Abstract
Polycomb group proteins have long been linked to the occurrence of different forms of cancer. Polycomb proteins form at least two distinct complexes, the Polycomb-repressive complexes 1 and 2 (PRC1 and PRC2). Some of the PRC complex subunits have been found to be overexpressed in a variety of different tumors. Epigenetic perturbations are likely to be the cause for transcriptional misregulation of tumor suppressor genes and of certain cell fates. It is especially critical for stem cells that their potential to self-renewal and to differentiate is tightly controlled and properly orchestrated. Misregulation of Polycomb protein levels often leads to either a block or unscheduled activation of developmental pathways, thereby enhancing the proliferation capability of a cell. The consequences of this misregulation have been linked to the establishment of cancer stem cells, which can produce tumors through a combination of increased self-renewal and the lack of complete cellular differentiation. Cancer stem cells are believed to persist within tumors and to elicit relapse and metastasis. In this review, we recapitulate the roles of Polycomb proteins in stem cell biology, and the impact their misregulation can have on cancer.
Keywords: Polycomb group proteins, embryonic stem cells, cancer stem cells
Mechanisms of Gene Regulation by Polycomb and Trithorax Complexes
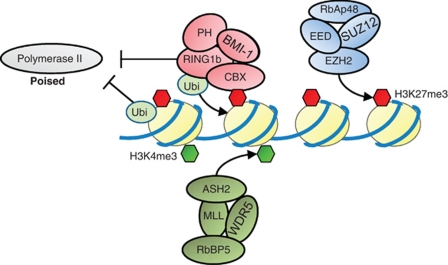
Polycomb proteins were initially identified in Drosophila melanogaster as genes important for developmental pathways. Mutations in Polycomb proteins cause developmental defects due to misregulation of specific transcriptional cascades that involve key transcription factors, such as the Homeobox protein; this leads to typical homeotic phenotypes.1, 2 Recent research has expanded our understanding of the mechanistic basis of Polycomb functions, explaining, for example, how such homeotic phenotypes occur.3, 4 However, many aspects of the molecular mechanism are still elusive. In flies, the Polycomb complexes that are recruited to chromatin at the Polycomb responsive elements work to silence genes, whereas proteins of the Trithorax family counteract this to activate the same genes.2 It is still unclear how Polycomb proteins are recruited to chromatin in mammalian cells. However, the epigenetic mechanisms of gene silencing by Polycomb-repressive complexes (PRCs) is conserved in eukaryotes (Figure 1).4, 5 The two Polycomb complexes, PRC1 and PRC2, have been characterized in depth. It has been demonstrated that the two complexes can silence genes either synergistically or independently of each other.6, 7 However, existing evidence indicates that PRC2 is involved in recruiting the PRC1 complex to promoters of their common target genes.8, 9 In mammals, PRC2 consists of at least four subunits, one of which is EZH2, a histone methyltransferase (HMT) that catalyzes the trimethylation of the histone H3 at lysine 27 (H3K27me3) via its SET domain.10 The trimethyl modification at H3K27 is a hallmark for gene repression and is usually found in the promoter regions of developmental genes.11 It has been demonstrated that this modification is the docking site for proteins harboring a chromobox (CBX) domain. The CBX protein family can function as subunits of the PRC1 complex, and it is believed that the CBX proteins help to recruit the PRC1 complex to chromatin through their interactions with H3K27me3 modifications set by PR2.12 PRC1 catalyzes the subsequent monoubiquitylation of lysine 119 at histone H2A (H2AK119ub) by the enzymatic action of the two ring domain-containing proteins, RING1B and Bmi-1.13, 14 Although this hierarchical model of Polycomb recruitment to genes seems to apply in many cases, it was recently challenged by the finding that PRC1 and PRC2 are not always located at the same genomic loci.7
Figure 1.
Epigenetic modifications at bivalent genes. Bivalent genes are earmarked by activating and repressive epigenetic marks. MLL complexes decorate chromatin with an activating H3K4me3 mark. The consecutive action of PRC2 and PRC1 complexes leads to disposition of repressive marks at chromatin. PRC2 carries out the specific methylation (H3K27me3), which is thought to recruit PRC1 via proteins of the CBX family. PRC1 complexes subsequently transfer a monoubiquitin residue to histone H2A (H2AK119). Both, PRC1 and monoubiquitinated histone H2A (H2Aub) lock chromatin in a silenced state and force Pol II to remain in a paused state
The monoubiquitin mark represents one of the most abundant epigenetic modifications and decorates about 10% of endogenous H2A proteins. The original report that ubiquitinated H2A protein helps to maintain gene silencing has been controversial, as this mark was also found in transcribed chromatin regions of D. melanogaster.15 A recent study has now demonstrated that chromatin condensation is not dependent on the monoubiquitin mark per se, but rather on PRC1 itself,16 supporting previous in vitro results that revealed that PRC1 helps to compact chromatin arrays.17 Indeed, we have recently found that the ubiquitin residue has a functional role as a platform for transcriptional activation.18
PRC1 also seems to block the transcriptional elongation of polymerase II (Pol II) at so-called bivalent genes, which are decorated with both repressive and activating trimethyl marks (H3K27me3 and H3K4me3, respectively; Figure 1).19 At these bivalent genes, RNA Pol II (phosphorylated in its C-terminal domain (CTD) at serine 5) is paused within the gene body, poised for activation upon differentiation stimuli. Pol II pausing most likely is due to the occupancy of PRC1 and the incorporation of the monoubiquitin mark at these loci;20 however, the exact molecular mechanism of this still needs to be elucidated.
