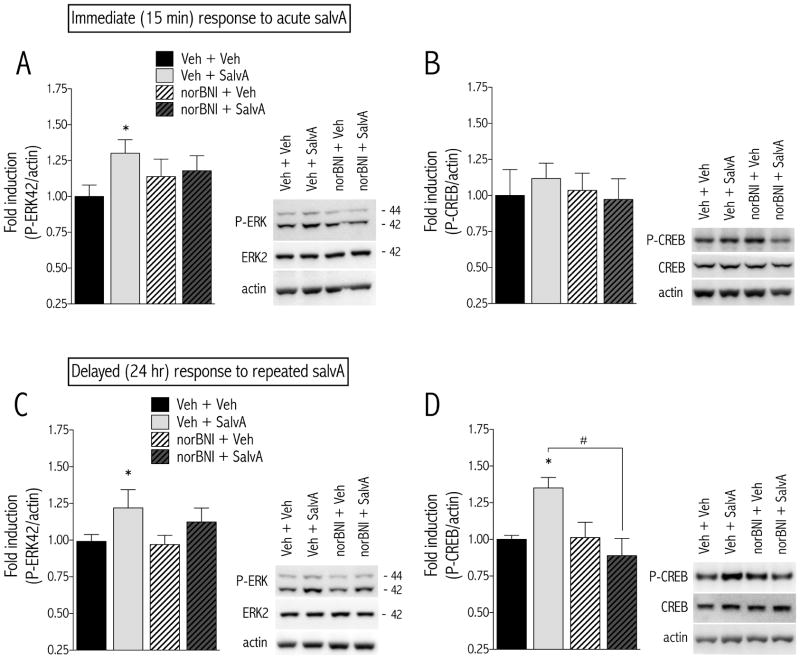
We measured the immediate and delayed effects of salvA on ERK and CREB in the NAc and caudate putamen (CPu). The immediate effects of acute salvA on ERK proteins in the NAc and CPu depended on treatment [F(3,20) = 5.00, p<0.01; Figure 6A and Table 1]. Post hoc analysis showed that rats treated with salvA had significantly higher levels of P-ERK in the NAc, but not the CPu, compared to rats treated with vehicle (p<0.05). Acute salvA did not change CREB protein levels in either the NAc or CPu [F(3,20) = 0.59, ns; Figure 6B and Table 1]. The effects of prior, repeated salvA on ERK proteins in the NAc and CPu depended on an interaction between treatment, brain region, and protein [F(3,24) = 4.37, p<0.05; Figure 6C, Table 1]. Rats treated with salvA had significantly higher levels of P-ERK in the NAc, but not the CPu, compared to rats treated with vehicle (p<0.05). The effects of prior, repeated salvA on CREB proteins in the NAc and CPu depended on an interaction between treatment, brain region, and protein [F(3,16) = 6.07, p<0.01; Figure 6D, Table 1]. Rats treated with salvA had significantly higher levels of P-CREB in the NAc, but not the CPu, compared to rats treated with vehicle (p<0.05). Furthermore, rats pretreated with norBNI—regardless of salvA treatment—had significantly higher levels of P-CREB in the CPu (Table 1).
Figure 6.
Temporal effects of acute and repeated salvA on P-ERK and P-CREB in the NAc. Rats were treated with vehicle (H2O) or norBNI (20 mg/kg) 24 hr prior to administration of acute or repeated (d1–5 and 8) vehicle (75% DMSO) or salvA (2.0 mg/kg/d) and were sacrificed 15-min (acute) or 24-hr (repeated) after the last salvA treatment. Tissue punches from the NAc and CPu were extracted for measurement of ERK and CREB activation. (A) Acute salvA significantly increased the level of P-ERK, and this effect was blocked by norBNI pretreatment. (B) Acute salvA had no effect on P-CREB. (C) Prior, repeated salvA significantly increased P-ERK and P-CREB (D) in the NAc and this effect was blocked by norBNI pretreatment (d0). Representative Western blots are shown to the right of each graph. Data are expressed as mean (±SEM) fold induction of protein of interest relative to Veh + Veh control group. *p<0.05, compared to Veh + Veh group, #p<0.05 comparing groups under the bar. Bonferroni/Dunn’s correction N=5–9 rats/group.
