Abstract
Purpose
Given the unprecedented efficacy of EGFR tyrosine kinase inhibitors (TKI) in advanced EGFR-mutant lung cancer, adjuvant TKI therapy is an appealing strategy. However, there are conflicting findings regarding the potential benefit of adjuvant EGFR-TKI in patients with lung cancer harboring EGFR mutations. To better understand these results, we studied the natural history of lung cancers which recurred despite adjuvant TKI.
Experimental design
Patients with recurrent EGFR-mutant lung cancer following adjuvant TKI were identified using an IRB approved mechanism. Recurrent cancer specimens were tested for resistance mutations. Sensitivity to re-treatment with EGFR-TKI was evaluated.
Results
Twenty-two patients with cancers harboring an EGFR sensitizing mutation received adjuvant erlotinib or gefitinib for a median of 17 months (range 1–37 months). T790M was more common in cancers which recurred while receiving TKI than in those which recurred after stopping TKI (67% vs. 0%, p=0.011). Fourteen patients who developed recurrence after stopping EGFR-TKI were re-treated, with a median time to progression of 10 months and radiographic response seen in 8 of 11 patients with evaluable disease (73%).
Conclusions
Recurrence of EGFR-mutant lung cancer after stopping adjuvant TKI should not preclude a trial of TKI re-treatment; a phase II trial of erlotinib in this setting is underway. Studies of adjuvant EGFR-TKI will underestimate the potential survival benefit of adjuvant TKI for patients with EGFR-mutant lung cancers if re-treatment at recurrence is not given.
Keywords: Non-small cell lung cancer, adjuvant, EGFR, tyrosine kinase inhibitor, T790M
Introduction
The remarkable efficacy of EGFR tyrosine kinase inhibitors (TKIs) against non-small cell lung cancers (NSCLC) harboring EGFR activating mutations has transformed lung cancer management. EGFR-TKIs like erlotinib and gefitinib are now a standard first-line therapy for patients with advanced lung cancer harboring EGFR mutations, after multiple randomized studies have confirmed their efficacy in this population (1–3). This success has led to investigations of whether erlotinib or gefitinib may have a role in early stage disease, to improve outcomes following definitive therapy.
Our group recently reported our experience using adjuvant TKI treatment in 167 patients with EGFR-mutant lung cancer and identified an improved 2-year disease free survival (DFS) in patients who received perioperative EGFR-TKI (adjusted HR 0.53, p=0.06) when compared to no adjuvant TKI (4). However, two prospective trials of adjuvant gefitinib delivered to unselected patients (i.e. both EGFR mutant and EGFR wild-type patients) have had disappointing results. A randomized study by the Southwest Oncology Group (SWOG) evaluated daily gefitinib maintenance following chemoradiation for stage III NSCLC and found that patients randomized to placebo lived a median of 14 months longer than those receiving gefitinib (5). More recently, results from the randomized placebo-controlled BR.19 study showed no survival benefit for adjuvant gefitinib (6). Neither of these studies selected for patients with tumors harboring EGFR mutations (7, 8). In the BR.19 study, where EGFR mutation status was tested post-hoc in a subset of patients, no survival benefit was identified for gefitinib over placebo (HR 1.58, p=0.16) in 76 patients whose tumors harbored EGFR mutations (6). Several ongoing studies are prospectively evaluating whether selected lung cancer cohorts gain benefit from adjuvant erlotinib. One such study (RADIANT) has completed accrual after randomizing more than 900 patients to adjuvant erlotinib versus placebo, but only 12% of patients are known to have tumors harboring EGFR mutations (9).
Importantly, adjuvant therapy with TKIs has been also prospectively evaluated in the treatment of gastrointestinal stromal tumor (GIST), where adjuvant imatinib improved DFS when compared to placebo but did not prolong overall survival (OS) (10). One hypothesis for why adjuvant TKI might improve disease free but not overall survival is that the TKI is merely delaying recurrence by suppressing the growth of residual disease after surgery, but not eradicating minimal residual disease. Thus, patients who do not receive adjuvant TKI may garner equal benefit by receiving TKI at recurrence. In a best case scenario, adjuvant TKI would eliminate minimal residual disease, preventing recurrence and curing a subset of patients. In a worst case scenario, adjuvant TKI might alter the biology of the disease in such a way that the recurrent cancer is somehow more virulent or resistant to TKIs, thereby worsening survival. Understanding the characteristics of patients with recurrent cancer is one strategy for evaluating the effect of the adjuvant therapy on the disease.
