Abstract
The aim of this study was to examine the early effects of low dose 12C6+ irradiation or X-ray on peripheral blood lymphocytes (PBL) of patients with alimentary tract cancer and to explore mechanisms that may be involved in an antitumor immune response. We found that the percentage of T lymphocyte subsets, the mRNA expression levels of IL-2 and IFN-γ in PBL, and their protein levels in supernatant were significantly increased 24 hours after exposure to low dose radiation. The effects were more pronounced in the group receiving 0.05Gy 12C6+ ion irradiation than the group receiving X-ray irradiation. There was no significant change in the percentage of NK cell subsets and TNF-α production of PBL. Our study suggests that low dose irradiation could alleviate immune suppression caused by tumor burden and that the effect was more pronounced for 0.05Gy high linear energy transfer (LET) 12C6+ irradiation.
Keywords: low dose irradiation, 12C6+, X-ray, peripheral blood cells, alimentary tract cancer
INTRODUCTION
Heavy-ion beams are generally characterized by a high linear energy transfer (LET), an energy deposition peak (Bragg peak) at the end of their tracks, and increased relative biological effectiveness (RBE) within the peak region (Blakely and Kronenberg 1998; Gerlach et al. 2002). Recent growing evidence suggests that such irradiation can influence immune system function (Gridley et al. 2006; Hayashi et al. 2005; McDunn et al. 2009). It is subsequently important to investigate the effect of heavy ions on peripheral blood lymphocytes (PBL), a major component of the human immune system.
Conventional lymphocytes are hypersensitive to irradiation, even in the case of low dose irradiation (LDI) (Ren et al. 2006). The percentage of PBL subgroups, including T and NK cells, is often used as a clinical indicator of functional status of the human immune system. In addition, the production of cytokines such as IL-2, IFN-γ and TNF-α has also been suggested to play an important role in host immune defense against infection and cancer (Dinarello 1996; Galon et al. 2006; Luster et al. 1999; Young and Hardy 1990). By examining the levels of lymphocytes and cytokines, such as T and NK subgroups, the design and direction of immunotherapy may be improved as well as used in the prediction and evaluation of the efficacy of LDI.
Furthermore, radiation hormesis, or the notion that chronic low doses of ionizing radiation are beneficial through the stimulation of repair mechanisms that protect against disease, has become an emerging research area in recent years (Calabrese and Baldwin 2000; Olivieri et al. 1984; Pollycove and Feinendegen 2003; Rattan 2004; Sagan and Cohen 1990; Shadley and Wolff 1987; Wang et al. 1991; Zhou et al. 2004). Hormesis effects on immune system following LDI have been shown to alleviate the suppression of immunity caused by tumor burden. Specifically, LDI treatment has been shown to stimulate the cancer patients’ immune system to attack and kill cancer cells (Jerry and Myron 2003; Liu 2003). This therapy has been used to treat many types of cancer (i.e., colon cancer, ovarian cancer, hematologic cancer, etc.) with less severe side effects (Cuttler et al. 2000; Safwat 2000; Safwat et al. 2003; Sakamoto et al. 1997). While the majority of these studies have been performed using X-ray or γ-ray irradiation (Cuttler et al. 2000; Safwat 2000), very few studies have employed high linear energy transfer (LET) radiation.
We previously reported that low-dose 12C6+ irradiation has a stimulatory effect on mouse immunity, especially at a dose of 0.05 Gray (Gy) (Xie et al. 2007). Our experimental animal study showed that low dose (0.05Gy) 12C6+ ions irradiation could induce adaptive hormetic responses to the harmful effects on the pituitary by subsequent high-dose exposure, and have greater RBE value than low LET radiation (60Co γ-ray) (Zhang et al. 2006). We also found that exposure to 0.05 Gy 12C6+ ions radiation increases cytotoxic activity of human peripheral blood lymphocytes (HPBL) from healthy volunteers (Chen et al. 2010). The intriguing findings suggest that it might be possible to employ low-dose 12C6+ irradiation to treat cancer patients. We subsequently conducted an experimental study to investigate the acute response of immune cells in alimentary tract cancer patients after low-dose 12C6+ irradiation by measuring the percentage of various subsets of T lymphocytes and NK cells, mRNA expression of IL-2, IFN-γ and TNF-α, protein levels of these cytokines.