In addition to these rather direct actions of the Polycomb complexes, another layer of epigenetic silencing is brought about by their interaction with the DNA methylation system. Methylation of DNA at cytidine residues within CPG islands is one of the most well-characterized mechanisms of gene silencing.21 The PRC2 subunit EZH2 was demonstrated to interact with DNA methyltransferases (DNMTs). Furthermore, PRC2 and DNMTs have been shown to be recruited to chromatin by the oncogenic fusion protein PML-RARa, which originates from a genetic translocation that causes acute leukemia.22, 23 Likewise, Bmi-1 (within the PRC1 complex) is recruited by the transcription factor promyelocytic leukemia zinc finger (PLZF), a member of the BTB/POZ-ZF family of transcription factors.24 In leukemia, the oncogenic fusion-protein PLZF-RARa causes aberrant recruitment of PRC1.25 Thus, Polycomb complexes are often recruited by transcription factors that regulate cell fate and thereby lock genes into a silenced state, either directly or indirectly by further recruiting DNMTs.
Polycomb targets include tumor suppressor genes, which function to keep uncontrolled proliferation at bay. Polycomb-mediated silencing of those genes prevents the cell from undergoing senescence, which might ultimately lead to the occurrence of cancer. Indeed, Polycomb complexes silence the CDKN2A and CDKN2B loci, which encode the tumor suppressors p14 (ARF), p15 (INK4B) and p16 (INK4A).26, 27, 28 Overexpression of Polycomb proteins leads to transcriptional repression of those loci, thereby inhibiting senescence and enhancing proliferation. The PRC2 subunit EZH2 has also been reported to have a pivotal role in apoptosis. EZH2 represses the expression of the DAB2-interacting protein, and thus, prohibits apoptosis meditated by the tumor necrosis factor.29 More recently, it was demonstrated that EZH2 directly regulates the apoptotic process in cancer cells by repressing the E2F1 target Bim.30 Given the involvement of Polycomb proteins in senescence and apoptosis, it is understandable that their misregulation is strongly related to cancer.
Proteins that counteract Polycomb function are also often implicated in cancer. A broad body of work has demonstrated that the trithorax group (TrxG) proteins counteract the function of Polycomb proteins and thereby activate genes.2, 31 TrxG proteins form multi-protein complexes that, like the Polycomb proteins, deposit histone marks that are linked to transcriptional activation. TrxG complexes usually comprise of HMTs that contain SET-domains, which methylate lysine 4 of histone 3 (H3K4me3), an activating chromatin mark.2 In sum, misregulation of members of the Polycomb and TrxG families, and of proteins that either interact with them or have an effect on their occupancy at chromatin, usually leads to a cancer phenotype.
Polycomb Group Complexes and their Role in Embryonic Stem (ES) Cells
In the last few years, ES cells have emerged as an excellent model system for elucidating the mechanisms governing cell fate transition during mammalian development. ES cells are derived from the inner cell mass of the pre-implantation embryo at the stage of blastocyst (3.5 days post coitum (dpc)). A hallmark of ES cells is their pluripotency, which bestows them with the ability to give rise to all cell types of an embryo except the trophoectoderm.32, 33 When reintroduced into host blastocysts, ES cells contribute to generate all cell types, including germ cells, and give rise to chimeras. In ES cells, pluripotency is maintained by the prevention of differentiation and the promotion of proliferation. Thus, ES cells can self-renew continuously in vitro if they are cultured under the proper conditions. Differentiation of ES cells in vitro recapitulates the same hierarchical steps of embryo development that occurs in vivo. During development, lineage-specific transcription factors are regulated by epigenetic events that impose either a permissive or a repressive environment over cell fate.
PRC1 and PRC2 are key factors in the epigenetic regulation of developmental loci, impeding the transcription of these through repressive marks.11, 34 Still, in most of the PRC target loci in ES cells, the activating H3K4me3 mark is also present. As mentioned above, according to the current model, PRC1 and PRC2 colocalize to these so-called bivalent domains, regulating the expression of many important developmental genes.35, 36 Interestingly, bivalent domains have recently been identified that are exclusively bound by PRC2.7 These PRC2-containing bivalent domains are only weakly conserved and, in contrast to domains decorated by both PRC1 and PRC2, correspond to genes encoding for membrane proteins or unknown functions. The peculiar chromatin signature of bivalent domains renders the respective developmental genes poised for activation, so that induction of transcription upon differentiation stimuli can occur extremely rapidly.37 This ability to respond rapidly is supported by the RNA Pol II that is phosphorylated at serine 5 of its CTD, which is enriched at many PRC1/PRC2 targets in ES cells.37 Bivalent domains are present in embryos beginning at the eighth cell stage and last until the blastocyst stage, at which point the cells separate into two different populations, the inner cells, from which the ES cells arise, and the extra-embryonic layer.38 A subset of genes in extra-embryonic cells still retain the bivalent domains, but these loci no longer have PRC1 and RNA Pol II recruited to them, although PRC2 is still present. A key role in the extra-embryonic cells is now had by Suv39h1, which directly targets the bivalent genes and catalyzes the specific trimethylation of lysine 9 in histone H3 (H3K9me3). Thus, PRC1 and Suv39h1 are mutually exclusive in specifying the fate of the bivalent domains during blastocyst formation.
