Abstract
Platelets shed microparticles not only upon activation, but also upon ageing by an apoptosis-like process (apoptosis-induced platelet microparticles, PMap). While the activation-induced microparticles have widely been studied, not much is known about the (patho)physiological consequences of PMap formation. Flow cytometry and scanning electron microscopy demonstrated that PMap display activated integrins and interact to form microparticle aggregates. PMap were chemotactic for monocytic cells, bound to these cells, an furthermore stimulated cell adhesion and spreading on a fibronectin surface. After prolonged incubation, PMap promoted cell differentiation, but inhibited proliferation. Monocyte membrane receptor analysis revealed increased expression levels of CD11b (integrin αMβ2), CD14 and CD31 (platelet endothelial cell adhesion molecule-1), and the chemokine receptors CCR5 and CXCR4, but not of CCR2. This indicated that PMap polarized the cells into resident M2 monocytes. Cells treated with PMap actively consumed oxidized low-density lipoprotein (oxLDL), and released matrix metalloproteinases and hydrogen peroxide. Further confirmation for the differentiation towards resident professional phagocytes came from the finding that PMap stimulated the expression of the (ox)LDL receptors, CD36 and CD68, and the production of proinflammatory and immunomodulating cytokines by monocytes. In conclusion, interaction of PMap with monocytic cells has an immunomodulating potential. The apoptotic microparticles polarize the cells into a resident M2 subset, and induce differentiation to resident professional phagocytes.
Keywords: apoptosis, atherosclerosis, inflammation, platelet microparticles, phagocytes, resident macrophages
The presence of microparticles in platelet-rich plasma has first been described in the late 60s, where these were designated as platelet dust.1 Since that time, the role of platelet-derived microparticles in the (patho)physiology of thrombosis and haemostasis has been well established. Circulating platelet microparticles in normal peripheral blood support low-grade thrombin generation.2 After surgery and under thrombotic conditions, the levels of microparticles in plasma can significantly increase,3, 4 and this has been associated with the pathologies of cardiovascular diseases,5 inflammation and atherosclerosis. The formation of these microparticles is considered to be a consequence of platelet activation with potent agonists, which indeed give rise to massive shedding of ‘activation-induced' microparticles with a strong procoagulant potential in vitro.6
Platelets circulate in blood for 9–10 days, and then are cleared in the spleen or liver following an apoptotic-like process.7, 8 This age-induced platelet apoptosis also leads to shedding of microparticles but, markedly, this process is not accompanied by signs of platelet activation.9, 10 Accordingly, there is a continuous formation of apoptosis-induced platelet microparticles (PMap) in blood from ageing, seemingly resting platelets. Phagocytes, including monocytes, dendritic cells and macrophages, are responsible for the clearance of apoptotic platelets and also PMap from the circulation.11 Conditions stimulating platelet apoptosis or suppression of phagocytosis will hence increase the plasma levels of PMap, which may aggravate inflammation-linked disorders like atherosclerosis.
Much attention has been paid to the microparticles that are generated from activated platelets. For instance, their high procoagulant potential has been characterized,12 their proteome with many bioactive compounds – including CCL5, CXCL4 and CXCL7 – has been unraveled13, 14 and they have recently been shown to enhance the vasoregenerative potential of angiogenic early outgrowth cells.15 Instead, there is very little knowledge of biological effects of the ‘spontaneously' formed apoptosis-induced microparticles, the PMap. Here, we hypothesized that PMap have an immunomodulating role by interacting with leukocytes, in particular monocytes. We aimed to characterize this role by determining short- and long-term effects of the interactions of PMap with monocytic cells and primary monocytes.
Results
Interactions of monocytic cells with PMap
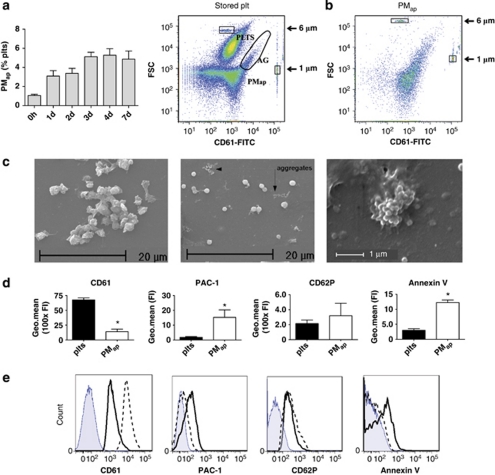
Microparticle-containing platelet-free plasma was isolated from stored platelet–plasma concentrates (5 days of storage) in which shed apoptotic microparticles accumulate time-dependently without platelet activation10 (Figure 1a, left panel), as visualized by flow cytometry using CD61 (glycoprotein (GP)IIIa) expression as a platelet-specific marker (Figure 1a, right panel). The plasma was microscopically checked for the presence of PMap and absence of intact platelets. Using a combination of micropore filtering (0.8 μm) and ultracentrifugation to remove intact platelets, PMap were isolated and characterized by flow cytometry, with freshly isolated platelets as a reference. The PMap fraction mostly contained particles of <1 μm in size, but also appreciable numbers of larger size particles (Figure 1b). These larger particles may be aggregated PMap, as scanning electron microscopy revealed the presence of single as well as aggregated microparticles (Figure 1c). Flow cytometry characterization further indicated that CD61 expression was lower in PMap than in resting platelets (compatible with the size difference), and that the activated conformation of GPIIb/IIIa (detected with PAC1 monoclonal antibody (mAb)) was higher in PMap (Figures 1d and e). In PMap the expression of markers of strong activation, for example, CD62P (P-selectin) and phosphatidylserine (annexin A5 binding), were increased in comparison to resting platelets. Together, this indicated that PMap assume an activation state with procoagulant phospholipids and activated integrins, thus explaining their ability to form aggregates.
