Abstract
Natural and synthetic glucocorticoids (GCs) have been used for decades to suppress inflammation. In this paper, we re-examine the role of the endogenous GC, cortisol, as a primary homeostatic regulator of the human inflammatory response to injury. Our data show that cortisol regulation of innate immunity can be both pro-inflammatory and anti-inflammatory. Using a human model of in vivo cortisol depletion, we first show that baseline (diurnal) cortisol concentrations do not exert an anti-inflammatory effect. This is the first clue that cortisol regulation of inflammation is not represented by a linear dose-response relationship. We next show in surgical patients that cortisol does exert an acute anti-inflammatory effect over a carefully regulated range of physiologic cortisol concentrations. Finally, transient pre-treatment of healthy humans with cortisol induces a bi-phasic response during a later, delayed systemic inflammatory response: an intermediate cortisol concentration augments inflammation while a high cortisol concentration is neither pro- nor anti-inflammatory. Based on these findings and the work of others, we propose a new paradigm that identifies cortisol regulation of human inflammation as both dualistic—it is pro- and anti-inflammatory—and dynamic, it evolves over time.
Keywords: Glucocorticoid, cortisol inflammation, innate immunity, bi-phasic, endotoxemia
INTRODUCTION
Glucocorticoids (GCs) have been widely used to suppress inflammation since 1949 when Hench et al surprised most of the biomedical community by reporting that GCs have anti-inflammatory effects in human disease (Hench et al. 1949). Since then, most GC research has focused on the anti-inflammatory and immune suppressive properties of GCs, while comparatively little research has examined the stimulatory properties of GCs that were discovered by Hans Selye and others and that were, in fact, widely acknowledged prior to 1949 (Selye et al. 1940; Selye 1944). Recently, investigators have re-examined the stimulatory effects of GCs on inflammatory defense mechanisms (Yeager et al. 2004, Sorrells and Sapolsky 2010). Recent reports show that GCs induce increased cellular expression of receptors for several pro-inflammatory cytokines including interleukin (IL)-1 (Spriggs et al. 1990), IL-2 (Wiegers et al. 1995), IL-4 (Paterson et al. 1994), IL-6 (Snyers et al. 1990), and IFN-g (Strickland et al. 1986), as well as GM-CSF (Hawrylowicz et al. 1994). GCs have also been shown to stimulate effector cell functions including phagocytosis by monocytes (van der Goes et al. 2000), effector cell proliferative responses (Spriggs et al. 1990), macrophage activation (Sorrells and Sapolsky 2010), and a delay of neutrophil apoptosis (Cox 1995).
The fundamental importance of GCs in human physiology is powerfully demonstrated by several observations including; regulation of GC synthesis at the brain diencephalic level, the rapid release of GCs in response to any external injury or threat of injury, and by the widespread distribution of intracellular receptors for GCs (GR) in virtually every somatic cell in the body. To account for the well-known suppressive and the less well-known stimulatory effects of GCs on inflammatory processes, we have proposed a model of GC action that describes a concentration-dependent, bi-phasic (both stimulatory and suppressive) GC regulation of in vivo defense mechanisms (Munck et al. 1984; Yeager et al. 2004) (Figure 1). The model is derived from work completed by many researchers who investigated, initially, the stimulatory properties of GCs and, since 1949, the suppressive actions of GCs. According to this model, normal diurnal concentrations of GCs support the activity of defense mechanisms in a permissive manner, while higher stress-induced concentrations act acutely to suppress inflammation and prevent tissue injury from an excessive or prolonged inflammatory response. Both activities are mediated by the GC intracellular receptor, GR, in a concentration-dependent agonist-receptor mechanism. The model further proposes that stress concentrations of cortisol also exert a delayed (time-dependent) preparative effect that is stimulatory. Recent studies have, in fact, demonstrated bi-directional effects of GCs on specific effector cell functions depending on GC concentration. Such effects have been shown for inflammatory cytokine release from monocytes, phagocytosis by macrophages, the acute phase protein response, delayed type hypersensitivity reactions and wound healing (Dhabhar and McEwen 1999; Dinkel et al. 2003; Yeager et al. 2004; Viswanathan and Dhabhar 2005; Lim et al. 2007). These reports identify a concentration- and time-dependent range of GC effects that are both pro- and anti-inflammatory. Due to the manifest clinical implications for pro-inflammatory GC regulation of the human injury response, we have used several clinical research paradigms to examine the clinical consequences of GC-induced enhancement of the human inflammatory response to injury.
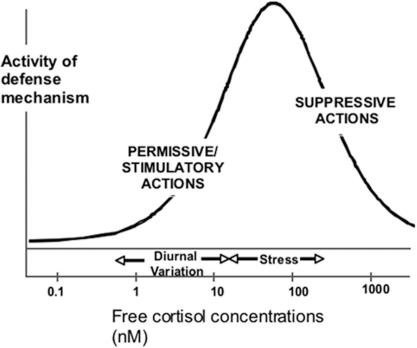
FIGURE 1.
The figure shows a bi-phasic model of GC regulation of in vivo defense mechanisms, including innate immune inflammation. According to this model, basal concentrations of cortisol are not anti-inflammatory but exert a supportive (permissive) action on various defense mechanisms. As cortisol concentrations increase to those associated with systemic stress or pharmacological administration of cortisol, a bi-phasic relationship is observed, especially when there is a time delay between the increase in cortisol concentration and the inflammatory stimulus.
