Abstract
Although intraocular tumors reside in an immune privileged site where immune responses are suppressed, some tumors are rejected nonetheless. An example of this is the rejection of intraocular adenovirus-induced (Ad5E1) tumors in C57BL/6 mice. We previously identified an Ad5E1 tumor clone in which the rejection is IFN-γ-dependent and culminates in the destruction of both the tumor and the eye. Although Ad5E1 tumors are not rejected when transplanted into the eyes of IFN-γ KO mice, they are rejected following subcutaneous transplantation (SC). Thus, outside of the eye Ad5E1 tumors elicit a form of tumor immunity that is IFN-γ-independent. Here, we demonstrate that IFN-γ-independent SC rejection requires both CD4+ and CD8+ T cells. Furthermore, SC tumor rejection requires IL-17, which is produced by IFN-γ-deficient CD4+ T cells in response to tumor antigens (TAs). Splenocytes from CD4-depleted IFN-γ KO mice produce significantly less IL-17 compared to splenocytes from isotype-treated IFN-γ KO animals in response to TAs. Furthermore, depletion of IL-17 decreases CTL activity against Ad5E1 tumor cells. In this model we propose that in the absence of IFN-γ, CD4+ T cells produce IL-17 in response to TAs, which increases CTL activity that mediates tumor rejection. However, this does not occur in the eye. IL-6 production within the eye is severely reduced, which is consistent with the failure to induce Th17 cells within the intraocular tumors. By contrast, the SC environment is replete with IL-6 and supports the induction of Th17 cells. Therefore, IFN-γ-independent tumor rejection is excluded from the eye and may represent a newly recognized form of ocular immune privilege.
Keywords: Eye, CTL, IL-17, Immune privilege, Intraocular tumors
Introduction
Ocular immune privilege is essential for maintaining vision by excluding or dampening immune-mediated inflammation, which can have devastating consequences on normal ocular cells that cannot regenerate. However, the eye invokes several mechanisms for maintaining immune privilege and reducing the risk of immune-mediated injury. The anterior chamber (AC) of the eye employs several mechanisms to restrain immune-mediated inflammation. One example is diminished expression or absence of MHC class I antigens on the corneal endothelium, which lessens the likelihood of injury due to CD8+ cytotoxic T lymphocytes (CTLs) (1). The aqueous humor contains a number of anti-inflammatory and immunosuppressive factors that function to quench inflammation (2–4). Another example of immune privilege is antigen-specific downregulation of Th1 and Th2 immune responses that occurs when antigens are introduced into the AC. This phenomenon, known as anterior chamber-associated immune deviation (ACAID), has been proposed as a key mechanism for inhibiting immune-mediated inflammation and preserving vision (4). Immune privilege sometimes allows the prolonged and even permanent existence of foreign tissues and tumors within the eye (5, 6).
It would seem that ocular immune privilege would provide an ideal environment for unrestrained ocular tumor growth. However, some experimental ocular tumors undergo immune rejection and there is evidence that retinoblastomas and uveal melanomas can occasionally undergo spontaneous resolution in human subjects (6–9). There is compelling evidence from experimental animal models indicating that ocular immune privilege can be circumvented resulting in the emergence of effector T cells that mediate the immune rejection of intraocular tumors (10, 11). These studies have revealed two fundamental patterns by which intraocular tumors can undergo T cell-dependent immune rejection. The first pattern is characterized by piecemeal necrosis of intraocular tumor cells and preservation of the architecture of the eye (7, 12). Animal studies using UV-induced fibrosarcomas and SV40 large T antigen-induced retinal pigment epithelial carcinomas have revealed evidence suggesting that piecemeal necrosis of intraocular tumors is mediated by tumor-specific CD8+ CTLs (13, 14). By contrast, murine embryonic tumors induced with the adenovirus gene (Ad5E1) undergo spontaneous immune rejection in the eyes of syngeneic C57BL/6 without damaging the ocular architecture (15). Rejection of Ad5E1 tumors occurs in CD8−/− and perforin −/− mice suggesting ocular tumor rejection that leaves the eye anatomically intact can be mediated by a CTL-independent process.
A second pattern of T cell-dependent rejection of murine intraocular tumors involves ischemic necrosis and extensive damage to both the tumor and innocent bystander cells within the eye (16). This pattern of rejection results in atrophy of the eye, a condition called phthisis (7). This form of immune rejection was first revealed in studies using a highly immunogenic clone of P815 mastocytoma (P91), which underwent an ischemic necrotizing form of T cell-dependent immune rejection in the eyes of syngeneic DBA/2 mice (16). The histopathological and immunological features of phthisical rejection of P91 tumors were reminiscent of a delay-type hypersensitivity (DTH)-mediated process (17).
In the present study we used an adenoviral-gene transformed embryonic cell tumor model (Ad5E1) that has been used to characterize the circumvention of ocular immune privilege to examine the role of IFN-γ in intraocular tumor rejection (18–20). We have isolated subclones of the original Ad5E1 tumor that undergo phthisical and non-phthisical patterns of tumor rejection respectively. The non-phthisical form of intraocular Ad5E1 tumor rejection requires IFN-γ, which acts directly on tumor cells by: a) inhibiting tumor cell proliferation; b) inducing tumor cell apoptosis; and c) down regulating pro-angiogeneic genes and upregulating anti-angiogeneic genes in the tumors (20, 21). Investigations on the phthisical form of intraocular tumor rejection have revealed that TNF-α is necessary for producing necrotizing injury to innocent bystander cells and phthisis, but is not needed for rejection of intraocular Ad5E1 tumors (22). By contrast, IFN-γ is not involved in the development of phthisis, but is necessary for the recruitment and activation of macrophages, which are intimately involved in the phthisical form of tumor rejection (22). The requirement of IFN-γ (for rejection) and TNF-α (for phthisis) is consistent with a DTH-mediated form of rejection resulting in phthisical rejection of intraocular tumors.
Although Ad5E1 tumors are not rejected when transplanted into the eyes of IFN-γ KO mice, they are rejected after subcutaneous (SC) transplantation (23). This suggests that IFN-γ-independent immune processes that eliminate SC tumors are excluded from the eye. The goal of this study was to determine the mechanisms that are responsible for this dichotomous expression of immune rejection processes. The present findings reveal a new form of immune deviation in which IFN-γ-independent immune processes can mediate tumor rejection at extraocular sites but are excluded from the eye.
Materials and Methods
Animals
C57BL/6 (H-2b) mice, interferon-γ (IFN-γ) knockout (KO) mice (B6.129S7-Ifngtm1Ts/J), interleukin 6 KO (IL-6) (B6.129S2-Il6tm1Kopf/J), severe combined immune deficiency mutation (SCID) (B6.CB17-Prkdcscid/SzJ), and nude (NU/J) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All animals were housed and cared for in accordance with the guidelines of the University of Texas Southwestern Medical Center Committee for the Humane Care of Laboratory Animals, National Institutes of Health Guidelines on Laboratory Animal Welfare, and the Association for Research in Vision and Ophthalmology statement about the Use of Animals in Ophthalmic and Vision Research.
Tumor cells
Ad5E1 tumor cells were kindly provided by Dr. Rene E.M. Toes (Leiden University Medical Center). The tumor cells were generated by the transformation of C57BL/6 mouse embryo cells with a plasmid encoding the human adenovirus type 5 early region 1 (Ad5E1) and propagated, as previously described (24). Mouse embryonic fibroblasts (MEF) were purchased from ATCC (Manassas, VA) (SCRC-1045). All cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM; GibcoBRL, Grand Island, NY) containing 10% heat-inactivated fetal calf serum, 1% L-glutamine, 1% sodium pyruvate, 1% nonessential amino acids, 1% HEPES buffer, and 1% antibiotic–antimycotic solution (GibcoBRL, Grand Island, NY).
Identification of tumor clones that undergo phthisical rejection
We suspected that the original Ad5E1 tumor cultures contained subpopulations that underwent phthisical, T cell-dependent immune rejection in C57BL/6 mice. Accordingly, monoclonal cell cultures were established from the parental Ad5E1 tumor cells by isolating single cells from bulk cultures using a MoFlo XDP cell sorter (Beckman Coulter, Fullerton, CA). Monoclonal cell cultures of Ad5E1 were established, expanded, and were screened for their rejection following transplantation into the anterior chamber (AC) of normal C57BL/6 mice. Four clones were found to consistently undergo phthisical rejection in C57BL/6 mice. One of these, designated as Ad5E1 clone 2.1, was used for the present study.
