Abstract
Objective
To evaluate risk factors for astigmatism in a population-based sample of preschool children.
Design
Population-based cross-sectional study
Participants
Population-based samples of 9970 children ages 6 to 72 months from Los Angeles County, California, and Baltimore, Maryland.
Methods
A cross-sectional study of children participating in the Multiethnic Pediatric Eye Disease Study and the Baltimore Eye Disease Study was completed. Data were obtained by clinical examination or by in-person interview. Odds ratios and 95% confidence intervals (95%CI) were calculated to evaluate potential associations between clinical, behavioral, or demographic factors and astigmatism.
Main Outcome Measures
Odds ratios (ORs) for various risk factors associated with astigmatism.
Results
Participants with myopia (≤−1.0 diopters) were 4.6 times more likely to have astigmatism (95%CI 3.56, 5.96) than those without refractive error, while participants with hyperopia (≥+2.00 diopters) were 1.6 times more likely (95%CI 1.39, 1.94). Children 6 months to <12 months of age were approximately 3 times more likely to have astigmatism than children 5 to 6 years of age (95%CI 2.28, 3.73). Both Hispanic (OR=2.38) and African-American (OR=1.47) children were more likely to have astigmatism than non-Hispanic white children. Further, children whose mothers smoked during pregnancy were 1.46 times (95% CI 1.14, 1.87) more likely to have astigmatism than children whose mothers did not smoke.
Conclusions
In addition to infancy, Hispanic and African-American race/ethnicity and correctable/modifiable risk factors such as myopia, hyperopia, and maternal smoking during pregnancy are associated with a higher risk of having astigmatism. While the prevalence of smoking during pregnancy is typically low, this association may suggest etiologic pathways for future investigation.
Astigmatism is an ocular condition in which the unequal curvature of one or more refractive surfaces of the eye prevents the formation of a clear image on the retina. This common form of refractive error accounts for approximately 13% of all refractive errors of the human eye. 1, 2 Identifying astigmatism in pediatric populations is particularly important because of its potential influence on normal visual development. High degrees of astigmatism are associated with the development of amblyopia 3, 4 and some associations have also been found between astigmatism and the development of myopia. 5-7 While the exact cause of astigmatism is unclear, factors such as high risk genes, eyelid pressure, extraocular muscle tension, gestational age, birth weight, and medical conditions such as cerebral palsy 2 also may play a role. Detection of modifiable risk factors for preschool age children that impact the prevalence of astigmatism requires further investigation.
In the current analysis, we explored the association between demographic and clinical characteristics of the children and behavioral characteristics of the mother during pregnancy as potential risk factors for astigmatism. We included data from a multiethnic (African American, Hispanic, and non-Hispanic white) population-based sample of preschool age children from Los Angeles, California, and Baltimore, Maryland. Data for the analyses were obtained by clinical examination at one of the study center clinics or by in-person interview on the same day as the clinical eye examination. Identifying the risk factors for astigmatism may help us recognize those children in need of early treatment and better understand the etiology of amblyopia. Finally, prevention of astigmatism may be possible if modifiable risk factors for the condition are identified.
Methods
Data for this manuscript were collected from two population-based studies of pediatric eye disease: the Multiethnic Pediatric Eye Disease Study (MEPEDS) and the Baltimore Pediatric Eye Disease Study (BPEDS). Details of these studies and data collection methods are described in an accompanying MEPEDS and BPEDS paper in this issue.8
Study Population
Children were eligible for inclusion in the current analysis if they were aged 6 months to 72 months at enrollment. MEPEDS participants were identified from 74 census tracts in and around the cities of Inglewood, Riverside, and Glendale in California. BPEDS participants were identified from 54 census tracts in and around the city of Baltimore, Maryland. The study design and sampling plans have been described in detail in previous publications. 9,10 After informed consent was obtained from the parent of an eligible child, a brief in-home interview was conducted to obtain basic demographic information and history of known eye conditions. Eligible children were then scheduled for a comprehensive eye examination at the local MEPEDS or BPEDS clinic. A more detailed in-person interview with the child’s parent was administered at the local clinic.
Eye Examination and Parental Interview
Eye Examination
All children underwent a comprehensive eye examination, performed by MEPEDS or BPEDS optometrists or ophthalmologists, trained and certified using standardized protocols. 9,10
Cycloplegic refraction was performed using the Retinomax Autorefractor (Right Manufacturing, Virginia Beach, VA) at least 30 minutes after cycloplegia with two drops (5 minutes apart) of 1% cyclopentolate. In MEPEDS 4.1% refused eye drops, while 4.7% refused eye drops in BPEDS. Cycloplegic retinoscopy was performed if Retinomax readings with confidence ratings of ≥8 in both eyes were not obtained after 3 attempts. Non-cycloplegic retinoscopy was performed if parents refused eye drops.