2. PATIENTS AND METHODS
2.1. Patients
A total of forty patients treated in the Tumor Hospital of Institute of Medical Science of Gansu province, China were included in the study (Table 1). The eligibility criteria for the study included: biopsy confirmed cancers of the esophagus, stomach, and colon, age less than 70 years, WHO performance status of 2 or better, a life expectancy of at least 3 months, laboratory tests of leukocytes>3.0×109/L and platelet count>100×109/L. Exclusion criteria included major organ metastases, major organ allografts, serious psychiatric diseases, and having previous systemic treatment for malignant tumors. The study was approved by the Ethical Committee of the Tumor Hospital of Institute of Medical Science of Gansu Province, China. Written consent form was obtained from each participant.
TABLE 1.
Patients characteristics (n=40)
| Characteristic | Esophageal cancer (n=14) | Gastric cancer (n=12) | Colon cancer (n=14) |
|---|---|---|---|
| Male to female ratio | 10/4 | 8/4 | 6/8 |
| Age (years, means±SD) | 58±3.36 | 42±5.34 | 48±3.15 |
| Performance status | |||
| 0 | 3 | 1 | 2 |
| 1 | 6 | 6 | 6 |
| 2 | 5 | 5 | 6 |
| Concurrent disease | 2 hypertension | 5 HBV; 1 HCV; 1 diabetes | 1 cardiac arrhythmia; 1 hypertension |
2.2. Isolation of PBL
After obtaining informed consent, a 30ml blood sample was drawn from each of the forty patients using vacuum tubes with heparin (Beijing Shuanghe Medicine Co. Ltd, Beijing, China). Peripheral blood lymphocytes (PBL) were isolated by a previously described method (Bellik et al. 2005). Briefly, PBL were isolated from patients with malignant tumor using a lymphocyte separation medium (LSM) (Shanghai Sangon Biological Engineering Technology and Service Co. Ltd, Shanghai, China) and density gradient centrifugation. The samples were then washed three times with phosphate buffered saline (PBS). All blood draws and PBL isolation experiments were finished within 1.5 hours. The PBL were then re-suspended in RPMI 1640 medium (Sigma, St Louis, MO, USA) with HEPES supplemented with 10% fetal bovine medium, 2 mM L-glutamine, 200 IU of penicillin per ml, 150 mg of streptomycin per ml, and 50 mg of gentamycin per ml to a concentration of 107 cells per ml. PBL from each patient were divided into three groups of equal number for subsequent irradiation treatment including sham, X-ray and 12C6+ irradiation.
2.3. Irradiation using heavy ion beams and X-ray
A carbon ion beam of 100 MeV/u was supplied by the Heavy Ion Research Facility in Lanzhou (HIRFL) at the Institute of Modern Physics, Chinese Academy of Sciences (IMP-CAS). The irradiation of cells was conducted at the therapy terminal of the HIRFL, which has a vertical beam line. Due to the energy degradation by the vacuum window, air gap, and Petri dish cover and medium, the energy of the ion beam on cell samples was calculated to be 89.63 MeV/u, corresponding to LET of 28.3 keV/...m and the dose rate was adjusted to be about 0.5Gy/min.
Low-LET irradiation was performed using SIEMENS Primus high energy electron linear accelerator operated at 6MV and at a source-to-surface distance of 100 cm, and the dose rate was approximately 0.5Gy/min. The dose used for each type of irradiation (carbon-ion or X rays) was 0.05Gy. All irradiations were performed once for each dish of cells at room temperature on the same day. The radiation dose selection was based on our previous results from animal data (Xie et al. 2007; Zhang et al. 2006).
2.4. Phenotype analysis
Twenty-four hours after radiation exposure, cells were obtained from PBL cultures for phenotype analysis using appropriate monoclonal antibodies, including CD3-Tc, CD3-FITC, CD4-FITC, CD8-PE, CD16-Tc, and CD56-PE (CALTAG, USA). T cells were detected by CD3, CD4 and CD8 antibodies, whereas NK cells were detected by CD3, CD16, CD56 antibodies. One million PBL were washed once in PBS containing 1% bovine serum albumin (BSA) and resuspended in 100ul of PBS buffer. The cells were incubated with various conjugated monoclonal antibodies for 20 min at 4° C, washed twice in PBS, and resuspended in 400ul of PBS. A flow cytometric analysis was performed on a FACSCalibur flow cytometry (CoulterEPICS XL, USA), and the data were analyzed using the SYSTEM statistical software. Forward and side scatter parameters were used to gate live cells (Han et al. 2005).
