Abstract
Cilostazol is a selective inhibitor of phosphodiesterase 3 that increases intracellular cAMP levels and activates protein kinase A, thereby inhibiting vascular smooth muscle cell (VSMC) proliferation. We investigated whether AMP-activated protein kinase (AMPK) activation induced by heme oxygenase-1 (HO-1) is a mediator of the beneficial effects of cilostazol and whether cilostazol may prevent cell proliferation and reactive oxygen species (ROS) production by activating AMPK in VSMC. In the present study, we investigated VSMC with various concentrations of cilostazol. Treatment with cilostazol increased HO-1 expression and phosphorylation of AMPK in a dose- and time-dependent manner. Cilostazol also significantly decreased platelet-derived growth factor (PDGF)-induced VSMC proliferation and ROS production by activating AMPK induced by HO-1. Pharmacological and genetic inhibition of HO-1 and AMPK blocked the cilostazol-induced inhibition of cell proliferation and ROS production.These data suggest that cilostazol-induced HO-1 expression and AMPK activation might attenuate PDGF-induced VSMC proliferation and ROS production.
Keywords: Cilostazol, Proliferation, ROS, AMPK, HO-1
INTRODUCTION
The proliferation of vascular smooth muscle cell (VSMC) plays a vital role in vascular diseases such as atherosclerosis and hypertension [1,2], which are most pronounced in coronary arteries, thereby producing acute coronary syndrome in affected subjects [3]. Consequently, anti-proliferation of VSMC is considered a marker of vascular health and can be used to gauge cardiovascular risk [4]. Therefore, strategies aimed at inhibiting VSMC proliferation might be of benefit in the prevention of atherosclerosis. This VSMC proliferation contributes to dominant cellular events in the re-narrowing of the vasculature, which causes the release of growth factors. Platelet-derived growth factor (PDGF) is a growth factor induced by VSMC, vascular endothelial cells, platelets or macrophages and plays an important role in intimal proliferation [5]. After stimulating cell growth, PDGF can induce proliferation of VSMC. Inhibition of PDGF-stimulated VSMC proliferation therefore represents an important point in the therapeutics for many vascular diseases.
ROS have been proposed to be important signaling molecules in many biological events such as cell proliferation which is important in the pathogenesis of intimal thickening in atherosclerosis [6]. One mechanism by which ROS provoked hypertension is via binding of nitric oxide (NO) and also increases intracellular free Ca2+ levels, which contribute to increased vascular tone [7]. ROS also has been shown to mediate the proliferative effects of many growth factors in VSMC. Several studies have demonstrated that PDGF can evoke intracellular oxidative stress and ROS may act as key mediator in the regulation of PDGF-induced VSMC proliferation [6,8].
AMP-activated protein kinase (AMPK) has an important function in cellular energy status. AMPK is activated by stress such as physical exercise and hypoxia, which increase the cellular AMP/ATP ratio. AMPK has also been implicated in the regulation of physiological signals, such as inhibition of cholesterol, fatty acid, and protein synthesis, and enhancement of glucose uptake and blood flow [9,10]. AMPK activation has several effects on vascular function and improves vascular abnormality. Therefore, AMPK may serve as an important target to inhibit protein synthesis and cell growth and to treat vascular proliferative diseases.
Heme oxygenase-1 (HO-1) is known for its cytoprotective effects against oxidative damage [11]. Activation of HO-1 has therapeutically beneficial effects in vascular narrowing caused by VSMC proliferation [12]. An increase in intracellular cAMP-coupled protein kinase A (PKA) activation also has been noted to induce HO-1 gene expression in cultured VSMC [13].
Cilostazol increases intracellular cAMP concentrations by selectively blocking phosphodiesterase 3 (PDE-3), inhibiting platelet aggregation and inducing peripheral vasodilation [14]. Cilostazol is a potent antiplatelet drug used in clinical practice to treat patients with chronic vascular obstruction [15]. Recent clinical studies indicate that cilostazol inhibits the proliferation of VSMC [16]. One mechanism by which cilostazol may inhibit VSMC proliferation is via an increase in intracellular cAMP, because cAMP inhibits the proliferation of VSMC.