Definitions of Astigmatism
Astigmatism was defined using a threshold level of cylindrical refractive error in the right or left eye of ≥1.50 D expressed in positive correcting cylinder form. Astigmatism type was defined by orientation as with-the-rule (WTR) (plus cylinder axis 90° ± 15°) and against-the-rule (ATR) (plus cylinder axis 180° ± 15°); all other orientations were considered oblique. The eye with the greater absolute amount of cylindrical refractive error was considered to be the worse eye.
Parental Questionnaire
A parental interview was conducted at the MEPEDS or BPEDS clinic by trained interviewers to collect information on demographic, medical, and ocular history variables. Details of the interviews have been described in previous publications. 9,10,8
Statistical Analysis
The frequencies of demographic and behavioral factors were evaluated for participating children with and without astigmatism at the time of the MEPEDS or BPEDS clinical examination. Tests of statistical significance were completed using chi-square tests for categorical variables and t-tests for continuous variables. All tests were two-sided; a P-value of < 0.05 was considered statistically significant.
Logistic regression was used to calculate odds ratios (OR) and 95% confidence intervals (95%CI) to evaluate the associations between demographic, behavioral, or clinical factors and astigmatism. Variables considered for inclusion in the models are described in an accompanying paper in this issue8 and shown in Table 1. Univariate models were completed to identify factors potentially associated with astigmatism in children overall and by orientation. Small for gestational age was defined by gender, weeks of gestation at birth, and birth weight based on a US national reference for birth weight 15. Stepwise multivariate modeling was completed such that demographic, behavioral, or clinical variables with a P-value <0.05 were kept in the multivariate model. Stratified analysis was used to further evaluate the association between maternal prenatal smoking and astigmatism by age of the child at diagnosis, gender, and race/ethnicity (African American, Hispanic, non-Hispanic white). Individuals with missing data were excluded from the univariate analysis for that variable; multivariate models were run first restricted to those with complete data for all variables entered into the model and re-run in the final analysis for all individuals with complete data for variables selected in the final step-wise regression. Formal tests of interaction were completed by including a product term in the multivariate model for maternal prenatal smoking with age, gender, and race/ethnicity.
Table 1. Frequency Distribution of Demographic, Behavioral, Clinical, and Ocular Risk Factors in Children With and Without Astigmatism in the MEPEDS and the BPEDS (N=8,579).
| Risk Factors* | Astigmatism N=859 n (%) |
No Astigmatism N=7720 n (%) |
P-Value** |
|---|---|---|---|
| Study Site | 0.03 | ||
| MEPEDS | 683 (10) | 5884 (90) | |
| BPEDS | 176 (9) | 1836 (91) | |
| Age (months) | <0.0001 | ||
| 06-11 | 185 (23) | 619 (77) | |
| 12-23 | 151 (10) | 1355 (90) | |
| 24-35 | 138 (9) | 1436 (91) | |
| 36-47 | 118 (8) | 1430 (92) | |
| 48-59 | 129 (8) | 1449 (92) | |
| 60-72 | 138 (9) | 1431 (91) | |
| Race/Ethnicity | <0.0001 | ||
| African American | 339 (9) | 3312 (91) | |
| Hispanic | 404 (13) | 2636 (87) | |
| Non-Hispanic White | 116 (6) | 1772 (94) | |
| Maternal age >=35 years at child birth a | 82 (8) | 993 (92) | 0.005 |
| Mean maternal age at child’s birth | 26.5 ( 6) | 25.7 (6) | 0.0002 |
| History of breast feeding – yesb | 569 (10) | 5178 (90) | 0.58 |
| Alcohol during pregnancy – yes c | 27 (10) | 233 (90) | 0.84 |
| Smoking during pregnancy - yes | 90 (12) | 671 (88) | 0.08 |
| Gestational age <33 weeks - yes d | 30 ( 12) | 218 (88) | 0.28 |
| Small for gestational age - yes e | 157 (10) | 1426 (90) | 0.87 |
| Cerebral Palsy – yes f | 7 (58) | 5 (42) | <0.0001 |
| Down Syndrome – yes g | 3 (19) | 13 (81) | 0.24 |
| Family history of strabismus – yes h | 57 (11) | 457 (88) | 0.40 |
| Family history of amblyopia - yes i | 7 ( 6) | 114 (94) | 0.12 |
| SE – right eye (Diopters) *** | <0.0001 | ||
| ≤ − 1.0 | 104 (31) | 236 (69) | |
| −1.0 to ≤ 0 | 168 (15) | 958 (85) | |
| 0 to < +1.0 | 163 (7) | 2,329 (93) | |
| +1.0 to ≤ + 2.0 | 189 (7) | 2,642 (93) | |
| +2.0 to ≤ +3.0 | 121 (11) | 966 (89) | |
| +3.0 to ≥ +5.0 | 114 (16) | 589 (84) | |
| Household income j | 0.002 | ||
| <$20,000 per year | 455 (11) | 3772 (89) | |
| Health Insurance – yes k | 822 (10) | 7405 (90) | 0.88 |
| Vision Insurance – yes l | 369 (9) | 3730 (91) | 0.002 |
Astigmatism: Right eye has 1.5 Diopters or greater astigmatism. MEPEDS: Multi-Ethnic Pediatric Eye Disease Study. BPEDS: Baltimore Pediatric Eye Disease Study. SE: Spherical equivalent
Chi-square test
children with SE of −1.0 Diopters or more extreme were considered myopes; children with SE of +2.0Diopters or more extreme were considered hyperopes.