Subcutaneous tumor cell injections
Single-cell suspensions of Ad5E1 clone 2.1 tumor cells were washed in Hanks’ Balanced Salt Solution (HBSS; Cambrex, East Rutherford, NJ) and resuspended in HBSS. Tumor cell suspensions (5 × 104/100 μl) were injected SC in the flank. Inoculation sites were palpated three times per week to assess SC tumor growth, and digital callipers (Fisher Scientific) were used to measure tumor size. Animals were considered to be tumor-bearing when tumors were 3 mm in diameter. SC tumor volumes (V) were determined as V = 1/2(ab2), where a is the largest and b is the smallest tumor diameter (25).
Intraocular tumor cell injections
Single-cell suspensions of Ad5E1 clone 2.1 tumor cells were washed in HBSS and suspended in HBSS for anterior chamber (AC) injections. Tumor cell suspensions were injected into the AC as previously described (24). Mice were anesthetized with 0.66 mg/kg of ketamine hydrochloride (Vetalar; Parke-Davis and Co., Detroit, MI) given i.p. The eye was viewed under a dissecting microscope (8×), and a sterile 30-gauge needle was used to trephene the cornea at the corneoscleral junction, parallel and anterior to the iris. A glass micropipette (diameter ~80 microns) was fitted onto a sterile infant feeding tube (5 French; Tyco Healthcare Group, Mansfield, MA) and mounted onto a 0.1-ml Hamilton syringe (Hamilton, Whittier, CA). A Hamilton automatic dispensing apparatus was used to inject 6 μl of a monocellular suspension of Ad5E1 clone 2.1 tumor cells (5 × 104 cells/6 μl). Eyes were examined three times per week, and the tumor volume was recorded as the percentage of AC occupied with tumor.
Mixed lymphocyte-tumor cell (MLTC) culture
Splenocytes (30 × 106) from either immunized or naïve mice were stimulated with 5 × 106 mitomycin-C treated tumor cells or MEF cells in complete RPMI 1640 medium (BioWhittaker) containing 10% heat-inactivated FBS (HyClone), as previously described (26). Cells were cultured for 5 days at 37°C. Restimulated lymphocytes were harvested, washed twice, and tested for cytolytic activity.
Chromium release assay
A standard 4 hr 51Cr- release assay, was used to measure CTL activity in vitro, as previously described (27). Effector cells were dispensed along with 2 × 104 51Cr-labeled Ad5E1 clone 2.1 cells/well in triplicate at two E: T ratios (100:1 or 50:1) in a 96-well U-bottom microtiter plate (Corning), in a total volume of 200 μl/well. Ad5E1 clone 2.1 tumor cells were also incubated alone (spontaneous release)or with 50 μl of Zapoglobin (Beckman Coulter) lytic reagent(total release). The plate was incubated at 37 °C for 4 h. The plate was then centrifuged at 800 rpm for 5 min before harvesting100 μl of the supernatant from each well for analysis using a Cobra Quantum gamma counter (Packard). Cytotoxicity was determined by the amount of 51Cr released by the target cells, and the specific lysis was calculated as follows: [(experimental cpm) – (spontaneous release cpm)] ÷ [(maximum release cpm) – (spontaneous release cpm)] × 100%.
In vitro stimulation of T cells and IL-17 ELISA
Tumor-bearing CD4-depleted IFN-γ deficient animals and non-tumor-bearing isotype antibody-treated IFN-γ deficient mice were euthanized and spleens were obtained. CD4+ T cells were isolated from spleens using mouse CD4 (L3T4) microbeads and magnetic cell sorting (Miltenyi Biotec). T cells (1 × 106) were incubated; a) alone (negative control), b) with25 μl of Mouse T-Activator CD3/CD28 Dynabeads (Invitrogen) (positive control), or c) with APCs pulsed with Ad5E1 clone 2.1 tumor antigens or MEF antigens (1 × 106) in a 24 well plate (BD Biosciences) in duplicate, as previously described (28). Cells were incubated for 5 days at 37 °C. Supernatants were harvested and levels of IL-17 in cell supernatants were determined using a mouse IL-17Quantikine ELISA kit (R&D Systems).
Antigen presenting cell (APC) isolation
APCs were obtained by mincing spleens from naïve animals and incubating them with 1 mg/ml collagenase D (Roche, Indianapolis, IN) at 37°C for 30 min. Cells were plated on Primaria tissue culture dishes (BD Biosciences) and incubated for 2 hours at 37°C. Non-adherent cells were aspirated and the plates were vigorously washed, leaving APCs (adherent macrophages and dendritic cells). APCs were incubated with tumor antigens (TA) from freeze-thawed and sonicated Ad5E1 clone 2.1 tumor cells or MEF cells for 24 hours and used as stimulator cells for in vitro assays.
Detection of intracellular IL-17 expression by flow cytometry
Spleen-derived CD4+ T cells from tumor rejector IFN-γ KO mice were isolated by CD4 microbead separation as described above. CD4+ T cells were incubated a) alone (negative control), b) with25 μl of Mouse T-Activator CD3/CD28 Dynabeads (Invitrogen) (positive control), or c) with APCs pulsed with Ad5E1 clone 2.1 tumor antigens in a 24 well plate (BD Biosciences). Cells were then harvested, washed, and stained with 1 μg/ml rat anti-mouse CD4-FITC antibody (R&D Systems) or 1 μg/ml rat IgG isotype-FITC control antibody (R&D Systems) for 30 min. at 4°C. Cells were then fixed, permeabilized and stained with 1 μg/ml rat anti-mouse IL-17A-PE antibody (ebiosciences) or 1 μg/ml rat IgG isotype-PE control antibody (ebiosciences) for 30 min. at 4°C Fluorescence was detected with a FACScan flow cytometer (BD Biosciences). Results were analyzed using CellQuest v.3.1f software (BD Biosciences).
Quantitative Real-time PCR
RNA was isolated from non-tumor bearing and tumor bearing eyes of C57BL/6, IFN-γ KO and SCID mice. RNA was also isolated from tumor-bearing skin samples. As positive controls for IL-17 and IL-6, CD4+ T cells were polarized into a Th17 phenotype by incubation with TGF-β (5 ng/ml), IL-6 (20 ng/ml) and anti-IFN-γ (10 μg/ml) for 5 days at 37 °C (29). RAW 264.7 cells were polarized into a M1 phenotype by incubation with LPS (125 ng/ml) and IFN-γ (10 ng/ml) for 24 hours at 37 °C (22). CD4+ T cells were isolated from spleens of C57BL/6 mice using mouse anti-CD4 (L3T4) microbeads and magnetic cell sorting (Miltenyi Biotec). Positive controls for CD8 and perforin were splenoctyes from a C57BL/6 spleen.
Expression of IL-17, IL-6, CD8 and perforin mRNA was assessed by quantitative real-time PCR using a MyiQ Single-Color Real-Time PCR Detection system (Bio-Rad). Briefly, 1 μg of total RNA was converted into first-strand cDNA using RT2 First Strand Kit (SA Biosciences) according to the manufacturer’s conditions. IL-6 and IL-17 RT2 qPCR primer assays were used (SA Biosciences). The PCR amplification reactions contained 1.0 μl of first-strand cDNA mixed with 12.5 μl of RT2 qPCR Master Mix (SA Biosciences), 10.5 μl ddH2O, and RT2 qPCR primers (SA Biosciences) in a final reaction volume of 25 μl. All reactions were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and performed in duplicate. The PCR profile consisted of an initial denaturation of 10 min at 95 °C, 40 cycles of 15 s at 95 °C denaturing, and 60 s at 60 °C annealing. A dissociation (melting) curve was performed to ensure proper quality control for each sample.
Statistics
A student’s t-test or a χ2 test was used to assess the statistical significance of the differences between experimental and control groups. A P-value of <0.05 was considered significant.
Results
Progressive growth of intraocular tumors in IFN-γ-deficient mice
Previous studies showed that the parental Ad5E1 tumor cell line grew progressively in the eyes of IFN-γ deficient mice (20). We confirmed that IFN-γ was also required for intraocular rejection of Ad5E1 clone 2.1 tumors, which undergo phthisical rejection in wild-type C57BL/6 mice. Ad5E1 clone 2.1 (5 × 104) tumor cells were injected into the AC of wild-type C57BL/6, IFN-γ KO and SCID mice. Whereas Ad5E1 clone 2.1 was rejected in C57BL/6 mice, tumors grew progressively in IFN-γ KO mice (Figure 1). To confirm that this form of immunity was dependent on T cells, SCID and nude mice were injected in the AC. Indeed, AC tumors grew progressively in T cell-deficient mouse strains (Table 1). Thus, like the parental Ad5E1 tumor line, clone 2.1 tumor rejection in the eye requires IFN-γ produced by T cells.