Given the interest in adjuvant EGFR-TKI for treatment of lung cancer and the disappointing preliminary results from BR.19, we undertook an analysis of recurrent EGFR-mutant lung cancers to better understand the impact of adjuvant TKI. Specifically, we were interested in exploring the relationship between adjuvant EGFR-TKI and the development of the T790M second-site mutation, which is highly prevalent in advanced cancers which develop acquired resistance to TKI (11, 12). Preclinical data suggests that when an EGFR-TKI is stopped, EGFR-mutant cell lines which have acquired the T790M resistance mutation revert to T790M-negative after a period of time without TKI exposure (13), perhaps because the slow growing clones carrying T790M are overgrown by the parental EGFR-mutant cells. Analogously, we hypothesized that those cancers which recur after stopping EGFR-TKI would be sensitive to TKI re-treatment.
Methods
Using an IRB-approved mechanism, patients with recurrent EGFR-mutant lung cancer were identified from a database of patients who received adjuvant or neoadjuvant gefitinib or erlotinib (4, 14, 15). Because we have previously found that EGFR mutations are consistently present over the course of TKI treatment (12), patients were considered eligible for this analysis if an EGFR mutation could be identified either at diagnosis or at recurrence. Patients were excluded if they received “adjuvant” TKI for stage IV disease after having had metastectomy or some other attempt at definitive therapy.
Pathologic specimens from biopsies performed following recurrence were studied for molecular characteristics of resistance, when available. EGFR genotyping was performed using fragment analysis or mass spectrometry, as previously reported (16, 17). We tested for the T790M second-site mutation using a highly sensitive locked nucleic acid (LNA)-based PCR/sequencing assay which uses an LNA probe to suppress the amplification of wild-type DNA, and allows the preferential amplification of the T790M-mutant allele (12). MET FISH analysis was performed to evaluate for MET copy number alterations when sufficient material was available (12).
Each patient’s clinical course was reviewed and patients were divided into two groups: those who developed recurrence while receiving adjuvant TKI, and those who developed recurrence after stopping adjuvant TKI. Patients developing recurrence after stopping adjuvant TKI were further divided into those who stopped due to toxicity and those who stopped after completing a planned course of adjuvant TKI (often 24 months, the treatment course given as part of several adjuvant protocols (6, 9, 18)). Date of recurrence was defined as the date of the suspicious imaging examination which led to biopsy or treatment for recurrence. Time to recurrence on adjuvant EGFR-TKI was defined as the time between the first dose of TKI (either neoadjuvant or adjuvant) and the date of recurrence. Time to progression on TKI re-treatment was defined as the period between restarting TKI and development of clinically determined disease progression. Time-to-event analyses were performed using a Kaplan-Meier method. Probability comparisons were performed using Fisher’s exact test.
Results
Sixty-five patients treated with adjuvant or neoadjuvant EGFR-TKI were identified from an institutional database of 222 patients with early-stage EGFR-mutant lung cancer (the details of this cohort are being reported separately (15)). Among these, the 22 patients who had developed disease recurrence were eligible for this analysis. The baseline and treatment characteristics of the 22 patients are shown in Table 1. 19 of the patients (86%) had an EGFR mutation detected in their primary tumor prior to adjuvant treatment; for the remaining 3 patients, baseline testing was not available but an EGFR mutation was identified at recurrence. Patients were started on neoadjuvant/adjuvant EGFR-TKI between 8/03 and 4/09, and received TKI for a median of 17 total months (range 1–37 months). Seven patients (32%) developed recurrence while receiving adjuvant TKI and 15 patients (68%) developed recurrence after having stopped adjuvant TKI. Of the 15 patients who developed recurrence after stopping TKI, 7 (47%) completed a planned course of therapy (median 25 months on TKI) and 8 (53%) stopped due to intolerance (median 5 months on TKI).
Table 1.