2.5. Isolation of RNA
Total RNA was extracted using Trizol reagent (Invitrogen, USA) from irradiated and unirradiated cells 24 hours after irradiation according to the manufacturer’s instructions. The quality of total RNA was determined by the Bioanalyzer (Petro 2005).
2.6. Preparation of cDNA
Five micrograms of total RNA from each sample was reversed transcribed to cDNA using PrimeScript™ reverse transcriptase kit (TaKaRa, Japan) according to the manufacturer’s protocol.
2.7. Analysis of mRNA expression by real time quantitative RT-PCR
Real-time quantitative RT–PCR analysis was performed using the Rotor-Gene RG-3000 Real-Time Sequence Detection System (Corbett Research, Australia). Reactions were carried out according to the manufacturer’s protocol of the SYBR Premix Ex Taq™ kit (Perfect Real Time) (TaKaRa, Japan). Oligonucleotides were used as primers and the predicted sizes of amplified PCR products are listed in Table 2. Using SYBR Premix Ex Taq™ kit (TaKaRa, Japan), the experiment was carried out in a final volume of 10μl of reaction mixture consisting of 5μl of SYBR Premix Ex Taq™, 0.4μl of the primers and 1μl cDNA according to the manufacturer’s instructions. The reaction mixture was then loaded into glass capillary tubes and subjected to denaturation at 95°C for 10 min, followed by 40 rounds of amplification at 95°C for 10 seconds for denaturation, 53°C for annealing, and 75°C for extension, with a temperature slope of 20°C per second, performed in the Rotor-Gene. The transcript amount for differentially expressed genes was estimated from the respective standard curves and normalized to the β-actin transcript amount which was determined in corresponding samples.
TABLE 2.
Primers used for qRT-PCR
| Gene | Forward primer | Reverse primer | Size (bp) |
|---|---|---|---|
| IL-2 | 5′-AACTCACCAGGATGCTCAC-3′ | 5′-CGTTGATATTGCTGATTAAGTCC-3′ | 168 |
| IFN-γ | 5′-TGGGTTCTCTTGGCTGTTAC-3′ | 5′-TGTCTTCCTTGATGGTCTCC-3′ | 252 |
| TNF-α | 5′-GTGAGGAGGACGAACATC-3′ | 5′-GAGCCAGAAGAGGTTGAG-3′ | 95 |
| β-actin | 5′-TGGCACCCAGCACAATGAA-3′ | 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ | 186 |
2.8. Cytokine protein production
Supernatants of PBL were harvested and assayed using enzyme-linked immunosorbent assays (ELISA) (ADL Co., USA) to quantify levels of IL-2, IFN-γ and TNF-α according to the manufacturer’s instructions.
2.9. Statistical analysis
Statistical analyses were performed using SPSS software (version 13.0). Each value was expressed as mean ± SD. An ANOVA analysis of variance was used to determine the level of any statistically significant differences between irradiated and unirradiated groups. A p-level of 0.05 was selected as a criterion for a statistically significant test.
RESULTS AND DISCUSSION
Lymphocytes flow analyses
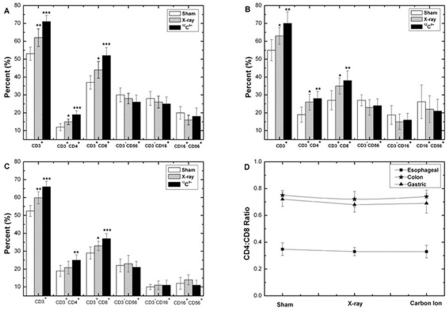
T lymphocytes and NK cells are an important aspect of host defense as they play a role in both tumor development and infection., Of particular interest is the impact of T cells in the control of human tumors (Alvaro et al. 2005; Clemente et al. 1996; Curiel et al. 2004; Galon et al. 2006; Parmiani 2005; Sato et al. 2005). This study demonstrated that the percentages of subgroups of CD3+ (mature T cells), CD3+CD4+ (T helper cells) and CD3+CD8+ (T cytotoxic cells) increased dramatically after low-dosage irradiation, and that the increase in the group treated by 12C6+ ion was higher than in the group by X-ray irradiation (Fig. 1). This was expected as 12C6+ ions are high LET radiation, characterized by a higher RBE than low LET radiation.
