Abstract
The search for a common cause of species richness gradients has spawned more than 100 explanatory hypotheses in just the past two decades. Despite recent conceptual advances, further refinement of the most plausible models has been stifled by the difficulty of compiling high-resolution databases at continental scales. We used a database of the geographic ranges of 2,869 species of birds breeding in South America (nearly a third of the world's living avian species) to explore the influence of climate, quadrat area, ecosystem diversity, and topography on species richness gradients at 10 spatial scales (quadrat area, ≈12,300 to ≈1,225,000 km2). Topography, precipitation, topography × latitude, ecosystem diversity, and cloud cover emerged as the most important predictors of regional variability of species richness in regression models incorporating 16 independent variables, although ranking of variables depended on spatial scale. Direct measures of ambient energy such as mean and maximum temperature were of ancillary importance. Species richness values for 1° × 1° latitude-longitude quadrats in the Andes (peaking at 845 species) were ≈30–250% greater than those recorded at equivalent latitudes in the central Amazon basin. These findings reflect the extraordinary abundance of species associated with humid montane regions at equatorial latitudes and the importance of orography in avian speciation. In a broader context, our data reinforce the hypothesis that terrestrial species richness from the equator to the poles is ultimately governed by a synergism between climate and coarse-scale topographic heterogeneity.
The staggering contrast in biotic diversity between equatorial and polar latitudes is one of Earth's most salient biological characteristics. Although this phenomenon has been recognized since the 19th century (1, 2), the proximate and ultimate causes of species richness gradients continue to galvanize scientific debate and drive hypothesis testing in macroecology and biogeography (3–11). Of the 120 hypotheses proposed thus far to explain regional variability in species richness (12), the majority are either vague, tautological and therefore untestable (e.g., interspecific competition begets diversity), implausibly contrived, or insufficiently supported by empirical evidence (5, 6). Research efforts during the past decade have winnowed the number of potential hypotheses to a credible few: (i) energy availability (4–6, 13); (ii) evolutionary time (3, 6); (iii) habitat heterogeneity (14–16); (iv) area (8); and (v) geometric constraints (11, 17–22).
Species richness gradients are affected undoubtedly by a combination of biotic and abiotic factors (23) but the chief obstacle to rigorous testing of competing hypotheses, and to the ranking of potential determinants, is the lack of high-quality data (16). Virtually all previous studies of terrestrial species richness at continental scales suffer from one to several methodological weaknesses, including limited taxonomic coverage, reliance on secondary and often tertiary sources for distributional data, coarse spatial resolution of biogeographic ranges (grid quadrants frequently >600,000 km2), and limited latitudinal range (seldom extending from equatorial to temperate latitudes). Qualitative deficiencies also extend to climatic variables used in testing ambient energy hypotheses. Until recently, published sources of climatic data for vast areas of the tropics were based on widely spaced ground stations (many in completely deforested regions) or on coarse extrapolations from satellite images that were inadequate for fine-scale geographic analyses. To compound matters, few papers have tested species richness hypotheses at different spatial scales or addressed multiple hypotheses with a given data set. As a consequence, and despite a century of study, it can be argued that the science of species richness gradients is still in its infancy.
In this paper, we systematically investigated the correlates of species richness gradients in South American birds, the most diverse group of terrestrial organisms in the Neotropics for which fine-scale distributional data are available. By using a multiscale database representing 2,869 breeding species, classified in 64 avian families (taxonomy of ref. 24), we sought to (i) characterize the relationship between species richness and potential determinants, including climate, quadrat area, topography, and ecosystem diversity; (ii) examine the power of regression models corresponding to causal hypotheses to predict species richness; (iii) investigate the degree of nonindependence among hypotheses; (iv) examine the variation in predictive power of models across 10 spatial scales (quadrat area, ≈12,300 to ≈1,225,000 km2); and finally, (v) evaluate continental patterns of species richness in context to postulated biotic and abiotic determinants.
Methods
Geographic Ranges.
South America supports the world's richest avifauna, constituting nearly a third of the living bird species. Although much remains to be learned about the distribution of birds in South America, the geographic ranges of species are better known and the taxonomic inventory is more complete than for any other specious group of organisms on the continent. We mapped geographic ranges of all land and fresh-water species breeding in South America at a resolution of 1° × 1° latitude-longitude quadrats from primary sources (museum specimens and documented sight records; see Acknowledgments). Final maps for each species (n = 2,869) represented a conservative “extent of occurrence” extrapolation based on confirmed records, spatial distribution of preferred habitat, and the consensus of taxonomic specialists (see refs. 25 and 26 for description of the methodology and sources). We used the worldmap computer program (version 4.19.12; ref. 27) to accommodate and overlay the distributional data. Species richness was calculated for latitude-longitude quadrats aligned at the equator and prime meridian at 10 spatial scales (1° × 1°, 2° × 2°, … 10° × 10°) spanning 2 orders of magnitude (≈12,300 to ≈1,225,000 km2). For brevity we abbreviate quadrat dimensions in the remainder of the paper (i.e., 1°, 2°, 3°, … ). Quadrat centroids were used as spatial coordinates. Fig. 1a was based on 533,627 species-in-quadrat records.
Figure 1.