Patient and treatment characteristics (N = 22)
| Characteristic | N (% total) | |
|---|---|---|
| Age | Median (Range) | 61 (37–88) |
| Stage | I II III |
5 (23%) 3 (14%) 14 (64%) |
| Histology | Adenocarcinoma | 22 (100%) |
|
EGFR sensitizing mutation |
Exon 19 deletion Exon 21 L858R Exon 19 insertion |
13 (59%) 8 (36%) 1 (5%) |
| Definitive treatment modality |
Surgery only Surgery and adjuvant chemotherapy Neoadjuvant chemotherapy Neoadjuvant chemoradiation Definitive chemoradiation |
8 (36%) 4 (18%) 7 (32%) 2 (9%) 1 (5%) |
| TKI timing | Neoadjuvant only Adjuvant only Neoadjuvant and adjuvant |
1 (5%) 17 (77%) 4 (18%) |
| TKI received | Gefitinib Erlotinib |
6 (27%) 16 (73%) |
| Recurrence timing |
While receiving TKI After completing TKI |
7 (32%) 15 (68%) |
Recurrence characteristics
The median time to recurrence on EGFR-TKI was 25 months for the entire cohort. Median time to recurrence was 16 months in patients who developed recurrence on TKI, 15 months in patients who stopped TKI due to intolerance, and 39 months in patients who completed a planned course of adjuvant TKI therapy. In all patients who stopped TKI, the median time off TKI until relapse was 13 months (range 1–48 months). All but one of the patients who developed recurrence while taking TKI had stage III disease (86%), while approximately half of the patients who developed recurrence after stopping TKI had stage III disease (53%, p=0.19). Recurrence in the brain tended to be more common in patients who had stopped TKI (40% versus 0%, p=0.12; Table 2), while recurrence only in lung and lymph nodes tended to be more common in patients receiving TKI (86% versus 47%, p=0.16).
Table 2.
Recurrence characteristics by subgroup
| Recurred while receiving TKI |
Recurred after stopping TKI |
p value | |
|---|---|---|---|
| Patients | N=7 | N=15 | |
| Recurrence in lung and/or lymph nodes only | 6 (86%) | 7 (47%) | 0.16 |
| Recurrence in CNS | 0 (0%) | 6 (40%) | 0.12 |
| Specimens with genotyping | N=6 | N=9 | |
| EGFR sensitizing mutation detected at recurrence | 5 (83%)* | 9 (100%) | 0.40 |
| EGFR T790M detected at recurrence | 4 (66%) | 0 (0%) | 0.011 |
One patient had a synchronous EGFR wild-type primary identified, believed to explain the EGFR wild-type recurrence
Eighteen of the 22 patients had a biopsy confirming recurrence and 15 of these were adequate for molecular studies. An EGFR sensitizing mutation could be identified in 14 of the 15 specimens; one patient with a history of resected stage IIIA disease harboring an EGFR exon 19 deletion subsequently underwent resection of a mediastinal lymph node which was found to be EGFR wild-type. In retrospect, an additional EGFR wild-type primary was identified in the initial resection specimen from this patient, potentially explaining the wild-type recurrence. T790M was detected in 4 of the 14 specimens harboring an EGFR sensitizing mutation (29%). T790M was common in cancers which recurred on TKI (67%, 95%CI: 22%–96%; Table 2) but was not detected in any of the cancers which recurred after TKI was stopped (0%, 95%CI: 0%–34%, p=0.011). MET FISH results were available for 6 specimens, and increased copy number was seen in 1 biopsy from a cancer which recurred on TKI.
