Abstract
We recently reported that young adults (YA) preferentially recruit cerebellar lobule HVI for symbolic motor sequence learning [3]. Learning magnitude in the symbolic condition was correlated with activation level in lobule HVI. Here, we evaluated age differences in the symbolic representation of motor sequence learning. Fourteen YA and 14 older adults (OA) performed the alternating serial reaction time task (ASRT) under conditions in which the spatial processing component was selectively eliminated from stimulus presentation (spatial versus symbolic), response execution (manual versus vocal), or both. Results showed that OA had reduced learning magnitudes relative to YA. Using the cerebellum lobule HVI as a region-of-interest, we found that OA had significantly lower activation in this region than YA during the symbolic learning conditions (FWE, P<0.05). Similar to YA, OA also showed a significant correlation between learning magnitude and cerebellar activation in the symbolic conditions. These results suggest that although YA and OA recruit similar neural networks during implicit learning, OA under-recruit relevant brain areas which may partially explain their implicit sequence learning deficits.
Keywords: implicit learning, spatial, fMRI, aging
Motor sequence learning refers to an ability to combine isolated movements into one smooth, coherent action. It can be either explicit, where participants are aware of the sequence and the goal of learning, or implicit, where learning occurs outside of conscious awareness. Previous studies have indicated that normal aging generally does not affect the implicit learning of sequences [8, 9]. Age-related differences in sequence learning emerge only when complex sequence structures are used, such as in the alternating serial reaction time task (ASRT) where sequential and random elements are presented in an alternating fashion [7, 10, 12]. Responses for this task can be made based on either spatial (e.g., responses are cued with different spatial locations) or symbolic cues (e.g., responses are cued with letters/colors) [4]. The latter condition requires more complex mapping between stimuli and responses [3, 4]. It has been reported that brain activation patterns do not generally differ between young (YA) and older adults (OA) performing sequence learning under spatial cueing [5]. However, several other studies have revealed differential activation patterns for young and older adults during sequence learning [6, 15]. For example, Rieckmann et al. [15] recently reported “compensatory” over-activations in the medial-temporal and frontal areas for OA. In contrast, Dennis & Cabeza [6] found that older adults had greater interactions between medial temporal regions and the striatum during both explicit and implicit learning, suggesting that the two learning systems become less distinct with age. Here, we specifically focus on age differences in symbolic representations of motor sequence learning.
We recently found that young adults selectively recruit the left cerebellum lobule HVI for ASRT learning under symbolic cueing, with the activation in lobule HVI correlating with learning magnitude [3]. Given that the cerebellum exhibits structural degeneration with age [14], we examined here whether age differences in cerebellar recruitment relate to sequence learning deficits typically seen in OA with the ASRT task. To examine the neural differences between symbolic and spatial sequence learning in YA and OA, we selectively eliminated spatial components from stimulus presentation (spatial versus symbolic), response execution (manual versus vocal), or both. We hypothesized that OA would show significantly less activation in the cerebellum during symbolically cued sequence learning. Further, we expected that this reduced activation would correlate with sequence learning magnitude in OA, suggesting that under-recruitment of the cerebellum contributes to sequence learning deficits in OA.
Fourteen OA (mean age = 72.7 years (± 4.0), 8 males and 6 females,) and 14 YA (mean age = 21.4 years (±2.5), 6 males and 8 females) participated in this study. All individuals were right-handed (determined by self-report and the Edinburgh handedness inventory [13]). The YA data have been previously published [13] and are included here for comparison purposes.