The bivalent domains tend to disappear upon further ES cell differentiation. However, depending on the expression of the gene in the particular cell context, the activating mark H3K4me3 or the repressive H3K27me3 can be maintained in the loci.39, 40 For instance, most of the bivalent domains present in ES cells resolve upon differentiation to neural stem cells (NSCs). The H3K4me3 mark remains only at the promoters of genes that are transcriptionally upregulated during neurogenesis, whereas marker genes of other lineages display increased levels of H3K27me3.41 On the other hand, Mohn et al.42 suggest that only a subset of Polycomb targets is determined in ES cells and that, upon cell fate commitment and establishment of progenitor cells, certain genes acquire bivalent domains. According to this study, the overall number of bivalent domains does not seem to change between ES cells and neural progenitors, as loss during differentiation is largely compensated by newly formed bivalent domains in differentiated cells at different states. This observation supports the fact that bivalent domains are not a unique feature of ES cells, but are also found in others stem cells, such as mesenchymal stem cells and hematopoietic stem and progenitor cells.39, 43 Taken together, these data underline the important role of the bivalent domains, and therefore of PRC1 and PRC2, in maintaining stem cell characteristics. Along these lines, Vastenhouw et al.44 provided evidence that bivalent domains are present at a subset of genes in early embryos of zebrafish, demonstrating that the function of these domains is evolutionary conserved.
Role of PRC2 in Embryonic Stem (ES) Cells
PRC2 is strongly involved in the early steps of embryogenesis, and both EZH2 and EED knockout mice display embryonic lethality soon after implantation (Table 1).45, 46, 47 However, PRC2 is not necessary for maintaining ES cell self-renewal, as each of the PRC2 components can be deleted without compromising the expression levels of pluripotent markers, such as Oct4 and Nanog.48, 49 As mentioned above, PRC2 is involved in the expression of developmental genes, and ES cells lacking Suz12, EED or EZH2 show aberrant de-repression of lineage-specific genes and are unable to properly differentiate. This is partially also due the lack of repression of pluripotent genes during differentiation steps. Accordingly, EED-knockout ES cells are able to give rise to chimeras, confirming that they are pluripotent in vivo. However, despite the robust occurrence of EED-depleted cells in chimeric embryos at 9.5 dpc, they are scarce at 12.5 dpc due to lethality of differentiated cells, as shown by Chamberlain et al.49
Table 1. Molecular and biological functions of PRC1 and PRC2 components.
| Human Polycomb protein | Function | Stem cell/cancer phenotype |
|---|---|---|
| PRC1 | ||
| RING1a, RING1b | Monoubiquitylation of histone H2A | RING1B KO ESCs show aberrant expression of differentiation markers and are unable to properly differentiate |
| CBX2, CBX4, CBX7, CBX8 | Binding of tri-methylated Lysines (H3K27me3) | Unknown |
| BMI1, MEL18, MBLR, NSPC1 | Unknown | Bmi1 depletion impairs neural, hematopoietic and prostate stem cell self-renewal Overexpressed in many types of cancer |
| PHC1, PHC2, PHC3 | Unknown | Unknown |
|
Human Polycomb protein |
Function |
Stem cell Phenotype |
| PRC2 | ||
| EZH2, EZH1 | Methylation of histone H3 (H3K27me3) | EZH2 KO ESCs show aberrant expression of differentiation markers and are unable to properly differentiate Overexpressed in many types of cancer |
| SUZ12 | Unknown | SUZ12 KO ESCs are unable to properly differentiate |
| EED | Binding of tri-methylated Lysines (H3K27me3 | EED KO ESCs are unable to properly differentiate |
| RBBP4, RBBP7 | Binding of Histone proteins | |
| PCL1, PCL2, PCL3, JARID2 | Unknown | PCL2 KO ESCs displays higher levels of pluripotency markers and are unable to properly differentiate Jarid2 KO ESCs are unable to properly differentiate |
In addition to the core subunits of PRC2, other PRC2-associated proteins affect the enzymatic activity of the core complex, at least in ES cells. Jarid2 is a member of the Jumonji proteins, which constitute a family of histone demethylases, although Jarid2 lacks the enzymatic activity. It was recently reported that Jarid2 associates with PRC2, and that it is required for proper embryo development.50 In contrast to EED knockout ES cells, Jarid2 knockout ES cells do not result in the de-repression of most of the PRC2 target genes, suggesting that Jarid2 is not essential in PRC2-mediated gene repression in ES cells. Yet Jarid2 knockout ES cells are unable to properly differentiate, probably because the recruitment of PRC1 and RNA polymerase to bivalent domains is strongly reduced.51
In D. melanogaster, the family of Polycomb-like (PCL) proteins forms a subset of PRC2 complexes. Of the three mammalian orthologs (PHF1, PHF19 and PCL2), PCL2 has been investigated in the context of stem cells. PCL2 knockdown in ES cells displays higher levels of Oct4 and Nanog, indicating that it is involved in the control of self-renewal.52, 53, 54 The increase of these pluripotent markers seems to be a direct consequence of PCL2-dependent transcriptional silencing of Tbx3 and Klf4, which are key factors involved in the maintenance of ES cells in an undifferentiated state, and in reprogramming somatic cells into induced pluripotent stem cells.55, 56 In addition, PCL2 knockdown cells are not able to properly differentiate, as they also maintain high levels of Oct4 and Nanog during neuroectodermal differentiation and show abnormalities in embryoid body formation.
Role of PRC1 in Stem Cell Self-renewal and Differentiation
Similar to the knockout of PRC2 proteins, knockout of the E3 ligase Ring1B causes gastrulation arrest and results in embryonic lethality,57 demonstrating that both PRC complexes are essential for development (Table 1). ES cells devoid of Ring1B are capable of maintaining the proper expression of some pluripotency markers, such as Oct4 and Nanog.58 Analogous to EED knockout ES cells, Ring1B knockout ES cells display a derepression of the PRC1-target genes and are impaired in proper differentiation.59 Furthermore, Ring1B knockout mice display the most severe phenotype among the members of PRC1.
