Abstract
The eye is an immunologically privileged and profoundly immunosuppressive environment. Early studies reported inhibition of T cell proliferation, IFN-γ production and generation of T regulatory cells (Treg) by aqueous humor (AH), and identified TGF-β as a critical factor. However, T cell subsets including FoxP3+ Treg and Th17 were unknown at that time, as was the role of retinoic acid (RA) in Treg induction. Consequently, the effect of the ocular microenvironment on T cell lineage commitment and function, and the role of RA in this process, had not been explored. We now use gene manipulated mice and highly purified T cell populations to demonstrate that AH suppresses lineage commitment and acquisition of Th1 and Th17 effector function of naïve T cells, manifested as reduction of lineage-specific transcription factors and cytokines. Instead, AH promoted their massive conversion to FoxP3+ Treg that expressed CD25, GITR, CTLA-4 and CD103 and were functionally suppressive. TGF-β and RA were both needed and synergized for Treg conversion by AH, with TGF-β enhancing T cell expression of RARα. Newly converted FoxP3+ Tregs were unstable, but were stabilized upon continued exposure to AH or by the DNA demethylating agent 5-AZA. In contrast, T cells already committed to effector function were resistant to the suppressive and Treg-inducing effects of AH. We conclude that RA in the eye plays a dual role: in vision and in immune privilege. Nevertheless, primed effector T cells are relatively insensitive to AH, helping to explain their ability to induce uveitis despite an inhibitory ocular microenvironment.
Introduction
Vision is without a doubt the single most important sense that we possess and the one most affecting survival ability. The process of inflammation, while important to eradicate infectious agents, can cause significant collateral damage to the tissue. Because even small perturbation of the integrity of the light sensing structures can have very deleterious consequences to vision, the eye resists inflammatory processes, a phenomenon known as immune privilege of the eye. These include: sequestration of retinal antigens behind an efficient blood-retina barrier (BRB), absence of lymphatic drainage of the interior of the intact globe (although once the BRB is breached, the eyes are drained by submandibular lymph nodes), a paucity of resident class II+ antigen presenting cells (APC) in the healthy retina and an immunosuppressive ocular microenvironment, composed of soluble and cell bound inhibitory factors. This includes 500-2250 pg/ml of TGF-β (mainly as TGF-β2) and immunoinhibitory neuropeptides in ocular fluids, as well as constitutive expression of FasL, PD-L1, galectins, CTLA-2α etc., on ocular cells (1-3). Finally, under some circumstances the eye is able to influence immunity at the systemic level, through anterior chamber associated immune deviation (ACAID) and post-recovery tolerance (2, 4). These evolutionary adaptations limit induction and expression of immunity in the eye in the event of influx into the eye of immune-competent cells from the circulation as a result of damage to retinal vasculature due to an abnormality or trauma (2). Nevertheless, despite immune privilege, the eye is subject to autoimmune inflammation triggered by retina-specific T cells activated in the periphery by innate or cross-reactive antigenic stimuli (5).
Early studies reported that AH, through the activity of TGF-β, could inhibit IFN-γ production in culture by T cells obtained from CFA-primed mice and converted them to TGF-β-producing Tregs (6). Additional factors identified in the AH that contribute to its immunosuppressive properties are the neuropeptides α-MSH, vasoactive intestinal peptide (VIP), calcitonin gene-related peptide (CGRP), and somatostatin (SOM) (7-10). These early experiments provided important evidence that the ocular fluids could promote local Treg generation, but tools were simply not available at that time to dissect the phenomenon at a mechanistic level made possible by today's state of knowledge. The studies predated the discovery of FoxP3 as a marker for Tregs. The experiments were done with mixed lymph node cell populations containing newly primed and naïve T cells as well as natural and induced Tregs. Thus, they could not distinguish whether Tregs arose from primed or from naïve precursors and could not distinguish proliferation of preexisting Tregs from de novo induction. Retinoic acid (RA) as a Treg inducer had not yet been recognized. RA is highly abundant in the eye due to its role in the visual cycle (25 pmoles/ml in human AH) (11) but its role, if any, in immune privilege is unknown. Finally, Th17 cells as pathogenic effectors with a central role in ocular pathology had not yet been discovered, so effects on Th17 induction could not be studied.
In the present study we fill these critical gaps in knowledge through the use of gene-manipulated mice and highly purified T cell populations. We demonstrate for the first time that not only Th1, but also Th17 lineage commitment is suppressed by AH, and instead these cells are shunted to the FoxP3+ Treg pathway. RA has a critical role in this process, as does TGF-β which upregulates RARα in the differentiating T cells and synergizes with RA. However, T cells that had initially been primed in the absence of AH are relatively resistant to these effects, and if anything, their effector function appears to even be stabilized by exposure to AH. This may explain why uveitogenic T cells, after being activated outside the eye and acquiring the ability to cross the BRB, are able to induce uveitis in the face of an inhibitory ocular microenvironment.
Materials and Methods
Mice
C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). FoxP3-GFP reporter mice on C57BL/6 background were a kind gift from Drs. V.K. Kuchroo and M. Oukka (Brigham and Women's Hosp., Cambridge, MA) (12). All mice were housed under SPF conditions, fed standard laboratory chow ad libitum and used at 6 to 10 weeks of age. Treatment of animals was in compliance with Institutional Guidelines and all animal study protocols were approved by the NEI Animal Care and Use Committee.
Cytokines and antibodies
Recombinant mouse IL-6 and human TGF-β1 were from R&D Systems (Minneapolis, MN); recombinant human IL-2 and mouse IL-12 were from PeproTech (Rocky Hill, NJ); anti-IFN-γ (clone R4-6A2) was from BioXCell (West Lebanon, NH); anti-IL-4 (11B11) was from National Cancer Institute-Frederick Biological Resources Branch Preclinical Repository (Frederick, MD). Antibodies used for flow cytometry analysis were all from eBioscience (San Diego, CA), labeled with fluorochromes as specified. All primary antibodies for Western Blotting were from Cell Signaling Technology (Danvers, MA).
Aqueous humor preparation
Fresh bovine eyes were purchased from J.W. Treuth & Sons (Baltimore, MD). AH was aspirated by syringe using 25-gauge needle. After being sterilized by filtration, AH was stored at -80°C. AH was “activated” by acidification with 1M HCl to pH ∼2 followed by neutralization with 1M NaOH to pH ∼7 before being used in the experiment. Unless otherwise indicated, AH was used at the final concentration of 20% (v/v).