METHODS
Human subjects
All research involving human subjects was approved by the Dartmouth College Committee for the Protection of Human Subjects (Institutional Review Board) and written; informed consent was obtained from all participants.
Laboratory Techniques
Monocyte isolation
Mononuclear cells were isolated by ficoll-hypaque density gradient (Histopaque 1077, Sigma-Aldrich, St. Louis, MO). Monocytes were positively selected from mononuclear cells with anti-CD14 magnetic beads (Miltenyi Biotec Inc. Auburn, CA); purity was always greater than 95%. Treatments and their duration were as noted in the Results.
Cytokine analysis
Samples were collected and frozen at −80°C for batched measurement of TNFa, IL-6 and IL-10 concentrations. TNFa was measured using a TNFa sandwich ELISA (paired antibodies, BD Biosciences Pharmingen, San Diego, CA). IL-6 levels were determined using an IL-6 ELISA kit (Peprotech, Rocky Hill, NJ). IL-10 was measured using an IL-10 ELISA kit (Biosource, Camarillo, CA).
Cell staining and flow cytometry
Following culture and treatments, washed monocytes were stained for surface expression of CD54, CD119 and CD163 (FITC-labeled anti-CD54 and FITC-labeled anti-CD119, R&D Systems, Minneapolis, MN; PE-labeled anti-CD163, Trillium Diagnostics, Bangor, ME. Cells were analyzed by flow cytometry on a FACSCAN flow cytometer and monocytes were gated on forward/side scatter to eliminate dead cells (<5%) from the analysis. Mean fluorescent intensity (MFI) was determined by the geometric mean of the fluorescence of gated monocytes.
Messenger RNA (mRNA) isolation and quantification
Total RNA was extracted from peripheral blood monocytes using RNeasy mini columns (Qiagen). RNA samples were treated with RNase-free DNase prior to amplification. RNA integrity and concentration were determined with RNA6000 Nano LabChip kit (Agilent). Using 500 ng of RNA as template, first-strand cDNA was synthesized using random hexamers and SuperScript II Moloney murine leukemia virus reverse transcriptase (Invitrogen). Expression of mRNA was quantified by Taqman PCR. cDNA (0.5 μl/well) was transferred into 96-well format plates, and Taqman Master Mix (Applied Biosystems) was added. Taqman-validated primers and Taqman MGB probes (labeled with fluorescent reporter dye 6FAM) were used for amplification of genes. Data were analyzed using ABI 7300 quantification software (Applied Biosystems) and are expressed as relative mRNA levels.
Cortisol
Total plasma cortisol was measured on an Immulite® analyzer using a DPC Cortisol kit (Los Angeles, CA). Salivary free cortisol, as a measure of active free cortisol at in vivo effector cell sites, was measured by Salimetrics® Expanded Range High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (State College, PA).
ACTH
Plasma ACTH was measured by solid-phase chemiluminscent assay (Immulite®, Diagnostic Products Corporation).
Statistical Analysis
The primary end-point for most studies was the cytokine response to endotoxin stimulation assessed by ELISA. Power analyses for clinical studies were calculated using differences in endotoxin-stimulated IL-6 concentrations between control and treatment conditions. Non-parametric rank tests and Student’s t-tests, with appropriate transformation to normality, were used to compare control and treatment conditions at each time period or treatment condition. To account for the correlated nature of the data that resulted from multiple measures when subjects were tested over time, we used generalized estimating equations (GEE) as the primary analytic tool for inter-group comparisons that consider all time points and to characterize changes over time. A p-value less than 0.05 was taken to indicate statistical significance without adjustment for multiple comparisons.
RESULTS
1. Basal (diurnal) cortisol concentrations do not exert an anti-inflammatory effect in vivo
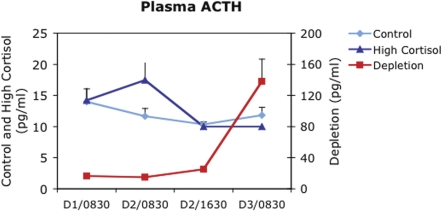
In this study, 8 healthy human subjects (6 male: 2 female), ages 19–47, were treated on Day 1 with a pharmacologically-induced depletion of endogenous cortisol activity for approximately 24 hours. Depletion of cortisol activity was achieved with 200 mg of the GR antagonist RU486 (Mifeprex®; Danco Laboratories, NY) orally at 0400 and 1600 hours and an intravenous infusion of the adrenal cortical synthesis inhibitor, etomidate, at 1.5mg/kg/hr from 0900hr to 2100hr to suppress endogenous synthesis of cortisol (Yeager et al. 2008). Peripheral blood samples were taken at 0900 the day before (Day 1) and the day after (Day 3) the cortisol depletion treatment. Total plasma and salivary free cortisol concentrations confirmed significant decreases in endogenous cortisol concentrations by 4 PM on Day 1 following the cortisol “depletion” (control 5.3 [+/− 1.0 S.E.] ug/dl vs. depletion 1.8 [+/−0.6 S.E.] ug/dl total plasma cortisol and control 3.9 [+/− 1.6 S.E.] nM vs. depletion 1.5 [+/− 0.6 S.E.] nM salivary free cortisol ;p<0.001 control vs. depletion for both). In addition, plasma adrenocorticotrophic hormone (ACTH) concentrations increased on the morning of Day 3 to approximately 10 times baseline values on Day −1 (16.5 pg/ml +/− 2.9 S.E. vs. 138 pg/ml +/− 28 pg/ml; p<0.001) indicating physiologically significant cortisol depletion (Figure 2). Monocytes were isolated on the day before cortisol depletion and the day after depletion. Monocytes were tested for inflammatory responsiveness by stimulating cells ex vivo with bacterial lipopolysaccharide (LPS) at a concentration of 1 ng/ml and measuring cellular release of tumor necrosis factor-alpha (TNF-a), interleukin-6 (IL-6) and inter-leukin-10 (IL-10), as well as cellular expression of the GC-inducible molecules CD54 and CD163. Monocytes showed no difference in their inflammatory responses to LPS following cortisol depletion (TNF-a = 1083pg/ml before vs. 911 pg/ml after depletion; IL-6 = 1734 pg/ml before vs. 1749 pg/ml after depletion; IL-10 = 171 pg/ml before vs. 57 pg/ml after depletion; p = n.s. for all).