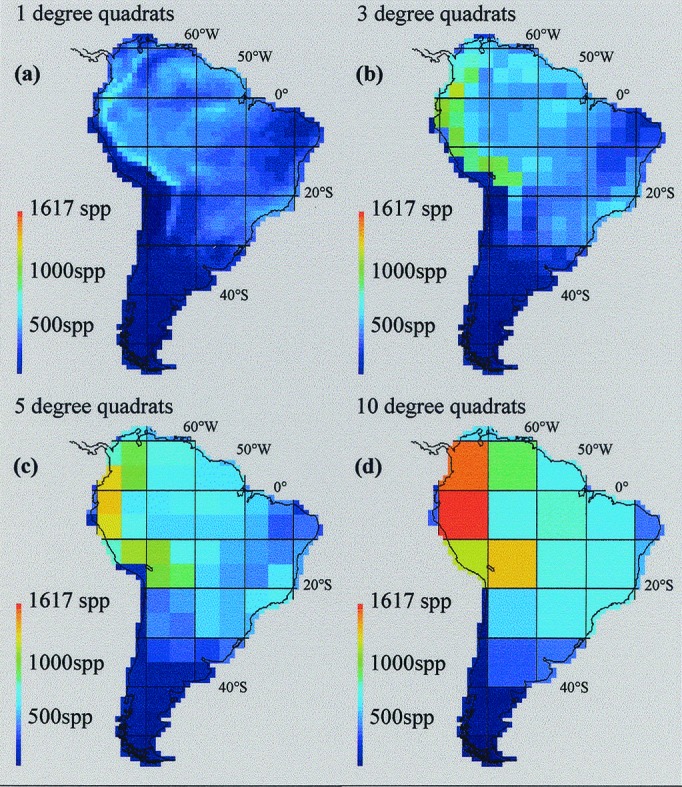
Spatial variation in species richness of 2,869 breeding land and fresh-water birds (Aves) of South America compiled at 1° × 1°, 3° × 3°, 5° × 5°, and 10° × 10° scales. Note the excessive loss of information and the spurious extrapolation of high species densities in species-poor localities at coarser spatial scales. spp, species.
Climatic Variables.
Past investigations of species richness patterns at equatorial latitudes were hampered by the unavailability of high-resolution climatic data. In this paper, we extracted climatic data (variables listed in Table 1) for quadrats of various dimensions from the mean monthly climatic database published by New et al. (32), which was compiled globally at a 0.5° latitude-longitude resolution for the period 1961–1990 (>3,000,000 data points for each variable). This source represents the most accurate published database on contemporary climate of the Neotropics available at this time. We included latitude in regression analyses as a nonspecific surrogate of global climate.
Table 1.
Independent variables used in stepwise regression analyses of species richness
| Independent variables | Mnemonic | Hypothesis |
|---|---|---|
| Precipitation (mm/yr−1) | PREC | A,B |
| Wet-day frequency (days) | WET | A,B |
| Mean annual temperature (C°) | Tmean | A,B |
| Mean daily maximum temperature (C°) | Tmax | A,B |
| Mean daily minimum temperature (C°) | Tmin | A,B |
| Mean daily temperature range (C°) | Trange | A,B |
| Mean vapor pressure (hPa) | VAPOR | A,B |
| Radiation (W/m2) | RAD | A,B |
| Cloud cover (Percent) | CLOUD | A,B |
| Frost frequency (days) | FROST | A,B |
| Mean wind speed (m/second) | WIND | A,B |
| Latitude | LAT | A,B,C |
| Quadrat area (km2) | AREA | A,B,C,D |
| Ecosystem diversity (number of ecosystems in quadrat) | ECO | A,C |
| Topographic relief (elevational range, m) | TOPO | A,D |
| Latitude × topographic relief | L × T | A,D |
Letters under hypothesis refer to regression models to which variables have been assigned: (A) ad hoc model; (B) contemporary climate model (4–6, 13, 28–30); (C) ecosystem diversity model (3, 5); and (D) topography × latitude model (14–16, 31). All variables are correlated significantly with avian species richness (P < 0.001, Bonferroni-adjusted for simultaneously testing) at the 1° × 1° latitude-longitude resolution (n = 1,679 quadrats).
Global patterns of species richness are widely believed to correlate with climate, particularly to energy-related variables (30). An alternate hypothesis posits that species richness gradients result from regional variability in effective evolutionary time. This idea is based in part on the observation that climatic constancy and ambient energy (6) vary regionally. Contemporary climate, however, is sometimes viewed as a surrogate for past climatic changes and history (refs. 33–35, but see ref. 29). Our data do not permit us to distinguish the effects of climate from those of evolutionary time (6, 13, 28).
Quadrat Area.
Area per se has an indisputable influence on species richness that must be dealt with in any analysis of species richness gradients (8, 18, 36–40). Accordingly, we included quadrat area adjusted for latitude as an independent variable in all regression models. In contrast to other studies (e.g., refs. 8 and 40), we retained in our analyses quadrats that intersected the continental shoreline, because the deletion of such quadrats also eliminates much of the important biological signal in South America, especially at coarser spatial scales (16). Land area within coarser-scale quadrats was estimated by summing the areas of nested 1° quadrats.
