Abstract
Proteins participating in immunological signaling have emerged as important targets for controlling the immune response. A multitude of receptor–ligand pairs that regulate signaling pathways of the immune response have been identified. In the complex milieu of immune signaling, therapeutic agents targeting mediators of cellular signaling often either activate an inflammatory immune response or induce tolerance. This review is primarily focused on therapeutics that inhibit the inflammatory immune response by targeting membrane-bound proteins regulating costimulation or mediating immune-cell adhesion. Many of these signals participate in larger, organized structures such as the immunological synapse. Receptor clustering and arrangement into organized structures is also reviewed and emerging trends implicating a potential role for multivalent therapeutics is posited.
Autoimmune disease
Autoimmunity results from the breakdown of mechanisms controlling immune tolerance and the failure of the host immune system to distinguish self from non-self antigens. Autoimmunity induces an attack by autoantibodies and/or autoreactive T cells. Central tolerance in primary lymphoid organs (thymus and bone marrow) provides the mechanism by which T cells and B cells are typically removed if they carry receptors that recognize self antigens. For self-reactive T cells or B cells that escape from the thymus or bone marrow, there is a backup mechanism of ‘peripheral tolerance’ that renders these cells inactive or anergic by several processes such as apoptosis or by the function of regulatory T cells [1,2]. Despite tight regulation, inappropriate humoral and cellular-mediated immune responses against self antigens occasionally occur and can lead to disease.
Immune reactions propagate a number of autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, Graves’ disease, Crohn’s disease, lupus erythematosus and insulin-dependent diabetes mellitus. Autoimmune diseases are chronic inflammatory diseases assumed to arise from a combination of genetic traits, environmental factors and/or the breakdown of regulatory mechanisms [1-5]. Both humoral (autoantibodies) and cellular (autoreactive lymphocytes) responses mediate autoimmune diseases. Some autoimmune diseases are mediated by cellular damage. Autoimmune diseases that are mediated by cellular damage occur when lymphocytes or antibodies bind to cell-membrane antigens and stimulate cellular lysis and inflammatory response. For example, insulin-dependent diabetes mellitus is caused by the attack of autoreactive T cells against insulin-producing cells (β cells) in the pancreas. Multiple sclerosis patients produce autoreactive T cells that infiltrate brain tissue and cause inflammatory lesions along the myelin sheath of nerve fibers [2]. Some autoimmune diseases are mediated by stimulation or suppression of autoantibody production. Two examples are Graves’ disease and Myasthenia gravis, respectively. In Graves’ disease, autoantibodies are produced and bind to the thyroid stimulating hormone (TSH) receptor without regulation, which leads to overstimulation of the thyroid and excess production of thyroid hormone. Myasthenia gravis is an autoimmune disease mediated by autoantibodies that block acetylcholine receptors on the motor end plate of muscles and inhibit the binding of acetylcholine to this receptor, hence weakening skeletal muscle.
Current therapies for autoimmune diseases only reduce symptoms to provide an acceptable quality of life; in many cases, these therapies include non-specific suppression of the immune system. A problem of using non-selective immunosuppressants, cytotoxic drugs or corticosteroids is that these drugs threaten the overall immune response, leaving the patient susceptible to opportunistic infections and other side effects [6]. Therefore, new therapeutic approaches that modulate the immune response in a specific manner without suppressing the general immune response are desperately needed.
Chronic inflammation is one target for autoimmune disease intervention [5]. The inflammatory pathway involves several steps including leukocyte transmigration and cytokine production. Discoveries of pathogenetic mechanisms of autoimmune diseases have contributed to the introduction of biologics as new therapies for autoimmune diseases. Inhibitors of the inflammatory cytokine TNF-α (infliximab, etanercept and adalimumab) have been used for treatment of rheumatoid arthritis, psoriasis and Crohn’s disease. There are several other cytokines that modulate inflammatory responses such as interleukins (IL-1, -6, -10, -17 and -23), and transforming growth factor-β (TGF-β). The rationale behind attempts to block the biological effects of cytokines is that an imbalance of proinflammatory cytokines and anti-inflammatory cytokines may be responsible for the induction of autoimmunity [5].