CD4+ T cell isolation and culture
CD4+ T cells were purified from pooled splenocytes and/or lymph nodes, either by sorting for CD4+GFP- T cells to ∼99% purity using FACS Aria (Becton Dickinson, San Jose, CA), or by immunomagnetic isolation (Miltenyi Biotec, Auburn, CA) for CD4+CD25- T cells (residual FoxP3+ T cells ∼1.5%). Cells in complete HL-1 medium (Cambrex, East Rutherford, NJ) were stimulated with plate-bound anti-CD3 (5μg/ml) plus soluble anti-CD28 (2 μg/ml) in the presence or absence of AH. Where indicated, cells were polarized under Th0 (neutral), Th1-polarizing conditions (10 ng/ml IL-12, 10 μg/ml anti-IL-4) or Th17-polarizing conditions (0.5 ng/ml TGF-β, 10 ng/ml IL-6, 10 μg/ml anti-IFN-γ, 10 μg/ml anti-IL-4) for 3 to 4 days as indicated. Cytokine concentration in culture supernatants was determined by ELISA (R&D).
T cell Proliferation
For thymidine uptake, CD4+ T cells were stimulated with anti-CD3/CD28 in the absence or presence of AH for 72 h. [3H]-thymidine (1μCi/well) was added for the last 6-8 h of culture. The incorporated radioactivity was measured by a MicroBeta TriLux scintillation counter (PerkinElmer Life Science, Boston, MA, USA). For proliferation dye dilution, CD4+ T cells were labeled with eFluor® 670 (5 μM, eBioscience) and activated with anti-CD3/CD28 in the presence or absence of AH for 72 h, or were injected into eyes of recipient mice that were harvested for analysis 4 days later. There was no difference in viability between control and AH-treated cells (data not shown).
Treg conversion
CD4+ T cells were stimulated with anti-CD3/CD28 in medium containing 10 ng/ml recombinant human IL-2 in presence or absence of AH for 72 h. Where indicated, RAR pan-antagonist LE540 (100 nM; Wako Chemicals, Richmond, VA), anti-TGFβ2 (20 μg/ml; R&D Systems), or anti-TGFβ1/2/3 (20 μg/ml; clone 1D11; BioExpress, West Lebanon, NH) were added 2 h before adding AH. For phenotypic analysis, cells were stained with PE-conjugated anti-CD25, anti-CD103, anti-GITR, or anti-CTLA-4. Dead cells were excluded by staining with 7-amino-actinomycin D (7-AAD; BD Biosciences, San Jose, CA).
Treg suppression assay
CD4+ T cells from FoxP3-GFP reporter mice were activated with anti-CD3/CD28 in the presence of AH. After 72 h, cells were sorted for CD4+GFP+ cells as AH-Tregs and co-cultured with eFluor670-labeled CD4+GFP- responder T cells (5×104cells/well) from Foxp3-GFP reporter mice in the presence of soluble anti-CD3 (0.5μg/ml) and irradiated T cell-depleted spleen cells (1×105cells/well) for another 72h. Cell proliferation was determined by eFluor 670 dilution.
Stability of FoxP3 expression
CD4+ T cells from FoxP3-GFP reporter mice were stimulated with anti-CD3/CD28 in the presence of AH for 72 h (1st culture). GFP+ cells were sorted and were re-stimulated (2nd culture) with anti-CD3/CD28 for another 72 h with or without AH or 5-Aza-2′-deoxycytidine (Aza, 0.3 μM, Sigma, Saint Louis, MO). Repeated cultures were performed as above, except that after the 3rd round cells were no longer sorted for FoxP3-GFP. Percent cells expressing FoxP3-GFP was determined after each round of stimulation.
Intracellular cytokine staining
Cells were stimulated with PMA (10 ng/ml) and Ionomycin (500 ng/ml) in the presence of Brefeldin A (GolgiPlug, BD Pharmingen, San Diego, CA) as previously described (13). After 4-5 h, cells were fixed with 4% paraformaldehyde, permeabilized with PBS containing 0.1% BSA and 0.05% Triton X-100 and stained with PerCP-Cy5.5-conjugated anti-CD4, PE-conjugated anti-IFN-γ, APC-conjugated anti-IL-17. Up to 100,000 events were acquired using BD CellQuest software and analyzed using FlowJo software (TreeStar Inc, Ashland, OR).
RNA isolation and Real-time PCR
Total RNA from cells or tissues was extracted with RNeasy Mini Kit (QIAGEN, Valencia, CA) and cDNA was synthesized using SuperScript III First-Strand Synthesis System (Invitrogen). Quantitative real-time PCR (qRT-PCR) was performed with TaqMan 7500 sequence detection system (Applied Biosystems, Foster City, CA) using endogenous control for 18S rRNA or GAPDH and primer/probe sets from Applied Biosystems. Data were normalized to 18S rRNA or GAPDH and expressed relative to Th0 or spleen control (set as 1).
Western blotting
After cell polarization for 12 h or 48 h, whole cell lysates were prepared in lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1mM EDTA, 1% Triton X-100, supplemented with a protease inhibitor mix). Lysates were centrifuged at 14,000 rpm for 5 min at 4°C, supernatants were collected and protein concentration was measured. A total of ∼5μg protein was resolved on a 10% SDS-PAGE gel (Invitrogen Life Technologies) and transferred to a nitrocellulose membrane (Whatman, Sanford, ME) using a semidry transfer cell apparatus (Bio-Rad, Hercules, CA). The membranes were treated with 5% nonfat milk for 2 h to block nonspecific binding, rinsed, and incubated with anti-phospho-STAT3 (Tyr705, p-STAT3), anti-STAT3, anti-phospho-STAT1 (Tyr701, p-STAT1), or anti-STAT1. Signals were detected with horseradish peroxidase-conjugated anti-rabbit IgG using the ECL system (Amersham Biosciences, Piscataway, NJ).
Statistics and experimental reproducibility
Values are presented as mean ± SD where indicated. Statistical differences were calculated with an unpaired Student's t-test, two-tailed (GraphPad Prism version 5.0b). Statistical significance was set at p ≤ 0.05. Experiments were repeated at least 3, and usually more times, with number of repetitions stated in the Figure Legend. Results were highly reproducible.
