Abstract
Metformin is the most widely used antidiabetic drug because of its proven efficacy and limited secondary effects. Interestingly, recent studies have reported that metformin can block the growth of different tumor types. Here, we show that metformin exerts antiproliferative effects on melanoma cells, whereas normal human melanocytes are resistant to these metformin-induced effects. To better understand the basis of this antiproliferative effect of metformin in melanoma, we characterized the sequence of events underlying metformin action. We showed that 24 h metformin treatment induced a cell cycle arrest in G0/G1 phases, while after 72 h, melanoma cells underwent autophagy as demonstrated by electron microscopy, immunochemistry, and by quantification of the autolysosome-associated LC3 and Beclin1 proteins. In addition, 96 h post metformin treatment we observed robust apoptosis of melanoma cells. Interestingly, inhibition of autophagy by knocking down LC3 or ATG5 decreased the extent of apoptosis, and suppressed the antiproliferative effect of metformin on melanoma cells, suggesting that apoptosis is a consequence of autophagy. The relevance of these observations were confirmed in vivo, as we showed that metformin treatment impaired the melanoma tumor growth in mice, and induced autophagy and apoptosis markers. Taken together, our data suggest that metformin has an important impact on melanoma growth, and may therefore be beneficial in patients with melanoma.
Keywords: melanoma, metformin, autophagy, apoptosis
Cutaneous melanoma deriving from the transformation of pigment-producing melanocytes is one of the most lethal cancers among young adults. Its incidence has increased at a dramatic rate during the last decades. Melanoma progression is characterized by an initial radial growth phase, encompassing in situ and minimally invasive tumors. This phase is followed by the development of vertical growth phase, which has been postulated to be the first point at which the tumor gains metastatic ability. Indeed, melanoma has a high capability of invasion and rapid metastasis to other organs. The prognosis of metastatic melanoma is extremely pejorative, as the various protocols of chemotherapy or immunotherapy have not shown, for the moment, real survival benefit.1 In addition to active prevention and early detection of melanomas, it appears necessary to develop new approaches enabling the discovery of new molecular target candidates for specific biotherapy treatment of this disease.
To this purpose, we have been interested in studying the effect of the oral antidiabetic drug, metformin, on melanoma. Metformin belongs to the family of biguanide and is the most widely used antidiabetic drug in the world.2 The effect of metformin on glucose homeostasis has been explained through reduced hepatic gluconeogenesis and increased glucose uptake in skeletal muscles.3, 4 This drug has the major clinical advantage of not inducing hypoglycemia and being tolerated very well. It is associated with only very low incidence of lactic acidosis (1/30 000) predominantly in patients with altered kidney or liver functions.5 The mechanism through which metformin reduces hepatic glucose production requires LKB1, which controls the AMPK (AMP-activated protein kinase)/mTOR (mammalian target of rapamycin) pathway and neoglucogenic genes.6
Metformin action on the AMPK/mTOR pathway leads to reduced protein synthesis and cell proliferation. These observations have given the impetus to numerous studies on the role of metformin in the regulation of tumor cell proliferation and apoptosis. Encouraging results emerged from these studies indicating that metformin can potentially be used as an efficient anticancer drug in various neoplasms such as prostate, breast, lung and pancreas cancers.7, 8 These results were confirmed by retrospective epidemiological studies that reported a decrease in cancer risk in diabetic patients treated with metformin.7, 9 Importantly, a recent work of Nakajima lab demonstrates that metformin diminishes the formation of rectal aberrant crypt foci, a marker of colorectal cancer, in non-diabetic patients.10 Despite compelling evidence of a role of metformin as an anticancer drug, its mode of action in cancer remains unelucidated. In a few studies, metformin induces apoptosis in cancer,11, 12, 13 and in one study performed on colon cancer, metformin triggers autophagy.14 Undoubtedly, in cancer there are multiple functional relationship reported between the apoptosis and autophagy, and these processes separately or/and jointly seal the fate of the cell.15 Thus, apoptosis or/and autophagy are interesting mechanisms to induce cancer cell death.
In this work, we wished to study the effect of metformin on melanoma cell viability, and to further investigate the molecular mechanisms by which metformin exerts its action on melanoma cells.
Results
Metformin exerts a deleterious effect on melanoma cell viability
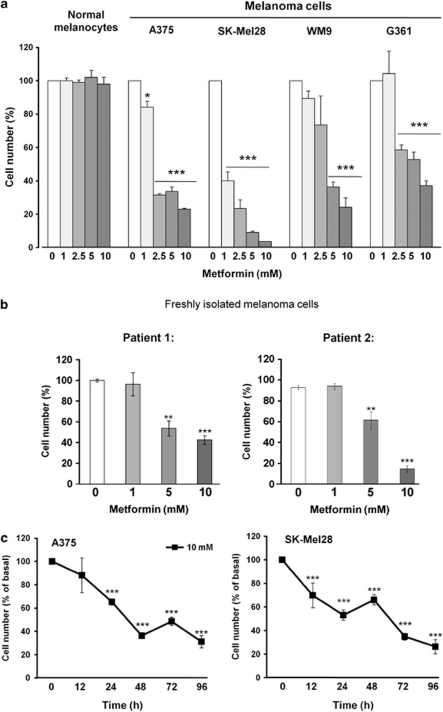
Metformin has been shown to affect the proliferation of several human cancer cell lines derived from the prostate, colon, gliomas and breast, in the 1–10 mM range. To investigate the effect of metformin on melanoma cells, we treated different human melanoma cell lines (A375, WM9, SKMel28 and G361) with metformin, and monitored cell number. As shown in Figure 1a, 72 h exposure of cells to various concentrations of metformin led to dose-dependent decrease in cell number. In contrast, human melanocytes were resistant to metformin treatment. In addition, metformin induced dose-dependent decrease in cell number of two melanoma cell samples freshly isolated from patient tumors (Figure 1b). Time-course experiments showed a rapid effect of 10 mM metformin, with an almost immediate and important decrease of cell number in comparison to phosphate-buffered saline (PBS) control (Figure 1c).