Table 1.
Immediate and delayed effects of salvA treatment on ERK and CREB activation
| Acute SalvA | NAc | CPu | NAc | CPu | ||||
|---|---|---|---|---|---|---|---|---|
| ERK | P-ERK | ERK | P-ERK | CREB | P-CREB | CREB | P-CREB | |
| Veh + Veh | 1.00 (0.05) | 1.00 (0.08) | 1.00 (0.05) | 1.00 (0.08) | 1.00 (0.07) | 1.00 (0.18) | 1.00 (0.06) | 1.00 (0.12) |
| Veh + SalvA | 1.04 (0.06) | 1.30* (0.09) | 1.05 (0.04) | 1.23 (0.07) | 0.94 (0.06) | 1.12 (0.11) | 0.98 (0.05) | 1.20 (0.24) |
| norBNI + Veh | 1.00 (0.02) | 1.14 (0.12) | 1.06 (0.06) | 0.99 (0.07) | 1.01 (0.08) | 1.04 (0.12) | 0.86 (0.04) | 1.62 (0.52) |
| norBNI + SalvA | 1.06 (0.06) | 1.18 (0.10) | 1.12 (0.09) | 1.06 (0.09) | 1.11 (0.12) | 0.97 (0.14) | 0.93 (0.04) | 0.96 (0.21) |
| Repeat SalvA | NAc | CPu | NAc | CPu | ||||
|---|---|---|---|---|---|---|---|---|
| ERK | P-ERK | ERK | P-ERK | CREB | P-CREB | CREB | P-CREB | |
| Veh + Veh | 1.00 (0.01) | 1.00 (0.07) | 1.00 (0.02) | 1.00 (0.07) | 1.00 (0.03) | 1.00 (0.03) | 1.00 (0.02) | 1.00 (0.05) |
| Veh + SalvA | 0.90 (0.04) | 1.22* (0.12) | 1.01 (0.01) | 0.96 (0.14) | 1.04 (0.04) | 1.35* (0.07) | 1.02 (0.07) | 1.02 (0.07) |
| norBNI + Veh | 0.98 (0.03) | 0.97 (0.06) | 1.05 (0.04) | 0.97 (0.06) | 1.13 (0.18) | 1.01 (0.08) | 1.08 (0.03) | 1.46 * (0.15) |
| norBNI + SalvA | 0.99 (0.04) | 1.12 (0.09) | 1.09 (0.09) | 1.12 (0.09) | 1.10 (0.14) | 0.89# (0.12) | 0.98 (0.07) | 1.22* (0.07) |
Rats were treated with vehicle (H2O) or norBNI (20 mg/kg) 24 hr prior to administration of acute or repeated (d1–5 and 8) vehicle (75% DMSO) or salvA (2.0 mg/kg/d) and were sacrificed 15-min (acute) or 24-hr (repeated) after the last salvA treatment. Tissue punches from the NAc and CPu were extracted for measurement of ERK and CREB activation. Data are expressed as mean (±SEM) fold induction of protein of interest relative to Veh + Veh control group.
Bonferroni (Dunn’s) post-hoc tests, p<0.05 compared to Veh + Veh of corresponding brain region and protein.
It has been shown that ERK phosphorylates and stabilizes the immediate early gene c-Fos, such that sustained c-Fos induction is indicative of sustained ERK activation (37). We performed double labeling immunohistochemistry for c-Fos and prodynorphin on tissue from rats treated acutely with salvA (Figure 7A). There was a significant interaction between salvA treatment and protein expression [F(1,19) = 47.05, p<0.01; Figure 7B]. Post hoc analysis showed that the number of cells positive for both Fos and prodynorphin was significantly less than for Fos alone, regardless of treatment. SalvA significantly increased the number of Fos-positive, but not Fos plus prodynorphin-positive, cells in the NAc compared to vehicle (p<0.01), suggesting that the acute effects of salvA on intracellular signaling in the NAc occur in in D2- and enkephalin-expressing, rather than dynorphin-expressing, cells.