Figure 1. IFN-γ is required for intraocular tumor rejection.
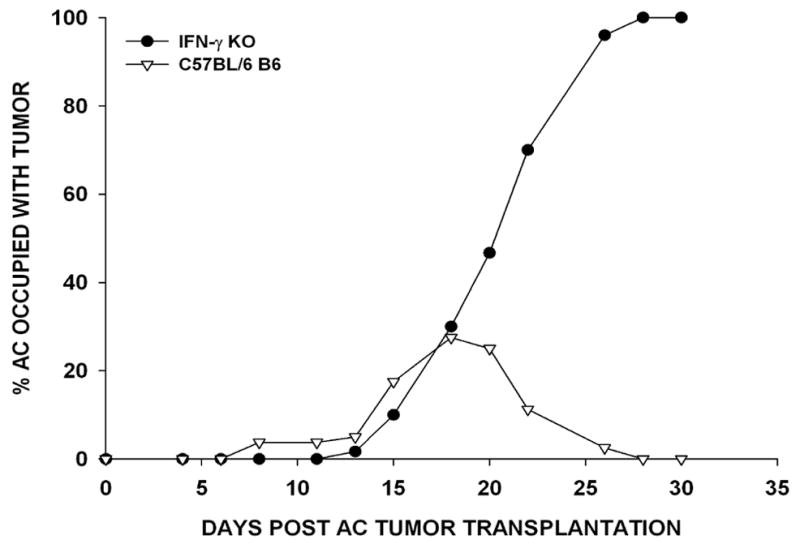
Ad5E1 clone 2.1 tumor cells (5 × 104) were injected into the AC of WT C57BL/6 (▽) and IFN-γ KO (●) mice. Two independent experiments were performed (N = 10/group/experiment).
Table 1.
IFN-γ-independent tumor rejection occurs at extraocular sites but is excluded from the eye
| Host | Anterior Chamber | Subcutaneous |
|---|---|---|
| C57BL/6 | Rejected (15/15) | Rejected (10/10) |
| IFN-γ KO | Not Rejected (20/20) | Rejected (22/25) |
| SCID | Not Rejected (10/10) | Not Rejected (9/10) |
| Nude | Not Rejected (10/10) | Not Rejected (10/10) |
Ad5E1 clone 2.1 tumor cells (5 × 104) were injected in the AC or SC. Tumor growth was observed three times/ week. Results shown above were recorded on day 30 post tumor transplantation.
IFN-γ-independent tumor rejection can be induced by subcutaneous tumors, but not by intraocular tumors
The requirement of IFN-γ for rejection of intraocular Ad5E1 clone 2.1 tumors raised the question as to whether IFN-γ is required for rejection of the tumor implanted at extraocular sites, or if this form of immunity is unique to the eye. Accordingly, panels of C57BL/6 and IFN-γ KO mice were injected with Ad5E1 clone 2.1 tumor cells (5 × 104) either SC or into the AC. As previously shown in Figure 1, Ad5E1 clone 2.1 tumor cells grew progressively in the eyes of IFN-γ KO mice. However, tumors were rejected when injected SC (Table 1). Thus, SC injection of Ad5E1 clone 2.1 tumor cells elicits a form of immunity that is IFN-γ-independent. In order to establish that this form of immunity is T cell-mediated, C57BL/6 SCID mice and C57BL/6 nude mice were injected either SC or in the AC with 5 × 104 Ad5E1 clone 2.1 tumor cells. Indeed, T cells were required for rejection of both AC and SC tumors (Table 1).
IFN-γ-independent tumor rejection requires both CD4+ and CD8+ T cells
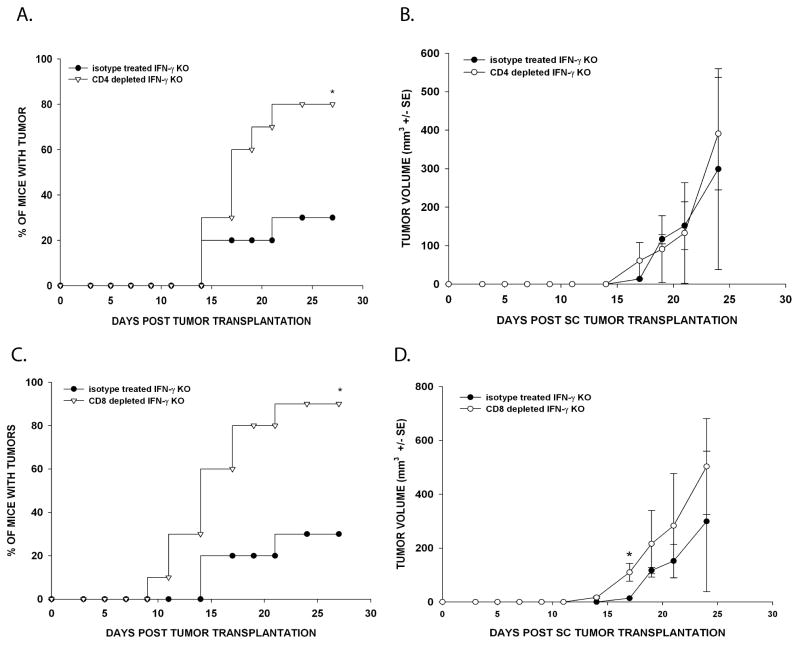
The requirement of T cells for IFN-γ-independent tumor rejection raised the question as to whether CD4+ or CD8+ T cells were required for SC tumor rejection. The T cell population that mediates IFN-γ-independent rejection was determined by depleting IFN-γ KO mice of either CD4+ or CD8+ T cells using monoclonal antibodies. Depletion of CD4+ T cells led to increased SC tumor incidence in anti-CD4 treated IFN-γ KO mice injected with Ad5E1 clone 2.1 tumor cells (80%) compared to the tumor incidence in IFN-γ KO mice treated with the isotype antibody and injected SC with Ad5E1 clone 2.1 tumor cells (30%) (Figure 2A). The rate of tumor growth was similar for both groups (Figure 2B). Similarly, depletion of CD8+ T cells led to increased Ad5E1 clone 2.1 SC tumor incidences. SC tumors grew in 90% of CD8-depleted IFN-γ KO animals compared to 30% in isotype control - treated IFN-γ KO mice (Figure 2C). Likewise, the rate of tumor growth was similar for both groups (Figure 2D). Thus, SC Ad5E1 clone 2.1 tumor rejection is dependent on both CD4+ and CD8+ T cells.
Figure 2. IFN-γ-independent SC tumor rejection requires CD4+ T and CD8+ T cells.
(A) IFN-γ KO mice were treated with either isotype control antibody (rat IgG) or anti-CD4 antibody twice per week. Ad5E1 clone 2.1 tumor cells (5 × 104) were SC injected in the right flank. The percentage of mice with tumor was determined. Data represents the combined results of two independent experiments (N = 10/group/experiment). * P < .01, as determine by χ2 test. (B) The volume of SC tumors that grew in (A) was calculated from measurements by calipers three times per week (mm3 +/− SE). 3/10 mice developed tumor in the isotype group compared to 8/10 mice in the CD4 depleted group. (C) Animals were treated with either isotype antibody or anti-CD8 antibody twice per week. Ad5E1 clone 2.1 tumor cells (5 × 104) were SC injected in the right flank. Tumor growth was observed three times per week. Data represents the combined results of two independent experiments (N = 10/group/experiment). * P < .01, as determine by χ2 test. (D) The volume of SC tumors that grew in (C) was calculated from measurements by calipers three times per week (mm3 +/− SE). 3/10 mice developed tumor in the isotype group compared to 9/10 mice in the CD8 depleted group. * P < .05, as determine by Student’s t test.
IFN-γ-independent tumor rejection requires IL-17
Reports by other investigators demonstrated that IFN-γ produced by Th1 T cells cross-regulates the development of Th17 cells that secrete IL-17 (30–32). Therefore, we hypothesized that the absence of IFN-γ favors the development of Th17 cells that elaborate IL-17, which is necessary for IFN-γ-independent rejection of SC-injected Ad5E1 clone 2.1 tumor cells. To address this hypothesis, IFN-γ KO mice were treated with either anti-IL-17 antibody or an isotype control antibody and injected SC with 5 × 104 Ad5E1 clone 2.1 tumor cells. The majority of the animals treated with anti-IL-17 had SC tumors three weeks after tumor injection (12/15) (Figure 3). By contrast, none of the mice (0/15) treated with the isotype antibody developed tumors, and all remained tumor-free for the duration of the experiment. Although IL-17 was required for tumor rejection in the absence of IFN-γ, it was not required in wild-type hosts that could produce IFN-γ; AC and SC tumor rejection remained unchanged in C57BL/6 mice depleted of IL-17 (data not shown).