FIGURE 2.
Plasma adrenocortiocotrophic hormone (ACTH) concentrations before, during and after a cortisol depletion treatment that included 2 doses of the glucocorticoid receptor antagonist, RU486 at 0400 and 1600 hours and an intravenous infusion of the adrenal cortical synthesis inhibitor, etomidate, from 0900 to 2100 hours. ACTH concentrations are compared to those observed with an intravenous saline treatment (Control) and treatment with pharmacological doses of cortisol (High Cortisol) (Note that intravenous cortisol treatment began at 0900 of Day 2, after the second ACTH measurement made at 0830 on that day). Note that, following the cortisol depletion treatment, ACTH concentrations increase by almost an order of magnitude (note different scales for ACTH) indicating significant depletion of effective cortisol concentrations.
These findings had 2 implications with regard to the design of further studies: First, the data show that basal (diurnal) concentrations of cortisol do not exert an anti-inflammatory effect on several pro-and anti-inflammatory mediators of the human immune inflammatory response. This finding supports the need for the innate immune system to maintain a ‘hair trigger’ response pattern in order to rapidly isolate and limit bacterial invasion following disruption of tissue integrity by physical injury. Most GC-mediated effects are genomic and take hours to find expression at the protein level. This is too long an interval for effective control of bacterial replication that can take place in a much shorter time span. Second, these data also provide a clue that GC regulation of inflammation may not be a simple matter of straightforward, concentration-dependent suppression of inflammatory responses since withdrawal of cortisol activity in vivo did not lead to increased inflammatory responsiveness of immune effector cells.
2. Cortisol exerts bi-directional regulation of monocyte mRNA for inflammatory mediators including GR, interleukin-10 (IL-10) and Suppressor of Cytokine Synthesis 3 (SOCS3)
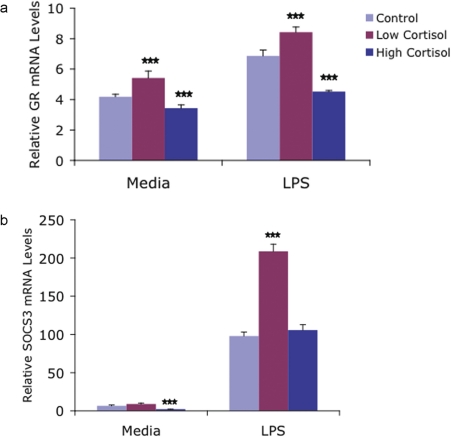
The same subjects also received, at separate times following a washout interval, either intravenous saline for 8 hours (Control) from 0900 to 1700 hours or a high dose of intravenous cortisol (hydrocortisone [SoluCortef, Upjohn] @ 8 ug//kg/min) for 8 hours (followed by oral hydrocortisone overnight) to raise plasma cortisol concentrations to high, pharmacological concentrations for approximately 24 hours. Monocytes were isolated from peripheral blood before and after each treatment, mRNA was extracted and analyzed for comparison between groups. In keeping with the well-known anti-inflammatory effects of GCs, mRNA for the anti-inflammatory cytokine IL-10 was regulated in concentration-dependent manner with decreased IL-10 mRNA following cortisol depletion and increased mRNA following high dose cortisol exposure. However, we also found regulation of other key molecules had more complex implications. We found, for example, that mRNA for GR alpha, the dominant GC receptor protein, was decreased following exposure to high dose cortisol, which has been previously reported (Burnstein et al. 1991), but that GR mRNA was also increased following the in vivo cortisol depletion treatment (Figure 3a). The latter result is an entirely new finding. Given the multiplicity of cellular events that are regulated by GCs, the clinical implications of a bi-directional regulation of GRa by cortisol are not only unexplored, they cannot be predicted to follow a linear dose-response relationship with in vivo cortisol concentrations. The latter point is underscored by our finding that mRNA for a recently described key suppressor of inflammatory cytokine synthesis (SOCS3) was increased in monocytes following the cortisol depletion treatment (Figure 3b). This finding would suggest that depletion of cortisol could lead to an inhibition of some inflammatory responses if a key suppressor of inflammatory mediators is increased. These findings again are consistent with, though they do not clearly demonstrate bi-phasic GC regulation of human inflammation.
FIGURE 3.
Following each of 3 in vivo treatments (Control, High Cortisol, Low Cortisol), monocytes were isolated from peripheral blood and incubated for 3 hours in either media alone or media supplemented with 1 ng/ml LPS. Messenger RNA levels were then determined for the glucocorticoid receptor (GR) (Figure 3a) and Suppressor of Cytokine Synthesis-3 (SOCS3) Figure 3b. GR mRNA levels were increased significantly by in vivo Low Cortisol and decreased by in vivo High Cortisol treatment. SOCS3 mRNA levels increased significantly following Low Cortisol treatment in stimulated cells. *** = p < 0.001 vs. control cells under similar conditions.