Figure 1.
Characterization of apoptosis-induced platelet microparticles. (a) Spontaneous generation of PMap in platelets stored in RPMI+5% FBS at 37 °C for 1–7 days (left panel) and flow cytometry visualization of platelets and PMap (right panel, marked areas, AG: aggregates) and (b) of PMap isolated from 5-day-old platelet concentrates. Staining was with FITC-labeled anti-CD61mAb, and 1 μm and 6 μm beads were added as size markers (in frames). (c) Electron microscopy images of platelets and of PMap, forming small aggregates (arrows and right panel). (d) Flow cytometry detection for resting platelets (plts) and PMap of expression levels of GPIIb/IIIa (FITC anti-CD61mAb), activated GPIIb/IIIa (FITC PAC1mAb), P-selectin (FITC-anti-CD62PmAb) and phosphatidylserine (APC-annexin A5). Mean±S.E.M. (n=4–6). *P<0.05 versus platelets. (e) Representative histograms of the flow cytometry analysis, filled histogram: (isotype) control, dashed line: platelets, solid line: PMap
We then examined the ability of PMap to bind to THP-1 cells, an established monocytic cell line. Electron microscopy detected multiple PMap on the surface of THP-1 cells coincubated with the microparticles (Figure 2a). Flow cytometry using anti-CD61mAb indicated that the PMap binding to cells increased between 30 min and 70 min (Figures 2b and c). At later time points, CD61 binding sites on the cell surface decreased again. This suggested that the PMap were internalized by endocytosis, which could be confirmed by confocal microscopy revealing PMap localized at the surface and in the cytoplasm of the THP-1 cells (Figure 2d and Supplemental movie). Binding was completely absent after heat treatment of PMap (56 °C for 30 min, data not shown), indicating the necessity for active adhesion molecules on the PMap surface for interaction with monocytic cells. To determine whether PMap contain biological activity towards blood cells, we used a Transwell migration assay detecting chemotactic effects on THP-1 cells. Strikingly, PMap evoked robust cell migration, which was comparable with the reference chemokine, CCL2 (Figure 2e), indicating a strong chemotactic potential of the microparticles. To further investigate the potential mediators derived from PMap, we employed blocking antibodies against CCL5 and CXCL7, two platelet-derived chemokines that have been shown to mediate mononuclear cell (MC) recruitment.16 The chemotaxis of THP-1 cells toward PMap could be effectively blocked with antibodies against CCL5, whereas anti-CXCL7 or isotype antibodies showed no effect (Figure 2e). This suggests that CCL5 is a major factor that controls monocyte migration in the direction of PMap.
Figure 2.
Influence and interaction of PMap with monocytes. (a) Electron microscopy image of multiple PMap bound to THP-1 cells after 30 min of coincubation. (b) Flow cytometry detection of PMap or resting platelets (plts) binding to THP-1 cells (FI). Cells (2 × 106 cells/ml) were incubated with vehicle control (ctrl), PMap (2 × 108 cells/ml), or platelets (2 × 108 cells/ml) for 30–180 min. Coaggregates with THP-1 cells were stained with FITC anti-CD61mAb. Mean±S.E.M. (n=4–6). *P<0.05 versus control, °P<0.05 versus 70 min time point. (c) Representative histograms of fluorescence changes in THP-1 cells due to PMap binding. Note secondary decrease after 180 min incubation, indicative for internalization. (d) Confocal microscopy of THP-1 cells coincubated with PMap for 4 h at RT. Scale bar: 10 μm, green fluorescence: CD61, red fluorescence: CD45. (e) In Transwell chambers, the number of THP-1 cells migrated during 24 h towards PMap (108 cells/ml) was measured in the lower chambers. CCL2 (50 ng/μl) was used as a positive control. Additionally, PMap were preincubated with anti-CCL5, anti-CXCL7 and isotype IgG1mAb (10 μg/ml, 40 min at RT), and cells were left for transmigration. Mean±S.E.M. (n=3). *P<0.05 versus control, °P<0.05 versus PMap and anti-CCL5mAb
PMap-induced adhesion of monocytic cells under static and flow conditions
Experiments were conducted to determine the functional consequences of monocytic cell interactions with PMap. Preincubation of THP-1 cells with PMap (44 h) caused a marked increase in stable adhesion to fibronectin-coated wells under static conditions. This effect was similarly effective to that of incubation with phorbol myristate acetate (PMA; Figure 3a). It tended to increase with higher counts of PMap, whereas supernatants from PMap preparations were inactive. Also, preincubation with resting platelets, added at similar counts, did not affect the adhesion. As platelets spontaneously shed PMap, the generation of PMap during the course of the coincubation experiments with resting platelets might influence our findings (Figure 1a). In order to exclude potential effects from these spontaneously generated PMap, we first determined the amount of PMap formed after 7 days relative to the 10/1 ratio of PMap that we commonly used in our experiments (Figure 3b). In a subsequent adhesion assay, we compared the functional effects of this amount of PMap with the 10/1 ratio of PMap. As expected, no effects of this minor amount of PMap were observed (Figure 3c).
Figure 3.