Figure 1.
Metformin represses melanoma cell proliferation (a) Primary human melanocytes and melanoma cell lines A375, SKMel28, WM9 and G361 were treated with indicated concentrations of metformin. After 72 h, viable cells were counted using trypan blue dye exclusion method. (b) Human melanoma cells freshly isolated from tumor were stimulated for 72 h with indicated concentrations of metformin. Viability of the cells was estimated by trypan blue staining. (c) A375 and SKMel28 were treated with PBS and 10 mM metformin during different times, and cell viability was determined by trypan blue dye exclusion assay. For each experiment, cell number is expressed in percent of control (100%). Data are mean±S.D. of three independent experiments performed in triplicate. Significantly different from the corresponding control *P<0.05; **P<0.01; ***P<0.001
Metformin induces autophagy
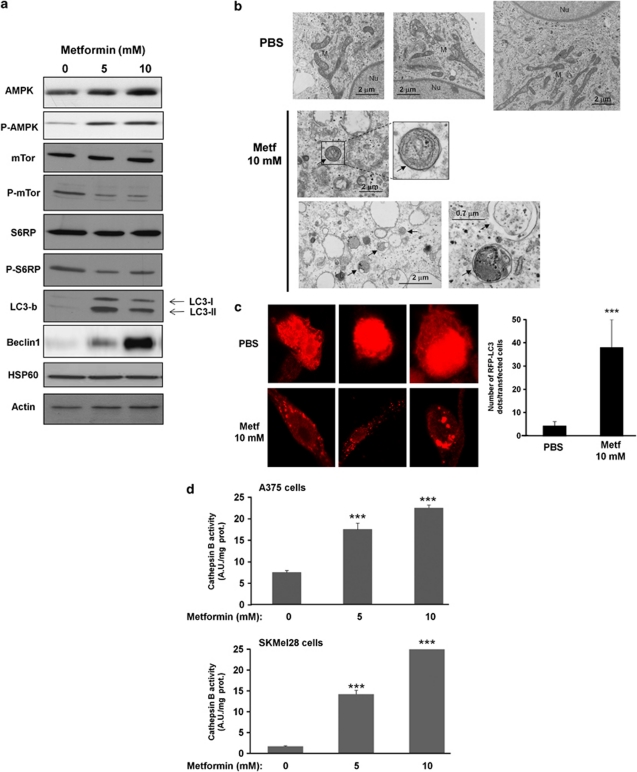
To elucidate whether AMPK activation is modulated in response to metformin, we studied the phosphorylation at Thr-172 of AMPK-α (AMPK T172) as a read-out of its activation state. Figure 2a shows that metformin induced a dose-dependent increase of AMPK T172 phosphorylation in A375 melanoma cell lines. We next examined the effect of metformin on the activity of mTOR, a downstream effector of AMPK. We observed a net decrease in the phosphorylation status of mTOR and S6 Ribosomal protein, suggesting a blockade of the mTOR pathway. As the mTOR pathway is a central regulator of multiple cellular responses to nutrient and growth factor signaling, including autophagy, we analyzed whether metformin might induce characteristic hallmarks of autophagy. During autophagy, LC3-I is converted to LC3-II through the ATG4-dependent insertion of a phosphoethanolamine moiety and recruited into the membrane of the forming phagophore, a double membrane required for the recycling of protein aggregates and organelles.16 Increased LC3 expression was detected for two doses of metformin. At the same concentrations, metformin also enhanced the expression level of Beclin 1, a protein required for the initiation of the formation of the autophagosome in autophagy.
Figure 2.
Metformin triggers autophagy in melanoma cells. (a) A375 melanoma cells were treated with indicated concentrations of metformin for 72 h. Cell lysates were separated by SDS-PAGE and analysed by western blot using the indicated antibody. HSP60 and actin were used as loading control. One representative experiment of three is shown. (b) Electron microscopy images presenting ultrastructure in representative control (PBS) and 10 mM metformin-treated melanoma cells for 72 h. Arrowheads, autophagosomes. Nu, nucleus; M, mitochondria. (c) Representative confocal microscopy images of RFP-LC3 staining in melanoma cells expressing RFP-LC3 and stimulated with or without metformin. In parallel (right panel), number of cells with RFP-LC3 dots were scored on 50 transfected cells. (d) Melanoma A375 and SKMel28 cells were incubated without or with metformin for 72 h, and cathepsin B activity was evaluated in the presence or absence of CA-074Me. Results are expressed as arbitrary units (A.U.) per mg of proteins. Data are mean±S.D. of representative experiment performed in triplicate. Significantly different from the corresponding control ***P<0.001
These data collectively strongly argue the induction of macroautophagy by metformin. Accordingly, electron microscopy images of A375 cells treated for 72 h with metformin showed typical images of autophagy including accumulation of numerous vesicles with distinct double membrane (Figure 2b). In addition, early autophagosomes sequestering cytosol, mitochondrion, or endoplasmic reticulum membranes were observed. By contrast, control melanoma cells (PBS) showed no autophagic vacuoles. Similar results were obtained with SKMel28 melanoma cells (Supplementary Figure S2).