Figure 3. IFN-γ-independent SC tumor rejection requires IL-17.
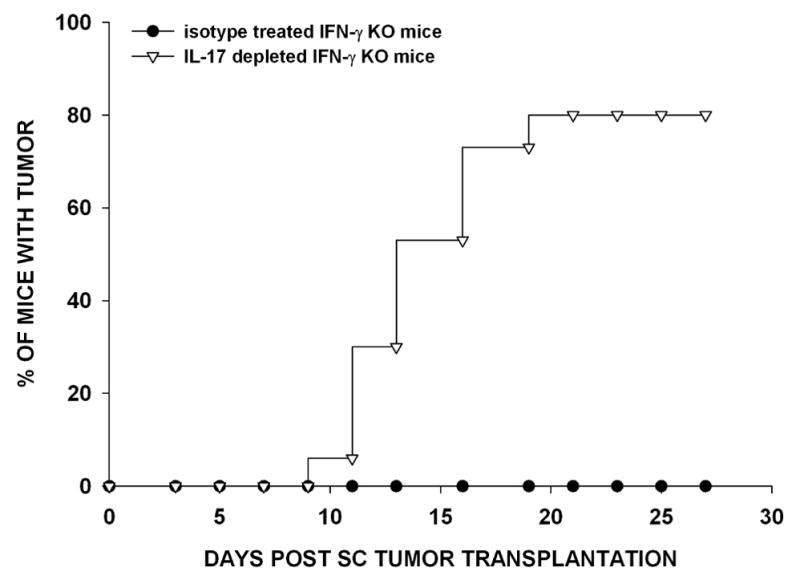
IFN-γ KO mice were treated with either isotype antibody or anti-IL-17 antibody twice per week. Ad5E1 clone 2.1 tumor cells (5 × 104) were SC injected in the right flank. Tumor growth was observed three times per week. Data are the combined results of three independent experiments (N=15 mice/group/experiment).
CD4+ T cells from SC tumor rejector IFN-γ KO mice produce IL-17 in response to tumor antigens
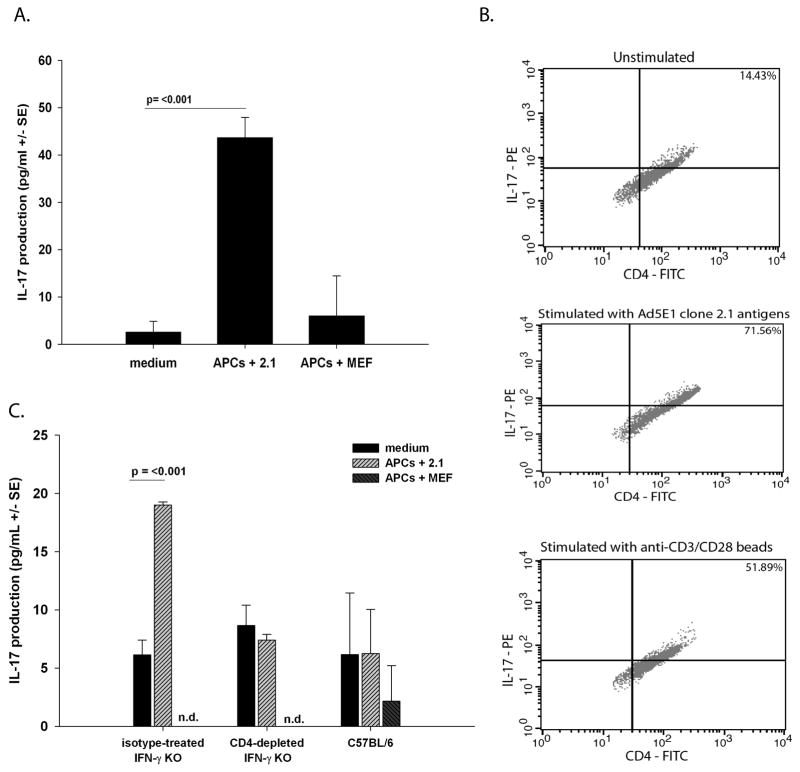
We hypothesized that CD4+ T cells produce IL-17 in response to Ad5E1 clone 2.1 tumor antigens. In order to address this hypothesis, CD4+ T cells were isolated from spleens of IFN-γ KO mice that had rejected SC inoculations of 5 × 104 Ad5E1 clone 2.1 tumor cells two weeks earlier. CD4+ T cells from IFN-γ KO SC tumor rejectors were incubated with Ad5E1 clone 2.1 tumor antigen-pulsed APCs or medium alone. To control for antigen specificity, APCs pulsed with mouse embryonic fibroblast (MEF) antigens were also incubated with CD4+ T cells from IFN-γ KO animals that rejected Ad5E1 clone 2.1 tumors. As a positive control, all splenocytes and T cells were found to produce IL-17 when incubated with anti-CD3/CD28 beads (data not shown). After five days, supernatants were harvested and IL-17 concentration was measured by ELISA. IFN-γ-deficient CD4+ T cells produced IL-17 at significantly higher levels when stimulated with Ad5E1 tumor antigen-pulsed APCs than compared to CD4+ T cells cultured with MEF-pulsed APCs or media alone (Figure 4A). In order to independently verify the production of IL-17 by CD4+ T cells, intracellular IL-17 expression was determined. CD4+ T cells isolated from tumor rejector IFN-γ KO mice were cultured with APCs pulsed with Ad5E1 clone 2.1 TAs or anti-CD3/CD28 dynabeads. As a control expression of IL-17 was also determined in unstimlated cells. Approximately 15% of unstimulated CD4+ T cells expressed IL-17 compared to 71% and 52% of CD4+ T cells stimulated with APCs pulsed with Ad5E1 clone 2.1 tumor antigens or anti-CD3/CD28 beads, respectively (Figure 4B).
Figure 4. Rejector CD4+ T cells produce IL-17 following stimulation with tumor antigens.
(A) T cells from SC tumor rejector IFN-γ KO mice were cultured with medium alone, Ad5E1 clone 2.1 tumor antigen-pulsed APCs, MEF antigen-pulsed APCs, or anti-CD3/CD28 dynabeads for 5 days at 37 °C. CD4+ T cells were harvested from isotype antibody treated IFN-γ KO mice. Production of IL-17 (pg/ml +/− SE) was determined by ELISA. Data are a representative of two independent experiments. P values < 0.05 were considered significant as determined by Student’s t test. (B) CD4+ T cells were cultured with medium alone (unstimulated) (upper panel), Ad5E1 clone 2.1 tumor antigen-pulsed APCs (middle panel), or anti-CD3/CD28 dynabeads (lower panel) and intracellular stained with anti-IL-17-PE and anti-CD4-FITC. Data are a representative of two independent experiments. (C) Splenocytes were harvested from isotype antibody-treated IFN-γ KO mice, from anti-CD4-treated IFN-γ KO mice or from WT C57BL/6 mice. Production of IL-17 (pg/ml +/− SE) was determined by ELISA. Data are a representative of two independent experiments. P values < 0.05 were considered significant as determined by Student’s t test.
To confirm that CD4+ T cells from IFN-γ KO mice produced IL-17, bulk splenocyte suspensions were isolated from CD4+ T cell-depleted or rat IgG isotype-treated IFN-γ KO mice and stimulated under the same conditions as described above. As a control, WT C57BL/6 splenocytes were harvested and cultured under the same conditions. Splenocytes from isotype-treated IFN-γ KO mice produced significantly more IL-17 than splenocytes from CD4+ T cell-depleted IFN-γ KO mice in response to Ad5E1 tumor cell lysate but not in response to MEF lysate (Figure 4C). By contrast, splenocytes from wild-type C57BL/6 mice produced IL-17 at similar levels as CD4+ T cell -depleted IFN-γ KO mice and significantly less than isotype control antibody-treated IFN-γ KO mice (Figure 4C). Together, these results provide evidence that CD4+ T cells are responsible for IL-17 production in the absence of IFN-γ, and the presence of IFN-γ suppresses IL-17-dependent immunity against SC Ad5E1 tumors.