1 child with astigmatism and 26 unaffected children missing maternal age at childbirth.
11 unaffected children missing history of breastfeeding.
9 unaffected children missing maternal alcohol during pregnancy.
4 children with astigmatism and 70 unaffected children missing gestational age.
23 children with astigmatism and 219 unaffected children missing gestational age.
3 unaffected children missing cerebral palsy data.
4 unaffected children missing Down Syndrome data.
6 children with astigmatism and 50 unaffected children missing family history of strabismus.
3 children with astigmatism and 123 unaffected children missing family history of amblyopia.
104 children with astigmatism and 762 unaffected children missing household income.
2 children with astigmatism and 8 unaffected children missing health insurance in last 12 months.
77 children with astigmatism and 670 unaffected children missing vision insurance in last 12 months.
Characteristics of participants were further evaluated comparing those children in the analysis dataset to those who had been excluded due to missing data; Breslow-Day tests of homogeneity and product interaction terms were used to evaluate significant differences by exclusion status.
To examine the possible nonlinear relationship between maternal prenatal smoking and astigmatism, a regression model was fitted conditioned on the mothers’ history of prenatal smoking; models were adjusted for age, race, and spherical equivalent of the right eye. A similar model was fitted for spherical equivalent (refractive error). Median smoking levels for 12 categories of smoking (pack years) with equal number of individuals per group were plotted against prevalence of astigmatism. An iterative, locally weighted, least squares method was used to generate lines of best fit (LOWESS fit line).16
Results
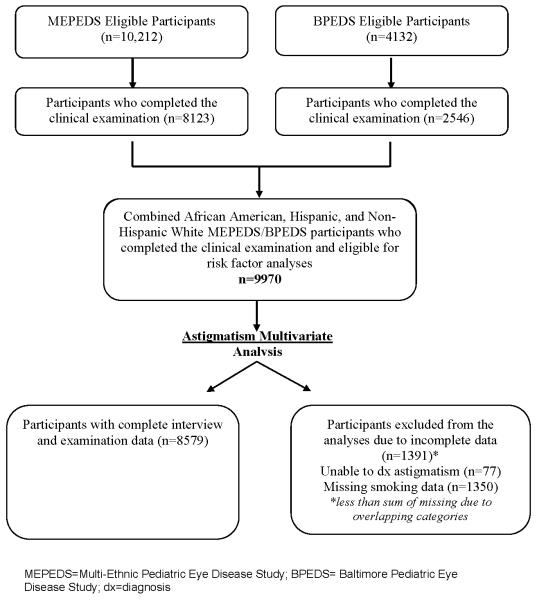
The sample for this analysis includes 8,479 children from Los Angeles, California, or Baltimore, Maryland (Table 1). The number of participants and reasons for exclusion are shown in figure 1. A total of 14,344 eligible children were identified, of whom 10,669 completed clinical eye examinations (2,546 in BPEDS and 8,123 in MEPEDS). The analysis was restricted to the three major racial/ethnic groups (African-American, Hispanic and non-Hispanic white) with completed recruitment and data collected at the two research centers as of August 2010, resulting in 9,970 children. Of the 9,970 children, 1,391 children were excluded because of missing information on astigmatism (n=77) or smoking history during the mother’s pregnancy with the index child (n=1,350). Therefore, the analysis includes 86% (N=8,579) of eligible participants (Fig 1). There were no significant differences in characteristics of children included in the data analysis compared to those excluded for missing data other than SE refractive error of the right eye (P<.0001). The distribution was similar except that 14% of kids with astigmatism in the analysis group were classified as having SE refractive error of the right eye in the highest range (+3 to ≥+5 D) compared to 8% in the excluded group. In the non-astigmatism group, 8% in the analysis group had SE refractive error of the right eye in the highest range (+3 to ≥+5 D) versus 7% in those excluded from the analysis (indicating no difference).
Figure 1.
Participant flowchart highlighting those children who were included and excluded from the final analysis sample in preschool children from both the Multi-Ethnic Pediatric Eye Disease Study (MEPEDS) and the Baltimore Pediatric Eye Disease Study (BPEDS).
The characteristics of children with and without astigmatism are shown in Table 1. Children with astigmatism were more likely to be <12 months of age and to be Hispanic than children without astigmatism. Families of children with astigmatism were more likely to have an income of less than $20,000 per year (P=0.002) and less likely to have vision insurance (P=0.002) than families who did not have a child with astigmatism. With respect to the clinical variables, children with astigmatism were also more likely to have other types of refractive error (i.e. myopia ≤ − 1.00 D spherical equivalent and hyperopia ≥ 2.00 D spherical equivalent) and more likely to have cerebral palsy. However, the number of children with cerebral palsy in either the astigmatism or no astigmatism group was very small. There was no difference in family history of strabismus or amblyopia for children with astigmatism or without astigmatism. Maternal smoking during pregnancy was slightly higher in the children with astigmatism (P=0.08).