Besides inhibition of cytokine production or signaling, blockade of cell trafficking, B-cell depletion and blocking of T-cell activation are used as therapeutic intervention strategies. Tysabri® (natalizumab), an α4β1 integrin blocker, acts as an inhibitor of leukocyte extravasation and was approved for the treatment of multiple sclerosis. The drug was withdrawn later due to cases of opportunistic infections in the brain, but was later reintroduced to treat relapsing forms of multiple sclerosis. Rituximab, a monoclonal antibody (mAb) against CD20 antigen presented on the surface of B cells, kills B cells via complement-mediated cytotoxicity, antibody-dependent cell-mediated cytotoxicity and apoptosis [5]. In the USA, it has been approved for use in combination with methotrexate (MTX) for reducing symptoms in patients with rheumatoid arthritis who have had an inadequate response to one or more anti-TNF-α therapies.
T-cell activation & costimulatory molecules
In autoimmune diseases, T-cell activation is initiated by presentation of self-antigen to T cells. Antigen-presenting cells (APCs) capture self-antigen and load it onto major histocompatibilty complex (MHC) class II. The antigen-primed APC then presents the self antigen to T-cell receptor (TCR) on T cells. T-cell activation requires two signals; the interaction of the TCR on T cells and antigenic peptide–MHC complex on APCs (signal 1) and a costimulatory signal (signal 2), to promote T-cell proliferation, cytokine secretion, and effector function [7,8]. Signal 1, which imparts specificity to the immune response, must occur through the antigen-specific receptor of T cells. Signal 2, the costimulatory signal, is composed of several receptor–ligand interactions including the binding of CD28 on T cells and B7 on professional APCs. The binding of leukocyte function-associated antigen-1 (LFA-1) primarily on T cells and intercellular adhesion molecule-1 (ICAM-1) primarily on APCs prolongs the interaction of the cells [2,9]. Activated T cells then either transmigrate to cells or tissue where antigen is located (i.e., β cells in Type 1 diabetes or the central nervous system [CNS] in multiple sclerosis) or T cells proceed to activate B cells to secrete antibodies (i.e., myasthenia gravis or systemic lupus erythematosis). TCR recognition of the antigen–MHC complex in the absence of the costimulatory signal can lead to T-cell anergy and interrupt the immune response. Abatacept, a CTLA-4-Ig fusion protein, blocks T-cell costimulation by blocking interaction of CTLA-4, a receptor expressed on CD4+ T cells, with its ligand. Abatacept has been approved to treat rheumatoid arthritis and is now in clinical trials for other autoimmune diseases [10].
The costimulaltory signal is important in directing T-cell activation since the TCR has low affinity for the antigen–peptide complex, has a small size, hindering engagement, and is only present in small quantities [6]. In the absence of the costimulatory signal, antigen-specific T cells fail to respond effectively and become anergic or die. Several costimulatory molecules have been identified and classified in families. Among these molecules, there are both stimulatory and inhibitory signals. T-cell costimulation plays an important role in the development of effective immune response including T-cell proliferation, differentiation and survival (Table 1) [6,8,11-86,201]. Here, the function of these signals is summarized and current therapeutic efforts are reviewed. Particular emphasis is placed on inhibition of signals to treat autoimmune diseases. Finally, emerging trends in multivalent therapeutics that aim to interrupt costimulation and/or cell adhesion within larger, organized structures such as the immunological synapse (IS) are discussed.