3. Cortisol exerts an acute, concentration-dependent, acute anti-inflammatory effect over the physiologic range of cortisol
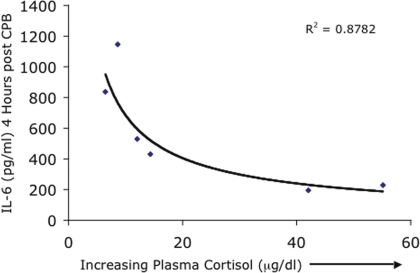
Based on our model of GC action that proposes a bell-shaped curve for GC regulation of in vivo defense mechanisms, we designed a study in which we carefully regulated cortisol concentrations from low to high during an acute systemic inflammatory stimulus. We studied patients scheduled to undergo cardiac surgery with cardiopulmonary bypass, which is known to induce a substantial and well-described systemic inflammatory response (Laffrey and Cheng 2002). We chose to measure plasma IL-6 as a marker of the pro-inflammatory response to surgery because it is released into the circulation in substantial quantities and because elevated IL-6 concentrations persist for several days after surgery. Sixty patients were randomly divided into equal groups of 10. Patients were administered 2 doses of etomidate during surgery to suppress endogenous cortisol synthesis while each group was then administered varying doses of cortisol immediately before and during surgery to control cortisol concentrations at pre-determined levels. A Control Group received no etomidate and intravenous saline. We were able to successfully regulate plasma cortisol concentrations during and immediately after cardiac surgery through the desired range from low (5–10 ug/dl) to ‘normal’ (15–20 ug/dl), to stress-associated concentrations (35–45 ug/dl), to high pharmacologic (non-physiological) concentrations (70–80 ug/dl). The results showed that cortisol exerted a concentration-dependent suppression of the systemic inflammatory IL-6 response to cardiac surgery (Figure 4). We also found, interestingly, that maximal suppression of inflammation was achieved by a stress-associated, but still physiologic, cortisol concentration. There was no greater anti-inflammatory effect at higher cortisol concentrations (Yeager et al. 2005) although IL-10 concentrations continued to increase with increasing cortisol concentrations as we and others have shown (Fillinger et al. 2002, Weis 2009).
FIGURE 4.
Cardiac surgical patients (n=60) were randomly divided into 6 groups before surgery. Treatment groups received intravenous etomidate during surgery to suppress endogenous cortisol synthesis. Varying doses of cortisol were then administered for 6 hours before and during surgery to regulate plasma cortisol concentrations over the physiologic range. Plasma cortisol concentration at the end of the cortisol infusion is plotted against peak plasma IL-6 concentrations that were observed 4 hours after surgery. The results show a classic dose-response relationship between the pro-inflammatory cytokine, IL-6, and the acute plasma cortisol concentration.
The well-described anti-inflammatory effects of GCs would indeed predict a concentration-dependent suppression of the pro-inflammatory response to cardiac surgery as shown here. However, in this study we carefully controlled endogenous cortisol release during an acute inflammatory stimulus in order to manipulate cortisol concentrations through the relevant physiological range from low to high. We were able to effectively control cortisol concentrations by suppressing endogenous synthesis and adding back varying doses of cortisol. This was an important step in defining the scope of cortisol effects on human inflammation. We found that, acutely, physiological cortisol concentrations are anti-inflammatory and, as proposed, act to limit over expression of an inflammatory response that could lead to tissue damage (Munck and Naray-Fejes-Toth 1994). We next looked for delayed, preparative (pro-inflammatory) effects of stress cortisol concentrations in vivo.
4. Cortisol exerts a delayed bi-phasic regulation of systemic inflammation with pro-inflammatory effects that are maximal at an intermediate cortisol concentration
Most of the in vivo animal studies that have shown pro-inflammatory effects of GCs have observed increased inflammation only when there is a time interval between the in vivo exposure to GCs and the subsequent pro-inflammatory stimulus. In this study, healthy human subjects (n=36) were randomly divided on Day 1 into 3 treatment groups: 1) A Control Group that received intravenous saline for 6 hours from 0900 to 1500 hours. 2) A Stress Cortisol group that received intravenous hydrocortisone at 1.5 ug/kg/min for the same time period. (This dose was designed to increase plasma cortisol concentrations to the range that is typically observed after a major physical stress [∼30–50 ug/dl]). 3) A Pharmacologic Cortisol group that received 3 ug/kg/min for 6 hours to raise plasma cortisol concentrations to a level that is usually observed only when exogenous cortisol is administered. The 6-hour time period corresponds to the approximate duration of elevated cortisol that is observed in humans after an isolated systemic inflammatory stimulus, in this case LPS (Rassias et al. 2005). The following day, subjects received 2ng/kg intravenous LPS to induce a transient systemic inflammatory response followed by serial sampling of peripheral blood. Subjects in all groups were matched for age, gender and weight. All subjects experienced a transient systemic inflammatory response as previously described (Suffredini et al. 1999) that included sequential increases in plasma concentrations of pro-inflammatory IL-6 and anti-inflammatory IL-10. In the Stress Cortisol group, the pro-inflammatory IL-6 response was significantly increased compared to both the Control and Pharmacologic Cortisol groups while the anti-inflammatory IL-10 response was decreased (Yeager et al. 2009). We compared the salivary free cortisol concentration that was achieved in vivo during the 6-hour intravenous infusion (Day1) with both the peak IL-6 concentrations (3 hours after LPS injection) and with the total area under curve (AUC) for IL-6 over 8 hours after LPS injection (Day 2). We found clear evidence that cortisol induced a bi-phasic regulatory effect on the pro-inflammatory response to LPS (Figure 5). Peak pro-inflammatory effects of cortisol were observed at the intermediate cortisol concentration that is typically observed during a major systemic stress. Higher and lower cortisol concentrations did not have the same effect. Notably, the higher dose cortisol exposure did not lead to a significantly greater pro- or anti-inflammatory effect compared to the control group. Clinically, the heart rate and temperature profiles suggested that a greater IL-6 response in the Stress Cortisol group led to a greater physical response to LPS, however there were no statistically significant differences between the 3 groups.