In this study, we investigated whether cilostazol suppressed proliferation of VSMC and ROS production via activation of AMPK and HO-1 expression. It is important to note how cilostazol inhibits VSMC proliferation. However, the effects of cilostazol mediated by activation of AMPK and HO-1 expression have not yet been clarified. These results may provide a better understanding of the anti-proliferation effect of cilostazol.
METHODS
Materials
Dulbecco's modified eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Hyclone (South Logan, UT, USA). RIPA protein extract buffer was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-AMPK, anti-phospho-AMPK and human platelet-derived growth factor BB (hPDGF-BB) were purchased from Cell Signaling Technology (Beverly, MA, USA). The HO-1 antibody was purchased from Stressgen Bioreagents (Ann Arbor, MI, USA). SNPP IX was purchased from Frontier Scientific (South Logan, UT, USA). Compound C was purchased from Calbiochem (San Diego, CA, USA). AMPK and HO-1 siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). 2',7'-dichlorofluorescein diacetate (DCF-DA) and lipofectamine 2,000 were purchased from Invitrogen (Carlsbad, CA, USA). Anti-β-actin and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cilostazol was donated by Otsuka Pharmaceuticals (Tokushima, Japan) and dissolved in DMSO.
Cell culture
Sprague-Dawley rats were anesthetized with pentobarbital (50 mg/kg). VSMCs were isolated from the thoracic aorta. The cells were processed using a 1 mm chop setting in a 10 cm culture dish, and cultured with 50% FBS-DMEM with 1% antibiotics-antimycotics (penicillin 10,000 U/ml, amphotericin B 25 µg/ml, streptomycin 10,000 µg/ml) in a CO2 incubator. Aortic VSMCs were maintained in DMEM with 10% FBS and 1% antibiotic-antimycotic. We used VSMC from passages 4 to 8 at 70~90% confluence in 10 cm dishes, and cell growth was arrested by incubation of the cells in serum-free DMEM for 24 hr prior to use.
Western blot analysis
Whole cell extracts were prepared by lysing the cells in RIPA protein extract buffer. The protein concentration was quantified with protein assay reagent from Bio-Rad (Hercules, CA, USA). Equal amounts of protein were mixed with sodium dodecyl sulfate (SDS) sample buffer and incubated for 5 min at 100℃ before loading. Total protein samples (30 µg) were subjected to 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) for 2 hr at 100 V. The separated proteins were electrophoretically transferred onto a PVDF membrane for 1 hr 20 min at 100 V. The membranes were blocked with 5% non-fat milk in phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T) for 2 hr at room temperature. The membranes were then incubated with the primary antibodies (1 : 1,000) in 5% skim milk in PBS overnight at 4℃. The membranes were then washed with four changes of wash buffer (0.05% Tween 20 in PBS) and incubated for 1 hr at room temperature in PBS-T containing anti-rabbit (Stressgen, Ann Arbor, MI, USA) or anti-mouse IgG (Santa Cruz, CA, USA) antibodies. Finally, after three more rinses with wash buffer, the membranes were exposed to ECL (Neuronex, Daegu, Korea) or ECL plus (GE Healthcare, Buckinghamshire, UK) western blot detection reagents.
Cell proliferation assay
Cell proliferation was analyzed using the MTT and bromodeoxyuridine (BrdU) assays. VSMCs were seeded on 24-well plates at 1×104 cells per well in DMEM supplemented with 10% FBS. After different treatments, 50 µl of 1 mg/ml MTT solution was added to each well (0.1 mg/well) and incubated for 4 hr. The supernatants were aspirated, and the formazan crystals in each well were solubilized with 200 µl of dimethyl sulfoxide (DMSO). An aliquot of this solution (10 µl) was placed in each well of 96-well plates. Cell proliferation was assessed by measuring the absorbance at 570 nm using a microplate reader. The incorporation of BrdU was also analyzed using a commercial kit (Roche Diagnostics GmbH, Mannheim, Germany).