In the multivariate analysis (Table 2), four variables remained significantly associated with astigmatism: spherical equivalent refractive error, age group, race/ethnicity, and maternal smoking during pregnancy. Children with spherical refractive error (myopia or hyperopia) were more likely to have astigmatism than children without spherical refractive error. Participants with myopia were 4.6 times as likely to have astigmatism (95%CI 3.56, 5.96) as children without refractive error and participants with hyperopia were 1.6 times as likely to have astigmatism as children without refractive error (95%CI 1.39, 1.94). Children in the youngest age group (6 months to <12 months) were nearly 3 times as likely to have astigmatism as children 5 to 6 years of age (60-72 months), but there were no significant differences for children in any of the other age groups from 12 months through 59 months of age when compared to children 5 to 6 years of age. Hispanic children were approximately 2.4 times as likely to have astigmatism as non-Hispanic white children. African-American children were also more likely to have astigmatism than non-Hispanic white children; however, the magnitude of effect was not as large (OR=1.47). After adjusting for the other variables in the model, maternal smoking during pregnancy was a significant risk factor for astigmatism. In this sample, children whose mothers smoked during pregnancy were 1.5 times as likely to have astigmatism as children whose mothers did not smoke during pregnancy.
Table 2. Independent Risk Factors for Astigmatism based on Multivariate Stepwise Regression in Participants from MEPEDS and BPEDS (N=8,579).
| Risk factors* | Order of Entry into Model |
OR | 95% CI |
|---|---|---|---|
| SE (Right eye) ** | 1 | ||
| ≤−1.0D vs. (>−1.0D <+2.0D) | 4.61 | (3.56, 5.96) | |
| ≥+2.0D vs. (>−1.0D <+2.0D) | 1.64 | (1.39, 1.94) | |
| Age group | 2 | ||
| 06-11 Months vs. 60-72 Months | 2.85 | (2.28, 3.73) | |
| 12-23 Months vs. 60-72 Months | 1.08 | (0.84, 1.38) | |
| 24-35 Months vs. 60-72 Months | 0.95 | (0.74, 1.23) | |
| 36-47 Months vs. 60-72 Months | 0.85 | (0.66, 1.11) | |
| 48-59 Months vs. 60-72 Months | 0.93 | (0.72, 1.20) | |
| Race/Ethnicity | 3 | ||
| African American vs. non-Hispanic White | 1.47 | (1.18, 1.85) | |
| Hispanic vs. non-Hispanic White | 2.38 | (1.91, 2.97) | |
| Smoking during pregnancy | 4 | ||
| Yes vs. No | 1.46 | (1.14, 1.87) |
MEPEDS: Multi-Ethnic Pediatric Eye Disease Study. BPEDS: Baltimore Pediatric Eye Disease Study.
Stepwise multivariate model: All risk factors with P-value < 0.1 at the univariate level were entered into the multivariate model. Risk factors with P-value < 0.05 were kept in the multivariate model.
SE: Spherical equivalent
OR = Odds Ratio; 95% CI = 95 percent Confidence Interval
children with SE of −1.0Diopter or more extreme were considered myopes; children with SE of +2.0Diopter or more extreme were considered hyperopes.
Model results were similar when we changed our threshold level of cylindrical refractive error in the right or left eye to ≥2.00D. When we compared children with cylindrical refractive error of ≥1.50 D to those of 0.00 D (i.e. excluding those children >0.00D and <1.5D, resulting in N=2,810 in the model) gestational age of <33 weeks became a significant risk factor in the multivariate model (P=0.0003, OR=2.55; 95%CI 1.52, 4.26).
When we examined these same four variables in a model restricted to children having WTR astigmatism, we observed similar results (data not shown). Comparing data for children with WTR astigmatism (N=649) to children without astigmatism we also found age < 12 months (OR=2.31, 95%CI 1.75, 3.03), race/ethnicity (Hispanic OR=2.86, 95%CI 2.21, 3.68 and African American OR=1.37, 95%CI 1.05, 1.79), maternal prenatal smoking (OR=1.48, 95%CI 1.11, 1.96), myopia (OR=4.39, 95%CI 3.25, 5.91), and hyperopia (OR=1.72, 95%CI 1.43, 2.08) were significantly associated. When restricting to ATR astigmatism we found the only significant associations were with myopia (OR=4.68, 95%CI 2.43, 9.00) and age (<12 months OR=6.79, 95%CI 2.20, 20.8; 12-23 months OR=6.83, 95%CI 2.38, 19.6; 24-25 months OR=3.78, 95%CI 1.26, 11.4). However, the ATR analysis was based on a limited number of cases (N=70).