Results
Aqueous humor favors FoxP3+ regulatory T cell induction at the expense of Th17 and Th1
Although lymphocytes are mostly excluded from the healthy eye, bleeding into the eye can occur as a result of a minor trauma or a vascular abnormality. T cells that find themselves in the eye would immediately be bathed in ocular fluids. The composition of ocular fluids is evolutionarily conserved among species (1, 14-16). Therefore, we used bovine aqueous humor (AH), transiently acidified to activate TGF-β, and highly purified CD4+Foxp3- T cells stimulated with anti-CD3/CD28, to examine direct effects of ocular fluid on T cell responses. Rabbit and rat AH had similar activity (not shown).
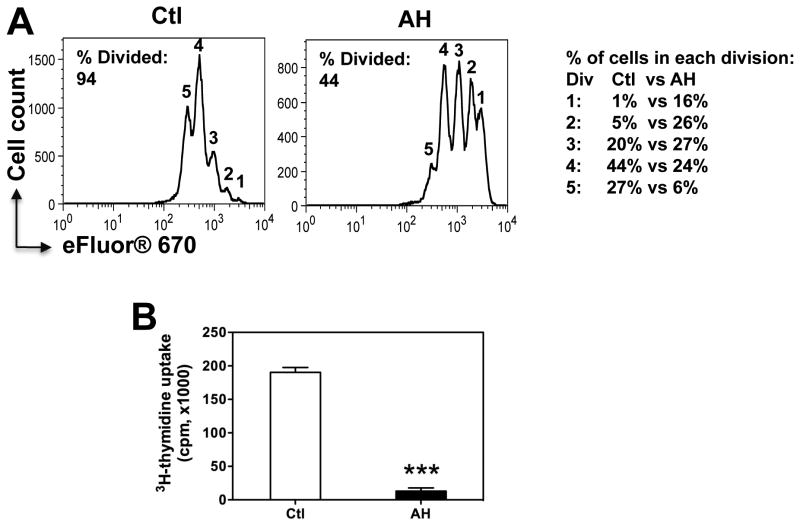
T cell proliferation and acquisition of effector function were markedly inhibited by AH. Comparison of proliferation dye eFluor® 670 dilution with 3H-thymidine uptake (Fig 1A and 1B) suggested that proliferation in presence of AH was induced, but ceased early, because despite the cells having undergone several divisions, there was little uptake of 3H-thymidine, which was added on the third day for the last 6 h of culture.
Figure 1. AH curtails TCR driven proliferation.
CD4+FoxP3- T cells (99.8% pure) from naïve FoxP3-GFP C57BL/6 reporter mice were activated with anti-CD3/CD28 with or without 20% AH. Proliferation was measured by dilution of eFluor 670 (A) and 3H-thymidine uptake (B) after 72h. Note the % of cells in each division, and the total percent of divided cells as calculated from the starting population.
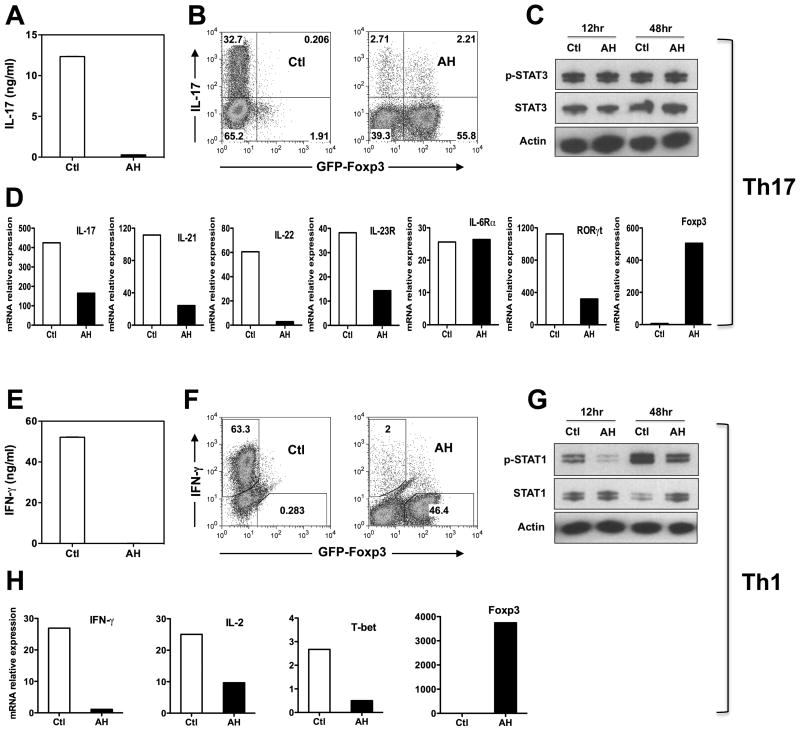
Acquisition of Th17 and Th1 effector function (measured as secretion of IL-17 and IFN-γ) was dramatically inhibited by AH (Fig 2A and 2E, respectively). Examination of Th17 and Th1 lineage-specific gene expression revealed that the respective effector programs were largely shut down. Message levels for IL-17A, IL-21, IL-22, IL-23R and the Th17-associated transcription factor RORγt were all reduced (Fig 2D). Interestingly, although IL-6-induced STAT3 plays a key role in Th17 development, IL-6Rα expression, phosphorylation of STAT3 and its total protein level were not affected (Fig 2C), suggesting that the effect of AH was downstream of STAT3 induction. Under Th1 conditions IFN-γ, IL-2, and the Th1 transcription factor T-bet were all reduced, as was the phosphorylation of STAT1 (Fig 2G and 2H). Instead, AH promoted massive FoxP3 expression, with 56% of the cells converting to FoxP3 positivity under Th17 and 46% under Th1 polarizing conditions (Fig 2B and 2F). There was also a strong FoxP3 induction at the mRNA level (Fig 2D and H). Under non-polarizing conditions conversion to FoxP3 positivity was even higher (approximately 80%). Because the CD4+FoxP3– T cells had been purified to virtual homogeneity (>99.8% FoxP3– cells), we would argue that proliferation of preexisting Tregs can be excluded as a reason for the accumulation of FoxP3+ cells.
Figure 2. AH induces FoxP3 expression at the expense of Th17 and Th1 differentiation.