Depletion of IL-17 reduces CTL activity against Ad5E1 clone 2.1 tumors in IFN-γ-deficient environments
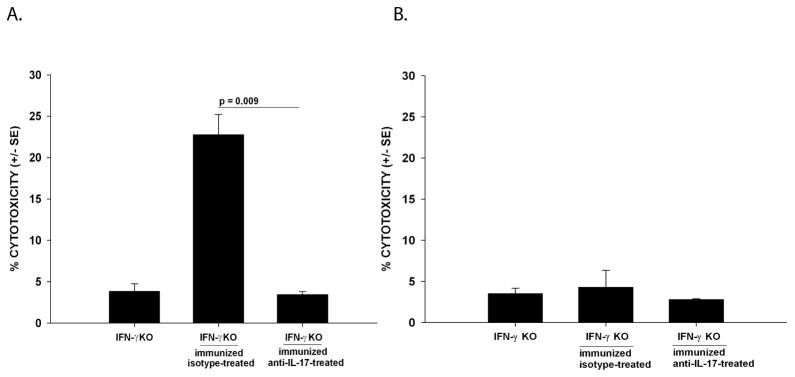
The next experiments examined the role of IL-17 in the rejection of extraocular Ad5E1 clone 2.1 tumors. First, we tested whether IL-17 was cytotoxic to Ad5E1 clone 2.1 tumor cells. Tumor cells were treated in vitro with rmIL-17 at varying doses (10 – 1000 ng/ml) for 24 and 48 hours. No cytotoxicity was observed at any IL-17 doses at either time point (data not shown) and thus suggested that IL-17 does not act directly on tumor cells. As presented above, IFN-γ-independent tumor rejection requires CD8+ T cells. The ability of CD8+ CTL to mediate tumor rejection is reflected by their ability to lyse tumor targets. Accordingly, we confirmed CD8+ CTL killing of Ad5E1 clone 2.1 tumor cells using a standard in vitro 51Cr- release CTL assay. Spleen cells from Ad5E1 clone 2.1 SC immunized IL-17-depleted or isotype-treated IFN-γ KO mice were used as tumor-specific effector cells against Ad5E1 clone 2.1 tumor target cells. Spleen cells from C57BL/6 mice injected SC with allogenic P815 mastocytoma cells were used as effector cells against P815 tumor cell targets and served as a positive CTL control (data not shown). To control for antigen-specificity of the CTL response to Ad5E1 clone 2.1 tumors this assay was also performed with control MEF cells. Depletion of IL-17 greatly reduced the ability of CTLs to kill Ad5E1 clone 2.1 tumor targets, compared to CTL killing by effector cells from isotype-treated IFN-γ KO mice (4% killing versus 22% killing, respectively) (Figure 5A). Cytotoxicity of Ad5E1 clone 2.1 targets was not observed in response to MEF antigens. Thus, the CTL-mediated cytotoxicity was specific for Ad5E1 clone 2.1 antigens (Figure 5B).
Figure 5. Depletion of IL-17 results in the reduction of CTL activity against Ad5E1 clone 2.1 in IFN-γ KO mice.
(A) Splenocytes from naïve or Ad5E1 clone 2.1 immunized C57BL/6 and IFN-γ KO mice (either treated with isotype-antibody or anti-IL-17 antibody) were harvested and cultured with mitomycin-C-treated Ad5E1 clone 2.1 tumor cells for 5 days at 37 °C. (B) Splenocytes from naïve or Ad5E1 clone 2.1 immunized C57BL/6 and IFN-γ KO mice (either treated with isotype-antibody or anti-IL-17 antibody) were harvested and cultured with mitomycin-C-treated MEF cells for 5 days at 37 °C, and tested for cytotoxicity against MEF target cells. Cytotoxicity (% cytotoxicity +/− SE) was determined by a standard 51Cr-release assay at a 100:1 E: T ratio. Data are the combined results of two independent experiments. P values < 0.05 were considered significant as determined by Student’s t test.
Putative lack of IL-6 in the ocular environment prevents Th17 induction of CTL
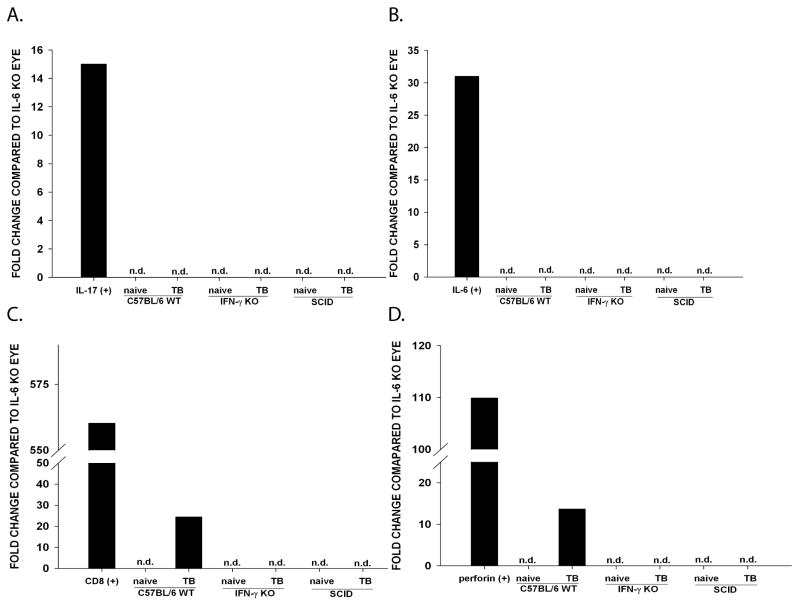
Kryczek et al. (33) demonstrated that Th17 cells predominately accumulate and differentiate in the tumor microenvironment and not in the tumor draining lymph node. Th17 cell differentiation requires TGF-β and IL-6 (34–36). Notably, the normal ocular environment is devoid of IL-6 unless there is ocular inflammation (37). The presence of IL-6 in the eye has been shown to abolish ocular immune privilege by inhibiting apoptosis of T cells entering the aqueous humor (AqH) or by antagonizing the effects of TGF-β (38). Therefore, the absence of detectable IL-6 in the AqH creates an intraocular environment that dramatically reduces the likelihood of Th17 cell generation in eyes bearing Ad5E1 tumors. The finding that Ad5E1 clone 2.1 tumors grew progressively in the eyes of IFN-γ KO mice but were rejected at extraocular sites led us to hypothesize that the absence of IL-6 within the eye prevents the induction of Th17 cells within the intraocular tumors. Accordingly, Ad5E1 clone 2.1 tumor-bearing eyes and non-tumor bearing eyes were collected from WT C57BL/6, IFN-γ KO, and SCID mice on day 14 post AC tumor injection (i.e., the peak time of intraocular tumor growth in WT C57BL/6 mice) and homogenized. To assess the IL-17 gene transcription levels in the eye, RNA was immediately isolated and quantitative PCR was performed to determine the expression of IL-17. All samples were compared to IL-6 KO eyes because it has been reported that IL-6 KO mice do not generate Th17 cells (39). IL-17 expression was not observed in normal eyes or tumor-bearing eyes of WT mice, IL-6 KO mice, IFN-γ KO mice, or SCID mice (Figure 6A). As a positive IL-17 control, CD4+ T cells were polarized in vitro into a Th17 phenotype and demonstrated elevated expression of IL-17 mRNA expression. Quantitative PCR was also performed to assess the level of intraocular IL-6 expression. IL-6 mRNA expression was not detected in either non-tumor-bearing or tumor bearing-eyes of C57BL/6, IFN-γ KO or SCID mice (Figure 6B). As a positive IL-6 control, RAW 264.7 cells were polarized into an M1 macrophage phenotype and displayed >4 fold increase in IL-6 expression.
Figure 6. IFN-γ-deficient naïve and Ad5E1 clone 2.1 tumor-bearing eyes do not express IL-17, IL-6, CD8, or perforin.
Non-tumor-bearing and Ad5E1 clone 2.1 tumor-bearing eyes of C57BL/6, IFN-γ KO and SCID mice were harvested. RNA was isolated and converted to cDNA. mRNA expression of IL-17 (A), IL-6 (B), CD8 (C) and perforin (D) was determined by qPCR. Samples were normalized to GAPDH and compared to IL-6 KO eye. As positive controls for IL-17 and IL-6, Th17-polarized CD4+ T cells and RAW 264.6 cells polarized into a Th17 or M1 phenotype, respectively. Data are a representative of two independent experiments. TB, tumor-bearing; n.d., not detected.