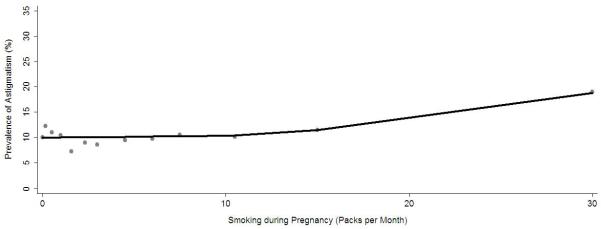
The association between maternal smoking during pregnancy and risk of astigmatism was further evaluated by gender, age group, and race/ethnicity using stratified analysis and formal tests of significance. The association was stronger for girls with astigmatism (OR=1.66, 95%CI 1.19, 2.31) than for boys (OR=1.37, 95%CI 0.96, 1.95) (Table 3), but the interaction term for maternal smoking during pregnancy and gender was marginal (P=0.11) (Table 4, available at http://aaojournal.org.). While an association was present in all 3 racial/ethnic groups (Table 3), the odds ratios were significant only for non-Hispanic white children. There was no statistical interaction for maternal smoking during pregnancy with race/ethnicity (p=0.42) or age group (p=0.21) (Table 4, available at http://aaojournal.org.). The relationship between pack-months of maternal smoking during pregnancy and prevalence of astigmatism shows a small increase in slope with increasing pack-months of tobacco use (Fig 2). The relationship between magnitude of spherical equivalent refractive error and prevalence of astigmatism indicates a U-shaped curve with greater astigmatism prevalence for children at higher levels of hyperopia and myopia values (Fig 3).
Table 3. Association Between Smoking and Astigmatism Stratified by Demographic Characteristics in Participants of the MEPEDS and BPEDS (N=8,579).
| Risk factors | OR | 95% CI |
|---|---|---|
| Smoking during pregnancy (yes vs. no)* | 1.46 | (1.14, 1.87) |
| Male | 1.37 | (0.96,1.95) |
| Female | 1.66 | (1.19, 2.31) |
| Age 6-11 months | 1.16 | (0.64, 2.11) |
| Age 12-23 months | 1.04 | (0.56, 1.96) |
| Age 24 months or higher | 1.74 | (1.30, 2.32) |
| African American | 1.37 | (0.99, 1.91) |
| Hispanic | 1.37 | (0.77, 2.43) |
| Non-Hispanic White | 1.95 | (1.24, 3.08) |
adjusted for age, race/ethnicity, gender, SE refractive error; MEPEDS: Multi-Ethnic Pediatric Eye Disease Study. BPEDS: Baltimore Pediatric Eye Disease Study. OR = Odds Ratio; 95% CI = 95 percent Confidence Interval
Figure 2.
Locally weighted regression line illustrating the independent relationship between maternal, prenatal smoking in packs per month and the estimated prevalence of astigmatism in preschool children in the Multi-Ethnic Pediatric Eye Disease Study and the Baltimore Pediatric Eye Disease Study after controlling for other risk factors. The estimated prevalence of astigmatism was obtained using the stepwise logistic regression procedure.
Figure 3.
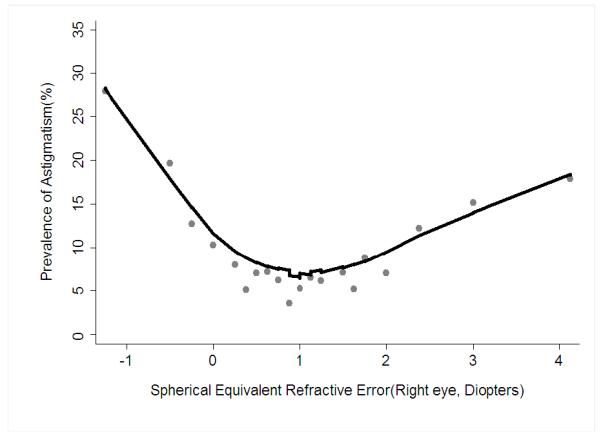
Locally weighted regression line illustrating the independent relationship between spherical equivalent refractive error and the estimated prevalence of astigmatism in preschool children in the Multi-Ethnic Pediatric Eye Disease Study and the Baltimore Pediatric Eye Disease Study after controlling for other risk factors. The estimated prevalence of astigmatism was obtained using the stepwise logistic regression procedure.
Discussion
While characteristics of children with astigmatism have been described in the literature, little is known about preventable risk factors for the condition. In the current analysis, we examined potential clinical, behavioral, and demographic factors that may be associated with astigmatism using data from 2 co-designed, population-based studies. In this sample of preschool age children, age, race, spherical equivalent refractive error, and maternal smoking during pregnancy were associated with astigmatism. Specifically, children with myopia and hyperopia (defined as spherical equivalent ≤ − 1.00 D and ≥ 2.00 D, respectively) were more likely to have astigmatism than children without these refractive errors. Both Hispanic and African-American children were more likely to have astigmatism than non-Hispanic white children and the odds of having the condition were substantially higher in Hispanic children than in the other two racial/ethnic groups. Infants (<12 months of age) were at significantly higher increased risk of astigmatism than older children (5-6 years of age); however, no significant difference in risk was found among children who were 12 months and older in a categorical analysis, suggesting that astigmatism in infancy diminishes as the child ages.