Mice deficient for Mel18 or Bmi-1 exhibit similar posterior transformations of the axial skeleton and display severe immune deficiency.60, 61, 62 This result supports the hypothesis that Mel18 and Bmi1 can act redundantly.63 However, this interpretation is in part challenged by the unique phenotypes observed in either Mel18 or Bmi-1 knockout mice, which is suggestive of different functions for Mel18 and Bmi-1. For instance, abnormalities in the cerebellum were observed only in Bmi-1 knockout mice, whereas intestinal obstruction due to hypertrophy of the smooth muscle layer was observed only in Mel-18 mutants.
The integrity of the PRC1 complex appears to be critical for stem cell maintenance. For instance, embryonic neural stem cells require Ring1B for maintaining proper undifferentiated features, as reported by Román-Trufero et al.64 Indeed, the Ring1B knockout NSCs had an upregulation of several differentiation markers and of the cell proliferation inhibitor p21. Likewise, Bmi1 was demonstrated to control p21 expression in embryonic NSCs.65 Moreover, Bmi1 is also important for self-renewal of post-natal NSCs, mediating the suppression of the Ink4/Arf cell cycle inhibitor proteins, p16 and p19.66 NSCs that overexpress Bmi1 show an increase in their proliferation and self-renewal potential without eliciting a neoplastic transformation.67 Bmi1 is important not only for NSCs, but also for a vast number of different stem cell lineages. Bmi1 is essential for multipotency of hematopoietic stem cells and multipotent progenitors, as it controls the B-cell lineage developmental regulators of Ebf1 and Pax5. Bmi1 depletion leads to an accelerated lymphoid specification and to a marked reduction in the stem cell compartment.68 Additionally, in prostate stem cells, Bmi1 is involved in the control of the self-renewal of p63-positive stem cells.69
Redundancy of the PRC Complex in ES Cells
The functional relevance of the PRC complex in ES cells has been highlighted by the fact that neither EED nor Ring1B knockout ES cells able to reprogram human B lymphocytes into pluripotent cells, suggesting that PRC complexes are necessary not only to maintain, but also to establish proper ES cell pluripotency.70 However, the mechanism of action of PRC complexes has not been fully elucidated. According to the proposed model of PRC action at chromatin, both complexes – and thus both epigenetic modifications – should decorate the nucleosomes of developmental target genes. This model has been challenged by Leebet al.,6 who suggest that the two complexes are, at least in part, independent and act redundantly. In Ring1B/EED double-knockout ES cells, the number of de-repressed genes, which are direct PRC targets, increased about two times, compared with the single EED or Ring1B knockout. Half of these genes were de-repressed only after the loss of both PRC1 and PRC2, and remained repressed in each single knockout, suggesting that PRC1 and PRC2 are redundant for repression of these genes. This observation is buttressed by the ‘rescue' of gene repression by inducing EED expression in the double knockout ES cells. Additionally, PRC1 is recruited to these redundantly silenced genes, albeit at low (but still detectable) levels, independently of PRC2, providing a further challenge to the current model of hierarchical PRC1 recruitment. This mechanism is gene specific, as some other PRC targets do not need the loss of both PRC1 and PRC2 complex, but are already de-repressed in the absence of either PRC1 or PRC2 alone.
Finally, the ability to differentiate is compromised in Ring1B and EED: the single knockout ES cells form teratomas much smaller than the control cells, but the capability of forming teratomas is completely abolished by the double knockout, supporting the functional redundancy of the two complexes.6
Polycomb Proteins and Cancer
Studying how Polycomb protein misregulation leads to a variety of different tumors has revealed in the last years the oncogenic potential of Bmi-1 (in PRC1) and EZH2 (in PRC2).71 Both proteins are often overexpressed in cancers. In bladder tumors, EZH2 displays increased expression levels,72 which directly correlate with the invasive potential of the carcinomas. Similar findings were obtained for prostate and breast cancers,73, 74 and deregulation of EZH2 was also directly linked to the emergence of colorectal cancer and oral squamous carcinomas.75, 76 Increased Bmi-1 protein levels are found in very different forms of cancer, which suggest that Bmi-1 has an important role in PRC1 action in addition to supporting the Ring1B-catalyzed enzymatic action at chromatin. Bmi-1 protein levels are elevated in squamous cell carcinomas,77 neuroblastomas58 and bladder tumors,78 and several studies describe its deregulation in leukemia.79 Furthermore, there is evidence for an increased co-expression of proteins from both Polycomb complexes in several types of tumors.80, 81 A plethora of publications links Polycomb proteins to cancer and, more recently, to the occurrence of cancer stem cells. Additionally, PRC complexes are important during embryogenesis, as has been demonstrated by the early embryonic lethality of Ring1b and EZH2 knockout mouse models.