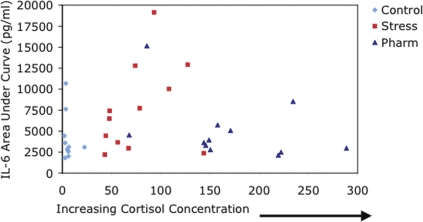
FIGURE 5.
Healthy human volunteers received 1 of 3 treatments the day before an intravenous injection of bacterial lipopolysaccharide (LPS): Intravenous saline for 6 hours from 0900 to 1500 hr, intravenous hydrocortisone @ 1.5ug/kg/min for 6 hours (Stress), or intravenous hydrocortisone @ 3.0 ug/kg/min (Pharm) for 6 hours. At the end of the 6 hour infusion, salivary free cortisol concentrations were measured. The next day, subjects received intravenous LPS to induce a transient systemic inflammatory response and serial testing of plasma IL-6 was performed for 8 hours. An intermediate (stress-associated) concentration of cortisol augmented the IL-6 response to LPS compared to lower control concentrations or higher, pharmacologic concentrations.
The results of this study are in accordance with animal studies that have shown both time- and concentration-dependent bi-phasic regulation of inflammation in animals in vivo. These results suggest, for the first time, a similar effect in humans. Acutely, cortisol has anti-inflammatory effects following a systemic inflammatory stimulus (Figure 4). However, a cortisol concentration that acts acutely to suppress systemic inflammation also has a delayed effect of augmenting the inflammatory response to subsequent, delayed stimulus (Figure 5). These data provide strong evidence of an adaptational role for stress-associated concentrations of cortisol in humans. Notably, higher cortisol concentrations (pharmacologic concentrations) did not have the same effect.
DISCUSSION
Research on GCs has traditionally been guided by a uni-dimensional paradigm in which GCs are considered to exert an anti-inflammatory action with a linear dose-response relationship: too little GC leads to excess inflammation and too much GC impairs inflammatory processes necessary to prevent infection and repair injured tissue. Recent work conducted primarily in vitro and in animals, has demonstrated a more complex relationship between GCs and immune-mediated inflammation. It is now increasingly clear that; 1) GCs can exert pro-inflammatory effects on key inflammatory processes and, 2) GC regulation of inflammation can vary from anti- to a pro-inflammatory in a time-dependent manner. These observations add a new perspective that is critical to understanding the regulation of inflammation in humans. The goal of this research was to identify and assess the potential clinical implications of GC enhancement of innate immune inflammation. Inflammatory processes and inflammatory symptoms account for a substantial proportion of human disease. Given the central role that GCs have in the regulation of inflammation, this new paradigm of GC-mediated inflammatory regulation could have widespread implications for understanding and treating human disease.
We previously proposed a unifying physiological model of GC action that integrates the well-known anti-inflammatory properties of GCs with the emerging data on their ‘preparative’ effects (Figure 1). The model predicts that GC effects on a stimulus-induced inflammatory response can be EITHER suppressive OR stimulatory in a concentration- and time-dependent manner. The immediate in vivo effect of both stress-induced and pharmacological GC concentrations is to suppress concurrent inflammation and protect the organism from an excessive or prolonged inflammatory response. Time is integral to the model, which predicts delayed preparative effects of GCs that will be maximal following intermediate increases in GC concentration and less prominent at higher or lower concentrations. These preparative effects would be expected to enhance an organism’s response to a subsequent inflammatory stimulus and thus increase resistance to disease in an adaptational manner. The data cited above clearly support this model of GC control of inflammation with regard to the function of monocytes and perhaps other effector cells as well.
GCs alone, in the absence of an inflammatory stimulus, up-regulate monocyte mRNA and/or receptors for several molecules that participate in pro-inflammatory signaling, as noted above and in the studies presented here. However, these upregulatory effects are typically not observed at the protein level in the absence of a succeeding inflammatory stimulus, presumably to avoid needless expenditure of cellular resources. The distinction between GC effects in the presence or absence of an inflammatory stimulus is important because it defines the distinction between acute GC effects on inflammation that are anti-inflammatory and delayed (preparative) effects that can be stimulatory or pro-inflammatory. In humans, as shown here, if in vivo GC concentrations are elevated concurrent with an inflammatory stimulus, anti-inflammatory effects are observed. In sharp contrast, with a time delay of 12 or more hours between an increased GC concentration and the onset of an inflammatory stimulus, enhancing effects on inflammation are observed. These effects have been shown to persist in humans for up to 6 days (Barber et al. 1993). Similarly, in rats, GCs lead to enhanced LPS-induced release of inflammatory cytokines, but only during a defined time window after GC exposure (Fantuzzi et al. 1995, Frank et al. 2009). Also, betamethasone given to pregnant ewes increases LPS-induced fetal lung inflammation 5 days after, but not 2 days after the GC administration (Kallapur et al. 2003). In another interesting parallel, mice exposed to the stress of a sterile burn develop increased resistance to subsequent E. coli sepsis in a time-dependent manner that is first observed following a delay of 1 day and peaks at 7 days after the burn injury. This effect was associated with enhanced response of cellular LPS signaling mediators (Maung et al. 2008).