Figure 7.
Double label immunohistochemistry for c-Fos and prodynorphin in the NAc shell. (A) Sample photomicrograph from NAc shell showing cytosolic prodynorphin immunoreactivity (brown reaction product), nuclear Fos immunoreactivity (blue open arrow, dark purple reaction product), and Fos + prodynorphin double labeling (green arrows). (B) Fos- and Fos + prodynorphin-positive cells were counted in the NAc shell, and a cell density was determined (# cells/area). Acute salvA increased c-Fos expression, but not in prodynorphin-expressing cells. *p<0.05, **p<0.01 compared to Fos group of corresponding treatment; ##p<0.01 comparing groups under the bars. Bonferroni/Dunn’s correction, N=4–6 rats/group. Abbreviations: Fos, c-Fos; DYN, prodynorphin.
Discussion
In this study, we found that repeated administration of the potent and selective KOR agonist salvA decreased the reward-potentiating effects of a cocaine challenge in ICSS. This was due, in part, to a delayed increase in basal reward function, which produced decreases in reward thresholds that reduced the “net” effect of cocaine. These findings support the hypothesis that the rewarding valence of cocaine depends on the “delta” between hedonic states before and after cocaine treatment (25, 29). SalvA produced rightward shifts in ICSS response-frequency curves immediately after each daily administration, reflecting decreases in the rewarding impact of ICSS (i.e. anhedonia), consistent with prior reports (19, 35, 49). The consistency of responses over the repeated salvA treatment regimen suggests that neither tolerance nor sensitization to the acute anhedonic effects of KOR activation occurred in this paradigm. Delayed effects of salvA administration included leftward shifts in ICSS response-frequency curves 24 hr after drug administration, which reflects an increase in the rewarding impact of ICSS. The selective and long-lasting KOR antagonist, norBNI, normalized the magnitude of cocaine effects in salvA-treated rats, but also prolonged the duration of cocaine-potentiated reward. Finally, both acute and repeated salvA induced P-ERK in the NAc, although the unique pattern of downstream effects suggest that salvA triggers multiphasic molecular responses in distinct NAc output pathways that contribute to KOR-mediated alterations in reward function.
ICSS is unique in that it quantifies the effects of stimuli on reward function in “real-time”, and it complements other tests such as place conditioning and drug self-administration. Although the acute depressive-like effects of KOR activation have been well established (for review, see (50), less is known about how KORs modulate hedonic state over time. Here we demonstrate that 24 hr after salvA treatment, ICSS thresholds were reduced. This observation is broadly consistent with opponent process theory, in which an initial affective state “A” is followed by an affective state “B” that is opposite in sign and of decreased magnitude (51). Classically this theory has been used to explain how negative affective states follow the immediate, rewarding effects of most drugs of abuse (3). In this context, ICSS has been used to track the emergence of drug-induced negative affective states (52, 53). However, opponent process theory also applies to stimuli that are initially aversive, such as stress, fear, and anxiety (51).
Although highly selective for KORs (31), salvA has pharmacological properties unique from other KOR agonists (54), raising the possibility that the biphasic effects of salvA on reward function cannot be generalized to other KOR ligands. Regardless, our findings are consistent with anecdotal reports and controlled studies describing euphoria and anti-depressant-like effects post-salvA intoxication (55, 56). Indeed, it has been reported that very low doses of salvA can produce conditioned place preferences (47). However, salvA has a short half-life in brain (57), and when doses of salvA similar to those used in Braida (47) were tested in ICSS, there was no effect on thresholds.