Efficacy of TKI re-treatment
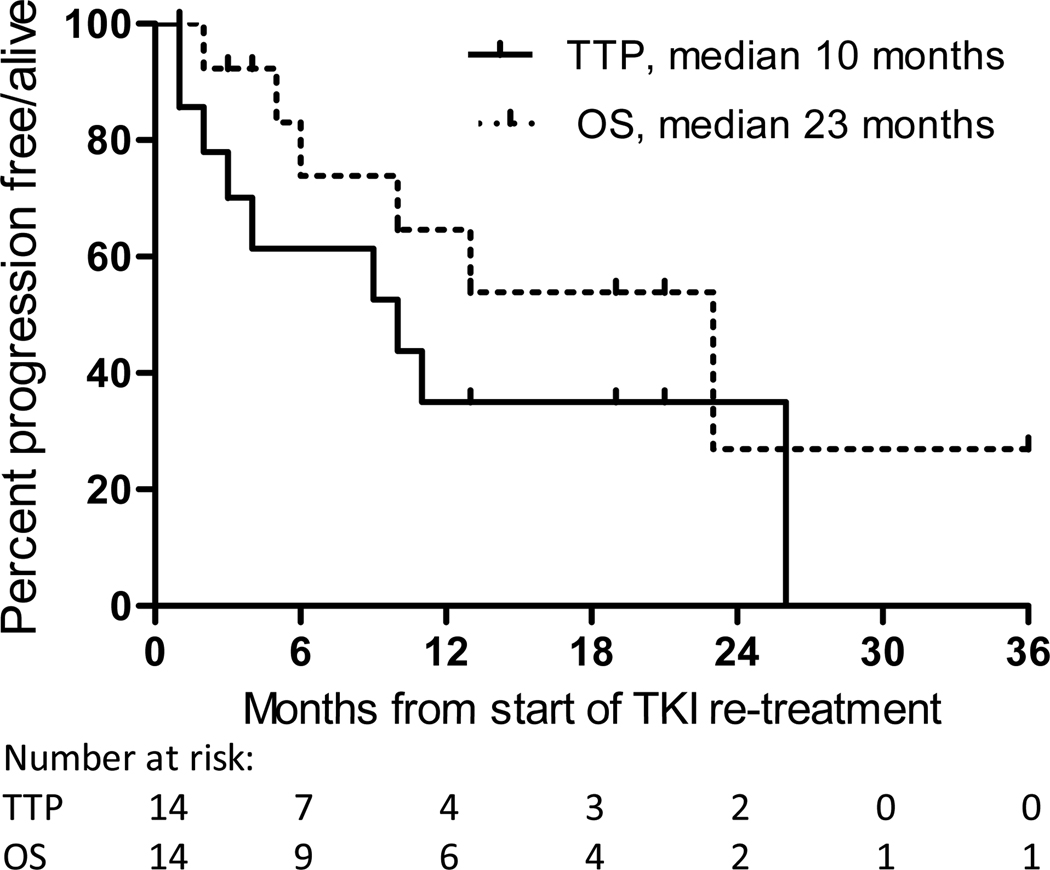
Of the 15 patients who developed recurrence after stopping EGFR-TKI, 14 received TKI re-treatment following recurrence (Table 3); the remaining patient received chemotherapy elsewhere. Radiographic response was seen in 8 of 11 patients (73%, 95%CI: 39%–94%) with evaluable disease (Supplementary Figure); the other 3 patients had no evaluable disease due to radiation or metastectomy. Median time to progression (TTP) on TKI re-treatment was 10 months and median survival was 23 months (Figure 1). Of the 4 patients with TTP of 3 months or less on TKI re-treatment, all had CNS involvement at time of recurrence, with 2 of these patients recurring less than 3 months after stopping adjuvant TKI therapy. One patient who developed progression on TKI re-treatment subsequently underwent a tumor rebiopsy; while T790M had not been present in the initial recurrence specimen, acquired T790M was detected in the rebiopsy performed after progression while receiving erlotinib for advanced disease.
Table 3.
Efficacy of re-treatment with an EGFR tyrosine kinase inhibitor (TKI) for patients who developed recurrence after having stopped adjuvant TKI
| Adjuvant TKI, months |
Reason TKI stopped |
Interval off TKI, months |
Recurrent sites |
Response to TKI re- treatment? |
TTP, months | |
|---|---|---|---|---|---|---|
| 29 | Completed | 2 | Brain, liver, bone, lung |
No: | Progressive liver metastases |
1 |
| 25 | Completed | 8 | Brain | NE: | NED after brain metastectomy |
>19 |
| 25 | Completed | 12 | LN | Yes: | Response in LN, consolidated with radiation |
26 |
| 27 | Completed | 13 | Pleura, LN | Yes: | Durable PET response, though CT stable |
>16 |
| 23 | Completed | 15 | Brain, CSF, bone |
NE: | Symptomatic decline, thought to be due to CNS radiation |
1 |
| 20 | Completed | 21 | Lung | Yes: | Resolution of multiple ground-glass opacities |
>3 |
| 22 | Completed | 21 | CSF, bone | NE: | Durable control of irradiated CSF disease |
10 |
| 6 | Toxicity | 1 | Brain | No: | Progression in the brain | 2 |
| 6 | Toxicity | 4 | Lung, bone | Yes: | CR of 1cm lung nodule, symptomatic bone response |
11 |
| 6 | Toxicity | 5 | Brain, lung | No: | Progressive brain metastases |
3 |
| 1 | Toxicity | 11 | Lung, LN | Yes: | Major response in lung and LN |
9 |
| 3 | Toxicity | 15 | Lung | Yes: | Brief response in lung, but erlotinib stopped due to toxicity |
4 |
| 4 | Toxicity | 30 | LN, bone | Yes: | Early response in LN | >1 |
| 19 | Toxicity | 48 | Lung | Yes: | Durable CR in lung nodules |
>13 |
CSF: cerebrospinal fluid; LN: lymph node; NE: non-evaluable; CR: complete response
Note: Nine of the above patients had recurrent tissue available for mutation testing; all harbored a sensitizing mutation and none harbored a T790M co-mutation
Figure 1.