To further address the hypothesis that CTLs are not generated in the eyes of IFN-γ KO mice, the expression of CD8 and perforin was examined by qPCR. Expression of CD8 and perforin was not detected in naïve or tumor-bearing eyes of IFN-γ KO or SCID mice (Figure 6C and 6D). As expected, CD8 and perforin were observed in tumor-bearing eyes of WT IFN-γ competent C57BL/6 mice.
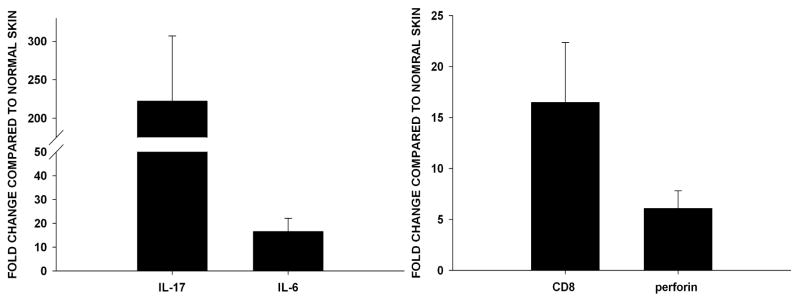
Additional experiments assessed the expression of IL-6 and IL-17 within the SC tumor site. Ad5E1 clone 2.1 tumor cells (5 × 104) were injected SC in the right flank of IFN-γ KO mice. After 7 days, skin surrounding the tumor injection site or from the contralateral flank (non tumor bearing) was excised and homogenized. RNA was isolated from skin samples and qPCR was performed to detect IL-6 and IL-17 mRNA expression. Skin samples from the Ad5E1 clone 2.1 tumor site expressed a 200-fold and a 10-fold increase in IL-17 and IL-6 expression, respectively, compared to tumor-free contralateral skin samples from the same mouse. (Figure 7A). The finding that extraocular (SC) tissues injected with tumor cells express IL-6 and IL-17, and that these cytokines are absent in tumor-bearing eyes, suggests that IL-17 is required for IFN-γ-independent tumor rejection at extraocular sites.
Figure 7. SC tumor sites express IL-17, IL-6, CD8 and perforin.
To determine the expression of IL-17, IL-6, CD8 and perforin in SC tumors Ad5E1 clone 2.1 tumor cells (5 × 104) were injected in the skin of IFN-γ KO mice. After 7 days, skin samples were excised and RNA was isolated. The same qPCR procedure was done as described above. mRNA expression of (A) IL-17 and IL-6, (B) CD8 and perforin was observed in skin samples injected with tumor cells. Samples were normalized to GAPDH and compared to normal (naïve) skin (fold change +/− SE). Data are the combined results of eight individual mice. P values < 0.05 were considered significant as determined by Student’s t test.
In order to confirm the role of CTLs in rejection of SC tumors, the expression of CD8 and perforin was examined. Messenger RNA expression of CD8 and perforin was increased 15-fold and 5-fold, repectively, in tumor-bearing skin compared to normal skin of IFN-γ KO mice (Figure 7B). By contrast, neither CD8 nor perforin expression was observed in tumor-bearing eyes of IFN-γ KO mice (Figure 6C and D). Thus, CTL activity only occurs in extraocular (SC) sites that are replete with IL-6.
Discussion
Ocular immune privilege is essential to maintaining vision. In order to limit sight-threatening inflammation, the eye employs multiple mechanisms to maintain an immunologically quiescent environment. In spite of immune privilege, the eye permits immune-mediated rejection of many experimental ocular tumors. In the present study we demonstrate that IFN-γ is required for the immune rejection of intraocular tumors, but is not needed for rejection of tumors at extraocular sites. Moreover, our results demonstrate that rejection of tumors at extraocular sites can be mediated by an alternate IFN-γ-independent rejection mechanism that is not available for rejection of intraocular tumors. We sought to determine the mechanisms of IFN-γ-independent tumor rejection and why this form of rejection is effective at eliminating extraocular tumors, but fails to reject intraocular tumors.
In previous studies, we have shown that rejection of intraocular Ad5E1 tumors requires IFN-γ, which acts directly on tumor cells by: a) inhibiting tumor cell proliferation; b) inducing tumor cell apoptosis; and c) downregulating pro-angiogeneic genes and upregulating anti-angiogeneic genes in the tumors (20, 21). We have also presented evidence that IFN-γ is required for the recruitment and activation of macrophages, which promote a phthisical form of tumor rejection at the expense of the eye (22). Although IFN-γ is necessary for Ad5E1 tumor rejection in the eye, it is not needed for the elimination of the tumor at extraocular sites, as IFN-γ KO mice reject SC tumors.
Rejection of Ad5E1 tumors is T cell-dependent, as nude mice fail to reject either SC or AC injected tumors. The present findings show that CD4+ T cells are required for rejection, as tumors grow in the majority of CD4-depleted IFN-γ KO mice. IFN-γ KO mice do not develop conventional Th1 cells, leaving either Th2 or Th17 cells as the possible candidates for CD4+ T cell-mediated tumor rejection. There are several studies that implicate Th2-mediated anti-tumor immunity as the primary effectors for tumor rejection (40–42). These studies demonstrate that anti-tumor activity of Th2 cells is in collaboration with tumor-infiltrating granulocytes, such as eosinophils (43, 44). Moreover, Th2 cells are present in the eye during allergic conjunctivitis (45) and corneal graft rejection in atopic hosts (46) and could be employed to reject tumors. This form of immunity appears to be dependent on the production of IL-4 (40) and recruitment of eosinophils (43, 44). However, we did not observe the presence of eosinophils in the tumor rejecting eyes.
This led us to test the hypothesis that Th17 cells are involved in the IFN-γ-independent rejection of subcutaneous SC Ad5E1 tumors. The weight of evidence reported here, indicates that IL-17 is required for rejection of SC Ad5E1 clone 2.1 tumors, as IL-17-depleted IFN-γ KO mice fail to reject SC tumors. We tested the simplest and most direct hypothesis, that IL-17 is cytotoxic to Ad5E1 clone 2.1 tumor cells, and found that high doses of rmIL-17 had no toxic effects when added to in vitro cultures of Ad5E1 clone 2.1 tumor cells. Since both CD4+ and CD8+ T cells are capable of producing IL-17 (47–49), we examined which T cell subset produced IL-17 in our tumor model. The data support the hypothesis that CD4+ T cells were the primary source of IL-17 associated with Ad5E1 clone 2.1 tumor rejection, as CD4+ T cells isolated from SC tumor immunized IFN-γ KO mice produced IL-17 when confronted with Ad5E1 clone 2.1 tumor antigens in vitro. Moreover, splenocytes from CD4+ T cell-depleted IFN-γ KO mice produced significantly less IL-17 than CD4+ T cells from isotype-treated IFN-γ KO mice. Interestingly, CD4+ T cells and bulk splenocytes from wild-type C57BL/6 mice made significantly less IL-17 in response to tumor antigens than IFN-γ KO mice. Thus, IL-17-associated tumor rejection is strongly inhibited by IFN-γ and probably does not occur in wild-type IFN-γ-competent mice. In WT C57BL/6 mice, Ad5E1 clone 2.1 tumor rejection (both intraocular and subcutaneous) occurs through IFN-γ-dependent Th1 responses (21).
Many studies have implicated IL-17 in the pathogenesis of autoimmunity (39, 50–52). However, the role of IL-17 in tumor immunity remains controversial. The response to IL-17 varies according to tumors originating from different tissue types and animal models. Reports have indicated that the population of Th17 cells increases within the tumor microenvironment in many animal models and in patients with melanoma, breast cancer, colon cancer, and ovarian cancer (53–56). Some studies demonstrate that IL-17 plays a pro-tumorigenic role by increasing angiogenesis and promoting metastasis (57–59). However, in other studies, Th17 cells have been shown to have anti-tumor properties (29, 60, 61). IL-17 might promote anti-tumor immunity by several mechanisms including: a) stimulating macrophages to produce IL-1β and TNF-α (62, 63); b) recruiting eosinophils and neutrophils (64, 65) and; c) increasing the expression of costimulatory molecules on maturing dendritic cells (66).