In addition, following transfection of melanoma cells with RFP-LC3 construct, the formation of RFP puncta in cells treated with metformin was observed, whereas the untreated cells exhibited a diffuse distribution of red fluorescence in the cytoplasm and nucleus (Figure 2c).
The observation that metformin affected the lysosomal compartment was further substantiated by the increase of cathepsin B activity mediated by metformin at 72 h in A375 and SKMel28 cells (Figure 2d). Importantly, increased cathepsin B activity has been associated with the late steps of autophagy.17
Metformin induces cell death
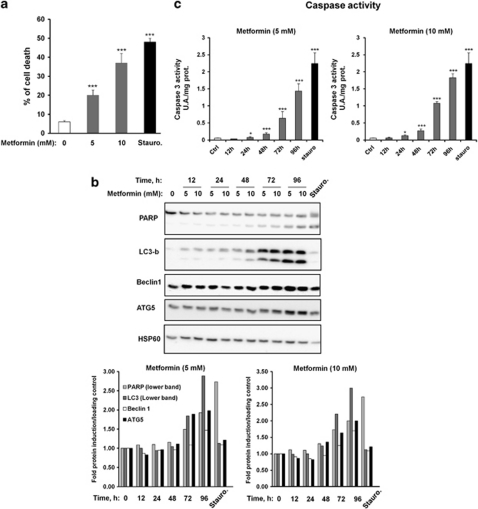
To determine whether autophagy mediated by metformin affects cell viability, we analyzed the percentage of cell death, by staining with propidium iodide (PI) in response to various metformin concentrations in A375 melanoma cells. Metformin induced a dose-dependent increase of cell death (Figure 3a). At a 10 mM metformin concentration, ∼40% of the cells were dead. This result was comparable to that obtained with the proapoptotic effector, staurosporine.
Figure 3.
Metformin induces melanoma cell death. (a) A375 melanoma cells were treated with indicated concentrations of metformin for 96 h. Cell death was measured by FACS analysis of propidium iodide uptake and results were expressed as a percentage of effective cell death. Staurosporine was used as a control of cell death. (b) A375 cells were incubated with metformin (5 or 10 mM) for the indicated times or with staurosporine as control of cell death. Cell lysates were separated by SDS-PAGE and analysed by western blot using the indicated antibody. HSP60 was used as loading control. One representative experiment of three is shown. Quantification of western blots were performed using Fuji Film, Multi Gauge. (c) A375 cells were incubated with metformin (5 or 10 mM) for the indicated times or with staurosporine as control of cell death. Caspase 3 activity was measured as indicated in Materials and Methods section. Significantly different from the corresponding control *P<0.05; **P<0.01; ***P<0.001
Previous studies have demonstrated that coregulation of apoptosis and autophagy can participate in mammalian cell death. To analyze the relationships between apoptosis and autophagy in melanoma cell death, we treated A375 melanoma cells for different times with 5 and 10 mM metformin. To monitor apoptosis, we evaluated both the cleavage of DNA repair enzyme poly(ADP-ribose) polymerase (PARP; Figure 3b) and the activation of caspase 3 (Figure 3c). In parallel, we determined autophagy induction by measuring the expression of LC3-b, Beclin 1 and ATG5. As shown in Figure 3b, PARP cleavage was significantly increased after 72 h of treatment with 5 or 10 mM metformin. At the same time, a clear activation of caspase 3 was detected (Figure 3c). In the same period of time, we also detected an increased expression of three proteins involved in autophagy process such as LC3, Beclin1 and ATG5. The apoptosis inductor staurosporine was found to induce apoptosis but not autophagy. These results were confirmed by quantification of western blots using a Fuji Film, Multi Gauge Ver 3.0 software (Fuji Film, Tokyo, Japan) (Figure 3b, lower part). Taken together, these results indicate that metformin induces a concomitant induction of autophagy and apoptosis processes in melanoma cells, both of which are involved in cell death. To identify the event sequence of autophagy and apoptosis, we have performed another kinetics of 6 h intervals. Supplementary Figure S3 clearly shows that the increase of expression and the conversion of LC3 precede the cleavage of PARP, suggesting that the autophagy is set up before apoptosis.
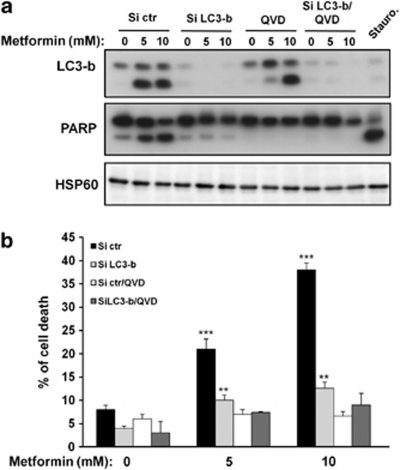
In an attempt to identify the contribution of autophagy and apoptosis or a switch between the two processes to induce cell death, we inhibited autophagy and apoptosis using LC3 or ATG5 small interfering RNA (siRNA) and pan caspase inhibitor Q-VD-OPH or caspase 3 siRNA, respectively. LC3 siRNA, which drastically reduced the expression of the protein (Figure 4a), prevented PARP cleavage (Figure 4a) and cell death induced by metformin (Figure 4b). The apoptosis inhibitor, Q-VD-OPH was capable of inhibiting PARP cleavage and cell death induced by metformin. In contrast, Q-VD-OPH failed to impair LC3-II expression mediated by metformin. As expected, combination of both LC3 siRNA and Q-VD-OPH abolished autophagy, apoptosis and cell death triggered by metformin. Similar results were found with ATG5 and caspase 3 siRNA (Supplementary Figure S4).
Figure 4.