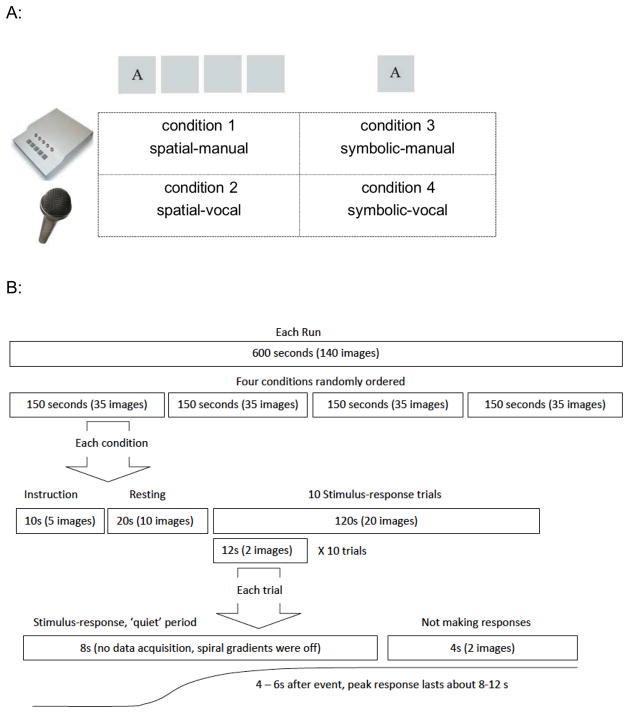
Completion of the experiment required two visits. During the first visit, participants lay supine in a mock scanner and performed finger tapping in response to randomly ordered stimuli across two runs to familiarize them with the task. During the second visit, participants were positioned in a 3.0 Tesla GE MRI scanner (General Electric, Waukesha, WI). They performed 7 runs; the first and last were runs of randomly ordered stimuli and the middle 5 were ASRT runs. For random runs, each trial was made up of 8 randomly generated elements. For ASRT runs, each trial contained an 8-element sequence in which fixed and random elements were alternated (i.e., D r B r A r C r). Each run consisted of four conditions (i.e., spatial-manual, spatial-vocal, symbolic-manual, and symbolic-vocal, Figure 1A) with 10 trials in each condition. Each condition began with a 10-second instruction and a 20-second resting period in which the participant visually fixated the center of the screen. Then, the 8-element sequence was presented element by element every 1000 ms (i.e., the stimulus stayed for 900 ms followed by 100 ms without the stimulus). This constant inter-stimulus interval (ISI) was used because, based on our previous study [4], it allowed the OA enough time to make responses and ensured that both groups performed the task at the same temporal pace. Participants were instructed to make the appropriate response as soon as they saw the stimulus. After each 8-second stimulus-response period (when the scanner remained quiet to allow for recording of vocal responses), participants looked at the fixation cross for 4 seconds (when two functional volumes were acquired, i.e. interleaved data collection method, Figure 1B and also see Bo et al., [3] for further details). After the 4-second data acquisition period, the fixation cross was replaced by the first element of the next sequence. Each complete 8-element sequence plus 4 seconds of scanning defined an individual trial. Each run consisted of four randomly ordered 10-trial conditions. Following the experiment, we interviewed participants to probe their awareness of the sequence using five increasingly specific questions [3].
Figure 1.
A) The four experimental conditions of the current study included 1) spatial-manual: four visual stimulus boxes were presented on the screen with one of four letters appearing in one of the stimulus boxes. The positions of these four letters were fixed to the four stimulus boxes, i.e. “A” always appeared at the leftmost; “B” was at the second leftmost, “C” appeared at the second rightmost box and “D” was at the rightmost position. The letters A, B, C and D were mapped onto the index, middle, ring and little fingers of the right hand; 2) spatial-vocal: participants were asked to make the vocal responses ant, bear, cat, and dog when seeing the stimulus letters, A, B, C, and D respectively. The stimulus presentation was identical to that of condition 1; 3)symbolic-manual: this condition involved only one centrally located stimulus box. The letter-finger mapping was the same as that in condition 1; 4) symbolic-vocal: participants made the same vocal responses as those in condition 2 in response to centrally located letter cues. B) Interleaved data collection method. There are four conditions randomly ordered within each run. Each condition began with a 10-second instruction, a 20-second resting period and 10 testing trials. Within each trial, the 8-element sequence was presented element by element every 1000 ms, followed by 4 seconds no response period. During the “quiet” 8-second stimulus-response period, the spiral imaging gradients were turned off to decrease the audio noise so that participants’ vocal responses could be recorded, while the slice selective RF pulse was kept on in order to preserve the magnetization effects throughout the scans. Then, two functional volumes were triggered to acquire the subsequent 4 seconds of data. This delayed interleaved method detects the peak hemodynamic response which typically occurs 5 to 6 seconds after an event [2].