Time to progression (TTP) and overall survival (OS) from start of TKI re-treatment, in patients who develop a recurrence of EGFR-mutant lung cancer after stopping adjuvant TKI. A portion of patients gain durable disease control on TKI despite prior adjuvant exposure.
Discussion
In this analysis of patients with EGFR-mutant lung cancers which recurred despite adjuvant EGFR-TKI therapy, we have found that patients who develop recurrence after stopping TKI are unlikely to harbor a detectable T790M mutation and can have durable responses to TKI re-treatment. This is in contrast to the convention in NSCLC, where patients who have recurrence after adjuvant cytotoxic chemotherapy are commonly believed to be refractory to the adjuvant drugs which were used, such that re-treatment is avoided. Our data indicate that re-treatment with EGFR-TKI should be considered in patients with EGFR-mutant lung cancer who develop recurrence after stopping adjuvant TKI, and may allow them to garner substantial therapeutic benefit from such agents.
Our findings are consistent with our improved understanding of acquired resistance to TKIs in EGFR-mutant lung cancer. In studies of EGFR-mutant cell lines, it has been found by multiple groups (11, 12, 19) that exposure to EGFR-TKI will, with time, lead to the acquisition of a secondary T790M mutation which restores EGFR phosphorylation in the presence of TKI. More recently, it has been shown that withdrawal of TKI from cell lines and patients with acquired resistance leads to gradual loss of the T790M mutation (13, 20), such that sensitivity to EGFR-TKI is reacquired. This phenomenon may be due to indolent growth of T790M-mutant cells (13), leading to overgrowth by parental cells harboring only the EGFR sensitizing mutation, or due to EGFR alleles lying in extra-chromosomal double-minutes which can be lost from cells without appropriate selection pressure (21). Our clinical dataset supports these preclinical findings – while micrometastases that survive adjuvant EGFR-TKI treatment may acquire T790M, this mutation is not detected after a period of TKI withdrawal, perhaps due to the slow growth of these clones. This indolent phenotype of T790M-mutant cells may also explain the trend toward a lower incidence of CNS recurrence in cancers that recurred while on TKI, since T790M-mediated acquired resistance has been found to be associated with later development of new metastatic disease sites (22). Interestingly, a similar finding of later brain metastasis has also been described in advanced EGFR-mutant lung cancer treated with TKI therapy (23, 24).
Our findings have potentially important implications for the future study and use of adjuvant TKI therapy in EGFR-mutant lung cancer. When considering the impact of adjuvant TKI, it is worth restating that the aim of adjuvant therapy in solid tumor oncology is to improve overall survival by eradicating minimal residual disease following definitive therapy. Historically, therapies with demonstrated efficacy against advanced cancer have been subsequently evaluated in the adjuvant setting, leading to the successful development of adjuvant doxorubicin for breast cancer, fluorouracil for colon cancer, and cisplatin-based chemotherapy for NSCLC. Targeted therapies have also had success in the adjuvant setting: trastuzumab improves overall survival after resection of HER2 positive breast cancer (25), while adjuvant imatinib improves DFS for resected GIST (10), and may also improve overall survival in high risk populations (26). However, the addition of bevacizumab to adjuvant chemotherapy did not significantly improve DFS in resected colon cancer (27), while adjuvant bevacizumab remains under investigation in NSCLC (28).
There are several possible reasons why an adjuvant targeted therapy could fail to improve overall survival, the simplest being that the therapy may have inadequate anti-tumor effect. In the case of adjuvant EGFR-TKI, one could hypothesize that the subset of lung cancers which recur after adjuvant TKI are those that were refractory to this targeted agent due to the de novo presence of resistance mutations. However, this study suggests that recurrent cancers infrequently harbor known resistance mutations and often demonstrate the expected sensitivity to TKIs seen in advanced EGFR-mutant lung cancers. A second explanation for why adjuvant targeted therapy may fail to improve survival is that it has deleterious effects, either on the patient or on the tumor biology. Patient toxicity is one reason why cytotoxic chemotherapy is avoided in resected stage IA NSCLC where any positive impact on survival may be outweighed by potentially dangerous side-effects (29). Alternatively, a harmful effect on tumor biology, leading to increased invasiveness, has been suggested to explain why adjuvant bevacizumab fails to improve survival in the treatment of resected colon cancer (27, 30, 31).