We did not investigate the geometric constraints model (17) of species richness, because an operational two-dimensional model has not yet been developed (11). This promising approach deals only with those species endemic to the region of analysis, which currently limits its use when it comes to patterns of distributional overlap of endemic as well as nonendemic species.
Ecosystem Diversity.
Although species richness is widely believed to correlate with the number of habitats at local scales, empirical support for this notion at regional scales (3) has been deemed insufficient by some authors (e.g., ref. 5). To explore the species richness-habitat hypothesis, we obtained a rough estimate of habitat diversity by enumerating the number of ecosystems per quadrat from a recently published map of global ecosystems (ref. 41, http://edcdaac.usgs.gov/glcc/sadoc1_2.html). This source recognizes 94 ecosystem classes derived from 1-km Advanced Very High-Resolution Radiometer (AVHRR) data spanning a 12-month period (April 1992–March 1993).
Topography.
We used topographic relief (maximum minus minimum elevation recorded in each quadrat) as a surrogate for topographic heterogeneity (16, 18). Elevational data, rounded to the nearest 100 m, were complied (1:1,000,000; ref. 42). These data are the most reliable estimates of elevational heterogeneity currently available in the public domain for the entire South American continent. Our use of topographic relief is conservative in that it tends to underestimate the true topographic heterogeneity at coarser spatial scales. We have avoided using extrapolation digital elevation models of topographic relief available from various sources on the Internet because of the unacceptably high error (≈300 m) associated with data point estimation (ref. 43, http://edcdaac.usgs.gov/gtopo30/papers/olsen.html).
Topographic relief seems to be a significant determinant of species richness at the regional scale in association with ambient energy (14, 31). Nevertheless, the effects of topographic relief usually are deemed secondary to, and independent of, those of energy (6). In a continental analysis of species richness in South American hummingbirds (n = 241 species), however, we showed that the importance of latitude fell precipitously to insignificance at coarser spatial scales when the influence of topography was removed (16). Accordingly, we entered an interaction term, topography × latitude, in selected regression models to determine its influence on species richness patterns for the complete spectrum of neotropical avian families.
Statistics.
We investigated the following hypotheses with stepwise multiple regression and partial correlation analysis at each of 10 spatial scales (Table 1): (i) contemporary climate model; (ii); ecosystem diversity model; and (iii) topography × latitude model. The predictive power of hypotheses was compared with that of a general ad hoc model, which included all 16 independent variables. Many characteristics of macroecological databases, such as spatial autocorrelation of quadrat values, are problematic in regression analyses and can lead to biased estimators and spurious biological conclusions. For this reason we emphasize the relative ranking of variables (F ratios) in regression models rather than P values (two-tailed). The performance of models was compared in terms of r2 values, partial r2 values, and the mapped distribution of residuals.
Results and Discussion
Ad Hoc Model.
A stepwise regression model incorporating all independent variables (n = 16) explained 77–94% of the variance in species richness (Table 2). Predictive power of the model exhibited a roughly monotonic increase from finer to coarser spatial scales. Topography, precipitation, topography × latitude, ecosystem diversity, and cloud cover emerged as the most important predictors of regional variability of species richness, although variable ranking depended on spatial scale. Precipitation was the best predictor of species richness at the finer scales of resolution. At coarser spatial scales (≥4°), topography was the dominant factor, often explaining more than twice the variance as the next most important variable in most analyses. Topography and the interaction term, topography × latitude, were the only variables retained in the ad hoc model at all spatial scales.
Table 2.
Spatial determinants of avian species richness
| Scale | n | Maximum quadrat size within scale, km2 | Model with all 16 variables | Contemporary climate model | Ecosystem diversity model | Topography X latitude model |
|---|---|---|---|---|---|---|
|
| ||||||