There is growing evidence that IL-17 promotes tumor rejection by increasing the induction and function of CTLs. Recent work by Martin-Orozco et al. has shown that Th17 cells participate in anti-tumor immunity by facilitating DC recruitment into tumor tissues and draining lymph nodes where they promote the activation of CTLs that eliminate the tumor (67). Benchetrit et al. reported that IL-17 increased the generation of tumor-specific CTLs directed against several different immunodominant antigens of P815 mastocytoma (61). The present findings show that in the absence of IFN-γ, both CD8+ T cells and CD4+ T cells are required for rejection of SC Ad5E1 clone 2.1 tumors. This led to the hypothesis that IL-17 increases CTL activity against Ad5E1 clone 2.1 tumors in the SC environment. Indeed, CTLs isolated from IL-17 antibody-depleted IFN-γ KO mice displayed decreased cytotoxicity against Ad5E1 clone 2.1 tumor cells compared to CTLs isolated from isotype antibody-treated IFN-γ KO mice. Increased expression of CD8 and perforin in the SC tumor environment further supports the notion that IFN-γ-independent rejection is CTL-mediated.
Recent work by Bos et al. reported that CD4+ T cells in the tumor microenvironment produce IL-2 and IFN-γ, which promote the recruitment and cytolytic function of tumor-specific CTLs (68). Similarly, we have determined that CD4+ Th17 cells produce IL-17 in the local tumor environment, which is important for rejection of extraocular Ad5E1 clone 2.1 tumors. In the absence of IFN-γ, tumor-specific Th17 cells are induced by the presence of IL-6 and TGF-β in the tumor microenvironment and promote the activation and/or function of CTLs. Gene expression analysis confirmed that the SC environment was replete with IL-6, which is necessary for IL-17-dependent generation of tumor-specific CTL. This conclusion is further supported by the findings of Kryczek et al. who reported that Th17 differentiation occurs primarily in the tumor microenvironment and not in the tumor-draining lymph node (33, 55). While the exact mechanism remains to be elucidated, it is possible that enhancement of CTL activity by Th17 cells is due to an increased expansion of DCs or, alternatively, by enhanced presentation of tumor antigens by individual DCs (67).
The present results show that there is an absence of IL-17 and IL-6 expression in normal eyes and tumor-bearing eyes of WT C57BL/6 and IFN-γ KO mice. However, IL-6 and IL-17 are both detected in SC tumors. Accordingly, a possible explanation as to why there is no IL-17 in the ocular tumor microenvironment is because IL-6, which is a crucial cytokine needed for induction and maintenance of Th17 cells (24), is normally absent in the ocular microenvironment, and thus, Th17 cells cannot differentiate in the IL-6-deficient intraocular environment. Other investigators have reported that the absence of IL-6 in the aqueous humor is required to maintain ocular immune privilege (37, 69). Moreover, several studies have confirmed that blocking IL-6 suppresses the inflammatory response of Th17 cells in autoimmune arthritis and uveoretinitis (52, 70, 71) and interferes with antigen-specific Th17 differentiation/expansion (39, 70).
The present results demonstrate two novel and important findings. First, we present further evidence for a role of Th17 cells in tumor rejection, particularly in the absence of IFN-γ-dependent anti-tumor responses. Second, the abrogation of a Th17 immune response pathway may represent a new mechanism that promotes ocular immune privilege in which IL-17-dependent immune responses are blocked within the intraocular milieu. This blockade may be a means of dampening immune responses, such as neutrophil recruitment and activation, that damage the eye (72, 73).
Acknowledgments
This was work supported by National Institutes of Health grants EY005631 and EY020799, and an unrestricted grant from Research to Prevent Blindness, New York, NY.
Abbreviations used in this paper
- Ad5E1
adenovirus type 5 early region 1
- AC
anterior chamber
- AqH
aqueous humor
- SC
subcutaneous
- TA
tumor antigens
References
- 1.Whitsett CF, Stulting RD. The distribution of HLA antigens on human corneal tissue. Investigative ophthalmology & visual science. 1984;25:519–524. [PubMed] [Google Scholar]
- 2.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 3.Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nature immunology. 2006;7:354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- 4.Niederkorn JY. Immune privilege in the anterior chamber of the eye. Critical reviews in immunology. 2002;22:13–46. [PubMed] [Google Scholar]
- 5.Medawar PB. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 6.Niederkorn JY, Wang S. Immunology of intraocular tumors. Ocular immunology and inflammation. 2005;13:105–110. doi: 10.1080/09273940490518586. [DOI] [PubMed] [Google Scholar]
- 7.Knisely TL, Luckenbach MW, Fischer BJ, Niederkorn JY. Destructive and nondestructive patterns of immune rejection of syngeneic intraocular tumors. J Immunol. 1987;138:4515–4523. [PubMed] [Google Scholar]
- 8.Niederkorn JY. The immunopathology of intraocular tumour rejection. Eye (London, England) 1991;5(Pt 2):186–192. doi: 10.1038/eye.1991.33. [DOI] [PubMed] [Google Scholar]
- 9.Niederkorn JY. Immunoregulation of intraocular tumours. Eye (London, England) 1997;11(Pt 2):249–254. doi: 10.1038/eye.1997.60. [DOI] [PubMed] [Google Scholar]
- 10.Niederkorn JY. Immunopathogenesis of intraocular tumors. Prog Retinal and Eye Res. 1995;14:505–526. doi: 10.1016/j.preteyeres.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niederkorn JY. Immune escape mechanisms of intraocular tumors. Prog Retin Eye Res. 2009;28:329–347. doi: 10.1016/j.preteyeres.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niederkorn JY, Knisely TL. Immunological analysis of a destructive pattern of intraocular tumor resolution. Current eye research. 1988;7:515–526. doi: 10.3109/02713688809031806. [DOI] [PubMed] [Google Scholar]
- 13.Knisely TL, Niederkorn JY. Emergence of a dominant cytotoxic T lymphocyte antitumor effector from tumor-infiltrating cells in the anterior chamber of the eye. Cancer Immunol Immunother. 1990;30:323–330. doi: 10.1007/BF01786881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma D, Alizadeh H, Comerford SA, Gething MJ, Sambrook JF, Anand R, Niederkorn JY. Rejection of intraocular tumors from transgenic mice by tumor-infiltrating lymphocytes. Curr Eye Res. 1994;13:361–369. doi: 10.3109/02713689409167300. [DOI] [PubMed] [Google Scholar]
- 15.Schurmans LR, Diehl L, den Boer AT, Sutmuller RP, Boonman ZF, Medema JP, van der Voort EI, Laman J, Melief CJ, Jager MJ, Toes RE. Rejection of intraocular tumors by CD4(+) T cells without induction of phthisis. J Immunol. 2001;167:5832–5837. doi: 10.4049/jimmunol.167.10.5832. [DOI] [PubMed] [Google Scholar]
- 16.Niederkorn JY, Meunier PC. Spontaneous immune rejection of intraocular tumors in mice. Investigative ophthalmology & visual science. 1985;26:877–884. [PubMed] [Google Scholar]
- 17.Knisely TL, Luckenbach MW, Fischer BJ, Niederkorn JY. Destructive and nondestructive patterns of immune rejection of syngeneic intraocular tumors. J Immunol. 1987;138:4515–4523. [PubMed] [Google Scholar]
- 18.Schurmans LR, den Boer AT, Diehl L, van der Voort EI, Kast WM, Melief CJ, Toes RE, Jager MJ. Successful immunotherapy of an intraocular tumor in mice. Cancer research. 1999;59:5250–5254. [PubMed] [Google Scholar]
- 19.Schurmans LR, Diehl L, den Boer AT, Sutmuller RP, Boonman ZF, Medema JP, van der Voort EI, Laman J, Melief CJ, Jager MJ, Toes RE. Rejection of intraocular tumors by CD4(+) T cells without induction of phthisis. J Immunol. 2001;167:5832–5837. doi: 10.4049/jimmunol.167.10.5832. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Boonman ZF, Li HC, He Y, Jager MJ, Toes RE, Niederkorn JY. Role of TRAIL and IFN-gamma in CD4+ T cell-dependent tumor rejection in the anterior chamber of the eye. J Immunol. 2003;171:2789–2796. doi: 10.4049/jimmunol.171.6.2789. [DOI] [PubMed] [Google Scholar]
- 21.Dace DS, Chen PW, Alizadeh H, Niederkorn JY. Ocular immune privilege is circumvented by CD4+ T cells, leading to the rejection of intraocular tumors in an IFN-{gamma}-dependent manner. Journal of leukocyte biology. 2007;81:421–429. doi: 10.1189/jlb.0806489. [DOI] [PubMed] [Google Scholar]