Autophagy is involved in apoptosis and antiproliferative effects of metformin. A375 cells were transfected with either control or LC3b-specific siRNAs. Cells were next preincubated with or without Q-VD-OPH (20 μM) and exposed to metformin (5 or 10 mM) for 96 h. (a) LC3-b expression and PARP cleavage were analyzed by western blotting. HSP60 was used as a loading control. (b) FACS analysis using propidium iodide was used to determine cell death. The results are expressed as percentage of cell death compared with control. Data are expressed as percentage of apoptotic cells and represent the mean±S.D. of six independent experiments. Significantly different from the corresponding control **P<0.01; ***P<0.001
The anti-melanoma effect of metformin is partially mediated by AMPK
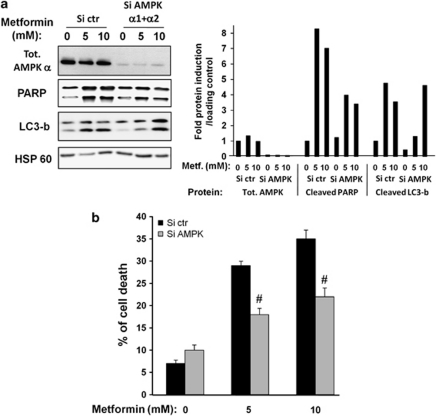
The anticancer effects of metformin have been essentially attributed to its ability to activate the AMPK pathway. To evaluate the function of AMPK in the effects of metformin, we knockdown both α1 and α2 catalytic subunits of AMPK with previously validated siRNAs.18 As shown in Figures 5a and b, inhibition of α1 and α2 AMPK subunits partially prevented cell death, and PARP cleavage induced by 5 and 10 mM metformin. Further, AMPK siRNA inhibited partially LC3 cleavage at 5 mM metformin but not at 10 mM metformin. These observations suggest that metformin mediates its anti-melanoma effects via both AMPK-dependent and -independent pathways.
Figure 5.
AMP kinase partially mediates cell death induced by metformin in melanoma. A375 cells were transfected with combination of α1 and α2 AMPK siRNAs or with control siRNA. The cells were next treated for 96 h with indicated concentration of metformin. (a) AMPKα subunit expression, LC3b expression and PARP cleavage were analyzed by western blotting. HSP60 was used as a loading control. Quantification of western blots were performed using Fuji Film, Multi Gauge. (b) FACS analysis using propidium iodide was used to determine cell death. The results are expressed as percentage of cell death compared with control. Data are expressed as percentage of apoptotic cells and represent the mean±S.D. of three independent experiments. Significantly different from the Si-control transfected cells #P<0.05
Metformin inhibits melanoma tumor development in mice
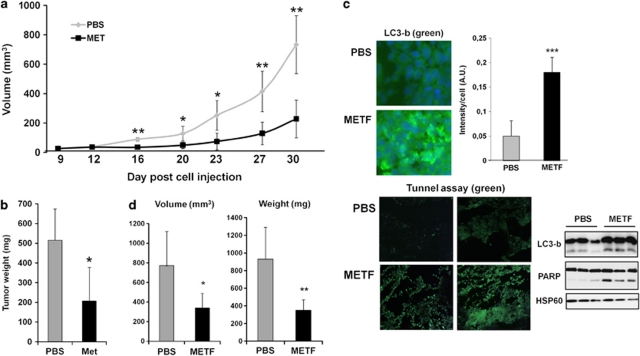
Finally, to assess a potential antineoplastic effect of metformin in vivo, A375 melanoma cells (2.5 × 106) were injected subcutaneously in 6-week-old female athymic nude mice and treated 5 days later by injection of vehicle or metformin (2 mg/mouse/day) over a period of 3 weeks. Untreated control mice rapidly developed visible tumors, and dramatic tumor growth was observed throughout the course of the study (Figure 6a). In contrast, treatment of mice with metformin markedly attenuated the ability of cells to develop visible tumors. Indeed, three of seven metformin-treated mice had no measurable tumors. Moreover, tumors derived from metformin-treated mice were consistently and significantly smaller than tumors from untreated control mice (Figure 6b). These data clearly demonstrate that metformin has anti-melanoma activity in vivo.
Figure 6.
Effect of metformin on melanoma tumor growth in mice. Female immune-deficient BALB/c nu/nu (nude) mice were inoculated subcutaneously with A375 melanoma cells (2.5 × 106), and after 5 days animals (n=6 in each group) were treated with metformin (2 mg/mice/day) or PBS over 3 weeks. (a) The growth tumor curves were determined by measuring the tumor volume. (b) Mice were euthanized and tumors were weighed. *P<0.05; **P<0.01. (c) Frozen sections of tumors from mice treated with PBS or metformin were fixed and stained using LC3-b (green) and DAPI (blue) or using TUNEL assay (green). Slides were examined with a Zeiss Axiophot fluorescence microscope. Representative sections are shown. The experiment was repeated on sections from four mice per condition. Images were treated and analyzed with ImageJ software for LC3 fluorescence quantification using DAPI fluorescence as internal control. LC3 expression and PARP cleavage were analyzed by western blotting. HSP60 was used as a loading control. (d) Female C57BL/6 mice were inoculated subcutaneously with B16F10 melanoma murine cells (2.5 × 105), and after 7 days animals (n=6 in each group) were treated with metformin (2 mg/mice/day) or PBS over 3 weeks. Mice were euthanized. The growth of tumors were determined by measuring the tumor volume, and tumors were weighed. Significantly different from the corresponding control *P<0.05; **P<0.01
To confirm the molecular mechanisms involved in the antineoplastic effects of metformin in vivo, LC3 expression was studied by immunofluorescent staining on tumor sections from mice treated with vehicle or metformin (Figure 6c, upper part). Sections of tumors from mice treated with metformin show a dramatic increase in LC3 staining compared with sections of tumors from control mice injected with vehicle. In addition, LC3 puncta, reminiscent of autophagosome formation in tumors of mice treated with metformin, were visualized.