| Variables entering model (F); model r2 | Variables entering model (F); model r2; (partial r2) | Variables entering model (F); model r2; (partial r2) | Variables entering model (F); model r2; (partial r2) | |||
| 1° grid | 1,679 | 12,308 | PREC (341), TOPO (202), CLOUD (113), ECO (89), L × T (75), LAT (51), Tmax (47), AREA (17), WIND (13), VAPOR (11), WET (6) | PREC (380), FROST (80), CLOUD (56), WET (36), LAT (28), AREA (17), RAD (14), Trange (9) | AREA (1,396), ECO (140) | LAT (597), L × T (86), TOPO (39) |
| r2 = 0.77*** | r2 = 0.70*** (r2 = 0.37***) | r2 = 0.50*** (r2 = 0.01**) | r2 = 0.51*** (r2 = 0.04***) | |||
| 2° grid | 451 | 49,225 | PREC (132), TOPO (119), ECO (72), CLOUD (58), Tmean (54), L × T (48), LAT (17), Trange (13), WET (4) | PREC (99), CLOUD (65), RAD (40), FROST (14), WET (13), AREA (10), Tmin (7), Trange (6), WIND (5) | AREA (112), ECO (67) | TOPO (102), L × T (85), AREA (41), LAT (29) |
| r2 = 0.80*** | r2 = 0.65*** (r2 = 0.38***) | r2 = 0.38*** (r2 = 0.07***) | r2 = 0.58*** (r2 = 0.04***) | |||
| 3° grid | 214 | 110,729 | ECO (108), TOPO (92), L × T (58), PREC (50), CLOUD (35), AREA (19), WET (8) | PREC (54), WIND (30), AREA (13), LAT (10), Trange (7), FROST (4) | AREA (48), ECO (48) | L × T (172), TOPO (153), AREA (83) |
| r2 = 0.83*** | r2 = 0.63*** (r2 = 0.38***) | r2 = 0.46*** (r2 = 0.15***) | r2 = 0.65*** (r2 = 0.07**) | |||
| 4° grid | 128 | 196,784 | TOPO (130), L × T (59), ECO (39), PREC (33), CLOUD (14), AREA (12) | PREC (39), WIND (28), LAT (22), AREA (16), Trange (7) | AREA (20), ECO (18) | L × T (166), TOPO (154), AREA (54) |
| r2 = 0.85*** | r2 = 0.65*** (r2 = 0.40***) | r2 = 0.37*** (r2 = 0.17***) | r2 = 0.71*** (r2 = 0.17***) | |||
| 5° grid | 90 | 307,338 | TOPO (146), L × T (49), ECO (24), PREC (23), CLOUD (15), AREA (8) | PREC (26), WIND (20), LAT (16), AREA (7), Trange (5) | AREA (12), ECO (12) | L × T (166), TOPO (160), AREA (61) |
| r2 = 0.90*** | r2 = 0.69*** (r2 = 0.37) | r2 = 0.43*** (r2 = 0.03) | r2 = 0.80*** (r2 = 0.25***) | |||
| 6° grid | 66 | 442,325 | TOPO (52), ECO (38), L × T (36), CLOUD (25), WET (10), WIND (10), PREC (5) | CLOUD (44), RAD (29), WET (20), AREA (16), PREC (8) | ECO (13), AREA (11) | L × T (84), TOPO (83), AREA (35) |
| r2 = 0.89*** | r2 = 0.72*** (r2 = 0.40***) | r2 = 0.51*** (r2 = 0.02) | r2 = 0.77*** (r2 = 0.11) | |||
| 7° grid | 48 | 601,674 | TOPO (50), L × T (33), ECO (33), CLOUD (19); | CLOUD (40), RAD (21), WET (15), PREC (6), AREA (5) | ECO (6), AREA (4); | L × T (106), TOPO (97), AREA (23) |
| r2 = 0.86*** | r2 = 0.72*** (r2 = 0.39***) | r2 = 0.34** (r2 = 0.14) | r2 = 0.80*** (r2 = 0.24**) | |||
| 8° grid | 40 | 785,268 | TOPO (132), L × T (87), ECO (9), PREC (8), RAD (8), AREA (7); | AREA (17), LAT (15) | AREA (26) | TOPO (121), L × T (107), AREA (40) |
| r2 = 0.93*** | r2 = 0.58*** (r2 = 0.46***) | r2 = 0.41** (r2 = 0.00) | r2 = 0.88*** (r2 = 0.36***) | |||
| 9° grid | 35 | 993,019 | TOPO (156), L × T (56), AREA (46), PREC (14) | AREA (16), LAT (12) | AREA (5), ECO (5) | TOPO (129), L × T (96), AREA (42) |
| r2 = 0.93*** | r2 = 0.60*** (r2 = 0.50***) | r2 = 0.52*** (r2 = 0.06) | r2 = 0.90*** (r2 = 0.35**) | |||
| 10° grid | 29 | 1,224,797 | TOPO (99), L × T (51), CLOUD (13), AREA (10), ECO (5) | AREA (19), LAT (14), WIND (4) | AREA (21) | L × T (93), TOPO (89), AREA (29) |
| r2 = 0.94*** | r2 = 0.66*** (r2 = 0.36*) | r2 = 0.44** (r2 = 0.05) | r2 = 0.89*** (r2 = 0.62***) | |||
| Mean | r2 = 0.87 | r2 = 0.66 (r2 = 0.42) | r2 = 0.44 (r2 = 0.07) | r2 = 0.75 (r2 = 0.23) | ||
| Coefficient of variation of | r2 = 6.7% | r2 = 7.3% (r2 = 14.6%) | r2 = 14.2% (r2 = 88.3%) | r2 = 17.9% (r2 = 81.1%) | ||
Stepwise regression of variables considered with forward selection (P to enter = 0.05) followed by a backward elimination (P to remove = 0.05) with tolerance = 0.01. Partial correlation analysis determining the predictive power of contemporary climate, ecosystem diversity, and topography × latitude hypotheses while controlling for the effects of variables not included in the models. P values were adjusted for error rate per variable: *, P < 0.05/10 = 0.005; **, P < 0.01/10 = 0.001; ***, P < 0.001/10 = 0.0001. See Table 1 for list of variables assigned to each model.
Contemporary Climate Model.
Climatic variables explained 58–72% of the variance in species richness. Precipitation was the most influential factor at finer spatial scales (≤5°), whereas quadrat area and cloud cover were the most important predictors of species richness at coarser spatial scales. Quadrat area was the only variable retained in the model at all spatial scales. Direct measures of ambient energy, such as mean and maximum temperature, were only minimally influential in explaining the observed pattern of species richness.
Ecosystem Diversity Model.