Experiments using RNA interference
Transfection of VSMCs with siRNA was performed using Lipofectamine 2,000, according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). Aliquots of 1×104 cells were plated on 6-well plates the day before transfection and grown to about 70% confluence. The cells were then transfected with 10 µM AMPK or HO-1 siRNA (Santa Cruz Biotechnology Inc., Santa Cruz, CA) plus100 pmol of Lipofectamine for 6 hr in Opti-MEM®I reduced serum medium (Invitrogen, Carlsbad, CA, USA). Following an incubation period of 48 hr, the protein level was measured using western blot analysis.
Measurement of intracellular ROS
Intracellular ROS production was measured by 2',7'-dichlorofluorescein fluorescence using live imaged laser scanning microscopy and fluorescence-activated cell sorting (FACS) analysis. Cells were grown in 60 mm dishesfollowing treatment with or without PDGF. Subconfluentcells were incubated in the dark for 10 min in the presence of 10 µM DCF-DA. ROS production was detected from the oxidation of DCF using a fluorescein isothiocyanate (FITC) filter set. For FACS analysis, cells were treated with cilostazol and incubated with DCF-DA at a final concentration of 10 µM for 30 min at 37℃. The treated cells were washed twice with PBS to remove the extracellular compounds and resuspended in 0.5 ml of PBS for analysis. The change in fluorescence intensity was monitored by flow cytometry (FACScan, Becton-Dickinson, San Jose, CA, USA).
Statistical analysis
All data are represented as the mean±S.E.M. Differences between data sets were assessed by one-way analysis of variance (ANOVA) or Bonferroni's test. A value of p<0.05 was considered statistically significant.
RESULTS
Cilostazol induces HO-1 expression and activates AMPK in VSMC
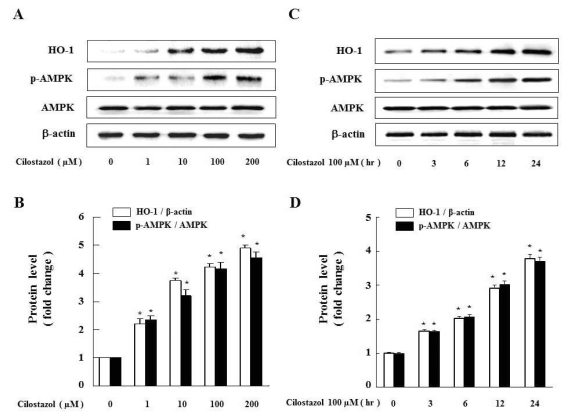
To examine whether cilostazol induces HO-1 and activates AMPK in VSMC, we measured the protein level after treatment with cilostazol at the indicated dose and time. As shown in Fig. 1A, cilostazol dose-dependently increased the levels of both HO-1 expression and AMPK phosphorylation. Cilostazol 100 µM time-dependently increased HO-1 expression and AMPK phosphorylation (Fig. 1C). Each graph represents the densitometry analysis of HO-1 expression and AMPK phosphorylation (Fig. 1B and D).
Fig. 1.
Cilostazol strongly induces HO-1 as well as AMPK activation in VSMC. (A) Cells were exposed to different concentrations (1~200 µM) of cilostazol for 24 hr and (C) treated with 100 µM of cilostazol for the indicated times. Expression of HO-1, p-AMPK and AMPK were analyzed by western blot. Each graph represents the densitometry analysis of HO-1 and p-AMPK (B, D). Data are represented as the mean±S.E.M (n=3). *p-value<0.01 compared with control.
