Abstract
Amphetamine, a synthetic psychostimulant, is transported by the dopamine transporter (DAT) to the cytosol and increases the exchange of extracellular amphetamine by intracellular dopamine. Recently, we reported that the phosphorylation levels of ezrin-radixin-moesin (ERM) proteins are regulated by psychostimulant drugs in the nucleus accumbens, a brain area important for drug addiction. However, the significance of ERM proteins phosphorylation in response to drugs of abuse has not been fully investigated. In this study, using PC12 cells as an in vitro cell model, we showed that amphetamine increases ERM proteins phosphorylation and protein kinase C (PKC) β inhibitor, but not extracellular signal-regulated kinase (ERK) or phosphatidylinositol 3-kinases (PI3K) inhibitors, abolished this effect. Further, we observed that DAT inhibitor suppressed amphetamine-induced ERM proteins phosphorylation in PC12 cells. These results suggest that PKCβ-induced DAT regulation may be involved in amphetmaine-induced ERM proteins phosphorylation.
Keywords: Amphetamine; ERM proteins (ezrin, radixin, moesin); PKCβ; PC12 cells; Dopamine transporter
INTRODUCTION
Drug addiction is a chronic disease, triggered by repeated exposure to substances such as opioids and psychostimulants, and it is generally defined as compulsive drug use and loss of control over drug intake. Exposure to drugs of abuse affects the function of several brain areas including the mesoaccumbens dopamine pathways, originating from the ventral tegmental area (VTA) and projecting to the nucleus accumbens (NAc) and some other forebrain regions, which play an essential role in the development of addictive behaviors [1-3].
At the cellular level, the drugs of abuse interfere with several neurotransmitter systems. However, among those, dopaminergic neurotransmission is known to be most importantly involved in mediating drug addiction [4]. For example, psychostimulants such as amphetamine (AMPH) contribute to the development of addiction by increasing the synaptic availability of dopamine (DA) either by inhibiting their reuptake or by enhancing their release from presynaptic nerve terminals [4,5]. In this process, AMPH is transported through the dopamine transporter (DAT) into the cytosol and increases the intracellular binding sites of the DAT for dopamine, resulting in the exchange of extracellular AMPH by intracellular dopamine, and eventually leading to an increase in extracellular dopamine levels and concomitantly enhance downstream dopamine signaling [6].
Although PC12 cells are not neurons, they contain DA which can be released in response to AMPH analogous to those found in the DA-containing brain structures such as the striatum and the NAc [7] and so they can be a useful single-cell model allowing investigation for the molecular mechanisms of AMPH-induced DA release and its related signaling pathway. For example, it has been reported that acute AMPH transiently and modestly increases either ERK or Akt phosphorylation levels in these cells [8].
The ezrin-radixin-moesin (ERM) proteins are a family of membrane-associated proteins that share a high degree of homology among themselves [9]. They have been implicated in playing important roles in cell-shape determination as well as in signaling pathways [10,11]. Recently it has been shown that the phosphorylation levels of ERM proteins in the NAc can be regulated by acute cocaine suggesting its possible contribution to the process of drug addiction [12].
However, the molecular mechanism for ERM proteins regulation by other signaling pathways in response to drugs of abuse has not been fully investigated. Thus, in this study, we examined whether ERM proteins phosphorylation level is regulated by acute AMPH treatment and which signaling pathway might be involved in this process in PC12 cells.
METHODS
PC12 cells culture
PC12 cells were obtained from the American Type Culture Collection (Rockville, MD, USA). Cells were maintained in a 75-cm2 tissue culture flask in growth medium composed of RPMI1640 medium supplemented with 10% (v/v) heat-inactivated horse serum, 5% heat-inactivated fetal bovine serum, 100 ug/ml of streptomycin and 100 U/ml of penicillin and incubated at 5% CO2. For amphetamine (Dextroamphetamine sulfate, U.S. Pharmacopeia, Rockville, MD, USA) treatment, the cells were cultured in RPMI 1640 containing 0.5% horse serum for 16 h, and then treated with 5 µM of AMPH. For the inhibition experiments, PC12 cells were incubated in resum reduced RPMI containing 30 µM of LY294002, 10 µM of PD098059, 10 µM of GBR12909, or 0.5 µM of Enzastaurin (Calbiochem, EMD Chemicals Inc., Darmstadt, Germany) for 30 min before AMPH treatment.