Adhesion of monocytic cells induced by PMap. (a) Static adhesion to a fibronectin surface after 44 h incubation of THP-1 cells (1.5 × 105 cells/ml) with vehicle control (ctrl), PMap (ratio per cell, 10/1 or 30/1), resting platelets (plts; ratio per cell, 10/1 or 30/1) or supernatant from centrifuged PMap (sup). As positive control, THP-1 cells were incubated with PMA (10 ng/ml). Note the increased adhesion with PMap in comparison to platelets. (b) The amount of PMap spontaneously generated during incubation with platelets (black bar) compared with the experimentally used 10/1 ratio of PMap. (c) Static adhesion to a fibronectin surface after 44 h incubation of THP-1 cells (1.5 × 105/ml) with vehicle (ctrl) and PMap (ratio per cell, 0.5/1 or 10/1). (d) Adhesion of primary monocytes during low-shear flow over a monolayer of confluent endothelial cells. Monocytes were preincubated for 44 h with vehicle control (ctrl), PMap (ratio, 10/1) or resting platelets (ratio, 10/1). Mean±S.E.M. (n=3–4). *P<0.05 versus control, °P<0.05 versus 0.5/1 ratio per cell (n=3–5)
The effect of PMap on adhesion of primary monocytes was studied under physiological conditions of low-shear flow over a confluent monolayer of human endothelial cells.17 Coincubation with PMap (44 h) dramatically stimulated the adhesion of isolated monocytes to the endothelium, whereas coincubation with platelets at similar counts had no effect (Figure 3d). Treatment with PMap thus stimulates the adhesive properties of both the monocytic cell line and primary monocytes.
PMap-induced differentiation to professional resident phagocytes
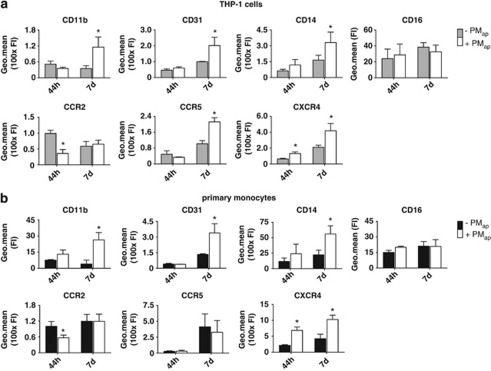
Changes in monocytic cell surface receptors were studied by flow cytometry after short-term (44 h) or long-term (7 days) incubation with or without PMap. The presence of PMap gradually increased the expression levels of CD11b (integrin αMβ2), CD14 (lipopolysaccharide co-receptor) and CD31 (platelet endothelial cell adhesion molecule-1) in similar ways for THP-1 cells (Figure 4a) and for primary monocytes (Figure 4b). The presence of PMap downregulated the expression of CCR2 (CCL2 receptor) both on THP-1 cells and monocytes after 44 h, although the expression of this receptor recovered after 7 days. By contrast, PMap significantly increased the level of CCR5 (CCL5 receptor) in THP-1 cells, but not in primary monocytes, and increased the level of CXCR4 (CXCL12 receptor) both in THP-1 cells and primary monocytes (Figures 4a and b). Jointly this suggested that PMap-treated monocytic cells tend to assume a resident (M2) monocyte phenotype, which previously has been defined as CD14+CD16+CCR2−CCR5+CXCR4++.18 Expression levels of CD16 (FcγRIII receptor) remained unchanged in our experiments (Figures 4a and b).
Figure 4.
Altered expression of adhesive and chemokine receptors induced by PMap. THP-1 cells (a) or primary monocytes (b) were incubated with vehicle control or PMap (ratio per cell, 10/1), basically as described for Figure 2. After 44 h or 7 days of incubation, expression levels (FI) of the adhesive GPs, CD11b, CD14 and CD31, and of the chemokine receptors, CCR2, CCR5 and CXCR4 were determined by flow cytometry. Mean±S.E.M. (n=4–7). *P<0.05 versus control
Prolonged incubation of THP-1 cells with PMap stimulated spreading of the cells on a fibronectin surface (Figure 5a). Spreading involved the formation of filopods and lamellipods, which appeared as actin filament bundles upon staining with the actin probe, phalloidin (Figure 5a). It was also found that the presence of PMap essentially annulled the proliferation of THP-1 cells (Figure 5b). Control staining with ethidium bromide showed that the non-viable cells remained at 6–7% after treatment with PMA, PMap or resting platelets (Figure 5c). Collectively, these findings supported the conclusion that THP-1 cells in the presence of PMap develop to a resident, non-proliferating phagocytic phenotype.
Figure 5.
Differentiation of monocytes into resident phagocytic cells induced by PMap. (a) Surface spreading and actin cytoskeleton rearrangements of THP-1 cells, cultured for 7 days with vehicle buffer or PMap. Bright-field images indicate cell spreading and filopod formation (arrows); images of FITC-phalloidin fluorescence show strands of actin filaments with PMap (scale bars: 100 μm, representative for three experiments). (b) Proliferation of THP-1 cells after 7 days of incubation with vehicle or PMap. Data are expressed as absolute cell numbers. (c) Relative amount (%) of necrotic cells after incubation with PMA (10 ng/ml), PMap, or resting platelets. (d) Uptake of oxLDL (labeled with DiI) by THP-1 cells, incubated for 7 days with PMap or resting platelets (plts). (e and f,) Expression of phagocytic cell markers on THP-1 cells (e) and primary monocytes (f). Cells were treated for 2 or 7 days with vehicle or PMap. Surface expression of CD36 was determined by flow cytometry, as was the intracellular expression of CD68 in permeabilized cells. Incubation conditions were as described for Figure 2. Mean±S.E.M. (n=4–5). *P<0.05 versus control
Further experiments were carried out to confirm the differentiation into professional phagocytic cells. Prolonged, 7-day incubation of THP-1 cells with PMap, but not with resting platelets, increased the uptake of oxidized low-density lipoprotein (oxLDL; Figure 5d). In agreement with this, the surface expression of the respective oxLDL and LDL receptors CD36 and CD68 (macrophage differentiation markers) was increased in the presence of PMap (Figure 5e). Similarly, incubation of primary monocytes with PMap for 7 days increased the CD36 level, but this period appeared to be too short to alter the CD68 expression (Figure 5f).