To determine the level of apoptosis in the tumors of mice treated with metformin, we performed a TUNEL assay on section of different tumors. We observed an increase in TUNEL-positive cells (green) in tumors sections from mice treated with metformin compared with tumor sections from mice treated with PBS.
In addition, to confirm these results, we made western blot from tumors of mice treated or not with metformin (Figure 6c, lower part). As expected, we showed an increase in expression and lipid conjugation of LC3b and cleavage of PARP in tumors of mice treated with metformin in comparison with control mice treated with PBS, suggesting that autophagy and apoptosis are also occurring in vivo.
Thus, the reduction of tumor volume and weight observed in metformin-treated mice seems to be, at least in part, related to the induction of autophagy and apoptosis.
Finally, we performed exactly the same experiment using a syngeneic tumor model in which B16 melanoma cells were injected in C57/Bl6 mice (Figure 6d). After 20 days of metformin treatment (2 mg/day/mouse), we observed a significant decrease in volume and weight of tumors for mice treated with drug compared with control mice, thereby confirming the antineoplastic effect of metformin in mice.
Discussion
The discovery of new therapeutic compounds is a very important challenge to treat advanced melanomas that are resistant to existing therapies. To this purpose, using in vitro and in vivo approaches, we demonstrate the potent anti-melanoma activity of the antidiabetic metformin. Interestingly, contrary to normal human melanocytes, drastic inhibition of cell viability mediated by metformin is observed in four different melanoma cell lines independent of the mutational status or melanoma development stage, and in two primary melanoma cell cultures freshly isolated from patients. These findings are consistent with results of Woodard et al.19 demonstrating AMPK activators AICAR and metformin reduced the proliferation of SKMel2 and SKMel28 melanoma cells.
Metformin action is mainly mediated by AMPK activation; however, several recent reports indicate that some effects of metformin in cancer cell lines could be mediated by an AMPK-independent pathway.20, 21, 22, 23 In our model, AMPK silencing by siRNA (Figure 5) or inhibition of AMPK activation by pharmacological inhibitor (data not shown) inhibits only partially the effect of metformin on melanoma cell death, suggesting that the effects of this drug are mediated, at least in part, through an AMPK-independent mechanism. In addition, AMPK knockdown protects partially the cleavage of PARP but fails to block the induction of LC3-b at 10 mM metformin. This result indicates that AMPK-dependent component of anti-melanoma action of metformin could be autophagy-independent. However, we cannot exclude that the low residual AMPK expression is sufficient to mediate metformin effect on cell death. Noteworthy, treatment of normal human melanocytes that express endogenous AMPK with metformin did not affect cell viability (Figure 1a), suggesting that metformin action is restrained to transformed cell lines and likely reflects a tumor-specific regulation.
The AMPK/mTOR pathway is under the control of LKB1, a serine-threonine kinase acting as a tumor suppressor. A recent report demonstrates that oncogenic V600E B-RAF, a common somatic mutation found in malignant melanoma (50–70%), negatively regulates LKB1 to promote melanoma cell proliferation.24 In our model, LKB1 seems not to be involved in metformin's anti-melanoma effect as G361 cells, which do not express LKB1, are also sensitive to the drug (Figure 1a). Accordingly, overexpression of a dominant negative form of LKB1 in melanoma cell lines did not prevent metformin sensitivity (data not shown).
Another protein that can mediate metformin effect is the tumor suppressor p53. Indeed, p53 functions as a transcriptional regulator of genes involved in cell cycle arrest, autophagy and apoptosis pathways.25 In addition, the AMPK-p53 pathway may represent a cell cycle checkpoint in response to energy limitation. AMPK has been shown to phosphorylate p53 on Ser-15 to induce G0/G1 phase arrest.26 In our melanoma cell lines, p53 is not phosphorylated on Ser-15 in response to metformin (data not shown). Further melanoma cells harboring a mutated TP53 gene (SKMel28 cells) exhibited the same sensitivity to metformin as other melanoma cell lines harboring wild-type p53 (Figure 1a), indicating that p53 is not involved in metformin effects in our cellular model.
Several recent reports have highlighted that metformin induces cell cycle arrest of cancer cell lines.7 We observed here that the proportion of cells in G0/G1 was significantly increased in melanoma cells treated for 24 h with metformin, while apoptotic cells with fragmented DNA (sub G1) could not be observed at 24 h (Supplementary Figures S1a and b). Cell cycle arrest in G0/G1 phase is associated at molecular level with a dramatic reduction of cyclin D1 expression, a marked increase in the expression of cyclin-dependent kinase inhibitor p21Cip1/Waf1, and hypophosphorylation of pRb (Supplementary Figure S1c).
However, the antiproliferative effect of metformin cannot be exclusively explained by cell cycle arrest as a strong increase in cell death was detected for longer time of drug treatment (>48 h; Figure 3a). Autophagy is a catabolic process for the degradation and recycling of macromolecules and organelles, which is activated during stress conditions.27, 28 Autophagy is initiated by the formation of double-membraned vesicles called autophagosomes, which fuse with lysosomes to form autophagolysosomes in which lysosomal hydrolases digest the vesicle contents for recycling.29 Autophagy is considered as a survival mechanism induced in adverse conditions to maintain cell integrity, but paradoxically, it is also involved in a particular mode of death called autophagic cell death or type II cell death.30 In our study, several compelling evidences indicate that metformin induces autophagy in melanoma cells (Figure 2). This finding is particularly interesting as until now, only one study has reported that metformin is able to induce autophagy in colon cancer.14 In this model and conversely to the present study, p53 seems to be required for autophagy after treatment with metformin. In agreement with this model, we observed that blockage of autophagy by siRNAs directed against LC3 and ATG5 (Figure 4a and Supplementary Figure S4) or pharmacological inhibition of autophagy by 3-methyl adenine (data not shown) promotes melanoma cell survival, suggesting that metformin does induce autophagic cell death.