Many studies have described the high prevalence of astigmatism in children younger than 12 months of age.17-29 One study reported that the prevalence of astigmatism (≥1.00 D) in children reached stable levels, similar to those observed in adults (<10%), by 18 months of age. 18 Decreasing astigmatism in early childhood has been described in numerous publications. 18, 20, 22, 24, 25, 30, 31 In a longitudinal study30 of astigmatism in infants, the authors also observed a high prevalence (~42%) of astigmatism (>1.00D) in early infancy that decreased with age. The authors noted that the reduction in astigmatism appeared to be due to a decrease in toricity of the cornea and the anterior lens, along with a decrease in the variation of the cornea and lenticular surfaces. 32 In our multivariate analysis, both race/ethnicity and age were significant predictors of astigmatism after controlling for all covariates simultaneously.
Our analysis dataset (N=8,579) included 649 children with WTR astigmatism and 70 with ATR astigmatism out of 859 total cases. All four variables associated with astigmatism in the overall model also were significantly associated with WTR astigmatism. For children with ATR astigmatism, only age (<12 months, 12-24 months, 25-35 months), and myopia remained significantly associated. However, the analysis of ATR astigmatism is limited by the small number of cases; therefore, it is not clear if the differences in findings were due to sample size or true variation in risk factors for astigmatism of different orientations.
The single environmental or behavioral factor identified as a risk factor for astigmatism in children was maternal smoking during pregnancy. It has been hypothesized that nicotine from tobacco smoke may activate nicotinic acetylcholine receptors, which are believed to be important in refractive development.33, 34 In animal models, drugs that block nicotinic acetylcholine receptors are associated with the development of myopia.35 We evaluated the association between astigmatism and maternal smoking during pregnancy as both dichotomous (yes/no) and continuous (pack-months) variables. Using regression analysis, we found a slight increase in slope of the LOWESS line with increasing pack-months of use; however, the data do not suggest a strong dose-response relationship between pack-months of smoking and astigmatism risk.
A few studies in the literature address parental smoking as a risk factor for refractive error.34, 36, 37 These studies focused on spherical equivalent refractive error, rather than astigmatism, and results from these studies were largely inconclusive. In the MEPEDS and BPEDS sample, maternal smoking during pregnancy was a risk factor for astigmatism and for spherical equivalent refractive error (see companion paper).
We also found that spherical equivalent refractive error, both hyperopia and myopia, were associated with astigmatism. Children with myopia (≤ −1.00D) were 4.6 times as likely to have astigmatism as children without significant refractive error (> −1.00D to <2.00D), while children with hyperopia (≥ 2.00D) were 1.6 times as likely to have astigmatism. Because our data were cross-sectional, we know that children with spherical equivalent refractive error were more likely to have astigmatism than children without spherical equivalent refractive error; but we cannot further evaluate the direction of the association. Previous studies have suggested that uncorrected astigmatism is associated with increased risk of myopia and more severe myopia,5-7, 38 while other studies have found no association between the 2 conditions.39, 40 When considering this additional information from the literature, it may be that astigmatism in infants and young children increases the risk of subsequent myopia, however, it may also be that these different forms of refractive error occur more frequently in the same children.
Several limitations to our analysis should be acknowledged. First, there are other possible risk factors that we did not evaluate, such as pressure of the eye lid on the cornea and genetic background. We do not currently have data to explore these factors. We also do not have quantitative data on maternal or paternal smoking in the child’s home. We were able to examine the association between maternal pack-months of smoking and astigmatism prevalence. We excluded 1,391 individuals from the analysis because of missing data; however, we did not find significant differences in characteristics of participants included in the analysis versus those who were excluded with the exception that a higher proportion of those with astigmatism in the analysis with SE refractive error of the right eye were classified in the highest category (+3 to ≥+5 D). Strengths of the study include a large sample from two population-based studies designed with identical protocols for key clinical and questionnaire data. All clinical examinations were conducted by trained examiners who were certified using standardized procedures by clinicians at both centers. The multiethnic nature of the sample also allowed us to explore the consistency of associations by race/ethnicity.
Astigmatism is a common refractive error for which the causes are largely unknown. Similar to reports in previous clinical and population-based studies, we found that astigmatism is common in infants but normalizes for many infants by 12 months of age. We also found significant variation in the presence of astigmatism by race/ethnicity; Hispanic and African-American children are more likely to have astigmatism than non-Hispanic white children, and this association remains even after correcting for the presence of spherical equivalent refractive error (myopia or hyperopia). Prenatal, maternal smoking was the single modifiable behavioral risk factor we identified for astigmatism. While the prevalence of smoking during pregnancy is typically low, this association may suggest etiologic or genetic pathways for future investigation.
Supplementary Material
Precis.
In a population based sample of 9970 preschool children, maternal smoking during pregnancy, myopia, and hyperopia are associated with a higher risk for astigmatism. Hispanic and African-American children, also, have a higher risk for astigmatism.