Protein extraction and immunoblotting
Cells were treated without or with 5 µM of AMPH and extracted with sampling buffer (50 mM Tris-pH 6.5, 10% glycerol, 10% SDS, 1 mM 2-mercaptoethanol, 0.1% bromophenol blue) at various times and boiled 5 min at 100℃. Protein concentration was determined by BCA (Thermo Fisher Scientific, USA) method before adding 1 mM 2-mercaptoethanol. Thirty micrograms of cell lysates were electrophoresed in SDS-polyacrylamide gels (SDS-PAGE) and transferred to nitrocellulose membranes, which were then incubated with anti-phospho ERM, anti-ERM, anti-phospho Akt, (Ser 473), Akt, anti-Akt, anti-phospho ERK, anti-ERK (Cell Signaling Technology, Beverly, MA, USA), anti-phospho PKCβ anti-PKCβ (Santa Cruz Biotech. Inc., CA, USA), anti-actin (Sigma, St. Louis, MO, USA) antibody for 16 h at 4℃. After washing with TBS-T (0.05%), blots were incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG and the bands were visualized using the ECL system (Thermo Fisher Scientific, USA). Band images were obtained by using a Molecular Imager ChemiDoc XRS+ (Bio-Rad, Hercules, CA, USA) and band intensity was analyzed using Image Lab™ software version 2.0.1 (Bio-Rad).
Statistical analysis
Results were given as mean±S.E.M. Data were analyzed using simple unpaired t-test. A p-value of 0.05 or less was considered as indicative of a significant difference.
RESULTS
Amphetamine induces ERM proteins phosphorylation in PC12 cells
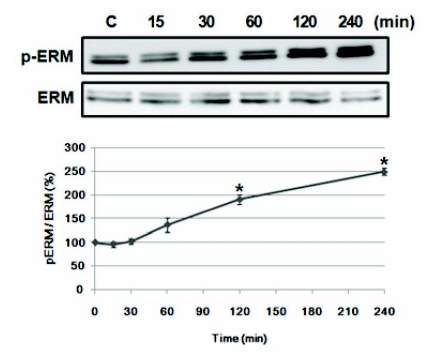
To find out proper concentration of AMPH, various concentration of AMPH (2, 5, 10, 50, or 100 µM) was treated in PC12 cells for 30 min and Akt and ERK phosphorylation was examined. Akt phosphorylation increased by two-fold at 2 to 50 µM of AMPH, but ERK phosphorylation increased at 5 and 10 µM in PC12 cells (data not shown). Thus, we selected 5 µM of AMPH to examine whether acute AMPH stimulation induces phosphorylation of ERM proteins in PC12 cells. After treatment, immunoblotting was performed at various times. As shown in Fig. 1, the ratio of the phosphorylated to total ERM protein was increased at 60 min (p<.05) after drug treatment and continued to increase up to 240 min, by 2.5 fold compared to basal level (p<.05). During this period, the total amount of ERM proteins was unchanged, which was confirmed by reprobing the membrane with an anti-ERM antibody (Fig. 1).
Fig. 1.
Amphetamine induces ERM proteins phosphorylation in PC12 cells. Serum reduced PC12 cells were treated with 5 µM of AMPH for the indicated time, and whole cell lysates were immunoblotted with anti-p-ERM or ERM antibody. The intensity of the phosphorylated ERM band was normalized to that of total ERM. The data represent the means±SE of three independent experiments. *p<0.05 vs. C.
Amphetamine-induced ERM proteins phosphorylation is not mediated by PI3K/Akt or MEK/ERK signal pathway
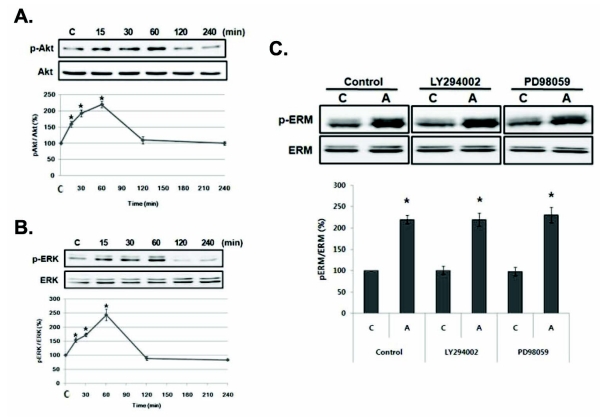
In order to find the upstream signaling molecule of ERM proteins, we first examined AMPH-induced Akt and ERK phosphorylation which is known to be activated by AMPH [13,14]. Results showed that phosphorylation of Akt or ERK was increased at 15 min (p<.05) and peaked at 60 min (p<.05) compared to basal level in PC12 cells, which precedes ERM proteins phosphorylation (Fig. 2A, B).