The molecular mechanisms underlying the effects of repeated salvA on reward function are not known. Given the rapid pharmacokinetics of salvA and dynorphin (57, 58), it is likely that the protracted behavioral effects of KOR activation are due to engagement of downstream molecular signaling pathways that take over behavioral responses after the KOR ligand itself is gone. One possibility involves within-system neuroadaptations similar to the development of supersensitive cAMP signal transduction pathways that occurs in response to chronic morphine (43, 59). However, given that neither P-ERK nor P-CREB displayed biphasic, opponent process-like patterns of activation in response to salvA, it is unlikely that these intracellular factors constitute an opponent process substrate per se. A second possibility is that the immediate effects of salvA might be due to decreased activation of dopamine receptors (35, 49) whereas a delayed effect of repeated salvA might be due to increased sensitivity of NAc dopamine receptors (22), although it is important to note that the depressive-like effects of KOR agonists are not totally dopamine-dependent (34). It has been shown that blockade of dopamine D2 receptors (comparable to salvA-mediated decreases in dopamine release in NAc) increases P-ERK in D2 receptor- and enkephalin-containing neurons, whereas activation of dopamine D1 receptors (comparable to delayed increases in dopamine receptor signaling) increases P-ERK in D1 receptor- and dynorphin-containing neurons (60). If the immediate effect of salvA is to activate ERK signaling pathways in D2 receptor-expressing cells through reduced dopamine signaling at D2 receptors, then salvA-induced ERK signaling cascades should be upregulated in this NAc neuronal population. We tested this by measuring the effect of salvA on the colocalization of c-Fos and prodynorphin-using double label IHC. We chose to determine the phenotype of c-Fos expressing cells because c-Fos is sensitive to sustained ERK activation (37). Our finding that acute salvA induces NAc c-Fos—but not in prodynorphin-expressing cells—suggests that salvA is activating ERK signaling in enkephalin and D2 receptor expressing cells. Although possible that salvA could be inducing c-Fos in NAc interneurons, a pilot study revealed only a few cells in which c-Fos colocalized with choline acetyltransferase (ChAT) or parvalbumin, and this did not increase with salvA treatment (data not shown).
NorBNI blocked the immediate and delayed effects of salvA on behavior and ERK signaling. NorBNI also prolonged the rewarding effects of cocaine, which is consistent with the hypothesis that dynorphin release counteracts cocaine-induced dopaminergic stimulation (61). One possible caveat with the effects of norBNI is that they may be due to compensatory neuroadaptations resulting from the drug’s extraordinarily long duration of action rather than from KOR blockade per se. For example, P-CREB is significantly increased in the CPu of rats exposed to norBNI for 9 days (see Table 1). We show that norBNI blocked the ICSS threshold-increasing effects of salvA challenges for at least 85 days after its administration, without altering baseline reward function on its own. While this finding is consistent with previous observations (46, 62), it extends the time course of this effect considerably. Additional procedural differences, such as rodent species (rat versus mouse) and behavioral endpoint (brain stimulation reward versus analgesia) may explain the difference in time course effects of norBNI in these different studies.
In conclusion, we have shown that prior, repeated KOR activation results in increased baseline reward function that contributes to decreases in the net reward-potentiating effects of cocaine. Although our study does not address whether the immediate and delayed effects of salvA would impact drug seeking or taking, it does connect the effects of repeated KOR activation on affective state and the reward-related effects of cocaine. We have also begun to dissect how activation of KORs regulates ERK signaling pathways in the NAc, which will inform future studies causally connecting ERK and affective states. These findings highlight the importance of understanding how KORs contribute to the effects of prolonged drug or stress exposure on mood states over time.
Supplementary Material
Acknowledgments
We thank Dr. Cécile Béguin (McLean Hospital) for providing the salvinorin A used in this study. Work supported by NARSAD (to EHC), the National Institute on Drug Abuse (Grant No. DA026552 to EHC), the National Institute of Mental Health (Grant no. MH063266 to WAC), and the Stanley Medical Research Institute (to BMC). A portion of this work has appeared in poster form at the Society for Neuroscience annual meeting (Potter DN et al., Society for Neuroscience. 2008; 34:564.14).