FIGURE 1.
Change in percentage of T and NK cells subsets of PBL and CD4+:CD8+ T lymphocytes ratio at 24 hours after irradiation with 0.05Gy X-ray or 12C6+ ions. (A) PBL of patients with esophageal cancer (B) PBL of patients with colon cancer (C) PBL of patients with gastric cancer (D) CD4+:CD8+ T lymphocytes ratio (*p<0.05, **p<0.01, ***p<0.001 vs. 0Gy; n=40). The significance among radiated groups was determined by ANOVA.
We did not, however, find significant differences in the CD3+CD4+/CD3+CD8+ ratio among sham, X-ray and 12C6+ ion groups in this study. Our results were similar to a clinical report in which no significant differences in CD3+CD4+/CD3+CD8+ ratio were observed in patients who were given total body irradiation before bone marrow transplantation (Clave et al. 1995). The data indicate that these two major T-lymphocyte subsets appeared equally radiosensitive.
Compared with the sham irradiation group, the percentages of NK cell subsets (CD3−CD16+, CD3−CD56+, CD16+CD56+) of PBL didn’t change significantly after LDI. Although the mechanisms are currently unclear, it is possible that NK cells might be relatively radioresistant compared to other lymphocyte subsets (Pecaut et al. 2003). It is also possible that since the analysis was conducted 24 hours after irradiation, the cells may have still been in the induction period. As such, data at later time points following exposure are needed.
The study suggested that 0.05Gy of high LET radiation (12C6+ ion) could stimulate a T cell-mediated adoptive immune response and enhance its anti-tumor function in patients with alimentary tract cancer, and that the effect of 12C6+ ion irradiation was more significant than that of low LET irradiation (X-ray). Our previous study using healthy HPBLs did not observe increases in the percentages of T-cell subgroup (CD3+, CD3+CD4+ and CD3+CD8+) (Chen et al. 2010). However, the percentages of these T-cell subgroups were much lower among patients with alimentary tract cancer compared with healthy individuals before irradiation. The findings suggest that cancer patients suffered immune suppression compared to healthy controls and the low dose irradiation could alleviate the immune suppression resulting from cancer burden.
Cytokine production
The proinflammatory cytokines, IL-2, TNF-α and IFN-γ, are involved in the generation of lymphokine-activated killer cells and cytotoxic T cells that are capable of destroying malignant tumors (Lauwerys et al. 1999; McAdam et al. 1995; Sadanaga et al. 1999; Saijo et al. 1999; Siegel et al. 1987; Yoneda et al. 1993). Progressive tumor growth is associated with reduced antitumor immunity and lower levels of IL-2 and IFN-γ (Ghosh et al. 1995; Kobayashi et al. 1998; Yamamura et al. 1993). Decreased serum levels of IL-2 and TNF-α were observed in cancer patients, and decreased levels of IFN-γ were found to be associated with poor survival (Martin et al. 1999). These studies provided strong evidence that the production of IL-2, TNF-α and IFN-γ was critical for antitumor immunity.
Experimental studies have previously suggested that low dose total body irradiation (LTBI) using low-LET radiation induces host immune enhancement (Galdiero et al. 1994; Hashimoto, 1997; Hashimoto et al. 1999; Liu et al. 1994; Nogami et al. 1993; Shen et al. 1991). For example, LDI using low-LET radiation can alter cytokine release, particularly the activation of IFN-γ and IL-2 (Galdiero et al. 1994; Hashimoto et al. 1999; Shen et al. 1991). In a clinical study, Yonkosky et al. treated nine non-Hodgkin lymphoma patients with fractionated LTBI after they failed previous chemotherapy (Yonkosky et al. 1978). The study showed an enhanced in vitro immune response after LTBI, which suggested that LTBI could induce a certain degree of immune enhancement in humans (Yonkosky et al. 1978). Our in vitro study showed an increase in mRNA expression of IFN-γ, IL-2, and TNF-α and their protein levels in healthy HPBLs (Chen et al. 2010). However, there is no data on effects of low doses of high LET irradiation on the immune response in cancer patients.