Regarding adjuvant EGFR-TKI, the SWOG study of gefitinib following chemoradiation for patients with stage III NSCLC found no increase in toxic death rate among patients randomized to gefitinib (5). While patients on the gefitinib arm had a trend toward a shorter progression free survival (8 versus 12 months, p=0.17), these patients were not selected on the basis of EGFR genotype and no genotyping from this trial has ever been reported. For BR.19, the available data does not include an analysis of disease free survival in the subset of patients with cancers harboring EGFR mutations, but a concerning incidence of toxic deaths from gefitinib was not identified (6). Thus, there is no clear indication that gefitinib fails as an adjuvant therapy for EGFR-mutant lung cancer due to toxicity or hastening progression, a conclusion supported by the published series showing a better disease free survival for EGFR-mutant lung cancer patients who had received adjuvant TKI (4).
We propose that there may be an additional reason why an active adjuvant therapy could fail to improve survival: specifically, by impacting subsequent treatment patterns without directly affecting disease biology. In the conventional management of recurrent NSCLC, agents given in the adjuvant setting are generally avoided because of the anticipated resistance. For small cell lung cancer, NCCN guidelines use duration of response as a marker of the value of re-treatment (32), however no such distinction is made for NSCLC (33). But avoidance of TKI re-treatment after “failing” a course of adjuvant TKI could have a major impact on the apparent effectiveness of such an adjuvant strategy in clinical trials. For example, in the subset of 76 patients with EGFR-mutant lung cancer treated on the BR.19 study (6), those patients randomized to gefitinib, though intended to receive a full 24 months of TKI, received a median of only 5 months of treatment because the study was closed early (5). At time of recurrence, patients who received adjuvant gefitinib (the study was unblinded when it was halted) may have received other therapies preferentially due to suspected resistance to TKI. In comparison, patients randomized to placebo would have been candidates for gefitinib or erlotinib following recurrence because both gefitinib and erlotinib were available for advanced NSCLC during the follow-up period. By receiving a full course of TKI until progression, these patients would have had a median time on TKI of 10–14 months (1, 34), and could have received TKI longer than patients on the adjuvant gefitinib arm of the study. In effect, patients with EGFR-mutant lung cancer who were randomized to adjuvant TKI could have been undertreated, receiving an abbreviated course of adjuvant treatment rather than treatment until progression following recurrence. Accurate interpretation of this aspect of the BR.19 trial results requires knowledge of whether patients randomized to gefitinib received subsequent TKI re-treatment.
This analysis provides little insight regarding the optimal duration of adjuvant TKI therapy. Although patients who completed a planned course of adjuvant TKI appear to have had a longer median time to recurrence than those who stopped TKI early, there is significant bias in attempting such a retrospective analysis; the former group inherently has the most favorable natural history, while the latter group stopped TKI due to greater vulnerability to toxicity. However, we note that responses to TKI re-treatment were seen both in patients who completed two years of adjuvant TKI as well as in those who stopped adjuvant TKI early (Table 3). Interestingly, while adjuvant cisplatin-based chemotherapy prolongs survival with just 3–4 months of treatment (35), no trial to date has studied the value of a few months of adjuvant TKI. The SWOG study of maintenance gefitinib planned a maximum of 5 years of therapy (5), the BR.19 study planned 2 years of adjuvant gefitinib (though was stopped early)(6), and the RADIANT study is evaluating 2 years of adjuvant erlotinib (9). Because the BR.19 study was halted early, patients received varying durations of adjuvant TKI – it would be useful to study in this unbiased cohort whether duration of TKI therapy influenced DFS. Additionally, future trials could consider studying a shorter course of adjuvant TKI for EGFR-mutant lung cancer, so long as re-treatment with TKI is incorporated at recurrence.
The retrospective nature of our analysis precludes us from determining whether sensitivity to TKI at recurrence is in any way diminished due to the adjuvant TKI exposure. One could hypothesize that, though initially responsive to TKI, these recurrent cancers may have a hidden resistance mechanism which could be revealed after a short period of re-treatment. Our data indicate, however, that durable responses can occur with TKI re-treatment. A phase II study of erlotinib in this setting is currently ongoing (NCT01189435), with the aim of prospectively evaluating the response rate and progression free survival with TKI re-treatment. We believe that adjuvant TKI therapy for EGFR-mutant lung cancer remains a potentially valuable treatment strategy deserving of future study, so long as future studies prospectively include recommendations for TKI re-treatment in appropriate patients.