Footnotes
Financial Disclosures
Dr. Carlezon has a patent (US 6,528,518; Assignee: McLean Hospital) related to the use of kappa-opioid antagonists for the treatment of depressive disorders. In addition, Dr. Carlezon and Dr. Cohen are part of a collaborative group (Assignees: McLean Hospital and Temple University) that has applied for a patent covering the synthesis and use of salvinorin derivatives. Ms. Diane Damez-Werno, Mr. David Potter and Dr. Elena Chartoff report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 4.Lu L, Shepard JD, Scott Hall F, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 5.Hollander JA, Im H-I, Amelio AL, Kocerha J, Bali P, Lu Q, et al. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466:197–202. doi: 10.1038/nature09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konradi C, Cole RL, Heckers S, Hyman SE. Amphetamine regulates gene expression in rat striatum via transcription factor CREB. J Neurosci. 1994;14:5623–5634. doi: 10.1523/JNEUROSCI.14-09-05623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muschamp JW, Van’t Veer A, Parsegian A, Gallo MS, Chen M, Neve RL, et al. Activation of CREB in the Nucleus Accumbens Shell Produces Anhedonia and Resistance to Extinction of Fear in Rats. J Neurosci. 2011;31:3095–3103. doi: 10.1523/JNEUROSCI.5973-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chartoff EH, Papadopoulou M, MacDonald ML, Parsegian A, Potter D, Konradi C, et al. Desipramine reduces stress-activated dynorphin expression and CREB phosphorylation in NAc tissue. Mol Pharmacol. 2009;75:704–712. doi: 10.1124/mol.108.051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- 11.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The Dysphoric Component of Stress Is Encoded by Activation of the Dynorphin {kappa}-Opioid System. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurd YL, Herkenham M. Molecular Alterations in the Neostriatum of Human Cocaine Addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- 13.Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- 16.Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- 17.Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 18.Shippenberg TS, LeFevour A, Heidbreder C. kappa-Opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J Pharmacol Exp Ther. 1996;276:545–554. [PubMed] [Google Scholar]
- 19.Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schenk S, Partridge B, Shippenberg TS. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 1999;144:339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- 21.Negus SS, Mello NK, Portoghese PS, Lin CE. Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1997;282:44–55. [PubMed] [Google Scholar]
- 22.Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., Jr Exposure to the selective kappa-opioid receptor agonist salvinorin A modulates the behavioral and molecular effects of cocaine in rats. Neuropsychopharmacology. 2008;33:2676–2687. doi: 10.1038/sj.npp.1301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heidbreder CA, Schenk S, Partridge B, Shippenberg TS. Increased responsiveness of mesolimbic and mesostriatal dopamine neurons to cocaine following repeated administration of a selective kappa-opioid receptor agonist. Synapse. 1998;30:255–262. doi: 10.1002/(SICI)1098-2396(199811)30:3<255::AID-SYN3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schindler AG, Li S, Chavkin C. Behavioral stress may increase the rewarding valence of cocaine-associated cues through a dynorphin/kappa-opioid receptor-mediated mechanism without affecting associative learning or memory retrieval mechanisms. Neuropsychopharmacology. 2010;35:1932–1942. doi: 10.1038/npp.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Negus SS. Effects of the kappa opioid agonist U50,488 and the kappa opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 2004;176:204–213. doi: 10.1007/s00213-004-1878-7. [DOI] [PubMed] [Google Scholar]
- 27.Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuentealba JA, Gysling K, Magendzo K, Andres ME. Repeated administration of the selective kappa-opioid receptor agonist U-69593 increases stimulated dopamine extracellular levels in the rat nucleus accumbens. J Neurosci Res. 2006;84:450–459. doi: 10.1002/jnr.20890. [DOI] [PubMed] [Google Scholar]