CD4+FoxP3- T cells (99.8% pure) from naïve FoxP3-GFP C57BL/6 reporter mice were activated with anti-CD3/CD28 with or without 20% AH. (A–D) Th17-polarizing conditions. (E–H) Th1-polarizing conditions. (A, D) IL-17 and IFN-γ in culture supernatants were analyzed by ELISA after 72h. (B, F) Intracellular IL-17 or IFN-γ vs. FoxP3, analyzed by flow cytometry after a PMA/Ionomycin pulse on day 3. (D, H) Expression of the lineage-specific genes was analyzed by qRT-PCR after 48h. (C, G) STAT1 and STAT3 phosphorylation, respectively, by Western blotting. Actin was used as loading control. Data are representative of three to five experiments. *** P ≤ 0.001.
It should be pointed out that although in our experiments the AH was transiently acidified to activate TGF-β FoxP3 was induced also by non-acid activated AH, albeit at lower levels (Supplementary Figure 1). For the purpose of this study, we therefore chose to activate the AH to achieve consistent and measurable results within a reasonable timeframe for an in vitro experiment, before cells would lose viability and make interpretation of the data difficult.
AH induced FoxP3+ T cells are functional Tregs
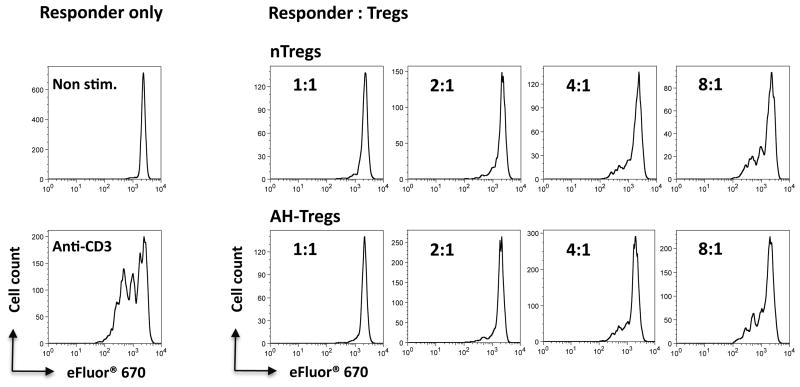
To confirm that the AH-converted T cells are functionally competent suppressor cells, we performed a standard proliferation inhibition assay using naïve T cells as targets and natural Tregs (nTregs) as positive controls. AH induced FoxP3+ T cells from FoxP3-GFP reporter mice were sorted from AH-induced cultures by FoxP3-GFP positivity. Positive control nTregs were similarly sorted from spleens of naïve FoxP3-GFP reporter mice. Both types of Tregs were then cultured at different suppressor-to-target cell ratios with naïve FoxP3-GFP– T cells in the presence of anti-CD3. The AH-induced FoxP3+ Tregs inhibited proliferation of conventional T cells in a dose-dependent manner as efficiently as did natural Tregs, demonstrating that they indeed are bona fide functional regulatory T cells (Fig 3).
Figure 3. AH-induced FoxP3+ cells are functionally suppressive.
CD4+ T cells from C57BL/6 FoxP3-GFP reporter mice were treated with AH. Converted CD4+GFP+ cells were sorted as AH-Tregs and were co-cultured at the indicated ratios with eFluor 670-labeled CD4+GFP– responder cells in the presence of soluble anti-CD3 and APC. nTreg control cells were sorted from FoxP3-GFP reporter mice as CD4+GFP+ cells. Cell proliferation was determined by eFluor 670 dilution. Data are from one representative experiment of three.
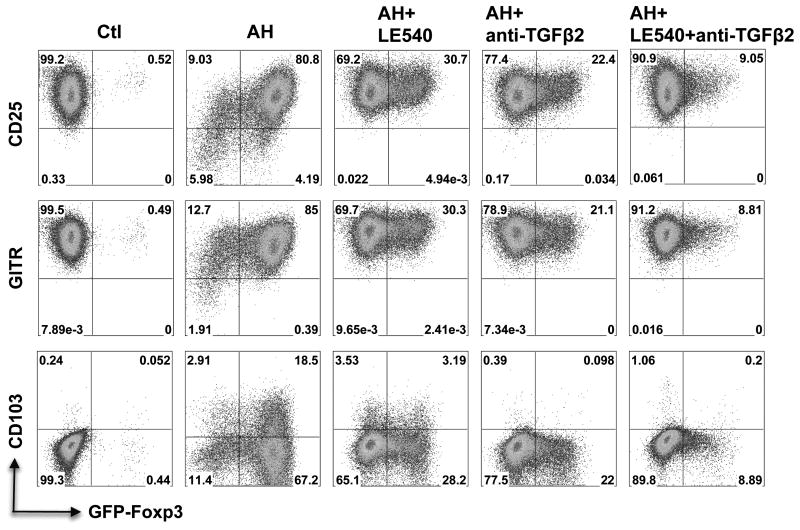
Induction of FoxP3 and Treg specific markers by AH is dependent on retinoic acid and requires TGF-β to upregulate RARα
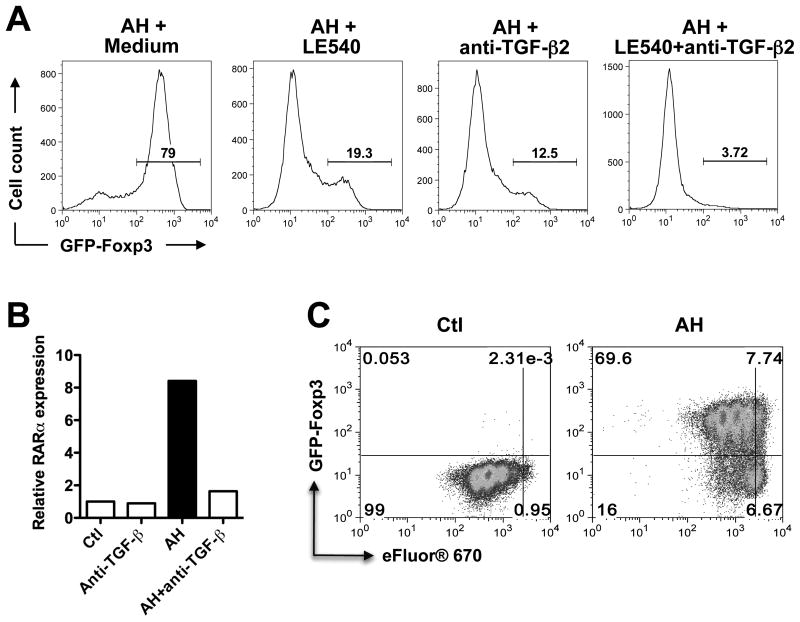
In addition to TGF-β, ocular fluids contain high levels of RA, which functions in the chemistry of the visual cycle (25 pg/ml in human AH, ref 11). Most of the TGF-β in ocular fluids is in the form of TGF-β2 (∼90% in rabbit AH) (1). It is now well established that in vitro, TGF-β converts conventional T cells into FoxP3+ Tregs and that RA strongly enhances this process (17-23). To address the respective roles of RA and TGF-β in AH-induced FoxP3 expression, retinoic acid receptor (RAR) antagonist LE540 and neutralizing antibodies to TGF-β2 were added to CD4+ T cells undergoing TCR stimulation in the presence of AH. Induction of FoxP3 by AH was reduced 4-fold by LE540 (from 79% to 19.3%) despite presence of TGF-β (Fig 4C). Similarly, there was a 6-fold reduction of FoxP3 expression in the presence of anti-TGF-β2 neutralizing antibody (from 79% to 12.5%), even though RA was present. A combination of LE540 with anti-TGF-β2 had a synergistic inhibitory effect. Neutralization of all TGF-β isoforms completely prevented induction of FoxP3 (not shown).