Images were acquired using a 3.0 Tesla GE MRI scanner at the University of Michigan’s Functional Magnetic Resonance Imaging Center. Functional images were acquired using a single-shot gradient-echo (GRE) reverse spiral pulse sequence. Pulse sequence parameters were repetition time/echo time/flip angle/field of view (TR/TE/FA/FOV) of 2000 ms/30 ms/90/220 mm respectively. Forty, 3.0 mm thick slightly oblique axial slices (no gap) were acquired. Our slice coverage captured the whole brain and all but the most inferior portion of the cerebellum (see Supplemental Figure S1 for a representative example).
High resolution anatomical images were also acquired using a T1-weighted gradient echo pulse sequence with the following parameters TR/TE/FA/FOV 300 ms/5 ms/90/220 mm and a voxel size of 1 mm × 1 mm × 1.2 mm.
Median reaction times (RT) for each condition within each run were computed separately for correct random and fixed trials. Then, the RT difference (random – fixed trials) was calculated by taking the difference between the random and fixed trials. We conducted a regression analysis on RT difference with learning runs (i.e. runs 2 – 6) as a continuous variable, and stimulus (spatial vs. symbolic), response (manual vs. vocal) and age group (YA vs OA) as categorical variables to assess learning.
FSL MCFLIRT was used for motion correction. The Brain Extraction Tool (BET) was used to strip the skull from the images. Using Statistical Parametric Mapping version 5 (SPM5), we registered the high-resolution T1 anatomical images to the functional images. Then, we acquired the transformation parameters to align the T1 to the MNI template and applied this transformation to the functional data.
Since we previously found that cerebellum HVI was selectively related to symbolic learning [3], the comparisons between spatial and symbolic conditions (pooling across response mode) during the learning runs (runs 2–6) in HVI (central coordinate: −8, −72, −20) were our particular interest. In addition, left putamen (central coordinate: −27, −15, 12) was chosen as another ROI since recent work suggested that activation in this striatal region correlated with learning performance in OA [6, 15]. The results of a whole brain analysis using a more liberal threshold (uncorrected, P < .005) are listed in the supplemental materials.
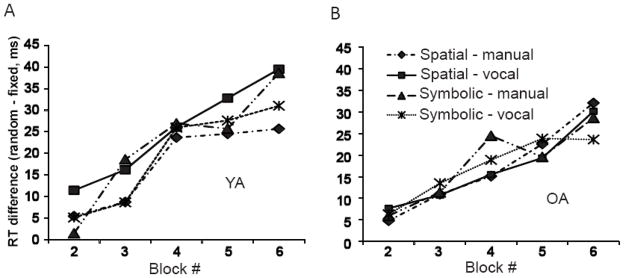
Figure 2A and 2B illustrates the RT differences between sequence and random elements across all 5 learning runs for YA and OA. A linear regression analysis on RT difference (with run as a continuous variable, age, stimulus and response as categorical variables) showed that both YA and OA improved sequence performance across runs (run effect: F(1, 520) = 50.01, P<0.01), but OA exhibited less learning overall (age effect: F(1, 520) = 9.44, P<0.01). None of the interactions, stimulus or response main effects were significant, suggesting that learning occurred equally across the experimental conditions. In order to further examine whether the significant learning across runs was not solely due to learning in the YA, a separate ANOVA analysis on the OA data set was performed. A significant run effect (F(3, 260) = 20.40, P<0.01) was found. None of the interactions, stimulus or response main effects were significant (all, P > 0.05)
Figure 2.
The reaction time differences (random-fixed) in four conditions during 5 learning runss for YA (A) and OA (B).
To determine the difference between spatial and symbolic cue effects, we performed main effect comparisons between the two spatially cued conditions and the two symbolically cued conditions during learning (runs 2–6). The whole brain analyses with a relatively liberal threshold (P < .005 uncorrected) revealed that YA had higher activation in the left hippocampus for the spatially cued conditions than the symbolically cued conditions while OA had higher activation in the right paracentral lobule (Supplemental Table S1a). In contrast, for the symbolically cued versus the spatially cued conditions, the left cerebellum lobule HVI showed higher activation in YA and the right superior temporal gyrus had higher activation in OA (Supplemental Table S1b). The contrasts between YA and OA (Supplemental Table S1a & b) revealed that there were no regions where OA showed significantly greater activation than the YA for either contrast.