Statement of Translational Relevance.
Adjuvant tyrosine kinase inhibitor (TKI) therapy for EGFR-mutant lung cancer is a biologically appealing treatment strategy; however preliminary clinical data is conflicting. While one large retrospective series has shown a disease free survival benefit, a post-hoc analysis of a prospective trial of adjuvant TKI demonstrated no survival benefit in the subgroup of cancers with EGFR mutations. In this analysis we studied a cohort of patients with EGFR-mutant lung cancers which recurred after adjuvant EGFR-TKI, and we found that cancers which recur after TKI is stopped do not harbor the T790M resistance mutation and can commonly have durable responses to re-treatment. Though it is not the convention in non-small cell lung cancer, patients with EGFR-mutant lung cancers which recur after stopping adjuvant EGFR-TKI should be offered subsequent re-treatment. Furthermore, trials of adjuvant TKI may significantly underestimate the potential benefit of TKI treatment if re-treatment at or after recurrence is not undertaken.
Supplementary Material
Acknowledgments
Funding: Supported by an ASCO Young Investigator Award and NCI grants R21-CA115051 and P01-CA129243
Footnotes
Previously presented: Material from this manuscript was presented as a poster presentation at the annual conference of the American Society of Clinical Oncology on 06/04/2011.
References
- 1.Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 2.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 3.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 4.Janjigian YY, Park BJ, Zakowski MF, Ladanyi M, Pao W, D'Angelo SP, et al. Impact on Disease-Free Survival of Adjuvant Erlotinib or Gefitinib in Patients with Resected Lung Adenocarcinomas that Harbor EGFR Mutations. J Thorac Oncol. 2011;6:569–575. doi: 10.1097/JTO.0b013e318202bffe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly K, Chansky K, Gaspar LE, Albain KS, Jett J, Ung YC, et al. Phase III Trial of Maintenance Gefitinib or Placebo After Concurrent Chemoradiotherapy and Docetaxel Consolidation in Inoperable Stage III Non-Small-Cell Lung Cancer: SWOG S0023. J Clin Oncol. 2008;26:2450–2456. doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]
- 6.Goss GD, Lorimer I, Tsao MS, O'Callaghan CJ, Ding K, Masters GA, et al. A phase III randomized, double-blind, placebo-controlled trial of the epidermal growth factor receptor inhibitor gefitinb in completely resected stage IB-IIIA non-small cell lung cancer (NSCLC): NCIC CTG BR.19. J Clin Oncol (Meeting Abstracts) 2010;28:LBA7005. [Google Scholar]
- 7.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 8.Riely GJ, Pao W, Pham D, Li AR, Rizvi N, Venkatraman ES, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839–844. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- 9.Richardson F, Richardson K, Sennello G, Young D, Orlov S, Papai-Szekely Z, et al. Biomarker analysis from completely resected NSCLC patients enrolled in an adjuvant erlotinib clinical trial (RADIANT) J Clin Oncol (Meeting Abstracts) 2009;27:7520. [Google Scholar]
- 10.DeMatteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PWT, Demetri GD, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired Resistance of Lung Adenocarcinomas to Gefitinib or Erlotinib Is Associated with a Second Mutation in the EGFR Kinase Domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arcila ME, Oxnard GR, Nafa K, Riely GJ, Solomon SB, Zakowski M, et al. Rebiopsy of Lung Cancer Patients with Acquired Resistance to EGFR Inhibitors and Enhanced Detection of the T790M Mutation Using a Locked Nucleic Acid-Based Assay. Clin Cancer Res. 2011;17:1169–1180. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Somwar R, Wang L, et al. Optimization of Dosing for EGFR-Mutant Non-Small Cell Lung Cancer with Evolutionary Cancer Modeling. Sci Transl Med. 2011;3:90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marks JL, Broderick S, Zhou Q, Chitale D, Li AR, Zakowski MF, et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. J Thorac Oncol. 2008;3:111–116. doi: 10.1097/JTO.0b013e318160c607. [DOI] [PubMed] [Google Scholar]
- 15.D'Angelo SP, Janjigian YY, Kris MG, Pao W, Riely GJ, Marks J, et al. Impact of EGFR and KRAS mutations on survival in 1,000 patients with resected lung adenocarcinoma. J Clin Oncol (Meeting Abstracts) 2010;28:7011. [Google Scholar]