There is increasing evidence that PRC complexes have a role in tumor progression and development by blocking differentiation and promoting stem cell self-renewal. A recent study demonstrated that enhanced EZH2 levels led to growth of Ewing tumors and a consequential inhibition of endothelial and neuroectodermal differentiation.82 Similarly, Bmi-1 seems to have a pivotal role in lung cancerogenesis. K-ras-initiated lung tumorigenesis was drastically compromised in Bmi-1 null mice, as loss of Bmi-1 caused a reduced self-renewal capacity and limited the expansion of cancer stem cells.83 In glioblastomas, both Bmi-1 and EZH2 have divisive roles: knockdown of EZH2 protein levels diminished the self-renewal ability and the tumor-initiating capacity of cancer stem cells,84 whereas a microRNA-mediated downregulation of Bmi-1 levels in glioblastomas inhibited the proliferation and reduced self-renewal of the glioma stem cells.85 In contrast to these findings, knockdowns of EZH2 and Bmi-1 did not inhibit osteosarcoma growth.86
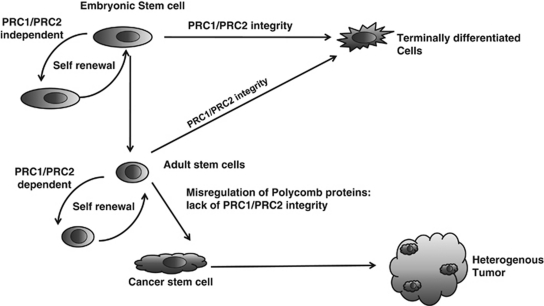
Regulation by the PRC complexes of pathways implicated in the emergence of cancer stem cells, such as the Hedgehog, Wnt, and Notch pathways,87, 88 has important implications. The Wnt protein was demonstrated to be a major factor in colorectal cancers,88 whereas Hedgehog was recently implicated in maintaining cancer stem cells in leukemia.89 Stem cells fine-tune their expression profiles, likely through bivalent domains, and a lack of Polycomb components leads to the aberrant upregulation of these mostly developmental loci. Therefore, to guarantee stem cell renewal and pluripotency, PRC proteins are essential. Cancer stem cells are believed to be a subpopulation of cells with self-renewal abilities, which reiterate the processes common to normal stem cell self-renewal. In fact, some tumors contain embryonic-like cells that likely originate from stem cells; this would explain the occurrence of heterogeneous tumors (Figure 2). Carcinomas that arise from stem cells, and accordingly show gene expression profiles similar to stem cells, appear to be quite aggressive and are prone to survive the cancer treatment.
Figure 2.
Role of Polycomb proteins in the emergence of cancer stem cells. ES cells undergo asymmetric cell divisions that give rise to daughter cells (self-renewal) and progenitor cells that ultimately differentiate into a distinct cell type. Integrity of Polycomb complexes is required for proper ES and adult stem cell differentiation. In addition, PRC1 and PRC2 are required for the self-renewal of adult, but not embryonic, stem cells. Misregulation of the levels of the Polycomb group proteins can lead to the formation of cancer stem cells, which maintain the ability to self-renew and undergo uncontrolled differentiation, leading to the generation of heterogenous tumors
It has thus become increasingly clear that epigenetic regulation has a critical role in determining stem cells fates. Elucidating the mechanisms by which the PcG and TrxG proteins act and interact to fine-tune the regulation of cell fate-specific genes is highly important for understanding this. As these regulatory pathways are often impeded on in cancer stem cells, this knowledge could offer us the possibility to directly target misregulated pathways with specific drugs.
Acknowledgments
We thank Luis Morey for discussions and VA Raker for help in preparing the manuscript. This work was supported by grants from the Spanish ‘Ministerio de Educación y Ciencia' (CONSOLIDER and BFU2010-18692), and from AGAUR to L. Di Croce.
Glossary
- CBX
chromobox
- CTD
C-terminal domain
- DNMT
DNA methyltransferase
- ES cells
embryonic stem cells
- HMT
histone methyltransferase
- NSCs
neural stem cells
- PCL
Polycomb-like
- PLZF
promyelocytic leukemia zinc finger
- PRC
Polycomb repressive complex
- Pol II
polymerase II
- TrxG
trithorax group
The authors declare no conflict of interest.
Footnotes
Edited by P Salomoni
References
- Dejardin J, Rappailles A, Cuvier O, Grimaud C, Decoville M, Locker D, et al. Recruitment of Drosophila Polycomb group proteins to chromatin by DSP1. Nature. 2005;43:533–538. doi: 10.1038/nature03386. [DOI] [PubMed] [Google Scholar]
- Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Bantignies F, Roure V, Comet I, Leblanc B, Schuettengruber B, Bonnet J, et al. Polycomb-dependent regulatory contacts between distant Hox loci in Drosophila. Cell. 2011;144:214–226. doi: 10.1016/j.cell.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey L, Helin K. Polycomb group protein-mediated repression of transcription. Trends Biochem Sci. 2010;35:323–332. doi: 10.1016/j.tibs.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Leeb M, Pasini D, Novatchkova M, Jaritz M, Helin K, Wutz A. Polycomb complexes act redundantly to repress genomic repeats and genes. Genes Dev. 2010;24:265–276. doi: 10.1101/gad.544410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku M, Koche RP, Rheinbay E, Mendenhall EM, Endoh M, Mikkelsen TS, et al. Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS Genet. 2008;4:e1000242. doi: 10.1371/journal.pgen.1000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhuis B, de Wit E, Muijrers I, Teunissen H, Talhout W, van Steensel B, et al. Genome-wide profiling of PRC1 and PRC2 Polycomb chromatin binding in Drosophila melanogaster. Nat Genet. 2006;38:694–699. doi: 10.1038/ng1792. [DOI] [PubMed] [Google Scholar]