PDGF-stimulated proliferation is inhibited by cilostazol-induced HO-1 and AMPK activation
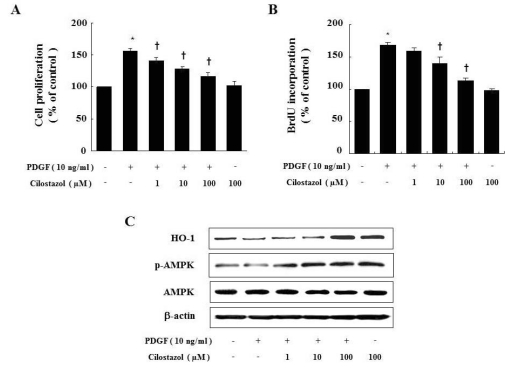
Initially, we examined the effect of cilostazol on cell proliferation and BrdU incorporation of VSMC under PDGF-stimulated conditions. Treatment of VSMC with PDGF (10 ng/ml) significantly increased cell proliferation, as shown in Fig. 2A and B. The inhibitory effect of cilostazol, a well-known treatment for peripheral arterial disease, on VSMC proliferation was examined. As shown in each graph, cilostazol inhibited PDGF-stimulated VSMC proliferation in a dose-dependent manner. However, cilostazol did not change the basal proliferation of VSMC in the absence of PDGF. Next, we examined whether cilostazol induces HO-1 expression and AMPK activation in PDGF-stimulated-condition. Cilostazol increased the levels of these molecules compared to those in the PDGF-treated group (Fig. 2C).
Fig. 2.
Cilostazol inhibits PDGF-stimulated VSMC proliferation via HO-1 and AMPK. Cell proliferation was determined by MTTassay (A) or BrdU incorporation assay (B) in the presence of indicated concentration of cilostazol for 24 hr. Dose-dependent inhibitory effect of cilostazol on percent change in VSMC proliferation is noted. (C) Protein expressions of HO-1 andp-AMPK were determined by western blot analysis. Data are represented as the mean±S.E.M (n=4). *p-value<0.01 compared with control, †p-value<0.05 compared with PDGF.
Cilostazol attenuates PDGF-generated intracellular ROS production
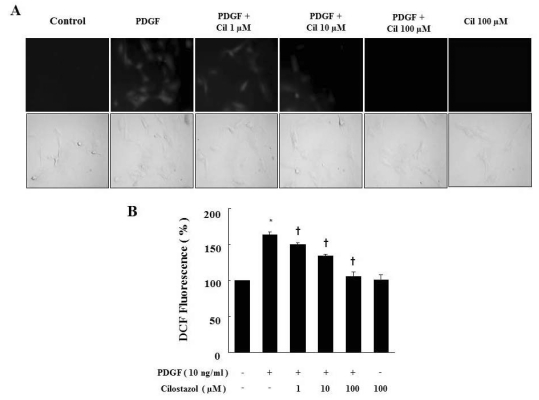
To examine whether cilostazol suppresses PDGF-generated ROS production, we examined the intracellular ROS after treatment with cilostazol at the indicated doses. We first observed intracellular ROS production by fluorescence microscopy after staining the cells with DCF-DA (Fig. 3A). After 2 hr from exposure to PDGF, cells showed morphological characteristics of ROS production. The intracellular ROS was decreased by cilostazol treatment. Next, the intracellular ROS production was determined by measuring the fluorescence intensity (Fig. 3B). Incubation of DCF-DA loaded cells in medium containing PDGF increased the fluorescence intensity. Cilostazol inhibited PDGF-induced ROS production.
Fig. 3.
Cilostazol suppresses ROS production generated by PDGF in VSMC. (A) ROS generated in viable cells produce a uniform bright green color in the cytoplasm and nuclei. For observation of intracellular ROS by fluorescence microscopy, cells were pretreated with cilostazol for 24 hr and then stimulated with PDGF (10 ng/ml) for 2 hr in the presence of 10 µM DCF-DA. (B) ROS production was assessed by FACS analysis. Data are represented as the mean±S.E.M (n=3). *p-value<0.01 compared with control, †p-value<0.01 compared with PDGF. Cil, cilostazol.