PMap-induced secretory properties of monocytic cells
Resident phagocytic monocytes are characterized by their capability to secrete matrix-degrading proteinases and reactive oxygen species.19 Indeed, incubation of THP-1 cells with PMap, but not resting platelets, for 2–7 days resulted in the production of functional matrix metalloproteinase (MMP)9, as was shown by zymography (Figure 6a). Quantification of the zymograms indicated a time-dependent increase of MMP activity after PMap incubation, which was similarly high as that of incubation with PMA after 7 days (Figure 6b). This time effect also indicated that MMP derived from PMap and platelets20 themselves did not measurably contribute to the zymographic activity of supernatants. Furthermore, PMap incubation markedly enhanced the release of hydrogen peroxide (H2O2) from both monocytic cells (Figure 6c) and primary monocytes (Figure 6d).
Figure 6.
Secreted products of monocytes induced by PMap. (a and b) Increase in MMP9 collagenolytic activity in supernatant of THP-1 cells, following incubation with vehicle control (ctrl), PMA (10 ng/ml), PMap or resting platelets (plts) for 44 h or 7 days. (a) Representative inverse zymograms of supernatants subjected to gel electrophoresis; note dark digestion bands at 260 kDa and 98 kDa. (b) Densitometric quantification of gelatinolytic activity of the 98-kDa band (mean gray value). Please note that gray value is inversely correlated with protease activity. (c and d) Quantification of H2O2 production after 20–44 h of incubation in supernatants from THP-1 cells (c) or monocytes after 44 h (d). Parallel tests gave fractions of non-viable cells of <0.3% (44 h) for THP-1 and monocytes, after 7 days 6–7% or 2–3%, respectively, under all conditions. Mean±S.E.M. (n=3). *P<0.05 versus control
To screen for the secretion of cytokines and chemokines, primary monocytes were incubated for 44 h with PMap or resting platelets, after which cell supernatants were analyzed for the presence of a panel of cytokines using a semi-quantitative commercial array kit (Figure 7a). Markedly, in comparison to platelets, the presence of PMap stimulated the release of several cytokines and other factors known to be proinflammatory, such as C5a, CCL5 and interleukin-23, and to a lesser extent of macrophage migration inhibitory factor, soluble CD40 ligand and soluble intercellular adhesion molecule 1. Furthermore, the monocytes produced several factors that participate in immunomodulating and regenerative processes rather than in inflammation, such as granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony-stimulating factor (GM-CSF). To determine the most abundantly secreted relevant cytokines in a more quantitative manner, we performed an enzyme-linked immunosorbent assay (ELISA) for C5a, GM-CSF, interferon-γ (IFN-γ) and tumor necrosis factor α (TNFα). Although secreted IFN-γ levels were below detection limits, the ELISA measurements largely confirmed the induction of monocyte C5a, GM-CSF and TNFα secretion by PMap, albeit that platelets more potently induced GM-CSF production in monocytes than PMap (Figure 7b).
Figure 7.
Secretome profile and concentration analysis of cytokine mediators produced by monocytes interacting with PMap or platelets. Monocytes on fibronectin-coated plates (3 × 105 cells/well, or 1.5 × 106 cells/ml) were coincubated with vehicle control (ctrl), PMap (1.5 × 107 cells/ml) or resting platelets (plts, 1.5 × 107 cells/ml) for 44 h. Supernatants were analyzed (a) for a panel of bioactive mediators using a cytokine array kit, and data were expressed as percentage differences compared with a vehicle control or (b) concentration determination of C5a, GM-CSF, IFN-γ and TNFα by conventional ELISA. Mean±S.E.M. (n=3), *P<0.05 versus control, °P<0.05 versus platelets
Discussion
In the present paper, we have identified a potent modulating role of PMap on monocyte functions. These microparticles, formed by ageing platelets in an apoptosis-like process, appear to display a characteristic ‘pro-adhesive' membrane surface with integrins (GPIIb/IIIa) in the activated conformation and exposure of phosphatidylserine, which may facilitate the interaction with monocytes. In the literature, the formation of platelet–leukocyte coaggregates is studied extensively, for example, following activation of the platelet protease-activated receptor (thrombin receptor) and P2Y12 receptors. Very little, however, is still known about the formation of platelet microparticle–leukocyte complexes. The present study is the first to demonstrate the role of platelet microparticles, produced from ageing, and apoptotic platelets in leukocyte function and differentiation. Jointly our results show that PMap interacting with monocytic cells and primary monocytes evoke potent effects in achieving cell polarization and differentiation. In our implemented model of coincubation of platelets and PMap with monocytic cells in vitro, we took effort to exclude confounding effects of PMap generated by platelets during the long-term coculture process. Indeed, the amount of spontaneously generated PMap by platelets was found to be functionally negligible.
The precise signaling pathways that are triggered in monocytes by PMap still need to be identified. One conceivable mechanism supported by our findings is triggering via chemokines such as CCL5, which is carried and secreted by activated platelets16, 21, 22 and activation-induced platelet microparticles.14, 23 Such a mechanism explains the chemotactic effect of PMap causing monocytic cell migration and has recently been implied in the modulation of T-cell differentiation by platelets.24 In addition, the PMap-induced activation of monocytes likely relies on specific molecular interactions with receptors on the microparticle surface, including lipids (phosphatidylserine),25, 26 adhesive receptors (for example, activated GPIIb/IIIa) and GP counter-receptors (P-selectin), similar to those identified for monocyte activation by platelets.22, 27 Furthermore, also endocytosis of PMap following adhesion may force the monocytes to enter specific differentiation pathways. Interestingly, it appeared that the PMap-induced adhesion to monocytic cells was abolished by cell treatment with wortmannin, suggesting a role of phosphoinositide 3-kinase signaling pathways in the monocyte activation (E. Vasina, unpublished data and Barry et al.28).