- Kuzmichev A, Nishioka K, Erdjument-Bromage H, Tempst P, Reinberg D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 2002;16:2893–2905. doi: 10.1101/gad.1035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14:155–164. doi: 10.1016/j.gde.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2006;20:1123–1136. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaustov L, Ouyang H, Amaya M, Lemak A, Nady N, Duan S, et al. Recognition and specificity determinants of the human cbx chromodomains. J Biol Chem. 2011;286:521–529. doi: 10.1074/jbc.M110.191411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–878. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- Cao R, Tsukada Y, Zhang Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol Cell. 2005;20:845–854. doi: 10.1016/j.molcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Levinger L, Varshavsky A. Selective arrangement of ubiquitinated and D1 protein-containing nucleosomes within the Drosophila genome. Cell. 1982;28:375–385. doi: 10.1016/0092-8674(82)90355-5. [DOI] [PubMed] [Google Scholar]
- Eskeland R, Leeb M, Grimes GR, Kress C, Boyle S, Sproul D, et al. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol Cell. 2010;38:452–464. doi: 10.1016/j.molcel.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis NJ, Kingston RE, Woodcock CL. Chromatin compaction by a polycomb group protein complex. Science. 2004;306:1574–1577. doi: 10.1126/science.1100576. [DOI] [PubMed] [Google Scholar]
- Richly H, Rocha-Viegas L, Ribeiro JD, Demajo S, Gundem G, Lopez-Bigas N, et al. Transcriptional activation of polycomb-repressed genes by ZRF1. Nature. 2010;468:1124–1128. doi: 10.1038/nature09574. [DOI] [PubMed] [Google Scholar]
- Stock JK, Giadrossi S, Casanova M, Brookes E, Vidal M, Koseki H, et al. Ring1-mediated ubiquitination of H2A restrains poised RNA polymerase II at bivalent genes in mouse ES cells. Nat Cell Biol. 2007;9:1428–1435. doi: 10.1038/ncb1663. [DOI] [PubMed] [Google Scholar]
- Brookes E, Pombo A. Modifications of RNA polymerase II are pivotal in regulating gene expression states. EMBO Rep. 2009;10:1213–1219. doi: 10.1038/embor.2009.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- Villa R, Pasini D, Gutierrez A, Morey L, Occhionorelli M, Viré E, et al. Role of the polycomb repressive complex 2 in acute promyelocytic leukemia. Cancer Cell. 2007;11:513–525. doi: 10.1016/j.ccr.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Barna M, Merghoub T, Costoya JA, Ruggero D, Branford M, Bergia A, et al. Plzf mediates transcriptional repression of HoxD gene expression through chromatin remodeling. Dev Cell. 2002;3:499–510. doi: 10.1016/s1534-5807(02)00289-7. [DOI] [PubMed] [Google Scholar]
- Boukarabila H, Saurin AJ, Batsché E, Mossadegh N, van Lohuizen M, Otte AP, et al. The PRC1 Polycomb group complex interacts with PLZF/RARA to mediate leukemic transformation. Genes Dev. 2009;23:1195–1206. doi: 10.1101/gad.512009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–530. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggeman SW, Valk-Lingbeek ME, van der Stoop PP, Jacobs JJ, Kieboom K, Tanger E, et al. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes Dev. 2005;19:1438–1443. doi: 10.1101/gad.1299305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumor suppressor locus: all for one or one for all. Nat Rev Mol Cell Biol. 2006;7:667–677. doi: 10.1038/nrm1987. [DOI] [PubMed] [Google Scholar]
- Chen H, Tu SW, Hsieh JT. Down-regulation of human DAB2IP gene expression mediated by polycomb Ezh2 complex and histone deacetylase in prostate cancer. J Biol Chem. 2005;280:22437–22444. doi: 10.1074/jbc.M501379200. [DOI] [PubMed] [Google Scholar]
- Wu ZL, Zheng SS, Li ZM, Qiao YY, Aau MY, Yu Q. Polycomb protein EZH2 regulates E2F1-dependent apoptosis through epigenetically modulating Bim expression. Cell Death Differ. 2010;17:801–810. doi: 10.1038/cdd.2009.162. [DOI] [PubMed] [Google Scholar]
- Kia SK, Gorski MM, Giannakopoulos S, Verrijzer CP. SWI/SNF mediates polycomb eviction and epigenetic reprogramming of the INK4b-ARF-INK4a locus. Mol Cell Biol. 2008;28:3457–3464. doi: 10.1128/MCB.02019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiani M, Scholer HH. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005;6:872–884. doi: 10.1038/nrm1744. [DOI] [PubMed] [Google Scholar]
- Niwa H. How is pluripotency determined and maintained. Development. 2007;134:635–646. doi: 10.1242/dev.02787. [DOI] [PubMed] [Google Scholar]
- Fisher CL, Fisher AG. Chromatin states in pluripotent differentiated and reprogrammed cells. Curr Opin Genet Dev. 2011;21:140–146. doi: 10.1016/j.gde.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Azuara V, Perry P, Sauer S, Spivakov M, Jørgensen HF, John RM, et al. Chromatin signatures of pluripotent cell lines. Nat Cell Biol. 2006;8:532–538. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- Surface LE, Thornton SR, Boyer LR. Polycomb group proteins set the stage for early lineage commitment. Cell Stem Cell. 2010;7:288–298. doi: 10.1016/j.stem.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Alder O, Lavial F, Helness A, Brookes E, Pinho S, Chandrashekran A, et al. Ring1B and Suv39h1 delineate distinct chromatin states at bivalent genes during early mouse lineage commitment. Development. 2010;137:2483–2492. doi: 10.1242/dev.048363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Jang MJ, Kang MJ, Han YM. Epigenetic signatures and temporal expression of lineage-specific genes in hESCs during differentiation to hepatocytes in vitro. Hum Mol Genet. 2011;20:401–412. doi: 10.1093/hmg/ddq476. [DOI] [PubMed] [Google Scholar]
- Golebiewska A, Atkinson SP, Lako M, Armstrong L. Epigenetic landscaping during hESC differentiation to neural cells. Stem Cells. 2009;27:1298–1308. doi: 10.1002/stem.59. [DOI] [PubMed] [Google Scholar]