Pharmacological inhibition of HO-1 and AMPK reverses the effect of cilostazol on proliferation and ROS production
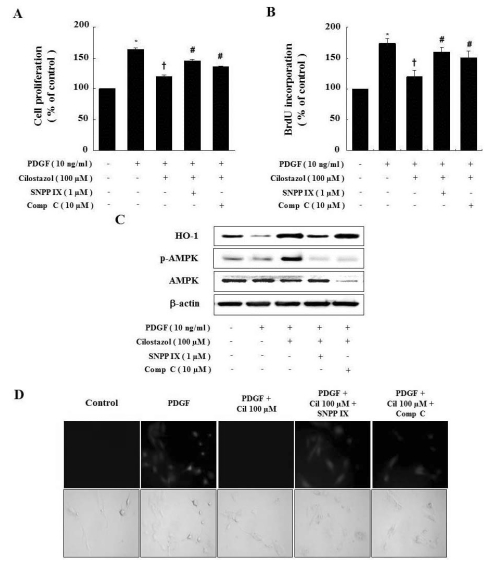
To further demonstrate the interaction of cilostazol, HO-1, and AMPK, we examined the effects of HO-1 and AMPK inhibitors on VSMC proliferation. Cilostazol decreased the PDGF-induced proliferation of VSMC. Abolishing HO-1 and AMPK activity with SNPP IX and compound C restored the cilostazol-induced inhibition of VSMC proliferation (Fig. 4A and B). Interestingly, SNPP IX, an inhibitor of HO-1, suppressed activation of AMPK, but compound C, an inhibitor of AMPK, did not affect HO-1 expression (Fig. 4C). Thus, the HO-1/AMPK signaling pathway is responsible for the anti-proliferative activity of cilostazol, and HO-1 is suggested that possible upstream controller of AMPK as shown in Fig. 4C.
Fig. 4.
Compound C and SNPP IX restore the cilostazol-induced inhibition of VSMC proliferation and ROS production. (A, B) Cells were pretreated with compound C or SNPP IX in the presence of cilostazol and then stimulated with PDGF. Cell proliferation was determined by the MTT assay and BrdU incorporation. (C) Protein expressions of HO-1 and p-AMPK were determined by western blot analysis. Representative blots from three independent experiments were shown. (D) For observation of intracellular ROS by fluorescence microscope, cells were pretreated with compound C or SNPP IX in the presence of cilostazol, and then stimulated with PDGF in the presence of 10 µM DCF-DA. Data are represented as the mean±S.E.M (n=4). *p-value<0.01 compared with control, †p-value<0.01 compared with PDGF, #p-value<0.05 compared with cilostazol+PDGF. Cil, cilostazol; Comp C, compound C.
To examine whether cilostazol-induced HO-1 and AMPK activation suppress PDGF-generated ROS production, we observed morphological levels of ROS by fluorescence microscopy after treating with SNPP IX and compound C (Fig. 4D). After 2 hr from exposure to PDGF, cells showed morphological characteristics of ROS production. The PDGF-generated ROS production was decreased by treatment with cilostazol, but SNPP IX and compound C restored PDGF-generated ROS production.
Genetic inhibition of HO-1 and AMPK reverses the effect of cilostazol on proliferation and ROS production
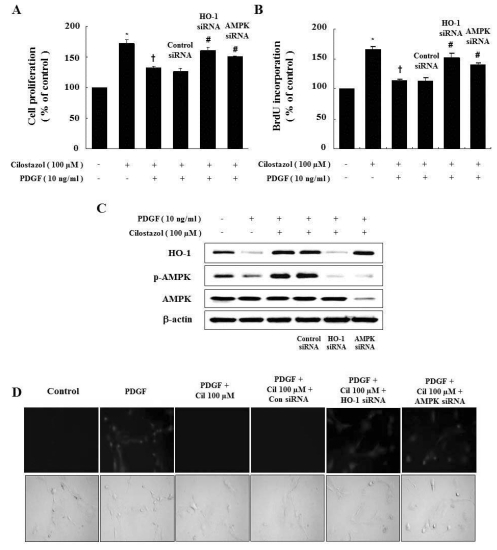
In order to determine if HO-1 expression and AMPK activation by cilostazol is required to inhibit cell proliferation, we carried out experiments with siRNA against HO-1 and AMPK. As shown in Fig. 5A and B, suppression of HO-1 and AMPK by siRNA blocked the anti-proliferative effect of cilostazol. Interestingly, HO-1 siRNA transfection suppressed activation of AMPK, but AMPK siRNA did not affect HO-1 expression based on western blot analysis (Fig. 5C). ROS production was also reversed by HO-1 and AMPK siRNA transfection (Fig. 5D). Therefore, the HO-1/AMPK signaling pathway is responsible for the effects of cilostazol, and HO-1 expression and AMPK activation by cilostazol suppress PDGF-induced cell proliferation and ROS production.