We also showed that GC-induced enhancement of inflammatory responses is maximal at an intermediate concentration, in our studies at a concentration that approximates that observed in vivo following a major systemic inflammatory stimulus. Similar data have been recently reported in vitro and in vivo in animals. For example, peritoneal macrophages show a 2- to 4-fold enhancement of Fc receptor-mediated phagocytosis when co-incubated with 10 to 100 nM hydrocortisone, but higher or lower concentrations were less effective (Warren and Vogel 1985). Co-incubation of bone marrow-derived macrophages with corticosterone (CS) leads to a dose-dependent increase in LPS-stimulated TNF-a and IL-6 release that is maximal (and only observed) at intermediated CS concentrations of 10–20 nM (Zhang and Daynes 2007a). A similar result was observed in mice implanted with CS pellets (serum CS concentrations ∼ 5–6 nM) and in 11B-hydroxysteroid dehydrogenase type-1 deficient knock-out mice that have increased circulating plasma CS compared to wild-type controls (Zhang and Daynes 2007b). Of note, the enhanced cytokine response was associated with augmented signaling by NFkB and MAPKs, prime mediators of LPS induced inflammation and targets for GC regulation of inflammation (Smyth et al. 2004). Other in vivo studies report tantalizing similarities but lack information on effective GC concentrations, effector cell populations involved, and mechanisms. For example, an intermediate CS concentration leads to enhanced wound healing (burst strength of intestinal anastamoses) after surgery in rats (Matsusue and Walser 1992). Similarly, low but not high GC pretreatment of rats leads to enhancement of a subsequent adaptive immune response that is associated with increased trafficking of leukocytes into inflammatory sites (Viswanathan and Dhabhar 2005).
In addition to enhanced responses to LPS, recently identified pro-inflammatory effects of GCs also show enhanced localization of effector cells at inflammatory sites. It has been known for some time that GCs alone induce a rapid and profound monocytopenia in humans (Calvano et al. 1987), but the potential mechanisms and consequences of GC-induced monocytopenia remained largely unstudied until recently. We now know that GCs, including cortisol, enhance expression of the chemokine receptor CCR2 on human monocytes in association with enhanced monocyte migration towards the chemotactic CCR2 ligand, CCL2 (MCP-1) (Penton-Rol et al. 1999; Okutsu et al. 2008). Related studies have shown GC enhancement of the monocyte migratory response to the complement fraction C5a (Pieters et al. 1995) as well as increased adherence to endothelial cells (Wenzel et al. 1996). Carefully conducted studies in animals have also revealed important consequences of these regulatory effects including enhanced adaptive immune responses to local antigen injection (Lyte et al. 1990; Dhabhar et al. 1996; Fleshner et al. 1998) and enhanced local inflammation in tissue injury models (Dinkel et al. 2003; Viswanathan and Dhabhar 2005). The in vivo inflammatory endpoints in all these studies have been tightly linked to GC-induced redistribution of effector cells, especially monocytes, to inflammatory sites. The significance of these findings in humans has yet to be established.
Based on the recent literature and the results reported here, we hypothesize that pre-exposure to stress-associated cortisol concentrations “prime” effector cells of the monocyte/macrophage lineage for an augmented pro-inflammatory response by; a) inducing preparative changes in key regulators of LPS signal transduction, and b) enhancing localization of inflammatory effector cells at potential sites of injury. These effects are maximal at an intermediate cortisol concentration, are only observed following a delay of 12–24 hours, and are most prominent in the presence of a delayed inflammatory stimulus. This hypothesis is the subject of ongoing investigations that focus on the cellular signaling pathways for LPS. The clinical implications of these findings have yet to be explored. In the setting of chronic inflammation or stress, where elevated GC concentrations are sustained, existing models of GC action are applicable. However, in the setting of repetitive stress or injury, these new data may provide an explanation for the development of resistance to disease and ultimately, functional decline, as originally shown by Selye.
Acknowledgments
Supported by National Institutes of Health, NIAID grant # AI051547 (PMG).
The authors gratefully acknowledge the expert technical assistance of Tracy Wynkoop in manuscript preparation.