Figure 4. FoxP3+ Treg induction by AH is dependent on retinoic acid (RA) and requires TGF-β.
(A) Blockade of AH-induced FoxP3 expression by LE540 and TGF-β2. CD4+ T cells were activated with anti-CD3/CD28 in the presence of IL-2 and pre-incubated with LE540 and/or anti-TGF-β2 for 2 h before adding AH. FoxP3 expression was examined after 72 h by flow cytometry. Data are representative of five repeat experiments. (B) Expression of RARα. CD4+ T cells were stimulated as in panel A and pre-incubated with anti-TGF-β for 2 h before adding AH. RARα mRNA expression was analyzed by qRT-PCR. Data are representative of four independent experiments. (C) Conversion vs. proliferation. Sorted CD4+FoxP3- T cells were labeled with eFluor® 670 and activated with anti-CD3/CD28 in the presence or absence of AH for 72 h. Cells were analyzed for FoxP3 expression and eFluor® 670 dilution. Data are representative of three independent experiments.
Because the complex effects of RA on T cells are at least in part mediated by the nuclear retinoic acid receptor-α (RARα) (24, 25), we examined the effect of AH on the expression of this receptor. There was a marked increase of RARα mRNA expression in cells activated in the presence of AH, compared to control. Notably, this induction was blocked by neutralization of TGF-β (Fig 4B), suggesting that TGF-β contributes to the effects of AH at least in part by upregulating RARα.
Proliferation accompanied acquisition of FoxP3 expression, but was not a prerequisite for conversion and FoxP3 expression was also induced on non-divided cells (Fig 4C). Compared to control cells stimulated in the absence of AH, the cells stimulated in presence of AH appeared to undergo fewer proliferations cycles, in agreement with data shown in Fig 1. Induction of activation markers shared by effector and by regulatory T cells, including CD25, GITR (Fig 5) as well as of CD44 and CTLA-4 (not shown) was unaffected by presence of AH. Expression shifted from FoxP3- to FoxP3+ population, and back to the FoxP3- population upon blockade of RA and/or TGF-β signaling, but levels of expression remained largely unaltered. AH promoted the induction of CD103 on FoxP3+ cells. Presence of LE540 or anti-TGF-β2 antibody significantly reduced or completely abrogated CD103 induction, respectively (Fig 5).
Figure 5. Expression of selected cell surface markers in AH-induced Tregs.
CD4+ T cells from Foxp3-GFP reporter mice were pretreated with LE540 and/or anti-TGF-β2 for 2 h, and then were activated with anti-CD3/CD28 and IL-2 in the presence or absence of AH for 72hr. The expression of Foxp3, CD25, GITR, and CD103 in CD4+ T cells was detected by flow cytometry. All assays were done under serum-free conditions to avoid possible contribution of RA and TGF-β from serum. Data are representative of four repeat experiments.
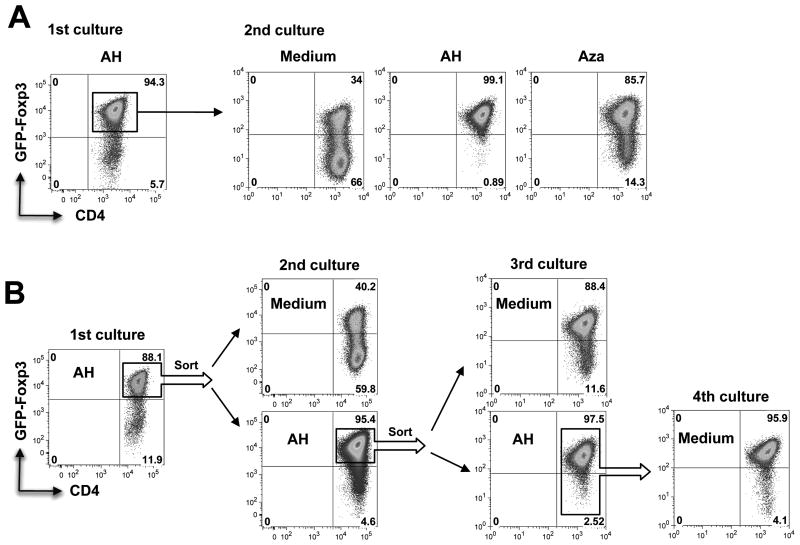
AH-induced FoxP3 expression is initially unstable but is stabilized by DNA demethylation or by continued exposure to AH
In contrast to natural Tregs, induced Tregs were reported to be unstable and to lose FoxP3 expression (26). We, therefore, examined the stability of the AH induced Tregs. Towards that end, CD4+ T cells were converted to FoxP3+ Tregs by AH (Fig 6A, left panel). FoxP3+ T cells were subsequently isolated and upon restimulation in medium alone, only 34% of the cells retained FoxP3 expression. However, FoxP3 expression continued to be stable (99.1%) in the presence of AH. FoxP3 expression could also be stabilized in the presence of the DNA demethylating agent 5-aza-2′-deoxycytidine (Aza), which interferes with DNA methylation by inhibiting the function of DNA methyltransferase-1 (27, 28). The presence of Aza largely prevented the loss of FoxP3 expression (Fig 6A), supporting the notion that the instability of FoxP3 expression was due to incomplete demethylation of the FoxP3 locus after a single exposure to AH.