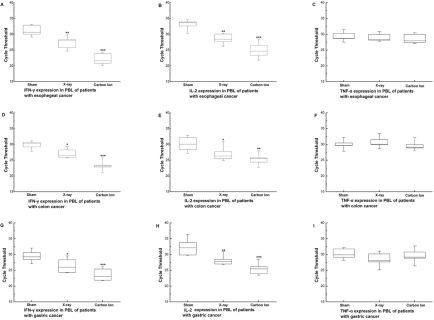
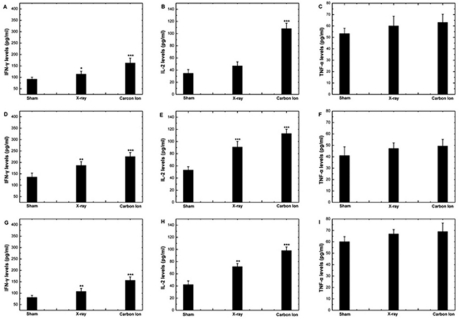
In this study, the mRNA expression levels of IFN-γ and IL-2 in PBL increased significantly after irradiation. The increase was more pronounced in the group irradiated by 12C6+ ion than in that irradiated by X-ray. The TNF-α only slightly increased with irradiation, but the difference was not significant (Figure 2). The results were further confirmed by ELISA assays assessing protein levels for IL-2, TNF-α and IFN-γ (Figure 3). These results indicated that low dose irradiation could alleviate the suppression of immunity in cancer patients with greater efficiency by high LET 12C6+-ion radiation.
FIGURE 2.
The change in Real-time PCR cycle threshold values in PBL at 24 hours after irradiation with 0.05Gy X-ray or 12C6+ ions. (A–C) Expression levels of IFN-γ, IL-2 and TNF-α in PBL of patients with esophageal cancer (D–F) Expression levels of IFN-γ, IL-2 and TNF-α in PBL of patients with colon cancer (G–I) Expression levels of IFN-γ, IL-2 and TNF-α in PBL of patients with gastric cancer were shown as medians (lines), 25th percentile to the 75th percentile (boxes) and ranges (whiskers) for 40 samples. (*p<0.05, **p<0.01, ***p<0.001 vs. 0Gy; n=40). The significance among radiated groups was determined by ANOVA.
FIGURE 3.
The change in protein levels of cytokines in supernatant of HPBL at 24 hours after irradiation with X-ray or 12C6+ ions. (A–C) Protein levels of IFN-γ, IL-2 and TNF-α in supernatant of HPBL of patients with esophageal cancer (D–F) Protein levels of IFN-γ, IL-2 and TNF-α in supernatant of PBL of patients with colon cancer (G–I) Protein levels of IFN-γ, IL-2 and TNF-α in supernatant of PBL of patients with gastric cancer (*p<0.05,**p<0.01,***p<0.001 vs. 0Gy; n=40). The significance among radiated groups was determined by ANOVA.
Our study provides the first evidence that exposure to 0.05Gy 12C6+ ion irradiation could alleviate the immune suppression in alimentary tract cancer patients. Low dose 12C6+ ions could therefore be considered a potential novel form of immunotherapy, with greater efficiency than conventional LDI using low LET radiation, such as X-ray. The T lymphocyte subsets and cytokine production levels might be used as potential bio-markers for the efficacy of LDI. We subsequently suggest that these findings be considered in future research for alimentary tract cancer as well as other cancers as the clinical application of these results may improve cancer outcomes.
Acknowledgments
We express our thanks to the accelerator crew at HIRFL, National Laboratory of Heavy Ion Accelerator in Lanzhou. The project was supported by Fogarty training grants 1D43TW008323-01 and 1D43TW007864-01 from the National Institute of Health (NIH). This work was also partially supported by grants from the National Science Foundation of China (30870364).