In addition, we observed that long-term treatment with metformin induces apoptosis as monitored by caspase 3 activation and PARP cleavage (Figures 3b and c). This result is consistent with the fact that metformin has been previously shown to promote apoptosis in several solid tumor cell lines.7
To determine whether autophagy and apoptosis are linked mechanisms, we performed several experiments following inhibition of apoptosis and/or autophagy. Inhibition of caspase activation by Q-VD-OPH or caspase 3 siRNA did not dampen LC3-II accumulation nor the melanoma cell death in response to metformin (Figure 4 and Supplementary Figure S4), suggesting that metformin-mediated apoptosis did not affect induction of autophagy. In contrast, inhibition of metformin-mediated autophagy prevented PARP cleavage, indicating that metformin-mediated autophagy contributes to metformin-induced apoptosis. This link between autophagy and apoptosis has been previously proposed for targeting melanoma cells in response to cytosolic delivery of dsRNA.31, 32
To the best of our knowledge, this is the first demonstration that metformin through concomitant regulation of autophagy and apoptosis favors elimination of cancer cells.
Beclin1 siRNAs were not able to block metformin-induced autophagy and cell death (Supplementary Figure S4), suggesting that metformin induced beclin1-independent autophagy as previously described.33, 34, 35 Several recent reports described the central role of the complexes, Beclin1/anti-apoptotic members of the Bcl-2 family in the cross-talk between autophagy and apoptosis.30 The fact that suppression of beclin1 did not modify autophagy and cell death suggested that this protein was not involved in the mechanism of cross-talk. Future studies in our lab will address the mechanism(s) through which metformin signaling induces a specific death in melanoma cells.
Finally, we have evaluated the anti-melanoma activity of metformin in a mouse model of melanoma xenograft (Figure 6). Importantly, we found that short-term administration (3 weeks) of metformin dramatically reduces the development of melanoma tumors in mice. In addition, metformin induces no apparent toxicity, as the body weight (data not shown) and overall appearance of mice given a metformin regimen were not different from those of controls. To determine whether the molecular events observed in cell lines were also found in mice, we performed immunohistological staining and TUNEL assay of mouse tumor sections. Experiments showed an increase in LC3 puncta corresponding to autophagosomes and an increase in TUNEL-positive cells in the tumors of metformin-treated mice. These results suggest that in vivo metformin induced autophagy and apoptosis. To circumvent partial immunity deficiency found in nude mice, we also performed the same experiment in a syngeneic model using allograft of B16 melanoma cells in C57Bl6 mice. We have previously checked that metformin decreased viability of melanoma B16 cells in vitro (data not shown). Also in this model, metformin diminished the volume and weight of tumor of treated mice, confirming the antineoplastic effect of this compound in vivo.
In summary, we demonstrate for the first time that metformin inhibits melanoma tumor growth through an autophagy-dependent mechanism. This finding brings new clues to the understanding of metformin action in melanoma cell death. Finally, taking into account the drastic effect of metformin on melanoma cell growth and survival in mice, it might be worth evaluating metformin treatment in patients suffering from metastatic melanoma.
Materials and Methods
Reagents and antibodies
Metformin, staurosporine, phenylmethylsulfonyl fluoride, PI, 3-isobutyl-1-methylxanthine, hydrocortisone, insulin, phorbol-12 myristate 13-acetate, MCDB 153 medium, sodium fluoride, dimethylacetamide, Hoechst 33258, sodium orthovanadate, 4-(2-aminoethyl)-benzene-sulfonyl fluoride, aprotinin and leupeptin were purchased from Sigma-Aldrich (Saint Quentin Fallavier, France). The caspase substrates and the caspase inhibitors were from MERCK Eurolab (Fontenay-sous-Bois, France). Trypan blue, Dulbecco's Modified Eagle's Medium (DMEM), penicillin/streptomycin and trypsin were from Invitrogen (Pontoise, France) and, fetal calf serum (FCS) from Hyclone (Brevieres, France). p21, p27, beclin1, HSP60 antibodies were purchased from Santa Cruz Biotechnology (TEBU; Le Perray en Yvelines, France). Anti-LC3-b, ATG5, AMPKα (T172), mTOR (S2448), S6 ribosomal (S235/236) and PARP, AMPKα, mTOR, S6 ribosomal antibodies were from Cell Signaling (Berverly, MA, USA). Antibodies against cyclin D1 and pRb were from BD Bioscience (Pont de Claix, France).
Cell cultures
Normal human melanocytes were prepared and maintained as described.36 Different melanoma cell lines were purchased from American Tissue Culture Collection (Molsheim, France). Cells were grown in RPMI 1640 (A375, WM9 and SKMel28) or in DMEM medium (G361 and B16) supplemented with 10% FCS and penicillin/streptomycin (100 U/ml/50 μg/ml) at 37°C and 5% CO2. Patient melanoma cells were prepared as described.37 Briefly, biopsy was dissected and digested for 1–2 h with collagenase A (0.33 U/ml), dispase (0.85 U/ml) and Dnase I (144 U/ml) with rapid shaking at 37°C. Large debris were removed by filtration through a 70-μm cell strainer. Viable cells were obtained by Ficoll gradient centrifugation.