Figure 6. Stability of AH-induced FoxP3 expression.
(A) FoxP3 expression is initially unstable, but can be stabilized by DNA demethylation or continued presence of AH. CD4+ T cells from C57BL/6 FoxP3-GFP reporter mice were activated with anti-CD3/CD28 in the presence of AH for 72 h (1st culture). GFP+ cells from 1st culture were re-stimulated for another 72 h in the absence or presence of AH or Aza (2nd culture). (B) FoxP3 expression stabilizes after three cycles of stimulation in AH. CD4+ T cells were stimulated as in panel A, with or without AH, for 3 cycles. After the 3rd stimulation cells were re-stimulated for the 4th time without AH. Expression of FoxP3 was determined by flow cytometry. Shown is a representative experiment of three.
To examine how long Tregs would need to remain in the presence of AH for FoxP3 expression to fully stabilize, FoxP3+ T cells were isolated after one, two or three rounds of culture with AH and each time were tested for stability by restimulation without AH. FoxP3 expression became progressively more stable and after 3 rounds of stimulation with AH FoxP3 expression was retained during the 4th round of stimulation in absence of AH (Fig 6B).
Unlike naïve cells, effector and memory T cells are relatively resistant to conversion by AH
The data described above are compatible with the interpretation that a T cell that enters the eye and sees its cognate Ag there, will be induced to adopt a Treg rather than a T effector fate. If so, the question immediately arises, how can uveitis develop? Because uveitogenic T cells are primed in the periphery before they encounter the ocular microenvironment, we decided to examine the effect of ocular fluid on already activated T cells.
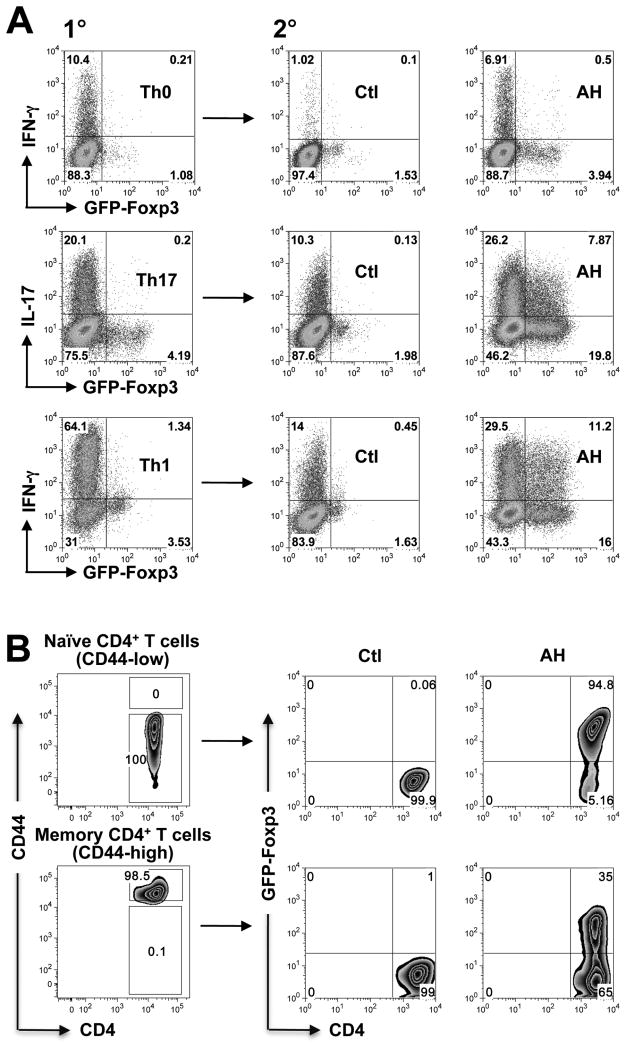
To examine the effect on activated effector T cells, polyclonal CD4+ T cells were polarized under either neutral (Th0), Th17 or Th1 conditions in the absence of AH. The cells were rested, and were exposed to AH during the 2nd stimulation (Fig 7A). Irrespective of the polarization conditions in primary culture, production of the respective effector cytokine was reduced under non-polarizing conditions in secondary control cultures. Strikingly, addition of AH to the secondary culture appeared to stabilize effector cytokine production, despite inducing measurable FoxP3 expression in a part of the population, in sharp contrast to the profoundly inhibitory effect of AH on effector cytokine production in naïve T cells (compare to Fig 2B and 2F). Stabilization of effector function was not observed in control cultures with a mix of TGF-β plus RA in place of AH (not shown), underscoring the much more complex effects of ocular fluids.
Figure 7. Effector and memory T cells are resistant to Treg conversion by the ocular microenvironment.
(A) CD4+ T cells from FoxP3-GFP reporter C57BL/6 mice were given a primary stimulation under Th0, Th17-polarizing, or Th1-polarizing conditions for 4 days (1°). Cells were rested in medium containing IL-2 for 4 days and reactivated with anti-CD3/CD28 in the absence or presence of AH under neutral conditions for 3 days in the presence of IL-2 (2°). Cells pulsed with PMA/ionomycin were analyzed for intracellular cytokines. Note: cells given 1° stimulation under non-polarizing conditions produced little or no IL-17, therefore, only IFN-γ staining is shown. (B) Memory or naïve CD4+FoxP3- T cells were purified from naïve FoxP3-GFP reporter C57BL/6 mice on the basis of CD44 expression. Cells were stimulated with anti-CD3/CD28 in the absence or presence of AH for 3 days. The level of FoxP3 expression was determined by flow cytometry. Data are representative of 5 experiments (A) and 3 experiments (B).
To examine the effect of AH on memory T cells, we used the criterion of high (memory) vs. low (naïve) CD44 expression to isolate polyclonal memory and naïve CD4 T cells from conventional FoxP3-GFP reporter mice. Both T cell populations were exposed to stimulation with CD3/CD28 in presence or absence of AH. Whereas the naïve population efficiently acquired FoxP3 expression, only one-third of the memory population was converted (Fig 7B). Notably, however, memory cells were less resistant to conversion than the more recently activated effector T cells (compare to panel A). That these pathways may be relevant to ocular immune privilege and to uveitis in vivo, is suggested by preliminary data that retina-specific T cells injected into the healthy eye behave similarly to T cells stimulated in vitro in presence of AH (Zhou et al., manuscript in preparation).