REFERENCES
- Alvaro T, Lejeune M, Salvado MT, Bosch R, Garcia JF, Jaen J, Banham AH, Roncador G, Montalban C, Piris MA. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11:1467–1473. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- Bellik L, Ledda F, Parenti A. Morphological and phenotypical characterization of human endothelial progenitor cells in an early stage of differentiation. FEBS Lett. 2005;579:2731–2736. doi: 10.1016/j.febslet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Blakely EA, Kronenberg A. Heavy-ion radiobiology: new approaches to delineate mechanisms underlying enhanced biological effectiveness. Radiat Res. 1998;150:S126–145. [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Radiation hormesis: the demise of a legitimate hypothesis. Hum Exp Toxicol. 2000;19:76–84. doi: 10.1191/096032700678815611. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li Y, Zhang H, Xie Y, Chen X, Ren J, Zhang X, Zhu Z, Liu H, Zhang Y. Early effects of low dose 12C6+ ion or X-ray irradiation on human peripheral blood lymphocytes. Adv Space Res. 2010;45(7):832–838. doi: 10.1016/j.asr.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clave E, Socie G, Cosset JM, Chaillet MP, Tartour E, Girinsky T, Carosella E, Fridman H, Gluckman E, Mathiot C. Multicolor flow cytometry analysis of blood cell subsets in patients given total body irradiation before bone marrow transplantation. Int J Radiat Oncol Biol Phys. 1995;33:881–886. doi: 10.1016/0360-3016(95)00213-6. [DOI] [PubMed] [Google Scholar]
- Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Cuttler J, Pollycove M, Welsh J. Application of low doses of radiation for curing cancer. Canadian Nuclear Society Bulletin. 2000:45–52. [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Galdiero M, Cipollaro de l’Ero G, Folgore A, Cappello M, Giobbe A, Sasso FS. Effects of irradiation doses on alterations in cytokine release by monocytes and lymphocytes. J Med. 1994;25:23–40. [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Gerlach R, Roos H, Kellerer AM. Heavy ion RBE and microdosimetric spectra. Radiat Prot Dosimetry. 2002;99:413–418. doi: 10.1093/oxfordjournals.rpd.a006821. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Komschlies KL, Cippitelli M, Longo DL, Subleski J, Ye J, Sica A, Young HA, Wiltrout RH, Ochoa AC. Gradual loss of T-helper 1 populations in spleen of mice during progressive tumor growth. J Natl Cancer Inst. 1995;87:1478–1483. doi: 10.1093/jnci/87.19.1478. [DOI] [PubMed] [Google Scholar]
- Gridley DS, Dutta-Roy R, Andres ML, Nelson GA, Pecaut MJ. Acute effects of iron-particle radiation on immunity. Part II: Leukocyte activation, cytokines and adhesion. Radiat Res. 2006;165:78–87. doi: 10.1667/rr3490.1. [DOI] [PubMed] [Google Scholar]
- Han SB, Moratz C, Huang NN, Kelsall B, Cho H, Shi CS, Schwartz O, Kehrl JH. Rgs1 and Gnai2 regulate the entrance of B lymphocytes into lymph nodes and B cell motility within lymph node follicles. Immunity. 2005;22:343–354. doi: 10.1016/j.immuni.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Hashimoto S. Effects of low-dose total body irradiation (TBI) on tumor-bearing rats. Nippon Igaku Hoshasen Gakkai Zasshi. 1997;57:418–424. [PubMed] [Google Scholar]
- Hashimoto S, Shirato H, Hosokawa M, Nishioka T, Kuramitsu Y, Matushita K, Kobayashi M, Miyasaka K. The suppression of metastases and the change in host immune response after low-dose total-body irradiation in tumor-bearing rats. Radiat Res. 1999;151:717–724. [PubMed] [Google Scholar]
- Hayashi T, Morishita Y, Kubo Y, Kusunoki Y, Hayashi I, Kasagi F, Hakoda M, Kyoizumi S, Nakachi K. Long-term effects of radiation dose on inflammatory markers in atomic bomb survivors. Am J Med. 2005;118:83–86. doi: 10.1016/j.amjmed.2004.06.045. [DOI] [PubMed] [Google Scholar]
- Jerry MC, Myron P. Can cacer be treated with low doses of radiation? Journal of American Physicians and Surgeons. 2003;8:108–111. [Google Scholar]
- Kobayashi M, Kobayashi H, Pollard RB, Suzuki F. A pathogenic role of Th2 cells and their cytokine products on the pulmonary metastasis of murine B16 melanoma. J Immunol. 1998;160:5869–5873. [PubMed] [Google Scholar]