- Mohn F, Weber M, Rebhan M, Roloff TC, Richter J, Stadler MB, et al. Lineage-specific polycomb targets and de novo DNA methylation define restriction and potential of neuronal progenitors. Mol Cell. 2008;30:755–766. doi: 10.1016/j.molcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Cui K, Zang C, Roh TY, Schones DE, Childs RW, Peng W, et al. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell. 2009;4:80–93. doi: 10.1016/j.stem.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastenhouw NL, Zhang Y, Woods IG, Imam F, Regev A, Liu XS, et al. Chromatin signature of embryonic pluripotency is established during genome activation. Nature. 2010;464:922–926. doi: 10.1038/nature08866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol. 2001;21:4330–4336. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini D, Bracken AP, Jensen MR, Lazzerini Denchi E, Helin K. Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity. EMBO J. 2004;23:4061–4071. doi: 10.1038/sj.emboj.7600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust C, Lawson KA, Schork NJ, Thiel B, Magnuson T. The Polycomb-group gene eed is required for normal morphogenetic movements during gastrulation in the mouse embryo. Development. 1998;125:4495–4506. doi: 10.1242/dev.125.22.4495. [DOI] [PubMed] [Google Scholar]
- Pasini D, Bracken AP, Hansen JB, Capillo M, Helin K. The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol Cell Biol. 2007;27:3769–3779. doi: 10.1128/MCB.01432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SJ, Yee D, Magnuson T. Polycomb repressive complex 2 is dispensable for maintenance of embryonic stem cell pluripotency. Stem Cells. 2008;26:1496–1505. doi: 10.1634/stemcells.2008-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini D, Cloos PA, Walfridsson J, Olsson L, Bukowski JP, Johansen JV, et al. JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature. 2010;464:306–310. doi: 10.1038/nature08788. [DOI] [PubMed] [Google Scholar]
- Landeira D, Sauer S, Poot R, Dvorkina M, Mazzarella L, Jørgensen HF, et al. Jarid2 is a PRC2 component in embryonic stem cells required for multi-lineage differentiation and recruitment of PRC1 and RNA Polymerase II to developmental regulators. Nat Cell Biol. 2010;12:618–624. doi: 10.1038/ncb2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Chang WY, Hunkapiller J, Cagney G, Garcha K, Torchia J, et al. Polycomb-like 2 associates with PRC2 and regulates transcriptional networks during mouse embryonic stem cell self-renewal and differentiation. Cell Stem Cell. 2010;6:153–166. doi: 10.1016/j.stem.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Manias JL, Chang WY, Stanford WL. PCL2 modulates gene regulatory networks controlling self-renewal and commitment in embryonic stem cells. Cell Cycle. 2011;10:45–51. doi: 10.4161/cc.10.1.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova M, Preissner T, Cerase A, Poot R, Yamada D, Li X, et al. Polycomblike 2 facilitates the recruitment of PRC2 Polycomb group complexes to the inactive X chromosome and to target loci in embryonic stem cells. Development. 2011;138:1471–1482. doi: 10.1242/dev.053652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Han J, Yuan P, Yang H, Zhang J, Soh BS, Li P, et al. Tbx3 improves the germ-line competency of induced pluripotent stem cells. Nature. 2010;463:1096–1100. doi: 10.1038/nature08735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voncken JW, Roelen BA, Roefs M, de Vries S, Verhoeven E, Marino S, et al. Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition. Proc Natl Acad Sci USA. 2003;100:2468–2473. doi: 10.1073/pnas.0434312100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak K, Kerl K, Fehr D, Kramps C, Gessner C, Killmer K, et al. BMI1 is a target gene of E2F-1 and is strongly expressed in primary neuroblastomas. Nucleic Acids Res. 2006;34:1745–1754. doi: 10.1093/nar/gkl119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb M, Wutz A. Ring1B is crucial for the regulation of developmental control genes and PRC1 proteins but not X inactivation in embryonic cells. J Cell Biol. 2007;178:219–229. doi: 10.1083/jcb.200612127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasaka T, Kanno M, Balling R, Mieza MA, Taniguchi M, Koseki H. A role for mel-18 a Polycomb group-related vertebrate gene during the anteroposterior specification of the axial skeleton. Development. 1996;122:1513–1522. doi: 10.1242/dev.122.5.1513. [DOI] [PubMed] [Google Scholar]
- Alkema MJ, van der Lugt NM, Bobeldijk RC, Berns A, van Lohuizen M. Transformation of axial skeleton due to overexpression of bmi-1 in transgenic mice. Nature. 1995;374:724–727. doi: 10.1038/374724a0. [DOI] [PubMed] [Google Scholar]
- van der Lugt NM, Domen J, Linders K, van Roon M, Robanus-Maandag E, te Riele H, et al. Posterior transformation neurological abnormalities and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 1994;8:757–769. doi: 10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]
- Akasaka T, van Lohuizen M, van der Lugt N, Mizutani-Koseki Y, Kanno M, Taniguchi M, et al. Mice doubly deficient for the Polycomb Group genes Mel18 and Bmi1 reveal synergy and requirement for maintenance but not initiation of Hox gene expression. Development. 2001;128:1587–1597. doi: 10.1242/dev.128.9.1587. [DOI] [PubMed] [Google Scholar]
- Román-Trufero M, Méndez-Gómez HR, Pérez C, Hijikata A, Fujimura Y, Endo T, et al. Maintenance of undifferentiated state and self-renewal of embryonic neural stem cells by Polycomb protein Ring1B. Stem Cells. 2009;27:1559–1570. doi: 10.1002/stem.82. [DOI] [PubMed] [Google Scholar]