- 22.Coursey TG, Chen PW, Niederkorn JY. Abrogating TNF-alpha expression prevents bystander destruction of normal tissues during iNOS-mediated elimination of intraocular tumors. Cancer research. 2011;71:2445–2454. doi: 10.1158/0008-5472.CAN-10-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dace DS, Chen PW, Niederkorn JY. CD4+ T-cell-dependent tumour rejection in an immune-privileged environment requires macrophages. Immunology. 2008;123:367–377. doi: 10.1111/j.1365-2567.2007.02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toes RE, Offringa R, Blom RJ, Brandt RM, van der Eb AJ, Melief CJ, Kast WM. An adenovirus type 5 early region 1B-encoded CTL epitope-mediating tumor eradication by CTL clones is down-modulated by an activated ras oncogene. J Immunol. 1995;154:3396–3405. [PubMed] [Google Scholar]
- 25.Fenton BM, Way BA. Vascular morphometry of KHT and RIF-1 murine sarcomas. Radiother Oncol. 1993;28:57–62. doi: 10.1016/0167-8140(93)90186-c. [DOI] [PubMed] [Google Scholar]
- 26.Chen PW, Uno T, Ksander BR. Tumor escape mutants develop within an immune-privileged environment in the absence of T cell selection. J Immunol. 2006;177:162–168. doi: 10.4049/jimmunol.177.1.162. [DOI] [PubMed] [Google Scholar]
- 27.Hegde S, Niederkorn JY. The role of cytotoxic T lymphocytes in corneal allograft rejection. Investigative ophthalmology & visual science. 2000;41:3341–3347. [PubMed] [Google Scholar]
- 28.Cunnusamy K, Chen PW, Niederkorn JY. IL-17 promotes immune privilege of corneal allografts. J Immunol. 2010;185:4651–4658. doi: 10.4049/jimmunol.1001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irmler IM, Gajda M, Brauer R. Exacerbation of antigen-induced arthritis in IFN-gamma-deficient mice as a result of unrestricted IL-17 response. J Immunol. 2007;179:6228–6236. doi: 10.4049/jimmunol.179.9.6228. [DOI] [PubMed] [Google Scholar]
- 31.Ivanov, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19:409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature immunology. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 34.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 35.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 36.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Mo JS, Streilein JW. Immune privilege persists in eyes with extreme inflammation induced by intravitreal LPS. Eur J Immunol. 2001;31:3806–3815. doi: 10.1002/1521-4141(200112)31:12<3806::aid-immu3806>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 38.Curnow SJ, Scheel-Toellner D, Jenkinson W, Raza K, Durrani OM, Faint JM, Rauz S, Wloka K, Pilling D, Rose-John S, Buckley CD, Murray PI, Salmon M. Inhibition of T cell apoptosis in the aqueous humor of patients with uveitis by IL-6/soluble IL-6 receptor trans-signaling. J Immunol. 2004;173:5290–5297. doi: 10.4049/jimmunol.173.8.5290. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimura T, Sonoda KH, Ohguro N, Ohsugi Y, Ishibashi T, Cua DJ, Kobayashi T, Yoshida H, Yoshimura A. Involvement of Th17 cells and the effect of anti-IL-6 therapy in autoimmune uveitis. Rheumatology (Oxford) 2009;48:347–354. doi: 10.1093/rheumatology/ken489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Modesti A, Masuelli L, Modica A, D’Orazi G, Scarpa S, Bosco MC, Forni G. Ultrastructural evidence of the mechanisms responsible for interleukin-4-activated rejection of a spontaneous murine adenocarcinoma. International journal of cancer. 1993;53:988–993. doi: 10.1002/ijc.2910530622. [DOI] [PubMed] [Google Scholar]
- 41.Pericle F, Giovarelli M, Colombo MP, Ferrari G, Musiani P, Modesti A, Cavallo F, Di Pierro F, Novelli F, Forni G. An efficient Th2-type memory follows CD8+ lymphocyte-driven and eosinophil-mediated rejection of a spontaneous mouse mammary adenocarcinoma engineered to release IL-4. J Immunol. 1994;153:5659–5673. [PubMed] [Google Scholar]
- 42.Tepper RI, Coffman RL, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science (New York, NY) 1992;257:548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 43.Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, Parish C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. The Journal of experimental medicine. 2003;197:387–393. doi: 10.1084/jem.20021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimura T, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, Sato T, Habu S, Tashiro H, Sato M, Ohta A. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. The Journal of experimental medicine. 1999;190:617–627. doi: 10.1084/jem.190.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reyes NJ, Mayhew E, Chen PW, Niederkorn JY. {gamma}{delta} T Cells are Required for Maximal Expression of Allergic Conjunctivitis. Investigative ophthalmology & visual science. 2011 doi: 10.1167/iovs.10-5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hargrave S, Chu Y, Mendelblatt D, Mayhew E, Niederkorn J. Preliminary findings in corneal allograft rejection in patients with keratoconus. Am J Ophthalmol. 2003;135:452–460. doi: 10.1016/s0002-9394(02)02055-x. [DOI] [PubMed] [Google Scholar]
- 47.Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J Immunol. 2006;177:6852–6858. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ivanov, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 50.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarkar S, Fox DA. Targeting IL-17 and Th17 cells in rheumatoid arthritis. Rheum Dis Clin North Am. 2010;36:345–366. doi: 10.1016/j.rdc.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 52.Serada S, Fujimoto M, Mihara M, Koike N, Ohsugi Y, Nomura S, Yoshida H, Nishikawa T, Terabe F, Ohkawara T, Takahashi T, Ripley B, Kimura A, Kishimoto T, Naka T. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9041–9046. doi: 10.1073/pnas.0802218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maniati E, Soper R, Hagemann T. Up for Mischief? IL-17/Th17 in the tumour microenvironment. Oncogene. 2010;29:5653–5662. doi: 10.1038/onc.2010.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 57.Charles KA, Kulbe H, Soper R, Escorcio-Correia M, Lawrence T, Schultheis A, Chakravarty P, Thompson RG, Kollias G, Smyth JF, Balkwill FR, Hagemann T. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. The Journal of clinical investigation. 2009;119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. The Journal of experimental medicine. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 62.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 63.Gu Y, Hu X, Liu C, Qv X, Xu C. Interleukin (IL)-17 promotes macrophages to produce IL-8, IL-6 and tumour necrosis factor-alpha in aplastic anaemia. Br J Haematol. 2008;142:109–114. doi: 10.1111/j.1365-2141.2008.07161.x. [DOI] [PubMed] [Google Scholar]
- 64.Benatar T, Cao MY, Lee Y, Li H, Feng N, Gu X, Lee V, Jin H, Wang M, Der S, Lightfoot J, Wright JA, Young AH. Virulizin induces production of IL-17E to enhance antitumor activity by recruitment of eosinophils into tumors. Cancer Immunol Immunother. 2008;57:1757–1769. doi: 10.1007/s00262-008-0502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183:4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 66.Antonysamy MA, Fanslow WC, Fu F, Li W, Qian S, Troutt AB, Thomson AW. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999;162:577–584. [PubMed] [Google Scholar]
- 67.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer research. 2010;70:8368–8377. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calabrese KS, Tedesco RC, Zaverucha do Valle T, Barbosa HS. Serum and aqueous humour cytokine response and histopathological alterations during ocular Toxoplasma gondii infection in C57BL/6 mice. Micron. 2008;39:1335–1341. doi: 10.1016/j.micron.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 70.Fujimoto M, Serada S, Mihara M, Uchiyama Y, Yoshida H, Koike N, Ohsugi Y, Nishikawa T, Ripley B, Kimura A, Kishimoto T, Naka T. Interleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory Th17 responses. Arthritis Rheum. 2008;58:3710–3719. doi: 10.1002/art.24126. [DOI] [PubMed] [Google Scholar]
- 71.Hohki S, Ohguro N, Haruta H, Nakai K, Terabe F, Serada S, Fujimoto M, Nomura S, Kawahata H, Kishimoto T, Naka T. Blockade of interleukin-6 signaling suppresses experimental autoimmune uveoretinitis by the inhibition of inflammatory Th17 responses. Exp Eye Res. 2010;91:162–170. doi: 10.1016/j.exer.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 72.Shi G, Ramaswamy M, Vistica BP, Cox CA, Tan C, Wawrousek EF, Siegel RM, Gery I. Unlike Th1, Th17 cells mediate sustained autoimmune inflammation and are highly resistant to restimulation-induced cell death. J Immunol. 2009;183:7547–7556. doi: 10.4049/jimmunol.0900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Z, Zhong W, Spencer D, Chen H, Lu H, Kawaguchi T, Rosenbaum JT. Interleukin-17 causes neutrophil mediated inflammation in ovalbumin-induced uveitis in DO11.10 mice. Cytokine. 2009;46:79–91. doi: 10.1016/j.cyto.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]