Fig. 5.
Genetic inhibition of HO-1 and AMPK restores the cilostazol-induced inhibition of VSMC proliferation and ROS production. (A, B) Cells were transfected with HO-1 or AMPK siRNA, and then stimulated with PDGF. Cell proliferation was assessed by the MTT assay and BrdU incorporation. (C) Protein expressions of HO-1 and p-AMPK were determined by western blot analysis. Representative blots from three independent experiments are shown. (D) For observation of intracellular ROS by fluorescence microscopy, cells were transfected with HO-1 siRNA or AMPK siRNA in the presence of cilostazol, and then stimulated with PDGF in the presence of 10 µM DCF-DA. Data are represented as the mean±S.E.M (n=4). *p-value<0.01 compared with control, †p-value<0.01 compared with PDGF, #p-value<0.05 compared with cilostazol+PDGF. Cil, cilostazol.
DISCUSSION
Cilostazol has been developed as a PDE-3 inhibitor and is used to treat patients with peripheral vascular disease [16]. In addition, inhibition of VSMC proliferation by cilostazol has been reported [17]. Therefore, it is important to understand how cilostazol inhibits VSMC proliferation. The main purpose of the present study was to demonstrate the anti-proliferative mechanism of cilostazol in VSMC, specifically focused on the AMPK activation and HO-1 expression. We examined that cilostazol-induced AMPK phosphorylation and HO-1 expression.
In the previous study, HO-1 is recognized as a beneficial molecule for protecting against oxidative stresses [18] and vascular constriction as well as proliferation [19]. We first examined whether cilostazol induced HO-1 expression in VSMC, because HO-1 increases the intracellular cAMP levels [20]. HO-1 expression is associated with increases in adiponectin and AMPK activation [21,22]. Our results indicated that cilostazol increased HO-1 and p-AMPK expression with dose-and time-dependent manners (Fig. 1). The present study also demonstrates that cilostazol plays a crucial role in the induction of HO-1 expression and HO-1 induces activation of AMPK in VSMC (Fig. 4C and Fig. 5C).
Weshowed that cilostazol caused phosphorylation of AMPK at Thr172 by inducing HO-1 in VSMC. The extent of AMPK phosphorylation at Thr172 strongly reflects its activity [23]. Cilostazol-induced AMPK activation has an attractive characteristic, because AMPK is reported to mediate beneficial and bio-protective effects of metformin [24] and adiponectin [25]. AMPK is a serine/threonine protein kinase, which serves as an energy sensor in eukaryotic cells. Several studies have revealed that AMPK activation strongly suppresses cell proliferation in normal cells as well as in tumor cells [26].
In the current study, we found that inhibition of PDGF-induced VSMC proliferation by cilostazol was mediated via AMPK phosphorylation and HO-1 expression. Compound C and AMPK siRNA strongly inhibited the cilostazol-induced AMPK phosphorylation, but did not affect expression of HO-1. Thus, inhibition of AMPK is irrelevant to HO-1 expression. However, AMPK phosphorylation and inhibition of VSMC proliferation were completely lost in the presence of the HO-1 inhibitor, SNPP IX, or HO-1 siRNA (Fig. 4A, B and Fig. 5A, B). These findings suggest that the anti-proliferative effect of cilostazol is mediated in part by an HO-1/AMPK signaling pathway. Compound C decreased AMPK expression (Fig. 4C). Although compound C is a well known inhibitor of AMPK and widely used to inhibit AMPK, Compound C also decreased AMPK expression in many experiments [27].