Acknowledgments
Support: Supported by the National Eye Institute, National Institutes of Health, Bethesda, MD (grant nos. EY14472 and EY03040), and an unrestricted grant from the Research to Prevent Blindness, New York, NY. Dr. Varma is a Research to Prevent Blindness Sybil B. Harrington Scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
“This article contains online-only material. The following should appear online-only: Table 4.”
Conflicts of Interest: The authors have no proprietary or commercial interest in any materials discussed in the manuscript.
References
- 1.Porter J, Guirao A, Cox IG, Williams DR. Monochromatic aberrations of the human eye in a large population. J Opt Soc Am A Opt Image Sci Vis. 2001;18:1793–803. doi: 10.1364/josaa.18.001793. [DOI] [PubMed] [Google Scholar]
- 2.Read SA, Collins MJ, Carney LG. A review of astigmatism and its possible genesis. Clin Exp Optom. 2007;90:5–19. doi: 10.1111/j.1444-0938.2007.00112.x. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamsson M, Sjostrand J. Astigmatic axis and amblyopia in childhood. Acta Ophthalmol Scand. 2003;81:33–7. doi: 10.1034/j.1600-0420.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- 4.Brown SA, Weih LM, Fu CL, et al. Prevalence of amblyopia and associated refractive errors in an adult population in Victoria, Australia. Ophthalmic Epidemiol. 2000;7:249–58. [PubMed] [Google Scholar]
- 5.Fulton AB, Hansen RM, Petersen RA. The relation of myopia and astigmatism in developing eyes. Ophthalmology. 1982;89:298–302. doi: 10.1016/s0161-6420(82)34788-0. [DOI] [PubMed] [Google Scholar]
- 6.Gwiazda J, Grice K, Held R, et al. Astigmatism and the development of myopia in children. Vision Res. 2000;40:1019–26. doi: 10.1016/s0042-6989(99)00237-0. [DOI] [PubMed] [Google Scholar]
- 7.Tong L, Saw SM, Carkeet A, et al. Prevalence rates and epidemiological risk factors for astigmatism in Singapore school children. Optom Vis Sci. 2002;79:606–13. doi: 10.1097/00006324-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Joint Writing Committee for the Multi-Ethnic Pediatric Eye Disease Study and Baltimore Pediatric Eye Disease Study Groups Risk factors for hyperopia and myopia in preschool children: the Multi-Ethnic Pediatric Eye Disease Study and the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2011 doi: 10.1016/j.ophtha.2011.06.030. Accepted for publication on June 3, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varma R, Deneen J, Cotter S, et al. Multi-Ethnic Pediatric Eye Disease Study Group The Multi-Ethnic Pediatric Eye Disease Study: design and methods. Ophthalmic Epidemiol. 2006;13:253–62. doi: 10.1080/09286580600719055. [DOI] [PubMed] [Google Scholar]
- 10.Friedman DS, Repka MX, Katz J, et al. Prevalence of decreased visual acuity among preschool-aged children in an American urban population: the Baltimore Pediatric Eye Disease Study, methods, and results. Ophthalmology. 2008;115:1786–95. doi: 10.1016/j.ophtha.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: adaptation of the Amblyopia Treatment Study visual acuity testing protocol. Am J Ophthalmol. 2001;132:903–9. doi: 10.1016/s0002-9394(01)01256-9. [DOI] [PubMed] [Google Scholar]
- 12.Holmes JM, Beck RW, Repka MX, et al. Pediatric Eye Disease Investigator Group The Amblyopia Treatment Study visual acuity testing protocol. Arch Ophthalmol. 2001;119:1345–53. doi: 10.1001/archopht.119.9.1345. [DOI] [PubMed] [Google Scholar]
- 13.Cotter SA, Tarczy-Hornoch K, Wang Y, et al. Multi-Ethnic Pediatric Eye Disease Study Group Visual acuity testability in African-American and Hispanic children: the Multi-Ethnic Pediatric Eye Disease Study. Am J Ophthalmol. 2007;144:663–7. doi: 10.1016/j.ajo.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Multi-Ethnic Pediatric Eye Disease Study (MEPEDS) Group Prevalence and causes of visual impairment in African-American and Hispanic preschool children: the Multi-Ethnic Pediatric Eye Disease Study. Ophthalmology. 2009;116:1990–2000. doi: 10.1016/j.ophtha.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander GR, Himes JH, Kaufman RB, et al. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 16.Cleveland WS, Grosse E. Computational methods for local regression. Stat Comput. 1991;1:47–62. [Google Scholar]
- 17.Abrahamsson M, Fabian G, Sjostrand J. Changes in astigmatism between the ages of 1 and 4 years: a longitudinal study. Br J Ophthalmol. 1988;72:145–9. doi: 10.1136/bjo.72.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkinson J, Braddick O, French J. Infant astigmatism: its disappearance with age. Vision Res. 1980;20:891–3. doi: 10.1016/0042-6989(80)90070-x. [DOI] [PubMed] [Google Scholar]