REFERENCES
- Barber AE, Coyle SM, Marano MA, Fischer E, Calvano SE, Fong Y, Moldawer LL, Lowry SF. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J Immunol. 1993;150:1999–2006. [PubMed] [Google Scholar]
- Burnstein K, Bellingham D, Jewell C, Powell-Oliver F, Cidlowski J. Autoregulation of glucocorticoid receptor gene expression. Steroids. 1991;56:52–8. doi: 10.1016/0039-128x(91)90124-e. [DOI] [PubMed] [Google Scholar]
- Calvano S, Albert J, Legaspi A, Organ B, Tracey K, Lowry S, Shires G, Antonacci A. Comparison of numerical and phenotypic leukocyte changes during constant hydrocortisone infusion in normal humans with those in thermally injured patients. Surg Gynecol Obstet. 1987;164:509–520. [PubMed] [Google Scholar]
- Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. J Immunol. 1995;154:4719–4725. [PubMed] [Google Scholar]
- Dhabhar F, Miller A, McEwen B, Spencer R. Stress-induced changes in blood leukocyte distribution. J Immunol. 1996:1638–1644. [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci USA. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkel K, MacPherson A, Sapolsky RM. Novel glucocorticoid effects on acute inflammation in the CNS. J Neurochem. 2003;84:705–716. doi: 10.1046/j.1471-4159.2003.01604.x. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Galli G, Zinetti M, Fratelli M, Ghezzi P. Short Communication. The upregulating effect of dexamethasone on tumor necrosis factor production is mediated by a nitric oxide-producing cytochrome P450. Cell Immunol. 1995;160:305–308. doi: 10.1016/0008-8749(95)80042-h. [DOI] [PubMed] [Google Scholar]
- Fillinger MP, Rassias AJ, Guyre PM, Sanders JH, Beach M, Pahl J, Watson RB, Whalen PK, Yeo KT, Yeager MP. Glucocorticoid effects on the inflammatory and clinical responses to cardiac surgery. J Cardiothorac Vasc Anesth. 2002;16:163–9. doi: 10.1053/jcan.2002.31057. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Nguyen K, Cotter C, Watkins L, Maier S. Acute stressor exposure both suppresses acquired immunity and potentiates innate immunity. Am J Physiol. 1998;275:R870–R878. doi: 10.1152/ajpregu.1998.275.3.R870. [DOI] [PubMed] [Google Scholar]
- Frank M, Miguel Z, Watkins L, Maier S. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2009;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Hawrylowicz C, Guida L, Paleolog E. Dexamethasone up-regulates granulocyte-macrophage colony-stimulating factor receptor expression on human monocytes. Immunology. 1994;83:274–280. [PMC free article] [PubMed] [Google Scholar]
- Hench P, Kendall E, Slocumb C, Polley H. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone: Compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis; Preliminary report. P Staff M Mayo Clin. 1949;24:181–197. [PubMed] [Google Scholar]
- Kallapur S, Kramer B, Moss TJM. Maternal glucocorticoids increase endotoxin-induced lung inflammation in perterm lambs. Am J Physiol Lung Cell Mol Physiol. 2003;284:L633–L642. doi: 10.1152/ajplung.00344.2002. [DOI] [PubMed] [Google Scholar]
- Laffrey JG BJ, Cheng DCH. The systemic inflammatory response to cardiac surgery. Anesthesiology. 2002;97:215–52. doi: 10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- Lim HY, Muller N, Herold MJ, van den Brandt J, Reichardt HM. Glucocorticoids exert opposing effects on macrophage function dependent on their concentration. Immunology. 2007 doi: 10.1111/j.1365-2567.2007.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M, Nelson S, Thompson M. Innate and adaptive immune responses in a social conflict paradigm. Clin Immunol Immunop. 1990;57:137–147. doi: 10.1016/0090-1229(90)90029-p. [DOI] [PubMed] [Google Scholar]
- Matsusue S, Walser M. Healing of intestinal anastomoses in adrenalectomized rats given corticosterone. Am J Physiol. 1992;263:R164–R168. doi: 10.1152/ajpregu.1992.263.1.R164. [DOI] [PubMed] [Google Scholar]
- Maung AA, Fujimi S, MacConmara MP, Tajima G, McKenna AM, Delisle AJ, Stallwood C, Onderdonk AB, Mannick JA, Lederer JA. Injury enhances resistance to Escherichia coli infection by boosting innate immune system function. J Immunol. 2008;180:2450–8. doi: 10.4049/jimmunol.180.4.2450. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre P, Holbrook N. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Munck A, Naray-Fejes-Toth A. Glucocorticoids and stress: permissive and suppressive actions. Ann N Y Acad Sci. 1994;746:115–30. doi: 10.1111/j.1749-6632.1994.tb39221.x. [DOI] [PubMed] [Google Scholar]
- Okutsu M, Suzuki K, Ishijima T, Peake J, Higuchi M. The effects of acute exercise-induced cortisol on CCR2 expression on human monocytes. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Paterson RK, Or R, Domenico J, Delespesse G, Gelfand E. Regulation of CD23 expression by IL-4 and corticosteroid in human B lymphocytes. J Immunol. 1994;152:2139–2147. [PubMed] [Google Scholar]
- Penton-Rol G, Cota M, Polentarutti N, Luini W, Bernasconi S, Borsatti A, Sica A, LaRosa GJ, Sozzani S, Poli G, Mantovani A. Up-regulation of CCR2 chemokine receptor expression and increased susceptibility to the multitropic HIV strain 89.6 in monocytes exposed to glucocorticoid hormones. J Immunol. 1999;163:3524–9. [PubMed] [Google Scholar]