Circulating monocytes appear to form different subsets with discrete properties and functions. A conventional division is into ‘proinflammatory' M1 monocytes, which secrete proinflammatory cytokines and have a cytotoxic role, and ‘resident' M2 monocytes, which participate in tissue remodeling, wound healing and angiogenesis.29 The M2 monocytes tend to differentiate into professional phagocytes characterized by potent endocytotic activity.18 Knowing that the formation of resident monocytes is accompanied by downregulation of CCR2 and upregulation of CCR5 and CXCR4 surface receptors, we observed a marked potential of PMap to differentiate monocytes in the direction of M2 cells and professional phagocytes. This was confirmed by an increased expression of adhesive integrins (CD11b), of the respective LDL and oxLDL receptors, CD68 and CD36, and a higher uptake of oxLDL. In support of the notion that M2 monocytes preferentially differentiate into dendritic cells,18 we detected elevated amounts of GM-CSF in the secretome of PMap-stimulated monocytes, a stimulus for differentiation to dendritic cells.30
The present findings likely have important implications for our understanding of the pathology of atherosclerosis. Initial support for a proatherogenic role of PMap-activated monocytes comes from their enhanced adhesion to endothelial cells under flow conditions that precedes plaque infiltration and is particularly relevant in early stages of atherosclerosis.16 This increased adhesion is likely mediated by receptors on the microparticles or by an increased expression of adhesive monocyte receptors (for example, CD11b, CD31)22, 31 and may be further augmented by the expression of chemokines such as CCL5.23 In addition, the monocyte subset LyCloCCR2−CCR5+ was shown to use the CCL5 receptor CCR5 when entering atherosclerotic plaques in mice,32 and the blocking of CCR5 led to a reduction of atherosclerosis in several studies.33 Analogously, in our experiments, the polarization of human monocytes by PMap into the CD14+CD16+CCR2−CCR5+CXCR4++ phenotype is suggestive for the formation of a proatherogenic monocyte subset, residing in human plaques and aggravating atherosclerosis. This suggestion is supported by the observation that PMap-activated monocytic cells upregulate the expression of phagocyte markers (CD14, CD36, CD68), and massively release MMP and H2O2, which are factors that constitute to plaque destabilization and eventual rupture, a clinically precipitating event in atherosclerotic disease.
Earlier studies have shown that the production of PMap is a continuous process in stored platelets in the apparent absence of platelet activation.10 In the circulation, like other apoptotic bodies, these microparticles will be actively removed by binding and phagocytosis.11 Conceivably, this removal is mediated by interactions of PMap with leukocytes, particularly monocytes. Indeed, coaggregates of platelet fragments and monocytes could be detected in blood from healthy subjects (E. Vasina, unpublished data). On the other hand, such interactions may contribute to long-term leukocyte differentiation, as we have pointed out in this paper, and can even be a polarizing factor in the development of resident monocytes. However, further work needs to be done to demonstrate that PMap-primed monocytes differentiate into M2 cells and resident macrophages/dendritic cells in vivo.
The present results even suggest that PMap have an immunomodulating potential by stimulating their own phagocytic removal, as CD11b, CD1434 and CD3625 all have been implicated in the uptake of apoptotic material. This suggestion is confirmed by the plethora of proinflammatory and non-inflammatory cytokines, including G-CSF, GM-CSF and TNFα, that are secreted by PMap contact with monocytes. In conclusion, this is the first study to demonstrate that microparticles spontaneously arising from apoptotic platelets are able to promote monocytes towards a resident phagocytic phenotype, changing their behavior and activation state, thus, presenting a novel mechanism in which platelets and their products might influence the progression of atherosclerotic disease.
Materials and Methods
Monocytic cells and primary monocytes
Human acute monocytic leukemia THP-1 cells were cultured in RPMI-1640 medium with -glutamine and 10% fetal bovine serum (FBS). MCs were obtained from buffy coats of peripheral blood from healthy human donors who gave informed consent. Monocytes were separated from neutrophils by Ficoll density gradient centrifugation and further isolated by negative selection using a MACS Monocyte isolation kit II (Miltenyi Biotech, Bergisch Gladbach, Germany), according to the manufacturer's protocol. Purity of the monocyte preparation was analyzed by flow cytometry based on cell forward scatter and side scatter and amounted to >97%.
Platelet-derived microparticles and washed platelets
PMap were isolated from platelet concentrates in plasma, which were stored for 5 days under standard blood bank conditions (Uniklinikum Aachen, Germany). Platelets were removed by rapid centrifugation at 4000 × g for 5 min. The platelet-free plasma preparations were centrifuged at 20 000 × g for 60 min. Pellets containing PMap were resuspended in Hepes buffer pH 7.45 (136 mmol/l NaCl, 10 mmol/l Hepes, 2.7 mmol/l KCl, 2 mmol/l MgCl2, 0.1% bovine serum albumin and 0.1% glucose), filtered through a 0.8-μm filter in order to remove residual platelets, and pelleted again at 20 000 × g for 40 min. Supernatants were used for control experiments. After resuspension of the pellet in Hepes buffer, PMap labeled with anti-CD61-FITC mAb (BD Biosciences, Heidelberg, Germany) were counted by flow cytometry in the presence of fixed numbers of 6 μm calibration beads (BD Biosciences). Event sizes were estimated by addition of 1 μm and 6 μm fluorescent beads (Polysciences, Eppelheim, Germany). The CD61-positive events <6 μm were counted as PMap. Suspensions of PMap were directly used or snap-frozen in liquid nitrogen. The isolated PMap were virtually negative for the exosome marker CD63.