Ecosystem diversity increased monotonically with spatial scale—the mean number of ecosystems per quadrat ranged from 12.3 ± 4.7 (1° quadrats) to 23.7 ± 4.0 (10° quadrats). A simple model incorporating quadrat area and the number of ecosystems per quadrat explained 34–51% of the variance in species richness.
Topography × Latitude Model.
Model performance increased almost monotonically from fine (r2 = 0.51) to coarse spatial scales (r2 = 0.89). Latitude was the dominant predictor of species richness at the 1° scale but was not retained in stepwise regression analyses of data compiled at quadrat sizes ≥3°. Topography and topography × latitude were significant at all spatial scales. This relatively simple model out-performed the contemporary climate model at quadrat sizes ≥3° and the ecosystem diversity model at all spatial scales.
Geographic Distribution of Regression Residuals.
Concordance between expected and observed values of species richness for all regression models was highest in regions exhibiting low to moderate degrees of topographic relief, ecosystem diversity, and species richness. The geographic distribution of large residuals (±3 standard deviations) highlighted substantive differences in the performance of regression models at the 1°-spatial scale (Fig. 2). Large residuals from the ad hoc model (n = 22) and contemporary climate model (n = 24) were clustered in the Andean region at low latitudes (<18°). In both models, the most extreme residuals occurred in the Chocó region of Colombia, where continental highs in precipitation and cloud cover resulted in excessive estimates of species richness (>800) for two coastal quadrats with fewer than 295 species. By comparison, the simple topography × latitude (n = 12) and ecosystem diversity models (n = 4) yielded far fewer large residuals. All models exhibited relatively poor predictive power in the species-rich Andes. Consequently, this region serves as a gauge for evaluating the performance of future generations of species richness models.
Figure 2.
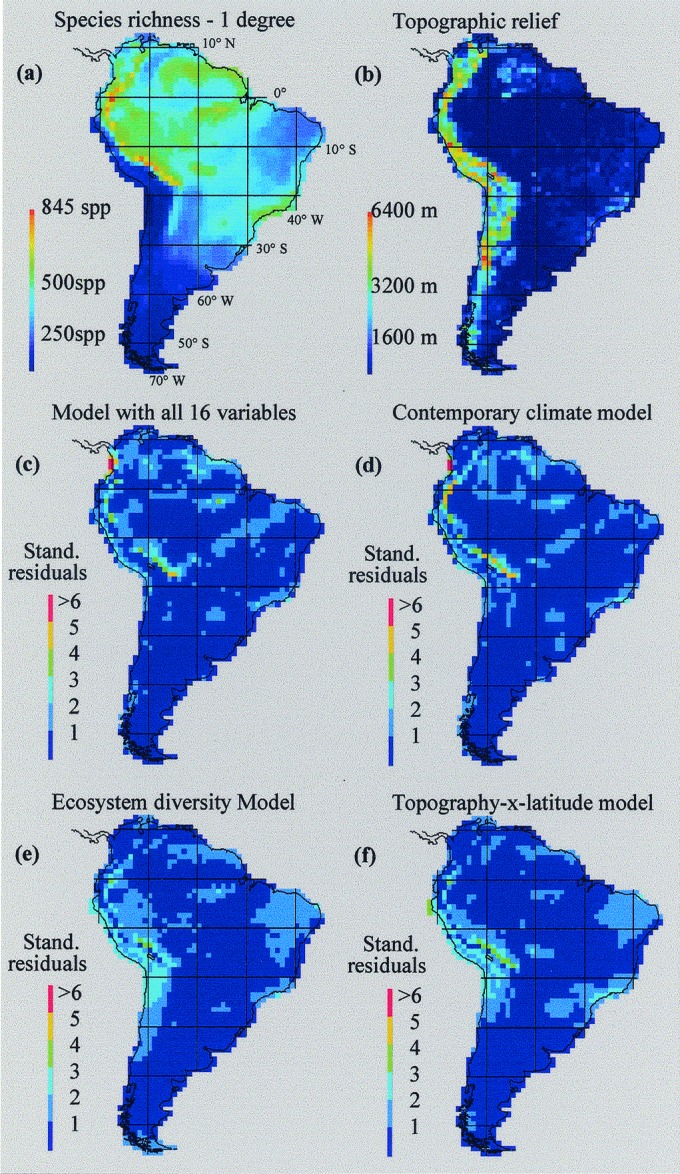
Species richness of 2,869 breeding land and fresh-water birds in South America (a), topographic relief (b), and spatial distribution of standardized residuals (c–f) from stepwise multiple regression models presented in Table 2. spp, species.
Partial Correlation and Scale Invariance.
Partial correlation values for the contemporary climate, ecosystem diversity, and topography × latitude models, which factor out the effects of other independent variables, clearly reflected the high degree of intercorrelation among subsets of potential determinants of species richness (Table 2).
Two patterns were notable. First, the predictive power of the ecosystem diversity model decreased to negligible levels when influence of other variables was removed. A second more interesting finding was that the predictive power of the topography × latitude model was scale-dependent. Whereas climatic variables influenced species richness at a wide range of spatial scales, the importance of topography increased dramatically at coarser scales.