For each experiment, cells were starved with or without 1% SVF in appropriate medium during 14 h before drug stimulation.
Immunofluorescence microscopy
Monolayers prepared for fluorescent staining were grown on glass coverslips. Immunofluorescence experiments were carried out as described.38
Electron microscopy
Electron microscopy experiments were performed as described.39
Caspase activity
Caspase activities were performed as described.38
Western blot assays
Western blot analyses were performed as described.40 Quantifications were made using Fuji Film Multi Gauge software.
Flow cytometry analysis
All flow cytometry analyses were performed using the FL2 channels of a FACScan (Becton Dickinson, Cowley, UK) and data were analyzed with CellQuest software (Becton Dickinson, Cowley, UK) as previously described.38 PI staining was performed using Staining Kit (Roche Diagnostics, Meylan, France) according to the manufacturer's protocol.
Transfection experiments
Transfections with RFP-LC3 construct were carried out by using the FuGENE 6 reagent (Roche Applied Sciences, Meylan, France) according to the manufacturer's specifications. Transfection of duplex siRNAs (50 nM) was carried out using Lipofectamine RNAiMAX (Invitrogen) in Opti-MEM (Invitrogen). The day after the transfection, metformin was added to the medium and proteins were extracted 96 h after the addition of metformin. Stealth siRNA targeting AMPKα1, AMPKα2, ATG5 and caspase 3 were purchased from Invitrogen, whereas LC3-b siRNA were from Dharmacon (Lafayette, CO, USA) and p21 siRNA were from Santa Cruz Biotechnology. As nonspecific control, a scramble sequence siRNAs were used.
Cathepsin B activity
Cathepsin B activity was determined as described.41
In vivo murine cancer model
Animal experiments were carried out in accordance with the Declaration of Helsinki and were approved by a local ethical committee. Female immune-deficient BALB/c nu/nu (nude) mice or female C57/Bl6 mice were obtained at 6 weeks of age from Harlan Laboratory (Gannat, France).
Nude mice or C57/Bl6 mice were inoculated subcutaneously with A375 melanoma cells (2.5 × 106 cells/mouse) or B16F10 melanoma murine cells (2.5 × 105), respectively. After 5 days, animals received intraperitoneal injection of metformin (2 mg/mouse/day) dissolved in PBS. The growth tumor curves were determined by measuring the tumor volume using the equation V=(L × W2)/2. At the end of the experiment, mice were euthanized by CO2 inhalation and tumors were taken for western blot and immunofluorescence experiments. TUNEL assay was performed using the In Situ Cell Death Detection Kit (Roche, Meylan, France).
Statistical analysis
Data presented are mean ±S.D. of three independent experiments performed in triplicate. Statistical significance was assessed using the Student's t-test except for in vivo experiments in which statistical significance was assessed using two-tailed Wilcoxon rank sum test. A value of P<0.05 was accepted as statistically significant.
Acknowledgments
This research was supported by the INSERM, University of Nice Sophia-Antipolis, Société Francaise en Dermatologie, ARC Grant (#5093) and the INCA Recherche Translationnelle 2011. Tijana Tomic is a recipient of a doctoral fellowship from the INSERM/Region PACA. Stéphane Rocchi is a recipient of ‘Contrat Hospitalier de Recherche Translationnelle du CHU de Nice'. INSERM U895, équipe 1 is ‘équipe labelisée ligue 2010'.
Glossary
- AMPK
AMP-activated protein kinase
- PBS
phosphate-buffered saline
- DMEM
Dulbecco's Modified Eagle's Medium
- FCS
fetal calf serum
- PARP
poly(ADP-ribose) polymerase
- pRb
retinoblastoma protein
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Stephanou
Supplementary Material
References
- Demierre MF. Epidemiology and prevention of cutaneous melanoma. Curr Treat Options Oncol. 2006;7:181–186. doi: 10.1007/s11864-006-0011-z. [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- Hundal HS, Ramlal T, Reyes R, Leiter LA, Klip A. Cellular mechanism of metformin action involves glucose transporter translocation from an intracellular pool to the plasma membrane in L6 muscle cells. Endocrinology. 1992;131:1165–1173. doi: 10.1210/endo.131.3.1505458. [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- Lalau JD, Race JM.Lactic acidosis in metformin therapy Drugs 199958(Suppl 155–60.discussion 75–82. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug. Mol Cancer Ther. 2010;9:1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- Pollak M. Metformin and other biguanides in oncology: advancing the research agenda. Cancer Prev Res (Phila) 2010;3:1060–1065. doi: 10.1158/1940-6207.CAPR-10-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- Hosono K, Endo H, Takahashi H, Sugiyama M, Sakai E, Uchiyama T, et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila) 2010;3:1077–1083. doi: 10.1158/1940-6207.CAPR-10-0186. [DOI] [PubMed] [Google Scholar]
- Liu B, Fan Z, Edgerton SM, Deng XS, Alimova IN, Lind SE, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8:2031–2040. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- Wang LW, Li ZS, Zou DW, Jin ZD, Gao J, Xu GM. Metformin induces apoptosis of pancreatic cancer cells. World J Gastroenterol. 2008;14:7192–7198. doi: 10.3748/wjg.14.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourelis TV, Siegel RD.Metformin and cancer: new applications for an old drug Med Oncol 2011. e-pub ahead of print. [DOI] [PubMed]
- Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conus S, Simon HU. Cathepsins: key modulators of cell death and inflammatory responses. Biochem Pharmacol. 2008;76:1374–1382. doi: 10.1016/j.bcp.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, et al. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010;70:2465–2475. doi: 10.1158/0008-5472.CAN-09-2782. [DOI] [PubMed] [Google Scholar]
- Woodard J, Platanias LC. AMP-activated kinase (AMPK)-generated signals in malignant melanoma cell growth and survival. Biochem Biophys Res Commun. 2010;398:135–139. doi: 10.1016/j.bbrc.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan R, Giri S, Hartmann L, Shridhar V. Metformin attenuates ovarian cancer cell growth in an AMP- kinase dispensable manner. J Cell Mol Med. 2009;15:166–178. doi: 10.1111/j.1582-4934.2009.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- Janjetovic K, Vucicevic L, Misirkic M, Vilimanovich U, Tovilovic G, Zogovic N, et al. Metformin reduces cisplatin-mediated apoptotic death of cancer cells through AMPK-independent activation of Akt. Eur J Pharmacol. 2011;651:41–50. doi: 10.1016/j.ejphar.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Kalender A, Selvaraj A, Kim SY, Gulati P, Brule S, Viollet B, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Jeong JH, Asara JM, Yuan YY, Granter SR, Chin L, et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell. 2009;33:237–247. doi: 10.1016/j.molcel.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilfou JT, Lowe SW. Tumor suppressive functions of p53. Cold Spring Harb Perspect Biol. 2009;1:a001883. doi: 10.1101/cshperspect.a001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004;36:2445–2462. doi: 10.1016/j.biocel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Scarlatti F, Granata R, Meijer AJ, Codogno P. Does autophagy have a license to kill mammalian cells. Cell Death Differ. 2009;16:12–20. doi: 10.1038/cdd.2008.101. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, et al. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ. 2009;16:87–93. doi: 10.1038/cdd.2008.131. [DOI] [PubMed] [Google Scholar]
- Morselli E, Galluzzi L, Kepp O, Vicencio JM, Criollo A, Maiuri MC, et al. Anti- and pro-tumor functions of autophagy. Biochim Biophys Acta. 2009;1793:1524–1532. doi: 10.1016/j.bbamcr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Alonso-Curbelo D, Soengas MS. Self-killing of melanoma cells by cytosolic delivery of dsRNA: wiring innate immunity for a coordinated mobilization of endosomes, autophagosomes and the apoptotic machinery in tumor cells. Autophagy. 2010;6:148–150. doi: 10.4161/auto.6.1.10464. [DOI] [PubMed] [Google Scholar]
- Tormo D, Checinska A, Alonso-Curbelo D, Perez-Guijarro E, Canon E, Riveiro-Falkenbach E, et al. Targeted activation of innate immunity for therapeutic induction of autophagy and apoptosis in melanoma cells. Cancer Cell. 2009;16:103–114. doi: 10.1016/j.ccr.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R. Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ. 2008;15:1318–1329. doi: 10.1038/cdd.2008.51. [DOI] [PubMed] [Google Scholar]
- Gao P, Bauvy C, Souquere S, Tonelli G, Liu L, Zhu Y, et al. The Bcl-2 homology domain 3 mimetic gossypol induces both Beclin 1-dependent and Beclin 1-independent cytoprotective autophagy in cancer cells. J Biol Chem. 2010;285:25570–25581. doi: 10.1074/jbc.M110.118125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Patel S, Raffoul F, Haller E, Mills GB, Nanjundan M. Arsenic trioxide induces a beclin-1-independent autophagic pathway via modulation of SnoN/SkiL expression in ovarian carcinoma cells. Cell Death Differ. 2010;17:1867–1881. doi: 10.1038/cdd.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larribere L, Khaled M, Tartare-Deckert S, Busca R, Luciano F, Bille K, et al. PI3K mediates protection against TRAIL-induced apoptosis in primary human melanocytes. Cell Death Differ. 2004;11:1084–1091. doi: 10.1038/sj.cdd.4401475. [DOI] [PubMed] [Google Scholar]
- Sarrabayrouse G, Corvaisier M, Ouisse LH, Bossard C, Mevel BL, Potiron L, et al. Tumor-reactive CD4(+)CD8alphabeta(+) CD103(+) alphabetaT cells: a prevalent tumor-reactive T-cell subset in metastatic colorectal cancers. Int J Cancer. 2010;128:2923–2932. doi: 10.1002/ijc.25640. [DOI] [PubMed] [Google Scholar]
- Botton T, Puissant A, Bahadoran P, Annicotte JS, Fajas L, Ortonne JP, et al. In vitro and in vivo anti-melanoma effects of ciglitazone. J Invest Dermatol. 2009;129:1208–1218. doi: 10.1038/jid.2008.346. [DOI] [PubMed] [Google Scholar]
- Colosetti P, Puissant A, Robert G, Luciano F, Jacquel A, Gounon P, et al. Autophagy is an important event for megakaryocytic differentiation of the chronic myelogenous leukemia K562 cell line. Autophagy. 2009;5:1092–1098. doi: 10.4161/auto.5.8.9889. [DOI] [PubMed] [Google Scholar]
- Botton T, Puissant A, Cheli Y, Tomic T, Giuliano S, Fajas L, et al. Ciglitazone negatively regulates CXCL1 signaling through MITF to suppress melanoma growth. Cell Death Differ. 2011;18:109–121. doi: 10.1038/cdd.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert G, Ben Sahra I, Puissant A, Colosetti P, Belhacene N, Gounon P, et al. Acadesine kills chronic myelogenous leukemia (CML) cells through PKC-dependent induction of autophagic cell death. PLoS One. 2009;4:e7889. doi: 10.1371/journal.pone.0007889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.