Platelets were isolated from freshly obtained human blood, as described.35
Flow cytometric determination of cell surface markers
Using flow cytometry on a fluorescence-activated cell sorting Canto II (BD Biosciences), platelets and PMap were characterized for surface protein levels with fluorescently labeled antibodies against CD61, CD62P or activated GPIIb/IIIa (PAC-1mAb; BD Biosciences). Expression of phosphatidylserine was probed with APC-annexin A5 (BD Biosciences). THP-1 cells or monocytes in suspension were stained (30 min, 4 °C) with mouse anti-human antibodies against CD11b, CD31 (Sigma, St. Louis, MO, USA); CD14, CD16, CCR2, CCR5, CXCR4 (BD Biosciences); or CD36 (ImmunoTools, Friesoythe, Germany) and prepared as described before.36 Intracellular CD68 was detected in cells fixed with paraformaldehyde, permeabilized with 0.5% Triton-X-100, and stained with mouse anti-human CD68mAb (BD Biosciences). Corresponding negative control antibodies were IgG1 (Sigma); IgG2b, κ (BD Biosciences) or IgG2a (ImmunoTools) and used at recommended dilutions. Expression levels are presented as geometric means of fluorescence histograms, corrected for the values obtained with isotype control antibodies and as representative histograms (FlowJo software, Tree Star, Inc., Ashland, OR, USA).
Adhesion of monocytic cells
Adhesion of THP-1 cells was measured in RPMI-1640 medium supplemented with 5% FBS, as described.37 Cells treated with vehicle, PMA (10 ng/ml, Calbiochem, Darmstadt, Germany), platelets or PMap, were left to adhere to fibronectin-coated wells at 37 °C. At indicated times, vital cells were labeled with BCECF acetoxymethyl ester (1 μg/ml, Sigma) for 1 h, after which fluorescence was measured of all cells and of adherent cells after a wash, using a Spectra FluoPlus reader (Tecan, Männedorf, Switzerland). Adhesion was expressed as percentage of fluorescence of all cells. Incubations were performed in triplicate. In parallel experiments, labeled cells were scraped from wells and counted via calibrated flow cytometry, with essentially the same results.
Adhesion of primary monocytes to a monolayer of human aorta endothelial cells (passage 6–7) under flow conditions (0.1 ml/min, 1.5 dynes/cm2) was measured by microscopic video imaging.17 Cells were preincubated with Hepes buffer (vehicle), PMap or platelets for 44 h at 37 °C. During perfusion adherent monocytes were counted in at least five random microscopic fields.
Transwell cell migration assay
In 5-μm pore Transwell plates (Corning Life Sciences, Lowell, MA, USA), bottom chambers were filled with vehicle, CCL2 (50 ng/ml, PeproTech, Hamburg, Germany) or PMap (108 cells/ml) alone, or coincubated with isotype control (IgG1) or blocking antibodies against CCL5 or CXCL7 (10 μg/ml, 40 min at RT, all from R&D Systems, Minneapolis, MN, USA) in RPMI-1640 medium. Upper chambers contained THP-1 cells in RPMI-1640 medium with 5% FBS (3 × 104 cells/well). After 24 h incubation at 37 °C, the cells in bottom and upper wells were counted by calibrated flow cytometry.38 Assays were run in triplicate. Percentages of transmigrated cells were calculated. Total cell counts remained unchanged at the various incubation conditions.
Monocyte cell function assays
Proliferation of THP-1 cells was measured after culturing in RPMI-1640 medium with 5% FBS at 37 °C. Cells were seeded into 96-well plates (3 × 104 cells/well, or 1.5 × 105 cells/ml), coated with fibronectin and stimulated, as described. At indicated times, adherent cells were labeled with DAPI (0.5 μg/ml for 1 h at 37 °C), and fluorescence was measured in a Spectra FluoPlus plate reader. Calibrations with known cell counts were performed to convert fluorescence values to cell numbers. In parallel, fractions of viable cells were determined microscopically by staining viable cells with calcein acetoxymethyl ester (1 μg/ml) and counter-staining leaky, non-viable cells with ethidium bromide (1 μg/ml). Fractions of non-viable THP-1 cells were <0.3% after 44 h and 6–7% after 7 days, regardless of the presence or absence of PMA, platelets or PMap.
Binding and uptake of oxLDL was determined in 24-well plates. THP-1 cells (8 × 105 cells/ml) were incubated with PMap (8 × 106 cells/ml), resting platelets (8 × 106 cells/ml) or PMA (10 ng/ml). At time points of interest, DiI (1,1′,di-octadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate)-labeled oxLDL (25 μg/ml) was coincubated for 4 h. After labeling, adherent cells were resuspended in phosphate-buffered saline containing 5 mmol/l ethylenediaminetetraacetic acid, and left for 15 min at room temperature (RT) to allow dissociation of surface-bound oxLDL. After a wash step (centrifugation at 300 × g for 10 min), fluorescence was analyzed by flow cytometry (phycoerythrin channel). Oxidized LDL and DiI-labeled oxLDL were prepared from pooled human plasma and labeled as described.39
Actin filaments in cultured THP-1 cells were determined after fixation, permeabilization and staining with fluorescently labeled FITC (fluorescein isothiocyanate)-phalloidin (1 mg/ml for 15 min, Invitrogen, Darmstadt, Germany), as described before.10
Determination of monocyte cell secretion products
To assess for secreted MMP, cell supernatants were subjected to acrylamide gel electrophoresis, and the collagenolytic activity in gels was determined by classical zymography. Produced H2O2 in cell supernatants was quantified from the conversion rate of tetramethylbenzidine substrate with a kit from Calbiochem.