A previous study suggests that the anti-apoptotic effects of cilostazol via cAMP-dependent protein kinase are ascribed to its antioxidant function and its ability to reduce production of ROS [28]. Furthermore, AMPK activation independently regulates ROS production [29]. Therefore, we demonstrated that cilostazol suppresses ROS production in VSMC through AMPK activation. In addition, cilostazol significantly inhibits PDGF-induced ROS production. Thus, cilostazol inhibits PDGF-induced VSMC proliferation and ROS production by phosphorylation of AMPK through HO-1 expression (Fig. 4D and Fig. 5D). These findings suggest that the effect of cilostazol might be mediated by the HO-1/AMPK signaling pathway.
In conclusion, we have demonstrated that cilostazol-induced AMPK activation inhibits PDGF-induced VSMC proliferation and ROS production via HO-1/AMPK pathway. The ability of cilostazol to activate AMPK is promising, since AMPK activations mediate vascular protective effect. Our observations indicate that cilostazol-induced AMPK activation in VSMC may have beneficial effects on vascular proliferative disorders such as atherosclerosis, in addition to its selective inhibition of PDE-3.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (2010-0007386) (2010).
ABBREVIATIONS
- AMPK
AMP-activated protein kinase
- HO-1
hemeoxygenase-1
- PDGF
platelet-derived growth factor
- VSMC
vascular smooth muscle cell
- ROS
reactive oxygen species
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Zhang C. MicroRNA-145 in vascular smooth muscle cell biology: a new therapeutic target for vascular disease. Cell Cycle. 2009;8:3469–3473. doi: 10.4161/cc.8.21.9837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruygrok PN, Muller DW, Serruys PW. Rapamycin in cardiovascular medicine. Intern Med J. 2003;33:103–109. doi: 10.1046/j.1445-5994.2003.00331.x. [DOI] [PubMed] [Google Scholar]
- 4.Braun-Dullaeus RC, Mann MJ, Dzau VJ. Cell cycle progression: new therapeutic target for vascular proliferative disease. Circulation. 1998;98:82–89. doi: 10.1161/01.cir.98.1.82. [DOI] [PubMed] [Google Scholar]
- 5.Yang X, Thomas DP, Zhang X, Culver BW, Alexander BM, Murdoch WJ, Rao MN, Tulis DA, Ren J, Sreejayan N. Curcumin inhibits platelet-derived growth factor-stimulated vascular smooth muscle cell function and injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2006;26:85–90. doi: 10.1161/01.ATV.0000191635.00744.b6. [DOI] [PubMed] [Google Scholar]
- 6.Park J, Ha H, Seo J, Kim MS, Kim HJ, Huh KH, Park K, Kim YS. Mycophenolic acid inhibits platelet-derived growth factor-induced reactive oxygen species and mitogen-activated protein kinase activation in rat vascular smooth muscle cells. Am J Transplant. 2004;4:1982–1990. doi: 10.1111/j.1600-6143.2004.00610.x. [DOI] [PubMed] [Google Scholar]
- 7.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Mesquita FS, Dyer SN, Heinrich DA, Bulun SE, Marsh EE, Nowak RA. Reactive oxygen species mediate mitogenic growth factor signaling pathways in human leiomyoma smooth muscle cells. Biol Reprod. 2010;82:341–351. doi: 10.1095/biolreprod.108.075887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 10.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 11.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Stocker R, Perrella MA. Heme oxygenase-1: a novel drug target for atherosclerotic diseases? Circulation. 2006;114:2178–2189. doi: 10.1161/CIRCULATIONAHA.105.598698. [DOI] [PubMed] [Google Scholar]
- 13.Durante W, Christodoulides N, Cheng K, Peyton KJ, Sunahara RK, Schafer AI. cAMP induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle. Am J Physiol. 1997;273:H317–H323. doi: 10.1152/ajpheart.1997.273.1.H317. [DOI] [PubMed] [Google Scholar]