Fig. 2.
Amphetamine-induced ERM proteins phosphorylation is not mediated by Akt or ERK activation in PC12 cells. (A) Serum reduced PC12 cells were treated with 5 µM of AMPH for the indicated time, and whole cell lysates were immunoblotted with anti-p-Akt or Akt antibody and (B) anti-p-ERK or ERK antibody. (C) Serum reduced PC12 cells were pre-incubated with 30 µM of LY294002 or 10 µM of PD098059 for 30 min, and then treated with AMPH for 4 h. The whole cell lysates were immunoblotted with anti-p-ERM or ERM antibody. The intensity of the phosphorylated ERM, Akt and ERK band was normalized to that of total ERM, Akt, and ERK, respectively. The data represent the means±SE of three independent experiments. *p<0.05 vs. C.C, unstimulated PC12 cells; A, AMPH-stimulated cells.
To investigate the involvement of PI3K/Akt or MEK/ERK in the AMPH-induced ERM proteins phosphorylation, we examined the effects of LY294002 or PD98059, inhibitors for PI3K or MEK, respectively, on the AMPH-induced ERM proteins phosphorylation in PC12 cells. Each inhibitor was added to the medium 30 min before the treatment of AMPH, and the ERM proteins phosphorylation was examined at 4 h. AMPH-induced ERM proteins phosphorylation was not suppressed by both inhibitors (Fig. 2C), indicating that this effect is not dependent on PI3K/Akt or MEK/ERK pathway in PC12 cells.
Amphetamine-induced ERM proteins phosphorylation is dependent on the PKCβ activation
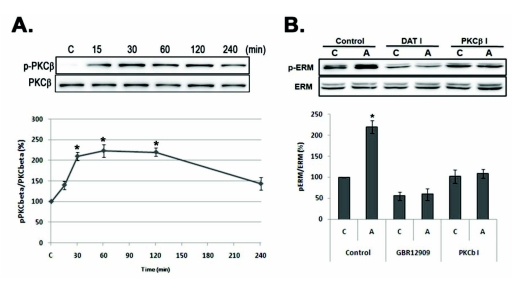
AMPH also activates PKC in PC12 cells [8] and PKCβ is a critical regulator of DAT trafficking and regulates the behavioral response to AMPH in mice [15]. Thus, we examined AMPH-induced PKCβ activation by using anti-phospho PKCβ antbody. As shown in Fig. 3A, AMPH-induced PKCβ phosphorylation increased at 15 min and lasted to 120 min (p<.05). Next, we examined whether AMPH-induced PKCβ activation is related with ERM proteins phosphorylation by using PKCβ inhibitor (PKCβ I), Enzastaurin. As shown in Fig. 3B, in the presence of PKCβ inhibitor, AMPH-induced ERM proteins phosphorylation was completely suppressed.
Fig. 3.
Amphetamine-induced ERM proteins phosphorylation is related with PKCβ and DAT activation in PC12 cells. (A) Serum reduced PC12 cells were treated with 5 µM of AMPH for the indicated time, and whole cell lysates were immunoblotted with anti-p-PKCβ or PKCβ antibody. (B) PC12 cells were pre-incubated with 10 µM of GBR12909 (DAT I) or 0.5 µM of Enzastaurin (PKCβ I) for 30 min, and then treated with 5 µM of AMPH for 4 h, and whole cell lysates were immunoblotted with indicated antibodies. The intensity of the phosphorylated PKCβ and ERM band was normalized to that of total ERM and PKCβ. The data represent the means±SE of three independent experiments. *p<0.05 vs. C, C, unstimulated PC12 cells; A, AMPH-stimulated cells.
Next, we examined the involvement of DAT trafficking using DAT inhibitor, GBR12909, which known to be regulated by PKC, in the AMPH-induced ERM proteins phosphorylation. Interestingly, DAT inhibitor suppressed basal phosphorylation level of ERM proteins and which was not increased by AMPH treatment in PC12 cells (Fig. 3A). These results suggest that AMPH-induced ERM proteins phosphorylation is mediated by PKCβ activation, which leads to regulation of DAT activity.
DISCUSSION
The dopamine transporter (DAT) is a key mediator of dopaminergic neurotransmission and a major target for AMPH, but to date, very little is known about the regulatory components involved in trafficking of the DAT. In the present study, we first demonstrated that the ERM proteins can be phosphorylated by AMPH treatment through PKCβ activation and may be one of key regulators of DAT trafficking in PC12 cells.