Human primary monocytes in fibronectin-coated well plates were incubated with PMap or platelets for 44 h (37 °C). Supernatants were analyzed for secreted mediators using a commercial human cytokine array kit (R&D Systems) for proteome profiling of secreted cytokines or conventional ELISA kits for C5a (R&D Systems), GM-CSF (PeproTech), IFN-γ and TNFα (both from eBioscience, San Diego, CA, USA).
Electron and confocal microscopy
Platelets or PMap, alone or coincubated with THP-1 cells, were immobilized on poly-lysine surfaces, and fixed with 4% paraformaldehyde. After overnight dehydration, samples were subjected to scanning electron microscopy, as described.40 PMap were labeled with anti-human CD61-FITC mAb (BD Biosciences) for 1 h and then coincubated with THP-1 cells (1 × 106 cells/ml) for 4 h. Afterwards, the cells were labeled with a leukocyte marker anti-human CD45-APC mAb (eBioscience) for 40 min (all incubations at RT). Finally, the cells were mounted on a coverslip without fixation and visualized using a 2100 MP (Bio-Rad, Hercules, CA, USA) microscope set in confocal mode.
Statistical analyses
Data are given as mean±S.E.M. Differences with P<0.05 were considered to be statistically significant (Student's t-test or analysis of variance where applicable).
Acknowledgments
This study was supported by the Deutsche Forschungs Gemeinschaft (DFG FOR809), the Euregio Cardiovascular International Research Training Group, GRK1508 (EuCAR) ‘Arterial Remodeling' and the Interdisciplinary Center for Clinical Research (IZKF Aachen, SP1 T5 and SP2 K1). We thank Dr. Sebastian Mause for helpful discussions and Martin Schmitt for assistance with the confocal microscopy.
Glossary
- APC
allophycocyanin
- BCECF
2,7-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein
- C5a
complement component 5a
- DiI
1,1′,di-octadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- FI
fluorescent intensity
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte macrophage colony-stimulating factor
- GP
glycoprotein
- H2O2
hydrogen peroxide
- IFN-γ
interferon-γ
- LDL
low-density lipoprotein
- oxLDL
oxidized low-density lipoprotein
- PMA
phorbol myristate acetate
- PMap
apoptosis-induced platelet microparticles
- RT
room temperature
- S.E.M.
standard error of the mean
- TNFα
tumor necrosis factor α
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Finazzi-Agró
Supplementary Material
References
- Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- Berckmans RJ, Nieuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85:639–646. [PubMed] [Google Scholar]
- Italiano JE, Jr, Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010;17:578–584. doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shantsila E, Kamphuisen PW, Lip GY. Circulating microparticles in cardiovascular disease: implications for atherogenesis and atherothrombosis. J Thromb Haemost. 2010;8:2358–2368. doi: 10.1111/j.1538-7836.2010.04007.x. [DOI] [PubMed] [Google Scholar]
- Leroyer AS, Tedgui A, Boulanger CM. Role of microparticles in atherothrombosis. J Intern Med. 2008;263:528–537. doi: 10.1111/j.1365-2796.2008.01957.x. [DOI] [PubMed] [Google Scholar]
- Heemskerk JW, Kuijpers MJ, Munnix IC, Siljander PR. Platelet collagen receptors and coagulation. A characteristic platelet response as possible target for antithrombotic treatment. Trends Cardiovasc Med. 2005;15:86–92. doi: 10.1016/j.tcm.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Grozovsky R, Hoffmeister KM, Falet H. Novel clearance mechanisms of platelets. Curr Opin Hematol. 2010;17:585–589. doi: 10.1097/MOH.0b013e32833e7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SP, Schoenwaelder SM. Procoagulant platelets: Are they necrotic. Blood. 2010;116:2011–2018. doi: 10.1182/blood-2010-01-261669. [DOI] [PubMed] [Google Scholar]
- Cauwenberghs S, Feijge MA, Harper AG, Sage SO, Curvers J, Heemskerk JW. Shedding of procoagulant microparticles from unstimulated platelets by integrin-mediated destabilization of actin cytoskeleton. FEBS Lett. 2006;580:5313–5320. doi: 10.1016/j.febslet.2006.08.082. [DOI] [PubMed] [Google Scholar]
- Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97:425–434. [PubMed] [Google Scholar]
- Vasina E, Heemskerk JW, Weber C, Koenen RR. Platelets and platelet-derived microparticles in vascular inflammatory disease. Inflamm Allergy Drug Targets. 2010;9:346–354. doi: 10.2174/187152810793938008. [DOI] [PubMed] [Google Scholar]
- Garcia BA, Smalley DM, Cho H, Shabanowitz J, Ley K, Hunt DF. The platelet microparticle proteome. J Proteome Res. 2005;4:1516–1521. doi: 10.1021/pr0500760. [DOI] [PubMed] [Google Scholar]
- Mause SF, Ritzel E, Liehn EA, Hristov M, Bidzhekov K, Muller-Newen G, et al. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation. 2010;122:495–506. doi: 10.1161/CIRCULATIONAHA.109.909473. [DOI] [PubMed] [Google Scholar]
- von Hundelshausen P, Koenen RR, Weber C. Platelet-mediated enhancement of leukocyte adhesion. Microcirculation. 2009;16:84–96. doi: 10.1080/10739680802564787. [DOI] [PubMed] [Google Scholar]