- Lauwerys BR, Renauld JC, Houssiau FA. Synergistic proliferation and activation of natural killer cells by interleukin 12 and interleukin 18. Cytokine. 1999;11:822–830. doi: 10.1006/cyto.1999.0501. [DOI] [PubMed] [Google Scholar]
- Liu S, Hann Z, Liu W. Changes in lymphocyte reactivity to modulatory factors following low dose ionising radiation. Biomed, Environ Sci. 1994;7:130–135. [PubMed] [Google Scholar]
- Liu SZ. Nonlinear dose-response relationship in the immune system following exposure to ionizing radiation: mechanisms and implications. Nonlinearity Biol Toxicol Med. 2003;1:71–92. doi: 10.1080/15401420390844483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster MI, Simeonova PP, Gallucci R, Matheson J. Tumor necrosis factor alpha and toxicology. Crit Rev Toxicol. 1999;29:491–511. doi: 10.1080/10408449991349258. [DOI] [PubMed] [Google Scholar]
- Martin F, Santolaria F, Batista N, Milena A, Gonzalez-Reimers E, Brito MJ, Oramas J. Cytokine levels (IL-6 and IFN-gamma), acute phase response and nutritional status as prognostic factors in lung cancer. Cytokine. 1999;11:80–86. doi: 10.1006/cyto.1998.0398. [DOI] [PubMed] [Google Scholar]
- McAdam AJ, Pulaski BA, Harkins SS, Hutter EK, Lord EM, Frelinger JG. Synergistic effects of co-expression of the TH1 cytokines IL-2 and IFN-gamma on generation of murine tumor-reactive cytotoxic cells. Int J Cancer. 1995;61:628–634. doi: 10.1002/ijc.2910610508. [DOI] [PubMed] [Google Scholar]
- McDunn JE, Muenzer JT, Dunne B, Zhou A, Yuan K, Hoekzema A, Hilliard C, Chang KC, Davis CG, McDonough J, Hunt C, Grigsby P, Piwnica-Worms D, Hotchkiss RS. An anti-apoptotic peptide improves survival in lethal total body irradiation. Biochem Biophys Res Commun. 2009;382:657–662. doi: 10.1016/j.bbrc.2009.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogami M, Huang JT, James SJ, Lubinski JM, Nakamura LT, Makinodan T. Mice chronically exposed to low dose ionizing radiation possess splenocytes with elevated levels of HSP70 mRNA, HSC70 and HSP72 and with an increased capacity to proliferate. Int J Radiat Biol. 1993;63:775–783. doi: 10.1080/09553009314552181. [DOI] [PubMed] [Google Scholar]
- Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223:594–597. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- Parmiani G. Tumor-infiltrating T cells—friend or foe of neoplastic cells? N Engl J Med. 2005;353:2640–2641. doi: 10.1056/NEJMp058236. [DOI] [PubMed] [Google Scholar]
- Pecaut MJ, Nelson GA, Moyers MF, Rabin B, Gridley DS. “Out-of-field” effects of head-localized proton irradiation on peripheral immune parameters. In Vivo. 2003;17:513–521. [PubMed] [Google Scholar]
- Petro TM. Disparate expression of IL-12 by SJL/J and B10.S macrophages during Theiler’s virus infection is associated with activity of TLR7 and mitogen-activated protein kinases. Microbes Infect. 2005;7:224–232. doi: 10.1016/j.micinf.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Pollycove M, Feinendegen LE. Radiation-induced versus endogenous DNA damage: possible effect of inducible protective responses in mitigating endogenous damage. Hum Exp Toxicol. 2003;22:290–306. doi: 10.1191/0960327103ht365oa. discussion 307, 315-297, 319-223. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Aging intervention, prevention, and therapy through hormesis. J Gerontol A Biol Sci Med Sci. 2004;59:705–709. doi: 10.1093/gerona/59.7.b705. [DOI] [PubMed] [Google Scholar]
- Ren H, Shen J, Tomiyama-Miyaji C, Watanabe M, Kainuma E, Inoue M, Kuwano Y, Abo T. Augmentation of innate immunity by low-dose irradiation. Cell Immunol. 2006;244:50–56. doi: 10.1016/j.cellimm.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Sadanaga N, Nagoshi M, Lederer JA, Joo HG, Eberlein TJ, Goedegebuure PS. Local secretion of IFN-gamma induces an antitumor response: comparison between T cells plus IL-2 and IFN-gamma transfected tumor cells. J Immunother. 1999;22:315–323. doi: 10.1097/00002371-199907000-00005. [DOI] [PubMed] [Google Scholar]