The activity and trafficking of DAT are regulated by a complicated protein network involving multiple protein kinases and DAT-interacting proteins [16]. Among them, PKC is the most characterized. Long-term activation of PKC leads to increased DAT endocytosis and reduced DAT recycling, resulting in reduced surface DAT expression and activity, as shown in rat synaptosomes and cultured cells [17]. Here, we first demonstrated that ERM proteins are involved in the AMPH-induced PKCβ activation, which leads to DAT internalization. Previously, Johnson and colleagues provided evidence that a protein complex formed by PKC and the DAT is important for maintaining amphetamine-stimulated outward transport of DA in the brain [18]. These authors showed that PKC-βI and PKC-βII, but not PKC-α or PKC-χ, co-immunoprecipitated with DAT from rat striatal membranes. Thus, it appears that an interaction of ERM proteins with PKC or DAT is responsible for the amphetamine-induced internalization of the DAT. Further studies are required to address this possibility.
In contrast to PKC activation, constitutive ERK and PI 3-kinase (PI3K) activations are known to serve to either inhibit translocation of DAT from the plasma membrane, speed the return of internalized DAT to the membrane, or both [19,20]. However, in the present study, although we examined that AMPH activates Akt and ERK in PC12 cells, AMPH-induced ERM protein phosphorylation was not altered in the presence of Akt or ERK inhibitor (Fig. 2). These discrepancies may be explained by duration of activity. Others used constitutively active mutant ERK or PI3K to examine the DAT regulation but, in the present study, AMPH-induced Akt and ERK activation was very transient. These imply that AMPH-induced Akt and ERK activation may not be involved in DAT regulation in PC12 cells.
Acute exposure of AMPH reduces DAT membrane expression, while brief treatment with transporter blockers increases DAT cell surface expression [21], suggesting that the mechanisms of action of AMPH or cocaine may be through the opposite way. These are confirmed by our observation that acute AMPH treatment induced ERM proteins phosphorylation, but cocaine decreased it [12].
Alterations of dendritic spine density in NAc by drugs of abuse are functionally significant in the development of synaptic plasticity mediating addictive behaviors [22]. ERM proteins link actin filaments to the membrane either directly via binding to cytoplasmic tail of transmembrane proteins or indirectly via scaffolding proteins attached to transmembrane proteins, which is essential for maintenance of cell shape, cell adhesion, migration and division [23]. Recently, it has been reported that glutamate-induced phosphorylation of ERM proteins in primary cultured differentiated hippocampal neurons is necessary for the formation of filopodial protrusion [24], and that the expression of constitutively active ezrin induces dendritic filopodia formation, which leads to subsequent synaptogenesis and establishment of functional neural circuitry in the developing brain [25]. Further, we also reported that nerve growth factor (NGF) induces moesin phosphorylation by PI3K and this process is required for neurite formation in PC12 cells [26], while others also showed that repeated and intermittent AMPH treatment induces neurite outgrowth [8]. These results suggest that ERM proteins might be involved in the drugs of abuse-induced synaptic plasticity including DAT regulation.
In conclusion, ERM proteins phosphorylation is regulated by AMPH and these effects are mediated through PKCβ and DAT activation in PC12 cells. These results suggest that phosphorylation of ERM proteins may play functionally important roles in regulating DAT activity in PC12 cells.
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation grant KRF-2008-314-E-00135.