- 16.Pan Q, Pao W, Ladanyi M. Rapid polymerase chain reaction-based detection of epidermal growth factor receptor gene mutations in lung adenocarcinomas. J Mol Diagn. 2005;7:396–403. doi: 10.1016/S1525-1578(10)60569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jurinke C, Oeth P, van den Boom D. MALDI-TOF mass spectrometry: a versatile tool for high-performance DNA analysis. Mol Biotechnol. 2004;26:147–164. doi: 10.1385/MB:26:2:147. [DOI] [PubMed] [Google Scholar]
- 18.Rizvi NA, Rusch V, Pao W, Chaft JE, Ladanyi M, Miller VA, et al. Molecular Characteristics Predict Clinical Outcomes: Prospective Trial Correlating Response to the EGFR Tyrosine Kinase Inhibitor Gefitinib with the Presence of Sensitizing Mutations in the Tyrosine Binding Domain of the EGFR Gene. Clin Cancer Res. 2011;17:3500–3506. doi: 10.1158/1078-0432.CCR-10-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 20.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ercan D, Zejnullahu K, Yonesaka K, Xiao Y, Capelletti M, Rogers A, et al. Amplification of EGFR T790M causes resistance to an irreversible EGFR inhibitor. Oncogene. 2010;29:2346–2356. doi: 10.1038/onc.2009.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, Kris MG, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR mutant lung cancer: Distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17:1616–1622. doi: 10.1158/1078-0432.CCR-10-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heon S, Yeap BY, Britt GJ, Costa DB, Rabin MS, Jackman DM, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16:5873–5882. doi: 10.1158/1078-0432.CCR-10-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heon S, Yeap BY, Lindeman NI, Rabin MS, Jackman DM, Johnson BE. Rates of central nervous system (CNS) metastases in patients with advanced non-small cell lung cancer (NSCLC) and somatic EGFR mutations initially treated with gefitinib or erlotinib versus chemotherapy. J Clin Oncol (Meeting Abstracts) 2011;29:7607. [Google Scholar]
- 25.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, et al. Trastuzumab plus Adjuvant Chemotherapy for Operable HER2-Positive Breast Cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 26.Joensuu H, Eriksson M, Hatrmann J, Sundby Hall K, Schutte J, Reichardt A, et al. Twelve versus 36 months of adjuvant imatinib (IM) as treatment of operable GIST with a high risk of recurrence: Final results of a randomized trial (SSGXVIII/AIO) J Clin Oncol (Meeting Abstracts) 2011;29:LBA1. [Google Scholar]
- 27.Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, et al. Phase III Trial Assessing Bevacizumab in Stages II and III Carcinoma of the Colon: Results of NSABP Protocol C-08. J Clin Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price K, Kris MG, Rusch V, Finley DJ, Azzoli CG, Downey RJ, et al. Phase II study of induction and adjuvant bevacizumab in patients with stage IB-IIIA non-small cell lung cancer (NSCLC) receiving induction docetaxel and cisplatin. J Clin Oncol (Meeting Abstracts) 2009;27:7531. [Google Scholar]
- 29.Besse B, Le Chevalier T. Adjuvant Chemotherapy for Non-Small-Cell Lung Cancer: A Fading Effect? J Clin Oncol. 2008;26:5014–5017. doi: 10.1200/JCO.2008.18.1081. [DOI] [PubMed] [Google Scholar]
- 30.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic Therapy Elicits Malignant Progression of Tumors to Increased Local Invasion and Distant Metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebos JML, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated Metastasis after Short-Term Treatment with a Potent Inhibitor of Tumor Angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalemkerian GP, Akerley W, Downey RJ, Ettinger DS, Fossella F, Grecula JC, et al. Small cell lung cancer. J Natl Compr Canc Netw. 2008;6:294–314. [PubMed] [Google Scholar]
- 33.Ettinger DS, Akerley W, Bepler G, Blum MG, Chang A, Cheney RT, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8:740–801. doi: 10.6004/jnccn.2010.0056. [DOI] [PubMed] [Google Scholar]
- 34.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 35.Arriagada R, Auperin A, Burdett S, Higgins J, Johnson D, Le Chevalier T, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375:1267–1277. doi: 10.1016/S0140-6736(10)60059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.