This pattern casts new light on the debate among advocates of the species richness-energy hypothesis (4, 5, 13, 44, 45) and those that stress the roles of history, biome area, and the large-scale steady state between allopatric speciation and extinction as determinants of regional variation in species richness (8, 33–35, 46, 47). The contrasting pattern of partial correlation values is consistent with the idea that climate is an important determinant of species richness at local and regional scales. It further suggests that the historical interaction between climate and topography, which is instrumental in generating the species pool from which local assemblages of species are drawn, becomes increasingly more important at coarser spatial scales.
Emergent Biogeographic Patterns.
The 1° map provides the most accurate illustration of species richness patterns yet produced for the immensely diverse South American avifauna (Figs. 1 and 2). Previous mapping efforts indicated that avian species richness at specific but widely scattered localities in lowland Amazonian forest (48, 49) increased westward as one approached the Andes. Our data generally confirm those observations, acknowledging the caveats of species list comparison (50). More importantly, our maps bring into focus a number of significant macroecological patterns that heretofore were unknown or poorly documented. Most notably, a conspicuous trough in species richness (≈320–420 species/1° quadrats) extends for ≈2,200 km across the central Amazon basin before hooking northward into the Llanos of the Rio Orinoco drainage. Although the number of avian species recorded from quadrats in this zone undoubtedly will be augmented with additional field research (e.g., ref. 51), the distribution of sampling localities (49, 52) suggests that the diversity trough is real rather than an artifact of uneven sampling. Species richness gradients on the periphery of the “trough” appear irregular, or even lumpy, south of the equator with significant richness peaks in lowland forest in the Rio Tapajos region and on the frontier between Bolivia and the Mato Grosso. North of the equator, species richness increases markedly in the Pantepui uplift in Venezuela and the Guianas, whereas the lowland forests to the west, along the headwater tributaries of the upper Amazon, are widely believed to support the richest floral and faunal assemblages in the world (53–56). As an exemplar, avian species richness at several lowland sites in eastern Ecuador and Peru exceeds 500 species (49).
In our study, 1° quadrats that exhibited the highest avian
diversity (>650 species) were restricted to the Andean arc,
particularly along the Amazonian versant of Ecuador (peaking at 845
species) and in southeastern Peru (peaking at 782 species) and southern
Bolivia (peaking at 698 species). These quadrats (n =
19) were physiographically complex (topographic relief,
1,700–5,700 m, 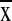 = 3, 897
± 1,228 m) and characterized by moderate precipitation (1,058–3,096
mm/yr−1,
= 3, 897
± 1,228 m) and characterized by moderate precipitation (1,058–3,096
mm/yr−1, 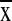 =
1,935 ± 655 mm) and maximum daily temperatures (16.9–25.3°C,
=
1,935 ± 655 mm) and maximum daily temperatures (16.9–25.3°C,
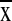 = 21.4 ± 2.3°).
= 21.4 ± 2.3°).
Comparable Data from the Central Amazonian Trough in Species Richness Are Illuminating.
Species richness values for 1° quadrats (n = 20),
sampled from a 2°-wide longitudinal band straddling the equator from
55° to 65° W, ranged from 331 to 383
(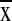 = 353 ± 14.4). Precipitation
values (1,869–2,607 mm/yr−1,
= 353 ± 14.4). Precipitation
values (1,869–2,607 mm/yr−1,
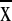 = 2133 ± 251 mm) were similar
to those of high-diversity quadrats along the Andean arc but
topographic relief was conspicuously lower (200–400 m,
= 2133 ± 251 mm) were similar
to those of high-diversity quadrats along the Andean arc but
topographic relief was conspicuously lower (200–400 m,
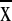 = 260 ± 60 m), whereas
maximum daily temperature (25.2–26.4°C,
= 260 ± 60 m), whereas
maximum daily temperature (25.2–26.4°C,
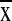 = 26.0 ± 0.3°) was
significantly higher.
= 26.0 ± 0.3°) was
significantly higher.
The upshot of this simple contrast was that neither area nor energy alone is sufficient to explain patterns of avian species richness in South America. The trough in β-diversity traverses the heart of the tropical moist forest biome (≈5 million km2), by far the largest in South America (57), at equatorial latitudes receiving maximal solar energy. If energy and biome area were the primary determinants of species richness, then continental peaks of species richness would occur in central Amazonia, which clearly is not the case.
On the other hand, species richness in neotropical birds seems to be
linked directly to habitat diversity, which in turn is correlated with
topographic heterogeneity. The number of ecosystems occurring in 1°
quadrats was correlated significantly
(r2 = 0.13, P <
0.0001, n = 1,080) with topographic relief at tropical
latitudes (<20°). Quadrats in the species-poor zone in central
Amazonia overlapped 5–16 distinctive ecosystems
(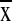 = 9.9 ± 3.7,
n = 20), whereas species-rich quadrats (>650 species)
overlapped 16–24 ecosystems (
= 9.9 ± 3.7,
n = 20), whereas species-rich quadrats (>650 species)
overlapped 16–24 ecosystems (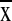 =
20.2 ± 2.3, n = 19). Most telling, quadrats
along the Andean arc supported ≈30–250% more species than
quadrats at equivalent latitudes in central Amazonia.
=
20.2 ± 2.3, n = 19). Most telling, quadrats
along the Andean arc supported ≈30–250% more species than
quadrats at equivalent latitudes in central Amazonia.