Taken together, these data suggest that effector T cells, and to a somewhat lesser extent memory T cells, are relatively resistant to the immunosuppressive and Treg-inducing effects of the ocular microenvironment. This can help to explain the ability of autoaggressive T cells that had been primed outside the eye to induce uveitis, as well as the chronicity of uveitis fueled by autoreactive memory T cells, despite ocular immune privilege.
Discussion
The present study uses highly purified T cells, FoxP3-GFP reporter mice and analyses at the cellular and molecular level to reexamine the paradigm of local immune privilege in the eye. Our current data considerably extend what has been known about the suppressive ability of ocular fluids and provide new information on the likely fate of a T cell that enters the eye and undergoes TCR ligation in the ocular environment. The entire differentiation program for Th1 as well as for Th17 was shut down and diverted towards de novo FoxP3+ Treg induction. Interestingly, although phosphorylation of STAT1 and its target, the Th1 lineage-specific transcription factor T-bet, were both inhibited, phosphorylation of STAT3, which is triggered by IL-6R ligation and induces the Th17 lineage-specific transcription factor RORγt (29), was not affected, and neither was expression of IL-6Rα. This suggests that inhibition of RORγt by AH was not through the IL-6-induced STAT3 pathway. Since FoxP3 was shown to bind to RORγt (30), high expression of FoxP3 induced by AH could have directly repressed RORγt activity, leading to the inhibition of Th17 responses. Our data also uncover a previously unappreciated role for RA, which functions in the visual cycle, in Treg induction by the eye. Although the effects of RA produced by DC on Treg induction has been well described in organs such as the gut (18-23), the case of the eye is a bit different, as RA is free in ocular fluids. In the present study we are reporting direct effects of RA on T cells, in the absence of DC and any modifying effects of RA on DCs. The RA receptor pathway may be important in EAU, as systemic treatment of mice with RA ameliorated induction of disease and its associated immunological responses (Supplementary Figure 2) and similar data were published by others (31, 32). We show that the massive switch in the AH-treated T cells from Th1 or Th17 programs to FoxP3+ Treg, required RA and was dependent on TGF-β-driven upregulation RARα. Proliferation accompanied acquisition of FoxP3 expression, but was not necessary for conversion and was curtailed after a few proliferation cycles. Expression of FoxP3 was initially unstable (apparently due to incomplete DNA methylation, as it could be maintained by 5-AZA) and required continued presence of AH through several cycles of TCR ligation to stabilize.
A question that our in vitro study is not able to address, is the identity of cells that might present Ag within the eye to support this differentiation process. The healthy eye has few mature APC (33). However, in a trauma situation local APC could become activated, and/or cells from the circulation with antigen presenting capability could enter the eye. An analogous situation on a more local scale with subsequent amplification might occur after entry of activated uveitogenic T cells. In support of this, indirect evidence suggests that Ag recognition in the eye does occur in that situation and is necessary for autoimmune uveitis to develop (34, 35).
Importantly, our data demonstrate a very different susceptibility to the inhibitory and Treg inducing effects of the ocular fluids of naïve as compared to effector T cells, supporting the conclusion that T cells which had been exposed to Ag shortly before entering the eye resist inhibition by ocular fluids. These findings can help to clarify the apparent contradiction that although immune privilege is effective in protecting the eye from consequences of minor insults and traumas and critically contributes to the success of corneal grafts that enjoy close to 90% success at the 1 year mark (2, 36), it is nevertheless unable to prevent an autoimmune attack on the eye. In trauma and first-time corneal transplantation the T cells with which the eye must deal are largely naïve, and thus easily converted. In contrast, autoimmune uveitis is elicited by activated autoreactive T cells that had encountered Ag outside the eye and acquired the ability to penetrate the BRB (37), which are resistant to conversion. Rather, some aspects of ocular immune privilege may even predispose to uveitis, which then paradoxically becomes the “price of privilege”: first, because sequestration of retinal antigens behind the BRB impedes peripheral tolerance (38), and second by “stabilizing” rather than inhibiting the effector function of recently activated effector T cells (present data, Fig. 7A). The relative resistance of in vivo generated memory cells to conversion might help to explain the chronic-relapsing nature of human uveitis in the face of ocular immune privilege, which is not abolished by ocular inflammation (39). Still, memory cells clearly are less resistant to conversion than recently primed effector cells, whose transcriptome and metabolome are in a highly activated state. It is therefore conceivable that, both in uveitis and in high-risk corneal transplantation, where memory cells might represent at least part of the population recruited to the eye, the ability to convert a proportion of them to Tregs might mitigate an inflammation that would otherwise be even more severe.
Our data do not exclude direct suppressive effects of ocular resident cells, such as ocular pigment epithelia and retinal glial Müller cells, on T cells that might be undergoing activation in the eye in vivo. These resident cells are in fact reported to act on already activated T cells and their function may be even enhanced by the inflammatory process itself (40, 41). However, whether and how often infiltrating T cells might come in direct contact with ocular resident cells, and therefore the extent of their contribution, would be difficult to estimate.
In summary, it is becoming increasingly clear that tissues, among them the eye, are not merely the passive victims of inflammatory and autoimmune attack, but that they can actively regulate and control immune responses taking place in their territory. A thorough understanding of immune privilege mechanisms as well as their limitations might point the way to modulating these processes to help control disease and limit tissue damage.
Supplementary Material
Acknowledgments
The authors thank Drs. Vijay K. Kuchroo and Mohamed Oukka (Harvard U) for the FoxP3-GFP reporter mice. We are grateful to Drs. Barry Rouse (Univ Tennessee), Yasmine Belkaid (NIAID, NIH) Joost Oppenheim (NCI, NIH), Xin Chen (NCI, NIH) and Igal Gery (NEI, NIH) for valuable critique of the manuscript. We thank the staff of the NEI Flow Cytometry Core Facility for cell sorting.
This work was supported by the intramural research grant of National Eye Institute, NIH.