- Sarabi A, Kramp BK, Drechsler M, Hackeng TM, Soehnlein O, Weber C, et al. CXCL4L1 inhibits angiogenesis and induces undirected endothelial cell migration without affecting endothelial cell proliferation and monocyte recruitment. J Thromb Haemost. 2011;9:209–219. doi: 10.1111/j.1538-7836.2010.04119.x. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Galt SW, Lindemann S, Medd D, Allen LL, Kraiss LW, Harris ES, et al. Differential regulation of matrix metalloproteinase-9 by monocytes adherent to collagen and platelets. Circ Res. 2001;89:509–516. doi: 10.1161/hh1801.096339. [DOI] [PubMed] [Google Scholar]
- Jurasz P, Chung AW, Radomski A, Radomski MW. Nonremodeling properties of matrix metalloproteinases: the platelet connection. Circ Res. 2002;90:1041–1043. doi: 10.1161/01.res.0000021398.28936.1d. [DOI] [PubMed] [Google Scholar]
- von Hundelshausen P, Weber KS, Huo Y, Proudfoot AE, Nelson PJ, Ley K, et al. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103:1772–1777. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
- Weyrich AS, Elstad MR, McEver RP, McIntyre TM, Moore KL, Morrissey JH, et al. Activated platelets signal chemokine synthesis by human monocytes. J. Clin. Invest. 1996;97:1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mause SF, von Hundelshausen P, Zernecke A, Koenen RR, Weber C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol. 2005;25:1512–1518. doi: 10.1161/01.ATV.0000170133.43608.37. [DOI] [PubMed] [Google Scholar]
- Gerdes N, Zhu L, Ersoy M, Hermansson A, Hjemdahl P, Hu H, et al. Platelets regulate CD4+ T-cell differentiation via multiple chemokines in humans. Thromb Haemost. 2011;106:353–362. doi: 10.1160/TH11-01-0020. [DOI] [PubMed] [Google Scholar]
- Tait JF, Smith C. Phosphatidylserine receptors: role of CD36 in binding of anionic phospholipid vesicles to monocytic cells. J Biol Chem. 1999;274:3048–3054. doi: 10.1074/jbc.274.5.3048. [DOI] [PubMed] [Google Scholar]
- Freyssinet JM, Toti F. Formation of procoagulant microparticles and properties. Thromb Res. 2010;125 (Suppl 1:S46–S48. doi: 10.1016/j.thromres.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Theilmeier G, Lenaerts T, Remacle C, Collen D, Vermylen J, Hoylaerts MF. Circulating activated platelets assist THP-1 monocytoid/endothelial cell interaction under shear stress. Blood. 1999;94:2725–2734. [PubMed] [Google Scholar]
- Barry OP, Pratico D, Savani RC, FitzGerald GA. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest. 1998;102:136–144. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa Martins PA, van Gils JM, Mol A, Hordijk PL, Zwaginga JJ. Platelet binding to monocytes increases the adhesive properties of monocytes by up-regulating the expression and functionality of beta1 and beta2 integrins. J Leukoc Biol. 2006;79:499–507. doi: 10.1189/jlb.0605318. [DOI] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen RR, Weber C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat Rev Drug Discov. 2010;9:141–153. doi: 10.1038/nrd3048. [DOI] [PubMed] [Google Scholar]
- Devitt A, Pierce S, Oldreive C, Shingler WH, Gregory CD. CD14-dependent clearance of apoptotic cells by human macrophages: the role of phosphatidylserine. Cell Death Differ. 2003;10:371–382. doi: 10.1038/sj.cdd.4401168. [DOI] [PubMed] [Google Scholar]
- Feijge MA, van Pampus EC, Lacabaratz-Porret C, Hamulyak K, Levy-Toledano S, Enouf J, et al. Inter-individual variability in Ca2+ signalling in platelets from healthy volunteers: effects of aspirin and relationship with expression of endomembrane Ca2+-ATPases. Br J Haematol. 1998;102:850–859. doi: 10.1046/j.1365-2141.1998.00844.x. [DOI] [PubMed] [Google Scholar]
- Hristov M, Gumbel D, Lutgens E, Zernecke A, Weber C. Soluble CD40 ligand impairs the function of peripheral blood angiogenic outgrowth cells and increases neointimal formation after arterial injury. Circulation. 2010;121:315–324. doi: 10.1161/CIRCULATIONAHA.109.862771. [DOI] [PubMed] [Google Scholar]
- Fraemohs L, Koenen RR, Ostermann G, Heinemann B, Weber C. The functional interaction of the beta 2 integrin lymphocyte function-associated antigen-1 with junctional adhesion molecule-A is mediated by the I domain. J Immunol. 2004;173:6259–6264. doi: 10.4049/jimmunol.173.10.6259. [DOI] [PubMed] [Google Scholar]
- Liehn EA, Piccinini AM, Koenen RR, Soehnlein O, Adage T, Fatu R, et al. A new monocyte chemotactic protein-1/chemokine CC motif ligand-2 competitor limiting neointima formation and myocardial ischemia/reperfusion injury in mice. J Am Coll Cardiol. 2010;56:1847–1857. doi: 10.1016/j.jacc.2010.04.066. [DOI] [PubMed] [Google Scholar]
- Groeneweg M, Vergouwe MN, Scheffer PG, Vermue HP, Sollewijn Gelpke MD, Sijbers AM, et al. Modification of LDL with oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (oxPAPC) results in a novel form of minimally modified LDL that modulates gene expression in macrophages. Biochim Biophys Acta. 2008;1781:336–343. doi: 10.1016/j.bbalip.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Siljander P, Farndale RW, Feijge MA, Comfurius P, Kos S, Bevers EM, et al. Platelet adhesion enhances the glycoprotein VI-dependent procoagulant response: Involvement of p38 MAP kinase and calpain. Arterioscler Thromb Vasc Biol. 2001;21:618–627. doi: 10.1161/01.atv.21.4.618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.