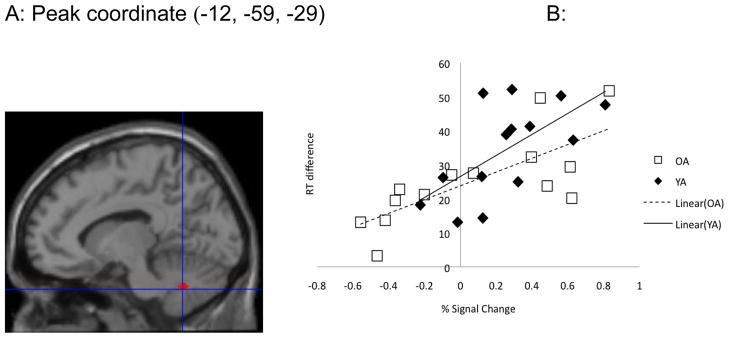
Using the cerebellum lobule HVI as a region of interest, we previously found that the left cerebellum lobule HVI showed stronger activation for the symbolically cued conditions than the spatially cued ones in YA. In addition, the percent signal change (i.e., percent change in the BOLD signal from the resting state to the stimulus/response condition) within the active region was significantly correlated with the RT difference between fixed and random trials in the symbolic conditions at the completion of sequence learning (r = .54, P < .05) [3]. This effect was absent when learning was spatially cued (P > .05). For OA, no areas showed stronger activation for the contrasts between symbolic and spatial conditions at the FWE-corrected .05 threshold. Comparing between YA and OA, we found that YA had significantly higher activation in the cerebellum lobule HVI region than OA during the symbolic learning conditions (FWE, P<0.05, Figure 3A). Similar to YA, OA also showed a significant correlation between learning magnitude (run 6) and cerebellar activation (r=.74, P<0.01) in the symbolic conditions. Figure 3B illustrates the correlations between the RT differences at run 6 in the symbolically cued conditions for both YA and OA. No group differences were found in the left putamen ROI for these contrasts.
Figure 3.
A) YA had significantly higher activation in cerebellum HVI than OA in the symbolic > spatial conditions; B) Significant correlation between percent signal change (i.e., percent change of the signal during the sequence trials relative to at rest) and RT difference at run 6 in the symbolic conditions for both YA and OA.
Areas that were activated across the whole time course of learning regardless of condition play a role in learning the more abstract features of the sequence. Results from the whole brain analyses (using a P < .005 uncorrected threshold) are listed in supplemental table S2. In general, OA showed very similar activation patterns to the YA, including activation in the left basal ganglia, cingulate gyrus, parietal cortex (BA 39) and bilateral cuneus (BA 17, 18) for sequence learning (Supplemental Table S2).
Similar to what we found previously for YA [3], the magnitude of activation in the cerebellar lobule HVI was correlated with the amount of learning that OA exhibited when movements were symbolically cued but not spatially cued. However, OA exhibited less sequence learning and reduced cerebellar recruitment in comparison to YA. It has been proposed that the cerebellum specifically encodes symbolic representations of action [1]. Spencer & Ivry [17] have demonstrated that deficits in sequence learning for patients with cerebellar damage are mitigated when responses are directly cued. The dissociation between symbolic and direct cueing suggests that the cerebellum is critical for maintaining sequential stimulus-response (S-R) associations [17]. Our current findings support that the cerebellum lobule HVI is the central site engaged in sequence learning when movements are symbolically cued.