Abbreviations
- AH
aqueous humor
- BRB
Blood-retinal barrier
- RA
retinoic acid
- Treg
regulatory T cells
- TCR
T cell receptor
- FoxP3
forkhead box P3 transcription factor
- RAR
retinoic acid receptor
- RORγt
retinoic acid receptor-related orphan receptor γt
- CFA
complete Freund's adjuvant
- CTLA-4
cytotoxic T lymphocyte antigen 4
- AZA
5-Aza-2′-deoxycytidine
- 7-AAD
7-aminoactinomycin D
References
- 1.Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32:2201. [PubMed] [Google Scholar]
- 2.Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7:354. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- 3.Sugita S, Horie S, Nakamura O, Futagami Y, Takase H, Keino H, Aburatani H, Katunuma N, Ishidoh K, Yamamoto Y, Mochizuki M. Retinal pigment epithelium-derived CTLA-2alpha induces TGFbeta-producing T regulatory cells. J Immunol. 2008;181:7525. doi: 10.4049/jimmunol.181.11.7525. [DOI] [PubMed] [Google Scholar]
- 4.Kitaichi N, Namba K, Taylor AW. Inducible immune regulation following autoimmune disease in the immune-privileged eye. J Leukoc Biol. 2005;77:496. doi: 10.1189/jlb.0204114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120:3073. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor AW, Alard P, Yee DG, Streilein JW. Aqueous humor induces transforming growth factor-beta (TGF-beta)-producing regulatory T-cells. Curr Eye Res. 1997;16:900. doi: 10.1076/ceyr.16.9.900.5043. [DOI] [PubMed] [Google Scholar]
- 7.Taylor AW, Yee DG, Streilein JW. Suppression of nitric oxide generated by inflammatory macrophages by calcitonin gene-related peptide in aqueous humor. Invest Ophthalmol Vis Sci. 1998;39:1372. [PubMed] [Google Scholar]
- 8.Taylor AW, Yee DG. Somatostatin is an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 2003;44:2644. doi: 10.1167/iovs.02-1216. [DOI] [PubMed] [Google Scholar]
- 9.Taylor AW, Streilein JW, Cousins SW. Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor. J Immunol. 1994;153:1080. [PubMed] [Google Scholar]
- 10.Taylor AW, Streilein JW, Cousins SW. Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr Eye Res. 1992;11:1199. doi: 10.3109/02713689208999545. [DOI] [PubMed] [Google Scholar]
- 11.Wakabayashi Y, Kawahara J, Iwasaki T, Usui M. Retinoic acid transport to lens epithelium in human aqueous humor. Jpn J Ophthalmol. 1994;38:400. [PubMed] [Google Scholar]
- 12.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 13.Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Aubin ML, Gionfriddo JR, Mama KR, Powell CC. Analysis of aqueous humor obtained from normal eyes of llamas and alpacas. Am J Vet Res. 2001;62:1060. doi: 10.2460/ajvr.2001.62.1060. [DOI] [PubMed] [Google Scholar]
- 15.Bours J. The protein distribution of bovine, human and rabbit aqueous humour and the difference in composition before and after disruption of the blood/aqueous humour barrier. Lens Eye Toxic Res. 1990;7:491. [PubMed] [Google Scholar]
- 16.Hazel SJ, Thrall MA, Severin GA, Lauerman LH, Jr, Lavach JD. Laboratory evaluation of aqueous humor in the healthy dog, cat, horse, and cow. Am J Vet Res. 1985;46:657. [PubMed] [Google Scholar]
- 17.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 20.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 24.Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37:2396. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 25.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H, Huehn J. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 28.Creusot F, Acs G, Christman JK. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2′-deoxycytidine. J Biol Chem. 1982;257:2041. [PubMed] [Google Scholar]
- 29.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keino H, Watanabe T, Sato Y, Okada AA. Anti-inflammatory effect of retinoic acid on experimental autoimmune uveoretinitis. Br J Ophthalmol. 2010;94:802. doi: 10.1136/bjo.2009.171314. [DOI] [PubMed] [Google Scholar]
- 32.Keino H, Watanabe T, Sato Y, Okada AA. Oral administration of retinoic acid receptor-alpha/beta-specific ligand Am80 suppresses experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2011;52:1548. doi: 10.1167/iovs.10-5963. [DOI] [PubMed] [Google Scholar]
- 33.McMenamin PG. Dendritic cells and macrophages in the uveal tract of the normal mouse eye. Br J Ophthalmol. 1999;83:598. doi: 10.1136/bjo.83.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thurau SR, Mempel TR, Flugel A, Diedrichs-Mohring M, Krombach F, Kawakami N, Wildner G. The fate of autoreactive, GFP+ T cells in rat models of uveitis analyzed by intravital fluorescence microscopy and FACS. Int Immunol. 2004;16:1573. doi: 10.1093/intimm/dxh158. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Vistica BP, Takase H, Ham DI, Fariss RN, Wawrousek EF, Chan CC, DeMartino JA, Farber JM, Gery I. A unique pattern of up- and down-regulation of chemokine receptor CXCR3 on inflammation-inducing Th1 cells. Eur J Immunol. 2004;34:2885. doi: 10.1002/eji.200425318. [DOI] [PubMed] [Google Scholar]
- 36.Vail A, Gore SM, Bradley BA, Easty DL, Rogers CA, Armitage WJ. Influence of donor and histocompatibility factors on corneal graft outcome. Transplantation. 1994;58:1210. [PubMed] [Google Scholar]
- 37.Prendergast RA, Iliff CE, Coskuncan NM, Caspi RR, Sartani G, Tarrant TK, Lutty GA, McLeod DS. T cell traffic and the inflammatory response in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 1998;39:754. [PubMed] [Google Scholar]
- 38.Caspi RR. Ocular autoimmunity: the price of privilege? Immunol Rev. 2006;213:23. doi: 10.1111/j.1600-065X.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 39.Mo JS, Streilein JW. Immune privilege persists in eyes with extreme inflammation induced by intravitreal LPS. Eur J Immunol. 2001;31:3806. doi: 10.1002/1521-4141(200112)31:12<3806::aid-immu3806>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 40.Caspi RR, Roberge FG, Nussenblatt RB. Organ-resident, nonlymphoid cells suppress proliferation of autoimmune T-helper lymphocytes. Science. 1987;237:1029. doi: 10.1126/science.2956685. [DOI] [PubMed] [Google Scholar]
- 41.Ke Y, Sun D, Jiang G, Kaplan HJ, Shao H. PD-L1hi retinal pigment epithelium (RPE) cells elicited by inflammatory cytokines induce regulatory activity in uveitogenic T cells. J Leukoc Biol. 2010;88:1241. doi: 10.1189/jlb.0610332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.