ABBREVIATIONS
- AMPH
amphetamine
- DA
dopamine
- DAT
dopamine transporter
- ERM
Ezrin/radixin/moesin
- NAc
nucleus accumbens
- VTA
ventral tegmental area
- ERK
extracellular signal-regulated kinase
- Akt
protein kinase B
References
- 1.Hooks SB, Martemyanov K, Zachariou V. A role of RGS proteins in drug addiction. Biochem Pharmacol. 2008;75:76–84. doi: 10.1016/j.bcp.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 2.Chen JC, Chen PC, Chiang YC. Molecular mechanisms of psychostimulant addiction. Chang Gung Med J. 2009;32:148–154. [PubMed] [Google Scholar]
- 3.White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 4.Kahlig KM, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur J Pharmacol. 2003;479:153–158. doi: 10.1016/j.ejphar.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 5.Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunha-Oliveira T, Rego AC, Oliveira CR. Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psychostimulant drugs. Brain Res Rev. 2008;58:192–208. doi: 10.1016/j.brainresrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Kantor L, Park YH, Wang KK, Gnegy M. Enhanced amphetamine-mediated dopamine release develops in PC12 cells after repeated amphetamine treatment. Eur J Pharmacol. 2002;451:27–35. doi: 10.1016/s0014-2999(02)02190-8. [DOI] [PubMed] [Google Scholar]
- 8.Park YH, Kantor L, Wang KK, Gnegy ME. Repeated, intermittent treatment with amphetamine induces neurite outgrowth in rat pheochromocytoma cells (PC12 cells) Brain Res. 2002;951:43–52. doi: 10.1016/s0006-8993(02)03103-7. [DOI] [PubMed] [Google Scholar]
- 9.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 10.Louvet-Vallée S. ERM proteins: from cellular architecture to cell signaling. Biol Cell. 2000;92:305–316. doi: 10.1016/s0248-4900(00)01078-9. [DOI] [PubMed] [Google Scholar]
- 11.Niggli V, Rossy J. Ezrin/radixin/moesin: versatile controllers of signaling molecules and of the cortical cytoskeleton. Int J Biochem Cell Biol. 2008;40:344–349. doi: 10.1016/j.biocel.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Kim WY, Shin SR, Kim S, Jeon S, Kim JH. Cocaine regulates ezrin-radixin-moesin proteins and RhoA signaling in the nucleus accumbens. Neuroscience. 2009;163:501–505. doi: 10.1016/j.neuroscience.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 13.Shi X, McGinty JF. Extracellular signal-regulated mitogen-activated protein kinase inhibitors decrease amphetamine-induced behavior and neuropeptide gene expression in the striatum. Neuroscience. 2006;138:1289–1298. doi: 10.1016/j.neuroscience.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Shi X, McGinty JF. Repeated amphetamine treatment increases phosphorylation of extracellular signal-regulated kinase, protein kinase B, and cyclase response element-binding protein in the rat striatum. J Neurochem. 2007;103:706–713. doi: 10.1111/j.1471-4159.2007.04760.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen R, Furman CA, Zhang M, Kim MN, Gereau RW, 4th, Leitges M, Gnegy ME. Protein kinase Cbeta is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. J Pharmacol Exp Ther. 2009;328:912–920. doi: 10.1124/jpet.108.147959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres GE. The dopamine transporter proteome. J Neurochem. 2006;97(Suppl 1):3–10. doi: 10.1111/j.1471-4159.2006.03719.x. [DOI] [PubMed] [Google Scholar]
- 17.Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem. 2003;278:22168–22174. doi: 10.1074/jbc.M301845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. J Biol Chem. 2005;280:10914–10919. doi: 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- 19.Morón JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, Shippenberg TS. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvelli L, Morón JA, Kahlig KM, Ferrer JV, Sen N, Lechleiter JD, Leeb-Lundberg LM, Merrill G, Lafer EM, Ballou LM, Shippenberg TS, Javitch JA, Lin RZ, Galli A. PI 3-kinase regulation of dopamine uptake. J Neurochem. 2002;81:859–869. doi: 10.1046/j.1471-4159.2002.00892.x. [DOI] [PubMed] [Google Scholar]
- 21.Little KY, Elmer LW, Zhong H, Scheys JO, Zhang L. Cocaine induction of dopamine transporter trafficking to the plasma membrane. Mol Pharmacol. 2002;61:436–445. doi: 10.1124/mol.61.2.436. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- 23.Niggli V, Rossy J. Ezrin/radixin/moesin: versatile controllers of signaling molecules and of the cortical cytoskeleton. Int J Biochem Cell Biol. 2008;40:344–349. doi: 10.1016/j.biocel.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Kim HS, Bae CD, Park J. Glutamate receptor-mediated phosphorylation of ezrin/radixin/moesin proteins is implicated in filopodial protrusion of primary cultured hippocampal neuronal cells. J Neurochem. 2010;113:1565–1576. doi: 10.1111/j.1471-4159.2010.06713.x. [DOI] [PubMed] [Google Scholar]
- 25.Furutani Y, Matsuno H, Kawasaki M, Sasaki T, Mori K, Yoshihara Y. Interaction between telencephalin and ERM family proteins mediates dendritic filopodia formation. J Neurosci. 2007;27:8866–8876. doi: 10.1523/JNEUROSCI.1047-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon S, Park JK, Bae CD, Park J. NGF-induced moesin phosphorylation is mediated by the PI3K, Rac1 and Akt and required for neurite formation in PC12 cells. Neurochem Int. 2010;56:810–818. doi: 10.1016/j.neuint.2010.03.005. [DOI] [PubMed] [Google Scholar]