- Safwat A. The role of low-dose total body irradiation in treatment of non-Hodgkin’s lymphoma: a new look at an old method. Radiother Oncol. 2000;56:1–8. doi: 10.1016/s0167-8140(00)00167-5. [DOI] [PubMed] [Google Scholar]
- Safwat A, Bayoumy Y, El-Sharkawy N, Shaaban K, Mansour O, Kamel A. The potential palliative role and possible immune modulatory effects of low-dose total body irradiation in relapsed or chemo-resistant non-Hodgkin’s lymphoma. Radiother Oncol. 2003;69:33–36. doi: 10.1016/s0167-8140(03)00247-0. [DOI] [PubMed] [Google Scholar]
- Sagan LA, Cohen JJ. Biological effects of low-dose radiation: overview and perspective. Health Phys. 1990;59:11–13. [PubMed] [Google Scholar]
- Saijo Y, Hong X, Tanaka M, Tazawa R, Liu SQ, Saijo K, Ohno T, Koike K, Ohkuda K, Satoh K, Nukiwa T. Autologous high-killing cytotoxic T lymphocytes against human lung cancer are induced using interleukin (IL)-1beta, IL-2, IL-4, and IL-6: possible involvement of dendritic cells. Clin Cancer Res. 1999;5:1203–1209. [PubMed] [Google Scholar]
- Sakamoto K, Myojin M, Hosoi Y, Ogawa Y, Nemoto K, Takai Y, Kakuto Y, Yamada S, Watabe N. Fundamental and clinical studies on cancer control with total or upper-half body irradiation. J Jpn Soc Ther Radiol Oncol. 1997;9:161–175. [Google Scholar]
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadley JD, Wolff S. Very low doses of X-rays can cause human lymphocytes to become less susceptible to ionizing radiation. Mutagenesis. 1987;2:95–96. doi: 10.1093/mutage/2.2.95. [DOI] [PubMed] [Google Scholar]
- Shen RN, Lu L, Feng GS, Miller J, Taylor MW, Broxmeyer HE. Cure with low-dose total-body irradiation of the hematological disorder induced in mice with the Friend virus: possible mechanism involving interferon-gamma and interleukin-2. Lymphokine Cytokine Res. 1991;10:105–109. [PubMed] [Google Scholar]
- Siegel JP, Sharon M, Smith PL, Leonard WJ. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987;238:75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Saigusa S, Sasaki MS. Adaptive response to chromosome damage in cultured human lymphocytes primed with low doses of X-rays. Mutat Res. 1991;246:179–186. doi: 10.1016/0027-5107(91)90120-d. [DOI] [PubMed] [Google Scholar]
- Xie Y, Zhang H, Wang YL, Zhou QM, Qiu R, Yuan ZG, Zhou GM. Alterations of immune functions induced by 12C6+ ion irradiation in mice. Int J Radiat Biol. 2007;83:577–581. doi: 10.1080/09553000701481774. [DOI] [PubMed] [Google Scholar]
- Yamamura M, Modlin RL, Ohmen JD, Moy RL. Local expression of antiinflammatory cytokines in cancer. J Clin Invest. 1993;91:1005–1010. doi: 10.1172/JCI116256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K, Osaki T, Yamamoto T, Ueta E. Effects of tumour necrosis factor-alpha (TNF-alpha), IL-1 beta and monocytes on lymphokine-activated killer (LAK) induction from natural killer (NK) cells and T lymphocytes. Clin Exp Immunol. 1993;93:229–236. doi: 10.1111/j.1365-2249.1993.tb07971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkosky DM, Feldman MI, Cathcart ES, Kim S. Improvement of in vitro mitogen proliferative responses in non-Hodgkin’s lymphoma patients exposed to fractionated total body irradiation. Cancer. 1978;42:1204–1210. doi: 10.1002/1097-0142(197809)42:3<1204::aid-cncr2820420325>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Young HA, Hardy KJ. Interferon-gamma: producer cells, activation stimuli, and molecular genetic regulation. Pharmacol Ther. 1990;45:137–151. doi: 10.1016/0163-7258(90)90012-q. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xie Y, Zhou Q, Liu B, Li W, Li X, Duan X, Yuan Z, Zhou G, Min F. Adaptive hormetic response of pre-exposure of mouse brain with low-dose 12C6+ ion or 60Co c-ray on growth hormone (GH) and body weight induced by subsequent high-dose irradiation. Adv Space Res. 2006;38:1148–1151. doi: 10.1111/j.1365-2605.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- Zhou H, Randers-Pehrson G, Waldren CA, Hei TK. Radiation-induced bystander effect and adaptive response in mammalian cells. Adv Space Res. 2004;34:1368–1372. doi: 10.1016/j.asr.2003.10.049. [DOI] [PubMed] [Google Scholar]