It is interesting though that Balsters & Ramnani [1] found that cerebellar lobule HVIIa (Crus I & II) was specifically related to symbolic cues. Based on the anatomical connections between the cerebellum and the cerebral cortex, cerebellar lobules HV, HVI, HVIIb and HVIII are part of the “motor” loop connected with the motor cortices while lobule HVIIa (principally Crus I and II) is part of the “cognitive” loop connecting with prefrontal areas [11]. It has been argued that S-R mapping is related to the dorsal prefrontal areas [16] and the prefrontal projecting areas of the cerebellum [1]. In the current study, we did not find prefrontal engagement for any of the contrasts for either YA or OA even when a more liberal threshold was used. Instead, the primary motor areas and premotor cortex (Supplemental table S2) were found to be involved in our YA data set for general sequence learning [3]. The discrepancy between the “motor” (i.e. lobule HVI from the current study) and “cognitive” (i.e. lobule HVIIa from Balsters & Ramnani [1]) sites of the cerebellum may be due to different experimental designs between the two studies. Balsters & Ramnani [1] employed a delayed stimulus-response task and found higher cerebellar activation when movements were cued with geometrical symbols compared to when movements were directly cued with fingers. Schwarb & Schumacher [16] focused on the role of dorsolateral prefrontal cortex on spatial S-R selection. They orthogonally manipulated spatial sequence structure (sequence versus random) and spatial S-R compatibility (i.e., spatial correspondence between stimuli and response fingers) and found that dorsal prefrontal areas are important for spatial S-R selection but not for sequence learning. Thus, it is not surprising that no “cognitive” sites were found in our current symbolic learning conditions. Furthermore, our results suggest that the “motor” areas of the cerebellum (lobule HVI) indeed contribute to symbolic sequence learning.
The current behavioral results replicated our previous finding that implicit sequence learning was not affected by spatial versus symbolic cueing for either YA or OA [3, 4]. Condition effects may have been weakened by our repeated measures approach. This seems unlikely though given the condition-specific correlations between cerebellar activation and learning. One may argue that less learning, regardless of condition, for OA in the current study may due to an insufficient number of learning runs or the fast paced task/response timing. Our previous study [4] did show equivalent learning outcomes between YA and OA after two days (60 minutes/day) of training. Thus, we cannot rule out the possibility that OA would eventually reveal compatible learning to YA if more learning runs were provided. However, we do not believe that task/response timing deteriorated the learning for OA in the current study. The fixed stimulus-response interval (SRI) length of 1s might be too slow for YA but should be appropriate for OA [4]. If the relatively longer ISI were to suppress learning, we should expect less learning in YA but not OA.
Future studies should explore the relationship between working memory capacity or response-selection load and symbolic learning in YA and OA. Our previous behavioral study [4] revealed that OA performance was more affected in tasks with higher response-selection load compared to YA. Other studies have suggested the importance of working memory in implicit sequence learning (e.g., [8]). If the cerebellum is indeed a critical site for symbolic learning, we may observe greater cerebellar activity in symbolic tasks with high response-selection loads.
Daselaar and colleagues reported that OA recruited the same neural network as YA during implicit sequence learning [5]. Other studies, however, found that healthy older adults recruited additional brain areas to perform a sequence task at the same level as young adults (e.g., [18]). Rieckmann et al. [15] reported that older adults relied on regions such as the medial-temporal and frontal areas to promote successful learning. The authors argued that the lack of age deficits in implicit learning might be due to compensatory processes. However, Dennis & Cabeza [6] recently reported that recruitment of the medial temporal areas by older adults during implicit learning was not related to performance, suggesting instead that over-recruitment during sequence learning reflects dedifferentiation mechanisms in aging. In the current study, we did not find additional recruitment of the temporal areas by OA. Rather, when we used a more liberal threshold (uncorrected, P < .005), YA had higher activation in the bilateral superior temporal gyri during the spatial implicit learning while OA did not.
Thus, our current findings do not demonstrate compensatory over-recruitment by OA. That is, there were no regions where OA showed greater activation than YA for any of the conditions. Rather, OA had significantly lower activation in the cerebellum lobule HVI, an area that we previously reported to be selectively recruited for symbolic learning in YA [3]. Both YA and OA showed a significant correlation between learning magnitude and cerebellar percent signal change. These findings suggest that although YA and OA recruit similar neural networks during implicit learning, OA under-recruit relevant brain areas which may partially explain their implicit sequence learning deficits.
Supplementary Material
Highlights.
We evaluated age differences in the symbolic representation of motor sequence learning.