- Fasano CA, Dimos JT, Ivanova NB, Lowry N, Lemischka IR, Temple S. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell. 2007;1:87–99. doi: 10.1016/j.stem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadirgi G, Leinster V, Acquati S, Bhagat H, Shakhova O, Marino S. Conditional activation of Bmi1 expression regulates self-renewal, apoptosis, and differentiation of neural stem/progenitor cells in vitro and in vivo. Stem Cells. 2011;29:700–712. doi: 10.1002/stem.614. [DOI] [PubMed] [Google Scholar]
- Oguro H, Yuan J, Ichikawa H, Ikawa T, Yamazaki S, Kawamoto H, et al. Poised lineage specification in multipotential hematopoietic stem and progenitor cells by the polycomb protein Bmi1. Cell Stem Cell. 2010;6:279–286. doi: 10.1016/j.stem.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Lukacs RU, Memarzadeh S, Wu H, Witte ON. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell. 2010;7:682–693. doi: 10.1016/j.stem.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira CF, Piccolo FM, Tsubouchi T, Sauer S, Ryan NK, Bruno L, et al. ESCs require PRC2 to direct the successful reprogramming of differentiated cells toward pluripotency. Cell Stem Cell. 2010;6:547–556. doi: 10.1016/j.stem.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Mills AA. Throwing the cancer switch: reciprocal roles of polycomb and trithorax proteins. Nat Rev Cancer. 2010;10:669–682. doi: 10.1038/nrc2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikert S, Christoph F, Köllermann J, Müller M, Schrader M, Miller K, et al. Expression levels of the EZH2 polycomb transcriptional repressor correlate with aggressiveness and invasive potential of bladder carcinomas. Int J Mol Med. 2005;16:349–353. [PubMed] [Google Scholar]
- Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA, et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium prostate and breast. J Clin Oncol. 2006;24:268–273. doi: 10.1200/JCO.2005.01.5180. [DOI] [PubMed] [Google Scholar]
- Collett K, Eide GE, Arnes J, Stefansson IM, Eide J, Braaten A, et al. Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer. Clin Cancer Res. 2006;12:1168–1174. doi: 10.1158/1078-0432.CCR-05-1533. [DOI] [PubMed] [Google Scholar]
- Mimori K, Ogawa K, Okamoto M, Sudo T, Inoue H, Mori M. Clinical significance of enhancer of zeste homolog 2 expression in colorectal cancer cases. Eur J Surg Oncol. 2005;31:376–380. doi: 10.1016/j.ejso.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Kidani K, Osaki M, Tamura T, Yamaga K, Shomori K, Ryoke K, et al. High expression of EZH2 is associated with tumor proliferation and prognosis in human oral squamous cell carcinomas. Oral Oncol. 2009;45:39–46. doi: 10.1016/j.oraloncology.2008.03.016. [DOI] [PubMed] [Google Scholar]
- He XT, Cao XF, Ji L, Zhu B, Lv J, Wang DD, et al. Association between Bmi1 and clinicopathological status of esophageal squamous cell carcinoma. World J Gastroenterol. 2009;15:2389–2394. doi: 10.3748/wjg.15.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafaroudi AM, Mowla SJ, Ziaee SA, Bahrami AR, Atlasi Y, Malakootian M. Overexpression of BMI1 a polycomb group repressor protein in bladder tumors: a preliminary report. Urol J. 2008;5:99–105. [PubMed] [Google Scholar]
- Mohty M, Yong AS, Szydlo RM, Apperley JF, Melo JV. The polycomb group BMI1 gene is a molecular marker for predicting prognosis of chronic myeloid leukemia. Blood. 2007;110:380–383. doi: 10.1182/blood-2006-12-065599. [DOI] [PubMed] [Google Scholar]
- van Leenders GJ, Dukers D, Hessels D, van den Kieboom SW, Hulsbergen CA, Witjes JA, et al. Polycomb-group oncogenes EZH2 BMI1 and RING1 are overexpressed in prostate cancer with adverse pathologic and clinical features. Eur Urol. 2007;52:455–463. doi: 10.1016/j.eururo.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Ikeda H, Itatsu K, Yamaguchi J, Sawada S, Minato H, et al. The overexpression of polycomb group proteins Bmi1 and EZH2 is associated with the progression and aggressive biological behavior of hepatocellular carcinoma. Lab Invest. 2008;88:873–882. doi: 10.1038/labinvest.2008.52. [DOI] [PubMed] [Google Scholar]
- Richter GH, Plehm S, Fasan A, Rössler S, Unland R, Bennani-Baiti IM, et al. EZH2 is a mediator of EWS/FLI1 driven tumor growth and metastasis blocking endothelial and neuro-ectodermal differentiation. Proc Natl Acad Sci USA. 2009;106:5324–5329. doi: 10.1073/pnas.0810759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovey JS, Zacharek SJ, Kim CF, Lees JA. Bmi1 is critical for lung tumorigenesis and bronchioalveolar stem cell expansion. Proc Natl Acad Sci USA. 2008;105:11857–11862. doi: 10.1073/pnas.0803574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvà ML, Riggi N, Janiszewska M, Radovanovic I, Provero P, Stehle JC, et al. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Res. 2009;69:9211–9218. doi: 10.1158/0008-5472.CAN-09-1622. [DOI] [PubMed] [Google Scholar]
- Godlewski J, Nowicki MO, Bronisz A, Williams S, Otsuki A, Nuovo G, et al. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008;68:9125–9130. doi: 10.1158/0008-5472.CAN-08-2629. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Setoguchi T, Matsunoshita Y, Gao H, Hirotsu M, Komiya S. The knock-down of overexpressed EZH2 and BMI-1 does not prevent osteosarcoma growth. Oncol Rep. 2010;23:677–684. doi: 10.3892/or_00000684. [DOI] [PubMed] [Google Scholar]
- Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt Notch and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Sousa E, Melo F, van der Heijden M, Cameron K, de Jong JH, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]