- 14.Kambayashi J, Liu Y, Sun B, Shakur Y, Yoshitake M, Czerwiec F. Cilostazol as a unique antithrombotic agent. Curr Pharm Des. 2003;9:2289–2302. doi: 10.2174/1381612033453910. [DOI] [PubMed] [Google Scholar]
- 15.Dawson DL, Cutler BS, Meissner MH, Strandness DE., Jr Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double-blind trial. Circulation. 1998;98:678–686. doi: 10.1161/01.cir.98.7.678. [DOI] [PubMed] [Google Scholar]
- 16.Stone WM, Demaerschalk BM, Fowl RJ, Money SR. Type 3 phosphodiesterase inhibitors may be protective against cerebrovascular events in patients with claudication. J Stroke Cerebrovasc Dis. 2008;17:129–133. doi: 10.1016/j.jstrokecerebrovasdis.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Kim MJ, Park KG, Lee KM, Kim HS, Kim SY, Kim CS, Lee SL, Chang YC, Park JY, Lee KU, Lee IK. Cilostazol inhibits vascular smooth muscle cell growth by downregulation of the transcription factor E2F. Hypertension. 2005;45:552–556. doi: 10.1161/01.HYP.0000158263.64320.eb. [DOI] [PubMed] [Google Scholar]
- 18.Ryter SW, Choi AM. Heme oxygenase-1: redox regulation of a stress protein in lung and cell culture models. Antioxid Redox Signal. 2005;7:80–91. doi: 10.1089/ars.2005.7.80. [DOI] [PubMed] [Google Scholar]
- 19.Choi HC, Lee KY, Lee DH, Kang YJ. Heme oxygenase-1 induced by aprotinin inhibits vascular smooth muscle cell proliferation through cell cycle arrest in hypertensive rats. Korean J Physiol Pharmacol. 2009;13:309–313. doi: 10.4196/kjpp.2009.13.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Immenschuh S, Hinke V, Ohlmann A, Gifhorn-Katz S, Katz N, Jungermann K, Kietzmann T. Transcriptional activation of the haem oxygenase-1 gene by cGMP via a cAMP response element/activator protein-1 element in primary cultures of rat hepatocytes. Biochem J. 1998;334:141–146. doi: 10.1042/bj3340141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.L'Abbate A, Neglia D, Vecoli C, Novelli M, Ottaviano V, Baldi S, Barsacchi R, Paolicchi A, Masiello P, Drummond GS, McClung JA, Abraham NG. Beneficial effect of heme oxygenase-1 expression on myocardial ischemia-reperfusion involves an increase in adiponectin in mildly diabetic rats. Am J Physiol Heart Circ Physiol. 2007;293:H3532–H3541. doi: 10.1152/ajpheart.00826.2007. [DOI] [PubMed] [Google Scholar]
- 22.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 23.Hardie DG. The AMP-activated protein kinase pathway--new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 24.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 26.Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation-AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung JY, Choi HC. Aspirin-induced AMP-activated protein kinase activation regulates the proliferation of vascular smooth muscle cells from spontaneously hypertensive rats. Biochem Biophys Res Commun. 2011;408:312–317. doi: 10.1016/j.bbrc.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 28.Kim MJ, Lee JH, Park SY, Hong KW, Kim CD, Kim KY, Lee WS. Protection from apoptotic cell death by cilostazol, phosphodiesterase type III inhibitor, via cAMP-dependent protein kinase activation. Pharmacol Res. 2006;54:261–267. doi: 10.1016/j.phrs.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Merry TL, Steinberg GR, Lynch GS, McConell GK. Skeletal muscle glucose uptake during contraction is regulated by nitric oxide and ROS independently of AMPK. Am J Physiol Endocrinol Metab. 2010;298:E577–E585. doi: 10.1152/ajpendo.00239.2009. [DOI] [PubMed] [Google Scholar]