In conclusion, the extraordinary abundance of species associated with humid montane regions at equatorial latitudes reflects the overwhelming influence of orography (58, 59) and climate on the generation and maintenance of regional species richness. In a broader context, our data reinforce the hypothesis that terrestrial species richness from the equator to the poles ultimately is governed by a synergism between climate and coarse-scale topographic heterogeneity.
Acknowledgments
We thank F. Skov for his Geographic Information Systems skills and for compiling the climatic and ecosystem data in a form suitable for analysis. P. Williams kindly provided the worldmap software used to manage the distributional data. Primary distributional data were derived from the collections of the Academy of Natural Sciences (Philadelphia); American Museum of Natural History (New York); Carnegie Museum of Natural History (Pittsburgh); Colección Ornitológica Phelps (Caracas, Venezuela); Delaware Museum of Natural History; Field Museum of Natural History (Chicago); L'Institute Royal des Sciences Naturalles (Brussels); Louisiana State University Museum of Natural Sciences, Moore Laboratory of Zoology (Los Angeles); Museo Argentino de Ciencias Naturales (Buenos Aires); Museo de Historia Natural “Javier Prado” de la Universidad Nacional Mayor de San Marcos (Lima, Peru); Museo de Historia Natural Universidad de Cauca (Popayán, Colombia); Museo Ecuatoriano de Ciencias Naturales (Quito, Ecuador); Museo Nacional de Ciencias Naturales (Bogotá, Colombia); Museo Nacional de Historia Natural (La Paz, Bolivia); Museo Nacional de Historia Natural (Santiago, Chile); Museu de Zoologia da Universidaded de São Paulo (Brazil); Museu Nacional (Rio de Janeiro, Brazil); Museu Paraense Emílio Goeldi (Belém, Brazil); Museum Alexander Humboldt (Berlin); Museum Alexander Köenig (Bonn); Museum of Comparative Zoology, Harvard University (Cambridge, MA); Museum of Natural History of Los Angeles County; Muséum d'Historie Naturelle (Neuchatel, Switzerland); Muséum National d'Histoire Naturelle (Paris); National Museum of Natural History (Washington, DC); Natural History Museum of Gothenburgh, Rijksmuseum van Natuurlijke Historie (Leiden, The Netherlands); Royal Ontario Museum (Toronto); Swedish Museum of Natural History (Stockholm); The Natural History Museum (London and Tring, United Kingdom); Western Foundation of Vertebrate Zoology (Los Angeles); and the Zoological Museum, University of Copenhagen (Denmark). The Smithsonian Research Opportunities Fund and the Danish Natural Science Research Council Grant 11–0390 supported travel to Copenhagen for G.R.G. and travel to Washington, DC, for C.R., respectively.
References
- 1.Bates H W. Trans Linn Soc London. 1862;23:495–566. [Google Scholar]
- 2.Wallace A R. Tropical Nature and Other Essays. London: Macmillan; 1878. [Google Scholar]
- 3.Pianka E R. Am Nat. 1966;100:33–46. [Google Scholar]
- 4.Currie D J. Am Nat. 1991;137:27–49. [Google Scholar]
- 5.Rohde K. Oikos. 1992;65:514–527. [Google Scholar]
- 6.Rohde K. Ecography. 1999;22:593–613. [Google Scholar]
- 7.Brown J H. Macroecology. Chicago: Univ. of Chicago Press; 1995. [Google Scholar]
- 8.Rosenzweig M L. Species Diversity in Space and Time. New York: Cambridge Univ. Press; 1995. [Google Scholar]
- 9.Rosenzweig M L, Ziv Y. Ecography. 2000;22:614–628. [Google Scholar]
- 10.Gotelli N J, Graves G R. Null Models in Ecology. Washington, DC: Smithsonian Institution Press; 1996. [Google Scholar]
- 11.Colwell R K, Lees D C. Trends Ecol Evol. 2000;15:70–76. doi: 10.1016/s0169-5347(99)01767-x. [DOI] [PubMed] [Google Scholar]
- 12.Palmer M W. Folia Geobot Phytotaxon. 1994;29:511–530. [Google Scholar]
- 13.Wright D H, Currie D J, Maurer B A. In: Species Diversity in Ecological Communities: Historical and Geographical Perspectives. Ricklefs R E, Schluter D, editors. Chicago: Univ. of Chicago Press; 1993. pp. 66–74. [Google Scholar]
- 14.Kerr J T, Packer L. Nature (London) 1997;385:252–254. [Google Scholar]
- 15.O'Brien E M, Field R, Whittaker R J. Oikos. 2000;89:588–600. [Google Scholar]
- 16.Rahbek C, Graves G R. Proc R Soc London Ser B. 2000;267:2259–2265. doi: 10.1098/rspb.2000.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colwell R K, Hurtt G C. Am Nat. 1994;144:570–595. [Google Scholar]
- 18.Rahbek C. Am Nat. 1997;149:875–902. doi: 10.1086/286028. [DOI] [PubMed] [Google Scholar]
- 19.Pineda J, Caswell H. Deep-Sea Res II. 1998;45:83–101. [Google Scholar]
- 20.Willig M R, Lyons S K. Oikos. 1998;81:93–98. [Google Scholar]
- 21.Lees D C, Kremen C, Andriamampianina L. Biol J Linn Soc. 1999;67:529–584. [Google Scholar]
- 22.Veech J A. Ecology. 2000;81:1143–1149. [Google Scholar]
- 23.Brown J H. J Mammal. 1999;80:333–344. [Google Scholar]
- 24.Sibley C G, Monroe B L., Jr . Distribution and Taxonomy of Birds of the World. New Haven, CT: Yale Univ. Press; 1990. [Google Scholar]
- 25.Fjeldså J, Rahbek C. In: Tropical Forest Remnants: Ecology, Management and Conservation of Fragmented Communities. Laurance W F, Bierregaard R O, editors. Chicago: Univ. of Chicago Press; 1997. pp. 466–482. [Google Scholar]
- 26.Fjeldså J, Rahbek C. In: Conservation in a Changing World. Mace G M, Balmford A, Ginsberg J R, editors. Cambridge, U.K.: Cambridge Univ. Press; 1998. pp. pp.139–160. [Google Scholar]
- 27.Williams, P. H. (1998) Worldmap iv Windows: Software and Help Document (Privately distributed, London), Version 4.1.