- Pieters W, Houben L, Koenderman L, Raaijmakers J. C5a-induced migration of human monocytes is primed by dexamethasone. Am J Respir Cell Mol Biol. 1995;12:691–696. doi: 10.1165/ajrcmb.12.6.7766432. [DOI] [PubMed] [Google Scholar]
- Rassias AJ, Holzberger PT, Givan AL, Fahrner SL, Yeager MP. Decreased physiologic variability as a generalized response to human endotoxemia. Crit Care Med. 2005;33:512–9. doi: 10.1097/01.ccm.0000155908.46346.ed. [DOI] [PubMed] [Google Scholar]
- Selye H. Role of the hypophysis in the pathogenesis of the diseases of adaption. Can Med Assoc J. 1944;50:426–433. [PMC free article] [PubMed] [Google Scholar]
- Selye H, Dosne C, Bassett L, Whittaker J. On the therapeutic value of adrenal cortical hormones in traumatic shock and allied conditions. Can Med Assoc J. 1940;43:1–8. [PMC free article] [PubMed] [Google Scholar]
- Smyth GP, Stapleton PP, Freeman TA, Concannon EM, Mestre JR, Duff M, Maddali S, Daly JM. Glucocorticoid pretreatment induces cytokine overexpression and nuclear factor-kappaB activation in macrophages. J Surg Res. 2004;116:253–61. doi: 10.1016/S0022-4804(03)00300-7. [DOI] [PubMed] [Google Scholar]
- Snyers L, DeWitt L, Content J. Glucocorticoid up-regulation of high-affinity interleukin 6 receptors on human epithelial cells. Proc Natl Acad Sci USA. 1990;87:2838–2842. doi: 10.1073/pnas.87.7.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Sapolsky RM. Glucocorticoids can arm macrophages for innate immune battle. Brain Behav Immun. 2010;24:17–8. doi: 10.1016/j.bbi.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs MK, Lioubin PJ, Slack J, Dower SK, Jonas U, Cosman D, Sims JE, Bauer J. Induction of an interleukin-1 receptor (IL-1R) on monocytic cells. J Biol Chem. 1990;265:22499–22505. [PubMed] [Google Scholar]
- Strickland RW, Wahl LM, Finblood DS. Corticoidsteroids enhance the binding of recombinant interferon-gamma to cultured human monocytes. J Immunol. 1986;137:1577–1580. [PubMed] [Google Scholar]
- Suffredini A, Hochstein H, McMahon F. Dose-related inflammatory effects of intravenous endotoxin in humans: Evaluation of a new clinical lot of Escherichia coli O:113 endotoxin. J Infect Dis. 1999;179:1278–82. doi: 10.1086/314717. [DOI] [PubMed] [Google Scholar]
- van der Goes A, Hoekstra K, van den Berg TK, Dijkstra CD. Dexamethasone promotes phagocytosis and bacterial killing by human monocytes/macrophages in vitro. J Leukoc Biol. 2000;67:801–807. doi: 10.1002/jlb.67.6.801. [DOI] [PubMed] [Google Scholar]
- Viswanathan K, Dhabhar F. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. PNAS. 2005;102:5808–13. doi: 10.1073/pnas.0501650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M, Vogel S. Opposing effects of glucocorticoids on interferon-_-induced murine macrophage Fc receptor and _a antigen expression. J Immunol. 1985;134:2462–2469. [PubMed] [Google Scholar]
- Weis F, Beiras-Fernandez A, Schelling G. Stress doses of hydrocortisone in high-risk patients undergoing cardiac surgery: effects on interleukin-6 to interleukin-10 ratio and early outcome. Crit Care Med. 2009;2009:1685–90. doi: 10.1097/CCM.0b013e31819fca77. [DOI] [PubMed] [Google Scholar]
- Wenzel I, Roth J, Sorg C. Identification of a novel surface molecule, RM3/1, that contributes to the adhesion of glucocorticoid-induced human monocytes to endothelial cells. Eur J Immunol. 1996;26:2758–2763. doi: 10.1002/eji.1830261131. [DOI] [PubMed] [Google Scholar]
- Wiegers GJ, Labeur M, Stec I, Klinkert W, Holsboer F, Reul J. Glucocorticoids accelerate anti-T cell receptor-induced T cell growth. J Immunol. 1995;155:1893–1902. [PubMed] [Google Scholar]
- Yeager M, Pioli P, Wardwell K, ML B, P M, HK L, AJ R, Guyre P. In vivo exposure to high or low cortisol has bi-phasic effects on inflammatory response pathways of human monocytes. Anesth Analg. 2008;107:1726–34. doi: 10.1213/ane.0b013e3181875fb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager MP, Guyre PM, Munck AU. Glucocorticoid regulation of the inflammatory response to injury. Acta Anaesthesiol Scand. 2004;48:799–813. doi: 10.1111/j.1399-6576.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- Yeager MP, Rassias AJ, Fillinger MP, Discipio AW, Gloor KE, Gregory JA, Guyre PM. Cortisol antiinflammatory effects are maximal at postoperative plasma concentrations. Crit Care Med. 2005;33:1507–1512. doi: 10.1097/01.ccm.0000164565.65986.98. [DOI] [PubMed] [Google Scholar]
- Yeager MP, Rassias AJ, Pioli PA, Beach ML, Wardwell K, Collins JE, Lee H, Guyre PM. Pre-treatment With Stress Cortisol Enhances the Human Systemic Inflammatory Response to Bacterial Endotoxin. Crit Care Med. 2009;37:2727–32. doi: 10.1097/ccm.0b013e3181a592b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TY, Daynes RA. Glucocorticoid conditioning of myeloid progenitors enhances TLR4 signaling via negative regulation of the phosphatidylinositol 3-kinase-Akt pathway. J Immunol. 2007a;178:2517–26. doi: 10.4049/jimmunol.178.4.2517. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Daynes RA. Macrophages from 11beta-hydroxysteroid dehydrogenase type 1-deficient mice exhibit an increased sensitivity to lipopolysaccharide stimulation due to TGF-beta-mediated up-regulation of SHIP1 expression. J Immunol. 2007b;179:6325–35. doi: 10.4049/jimmunol.179.9.6325. [DOI] [PubMed] [Google Scholar]