- 19.Cowen L, Bobier WR. The pattern of astigmatism in a Canadian preschool population. Invest Ophthalmol Vis Sci. 2003;44:4593–600. doi: 10.1167/iovs.02-0730. [DOI] [PubMed] [Google Scholar]
- 20.Dobson V, Fulton AB, Sebris SL. Cycloplegic refractions of infants and young children: the axis of astigmatism. Invest Ophthalmol Vis Sci. 1984;25:83–7. [PubMed] [Google Scholar]
- 21.Dobson V, Miller JM, Harvey EM. Corneal and refractive astigmatism in a sample of 3- to 5-year-old children with a high prevalence of astigmatism. Optom Vis Sci. 1999;76:855–60. doi: 10.1097/00006324-199912000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Ehrlich DL, Braddick OJ, Atkinson J, et al. Infant emmetropization: longitudinal changes in refraction components from nine to twenty months of age. Optom Vis Sci. 1997;74:822–43. doi: 10.1097/00006324-199710000-00022. [DOI] [PubMed] [Google Scholar]
- 23.Fulton AB, Dobson V, Salem D, et al. Cycloplegic refractions in infants and young children. Am J Ophthalmol. 1980;90:239–47. doi: 10.1016/s0002-9394(14)74861-5. [DOI] [PubMed] [Google Scholar]
- 24.Gwiazda J, Scheiman M, Mohindra I, Held R. Astigmatism in children: changes in axis and amount from birth to six years. Invest Ophthalmol Vis Sci. 1984;25:88–92. [PubMed] [Google Scholar]
- 25.Howland HC, Sayles N. Photokeratometric and photorefractive measurements of astigmatism in infants and young children. Vision Res. 1985;25:73–81. doi: 10.1016/0042-6989(85)90082-3. [DOI] [PubMed] [Google Scholar]
- 26.Ingram RM, Barr A. Changes in refraction between the ages of 1 and 3 1/2 years. Br J Ophthalmol. 1979;63:339–42. doi: 10.1136/bjo.63.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer DL, Hansen RM, Moore BD, et al. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001;119:1625–8. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- 28.Mohindra I, Held R, Gwiazda J, Brill J. Astigmatism in infants. Science. 1978;202:329–31. doi: 10.1126/science.694539. [DOI] [PubMed] [Google Scholar]
- 29.Woodruf ME. Cross sectional studies of corneal and astigmatic characteristics of children between the twenty-fourth and seventy-second months of life. Am J Optom Arch Am Acad Optom. 1971;48:650–9. [PubMed] [Google Scholar]
- 30.Abrahamsson M, Fabian G, Andersson AK, Sjostrand J. A longitudinal study of a population based sample of astigmatic children. I. Refraction and amblyopia. Acta Ophthalmol (Copenh) 1990;68:428–34. doi: 10.1111/j.1755-3768.1990.tb01671.x. [DOI] [PubMed] [Google Scholar]
- 31.Friling R, Weinberger D, Kremer I, et al. Keratometry measurements in preterm and full term newborn infants. Br J Ophthalmol. 2004;88:8–10. doi: 10.1136/bjo.88.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutti DO, Mitchell GL, Jones LA, et al. Refractive astigmatism and the toricity of ocular components in human infants. Optom Vis Sci. 2004;81:753–61. doi: 10.1097/00006324-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Lindstrom J. Nicotinic acetylcholine receptors in health and disease. Mol Neurobiol. 1997;15:193–222. doi: 10.1007/BF02740634. [DOI] [PubMed] [Google Scholar]
- 34.Stone RA, Wilson LB, Ying GS, et al. Associations between childhood refraction and parental smoking. Invest Ophthalmol Vis Sci. 2006;47:4277–87. doi: 10.1167/iovs.05-1625. [DOI] [PubMed] [Google Scholar]
- 35.Stone RA, Sugimoto R, Gill AS, et al. Effects of nicotinic antagonists on ocular growth and experimental myopia. Invest Ophthalmol Vis Sci. 2001;42:557–65. [PubMed] [Google Scholar]
- 36.Ip JM, Robaei D, Kifley A, et al. Prevalence of hyperopia and associations with eye findings in 6- and 12-year-olds. Ophthalmology. 2008;115:678–85. doi: 10.1016/j.ophtha.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 37.Saw SM, Chia KS, Lindstrom JM, et al. Childhood myopia and parental smoking. Br J Ophthalmol. 2004;88:934–7. doi: 10.1136/bjo.2003.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan DS, Rao SK, Cheung EY, et al. Astigmatism in Chinese preschool children: prevalence, change, and effect on refractive development. Br J Ophthalmol. 2004;88:938–41. doi: 10.1136/bjo.2003.030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goss DA, Grosvenor T. Rates of childhood myopia progression with bifocals as a function of nearpoint phoria: consistency of three studies. Optom Vis Sci. 1990;67:637–40. doi: 10.1097/00006324-199008000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Parssinen O. Astigmatism and school myopia. Acta Ophthalmol (Copenh) 1991;69:786–90. doi: 10.1111/j.1755-3768.1991.tb02061.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.