- 28.Rosenzweig M L, Abramsky Z. In: Species Diversity in Ecological Communities: Historical and Geographical Perspectives. Ricklefs R E, Schluter D, editors. Chicago: Univ. of Chicago Press; 1993. pp. 52–65. [Google Scholar]
- 29.Francis A P, Currie D J. Oikos. 1998;81:598–602. [Google Scholar]
- 30.Currie D J, Francis A P. Ecoscience. 1999;6:392–399. [Google Scholar]
- 31.Kerr J T, Vincent R, Currie D J. Ecoscience. 1998;5:448–453. [Google Scholar]
- 32.New M, Hulme M, Jones P. J Clim. 1999;12:829–856. [Google Scholar]
- 33.Latham R E, Ricklefs R E. Oikos. 1993;67:325–333. [Google Scholar]
- 34.McGlone M S. Global Ecol Biogeogr Lett. 1996;5:309–314. [Google Scholar]
- 35.Ricklefs R E, Latham R E, Qian H. Oikos. 1999;86:369–373. [Google Scholar]
- 36.Connor E F, McCoy E D. Am Nat. 1979;113:791–833. [Google Scholar]
- 37.Palmer M W, White P S. Am Nat. 1994;144:717–740. [Google Scholar]
- 38.Rahbek C. Ecography. 1995;18:200–205. [Google Scholar]
- 39.Pastor J, Downing A, Erickson H E. Oikos. 1996;77:399–406. [Google Scholar]
- 40.Lyons S K, Willig M R. Ecology. 1999;80:2483–2491. [Google Scholar]
- 41.Olsen J S. Global Ecosystem Framework-Definitions. Sioux Falls, IA: U.S. Geological Society Earth Resources Observation Satellite Data Center Internal Report; 1994. [Google Scholar]
- 42.U.S. Defense Mapping Agency Aerospace Center. Operational Navigation Charts. St. Louis; 1980–1994. [Google Scholar]
- 43.Olsen L M, Bliss N B. Environmental Systems Research Institute User Conference Proceedings. Inc., Redlands, CA: Environmental Systems Research Institute; 1997. , CD-ROM. [Google Scholar]
- 44.Rohde K. Oikos. 1997;79:169–172. [Google Scholar]
- 45.Rohde K. Oikos. 1998;82:184–190. [Google Scholar]
- 46.Rosenzweig M L, Sandlin E A. Oikos. 1997;80:172–176. [Google Scholar]
- 47.Terborgh J. Am Nat. 1973;107:481–501. [Google Scholar]
- 48.Vuilleumier F. Biota Bull. 1988;1:5–32. [Google Scholar]
- 49.Haffer J. Stud Neotrop Fauna Environ. 1990;25:157–183. [Google Scholar]
- 50.Remsen J V. Auk. 1994;111:225–227. [Google Scholar]
- 51.Cohn-Haft M, Whittaker A, Stouffer P C. Ornithol Monogr. 1997;48:205–235. [Google Scholar]
- 52.Haffer J. Nuttall Ornithol Club. 1974;14:1–390. [Google Scholar]
- 53.Dixon J. Mus Nat Hist Univ Kansas Monogr. 1979;7:217–240. [Google Scholar]
- 54.Duellman W E. Ann Missouri Bot Gardens. 1988;75:79–104. [Google Scholar]
- 55.Gentry A H. Proc Natl Acad Sci USA. 1988;85:156–159. doi: 10.1073/pnas.85.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Terborgh J, Robinson S K, Parker T A, III, Munn C A, Pierpont N. Ecol Monogr. 1990;60:213–238. [Google Scholar]
- 57.Dinerstein E, Olson D M, Graham D J, Webster A L, Primm S A, Bookbinder M P, Ledec G. A Conservation Assessment of the Terrestrial Ecoregions of Latin America and the Caribbean. Washington, DC: The World Bank in association with The World Wildlife Fund; 1995. [Google Scholar]
- 58.Graves G R. Auk. 1985;102:556–579. [Google Scholar]
- 59.Graves G R. Auk. 1988;105:47–52. [Google Scholar]