Older adults had reduced learning magnitudes relative to YA in the alternating serial reaction time task (ASRT).
Older adults had significantly lower activation in the cerebellum lobule HVI than young adults during the symbolic learning.
Older adults showed a significant correlation between learning magnitude and cerebellar activation in the symbolic learning.
Acknowledgments
This work was supported by NIH AG024106 (to RS) and the UM Pepper Center Human Subjects Core (NIH AG 08808). The authors wish to thank all of the research assistants who helped with data collection and the participants who gave willingly of their time and effort.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balsters JH, Ramnani N. Symbolic representations of action in the human cerebellum. Neuroimage. 2008;43:388–398. doi: 10.1016/j.neuroimage.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Hum Brain Map. 1999;7:106–114. doi: 10.1002/(SICI)1097-0193(1999)7:2<106::AID-HBM4>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bo J, Peltier SJ, Noll DC, Seidler RD. Symbolic representations in motor sequence learning. Neuroimage. 2011;54:417–426. doi: 10.1016/j.neuroimage.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bo J, Seidler RD. Spatial and symbolic implicit sequence learning in young and older adults. Exp Brain Res. 2010;201:837–851. doi: 10.1007/s00221-009-2098-5. [DOI] [PubMed] [Google Scholar]
- 5.Daselaar SM, Rombouts SA, Veltman DJ, Raaijmakers JG, Jonker C. Similar network activated by young and old adults during the acquisition of a motor sequence. Neurobiol Aging. 2003;24:1013–1019. doi: 10.1016/s0197-4580(03)00030-7. [DOI] [PubMed] [Google Scholar]
- 6.Dennis NA, Cabeza R. Age-related dedifferentiation of learning systems: an fMRI study of implicit and explicit learning. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennis NA, Howard JH, Jr, Howard DV. Implicit sequence learning without motor sequencing in young and old adults. Exp Brain Res. 2006;175:153–164. doi: 10.1007/s00221-006-0534-3. [DOI] [PubMed] [Google Scholar]
- 8.Frensch PA, Miner CS. Effects of presentation rate and individual differences in short-term memory capacity on an indirect measure of serial learning. Mem Cognit. 1994;22:95–110. doi: 10.3758/bf03202765. [DOI] [PubMed] [Google Scholar]
- 9.Howard DV, Howard JH. Age-Differences in Learning Serial Patterns - Direct Versus Indirect Measures. Psychol Aging. 1989;4:357–364. doi: 10.1037//0882-7974.4.3.357. [DOI] [PubMed] [Google Scholar]
- 10.Howard DV, Howard JH, Jr, Japikse K, DiYanni C, Thompson A, Somberg R. Implicit sequence learning: effects of level of structure, adult age, and extended practice. Psychol Aging. 2004;19:79–92. doi: 10.1037/0882-7974.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negash S, Howard DV, Japikse KC, Howard JH. Age-related differences in implicit learning of non-spatial sequential patterns. Aging Neuropsychol Cognit. 2003;10:108–121. [Google Scholar]
- 13.Oldfield RC. Assessment and Analysis of Handedness - Edinburgh Inventory. Neuropsychologia. 1971;9:97. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 14.Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle–aged and older adults: regional and individual differences. Neuroimage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieckmann A, Backman L. Implicit learning in aging: extant patterns and new directions. Neuropsychol Rev. 2009;19:490–503. doi: 10.1007/s11065-009-9117-y. [DOI] [PubMed] [Google Scholar]
- 16.Schwarb H, Schumacher EH. Neural evidence of a role for spatial response selection in the learning of spatial sequences. Brain Res. 2009;1247:114–125. doi: 10.1016/j.brainres.2008.09.097. [DOI] [PubMed] [Google Scholar]
- 17.Spencer RM, Ivry RB. Sequence Learning is Preserved in Individuals with Cerebellar Degeneration when the Movements are Directly Cued. J Cogn Neurosci. 2008;7:1302–1310. doi: 10.1162/jocn.2009.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu T, Hallett M. The influence of normal human ageing on automatic movements. Journal of Physiology-London. 2005;562:605–615. doi: 10.1113/jphysiol.2004.076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.