- 29.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nature protocols. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- 31.Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, et al. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci U S A. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svingos AL, Colago EE, Pickel VM. Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J Neurosci. 1999;19:1804–1813. doi: 10.1523/JNEUROSCI.19-05-01804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, et al. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 2010;210:241–252. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruchas MR, Xu M, Chavkin C. Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport. 2008;19:1417–1422. doi: 10.1097/WNR.0b013e32830dd655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci. 2006;31:268–275. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 40.Lee DY, Karnati VV, He M, Liu-Chen LY, Kondaveti L, Ma Z, et al. Synthesis and in vitro pharmacological studies of new C(2) modified salvinorin A analogues. Bioorg Med Chem Lett. 2005;15:3744–3747. doi: 10.1016/j.bmcl.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 41.Beguin C, Potter DN, Dinieri JA, Munro TA, Richards MR, Paine TA, et al. N-methylacetamide analog of salvinorin A: a highly potent and selective kappa-opioid receptor agonist with oral efficacy. J Pharmacol Exp Ther. 2008;324:188–195. doi: 10.1124/jpet.107.129023. [DOI] [PubMed] [Google Scholar]
- 42.Carlezon WA, Jr, Bèguin C, Knoll AT, Cohen BM. Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol Ther. 2009;123:334–343. doi: 10.1016/j.pharmthera.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chartoff EH, Mague SD, Barhight MF, Smith AM, Carlezon WA., Jr Behavioral and molecular effects of dopamine D1 receptor stimulation during naloxone-precipitated morphine withdrawal. J Neurosci. 2006;26:6450–6457. doi: 10.1523/JNEUROSCI.0491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 45.Berretta S, Parthasarathy HB, Graybiel AM. Local release of GABAergic inhibition in the motor cortex induces immediate-early gene expression in indirect pathway neurons of the striatum. J Neurosci. 1997;17:4752–4763. doi: 10.1523/JNEUROSCI.17-12-04752.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horan P, Taylor J, Yamamura HI, Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther. 1992;260:1237–1243. [PubMed] [Google Scholar]
- 47.Braida D, Limonta V, Capurro V, Fadda P, Rubino T, Mascia P, et al. Involvement of kappa-opioid and endocannabinoid system on Salvinorin A-induced reward. Biol Psychiatry. 2008;63:286–292. doi: 10.1016/j.biopsych.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 48.Endoh T, Matsuura H, Tanaka C, Nagase H. Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn Ther. 1992;316:30–42. [PubMed] [Google Scholar]
- 49.Carlezon WA, Jr, Beguin C, Dinieri JA, Baumann MH, Richards MR, Todtenkopf MS, et al. Depressive-Like Effects of the kappa-Opioid Receptor Agonist Salvinorin A on Behavior and Neurochemistry in Rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 50.Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solomon RL, Corbit JD. An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- 52.Barr AM, Markou A, Phillips AG. A ‘crash’ course on psychostimulant withdrawal as a model of depression. Trends Pharmacol Sci. 2002;23:475–482. doi: 10.1016/s0165-6147(02)02086-2. [DOI] [PubMed] [Google Scholar]
- 53.Goussakov I, Chartoff EH, Tsvetkov E, Gerety LP, Meloni EG, Carlezon WA, Jr, et al. LTP in the lateral amygdala during cocaine withdrawal. Eur J Neurosci. 2006;23:239–250. doi: 10.1111/j.1460-9568.2005.04538.x. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DYW, et al. Comparison of pharmacological activities of three distinct kappa ligands (salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther. 2005;312:220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- 55.Baggott M. A survey of Salvia divinorum users. Erowid Extracts. 2004;6:12–15. [Google Scholar]
- 56.Gonzalez D, Riba J, Bouso JC, Gomez-Jarabo G, Barbanoj MJ. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend. 2006;85:157–162. doi: 10.1016/j.drugalcdep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Hooker JM, Xu Y, Schiffer W, Shea C, Carter P, Fowler JS. Pharmacokinetics of the potent hallucinogen, salvinorin A in primates parallels the rapid onset and short duration of effects in humans. Neuroimage. 2008;41:1044–1050. doi: 10.1016/j.neuroimage.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wagner JJ, Terman GW, Chavkin C. Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature. 1993;363:451–454. doi: 10.1038/363451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 60.Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, et al. Opposing Patterns of Signaling Activation in Dopamine D1 and D2 Receptor-Expressing Striatal Neurons in Response to Cocaine and Haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci. 1993;13:5066. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, et al. Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem. 2007;282:29803–29811. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.