Table 1. Summary of costimulatory molecules.
| Molecule | Expression | Ligand(s) | Expression | Function | Drugs/mAb |
|---|---|---|---|---|---|
| CD28 | T cells, thymocytes, NK eosinophils and melanoma cells [6,8,11,12] |
CD80 (B7-1) CD86 (B7-2) |
Upregulated on activated DCs [13], B cells and macrophages [6] Upregulated on activated monocytes, DCs and B cells, T cells and APCs [6] |
Enhances T-cell proliferation and cytokine production through induction of IL-2 expression [6] Promotes T-cell differentiation into IL-4-producing T-helper 2 cells [6,14] Stimulates B cells for antibody production [8] Augments the kinetics and level of expression of OX40, 4–1BB and CD30 [6,15] Inhibition of CD28/B7 reduces specific Ab production, prolongs the survival of organ transplants and inhibits autoimmune diabetes and lupus |
Abatacept (CTLA-4-Ig) targeting CD80/86 has been approved by FDA to use in RA. It is also in Phase II clinical trials in psoriasis [16-19] Belatacept (CTLA-4-Ig) has clinical indication in renal transplantation [20,21] Galiximab is an anti-CD80 mAb. It has a clinical indications in non-Hodgkin’s B-cell lymphoma [22] and psoriasis [16]. It is now in Phase III clinical trials [201] |
|
| |||||
| PD-1 | B cells, monocytes/ macrophages and activated T cells [23] |
PDL-1 (B7-H1) PDL-2 (B7-DC) |
Found on nonhematopoietic cells [23] T cells, B cells, dendritic cells and macrophages [23,24] |
Transmits signals that inhibit T- and B-cell activation [23] |
PDL-1-Ig fusion protein reduced clinical score in collagen induced arthritis mouse model and prolonged graft surviva in mice [23,25] Anti-PD-1 antibody antagonists (MDX-1106, CT-011) are currently in Phase I clinical trials in melanoma [26,27,201] |
|
| |||||
| CTLA-4 (CD152) |
Activated T cells [14] | B7-1 (CD80) and B7-2 (CD86) |
T cells, B cells, macrophages, DCs, endothelial cells, epithelial cells and fibroblasts [14] |
Transmits an inhibitory signal to T cells and inhibits T-cell activation [14] Depletes the expression of IL-2 and IL-2R [8] CTLA-4 binds B7 with a much higher affinity than CD28 |
Anti-CTLA-4 mAb (Ipilimumab and tremelimumab) have clinical indications in melanoma and other tumors. They have been studied in Phase II/III clinical trials [28-31,201] |
|
| |||||
| ICOS (CD278) |
Activated T cells [32] | ICOSL (B7RP-1 or CD275) |
Constitutively expressed on B cells, dendritic cells, monocytes, endothelial cells, fibroblasts and epithelial cells [32] |
Enhances T-cell differentiation, cytokine production and survival [32] |
Anti-ICOSL mAb antagonist has been shown to reduce the severity of G6PI-induced arthritis [33], reduce lethality and symptoms in GVHD in mice [34] Anti-ICOS mAb prolongs cardiac and bone marrow graft survival in mice [35-37] |
|
| |||||
| BTLA (CD272) | B cells and inducible during activation of T cells [38,39] |
HVEM | Naive or resting T cells, NK cells, B cells and DCs, myeloid cells [38,39] |
Delivers the inhibitory signal of T-cell activation [38,39] Decreases IL-2 production [38,39] Balances activation and inhibition during an immune response [40] |
Anti-BTLA in a combination with CTLA-4-Ig has shown efficacy in allograft rejection [41,42] |
|
| |||||
| CD40 | B cells, monocytes, macrophages, DCs, platelets and inducibly expressed on non-hematopoietic cells such as epithelial and endothelial cells and fibroblasts [43] |
CD40L (CD154) |
Activated T cells, activated B cells and platelets [43] |
Promotes B-cell activation and DC maturation [8] Induces memory B cells maturation [43,44] CD40 ligation leads to the upregulation of B7-1 and B7-2 on APCs and enhances the ability of APCs to activate naive T cells [43,44] |
Dacetuzumab (anti-CD40 mAb) has been shown that it has an immunosuppressive effect in multiple myeloma, non-Hodgkin’s lymphoma (Phase I) [45,46], diffuse large B-cell lymphoma [47] and chronic lymphocytic leukemia (Phase I) [48] Anti-CD40L mAb significantly suppressed T-cell-mediated alloimmune responses and prolonged allograft survival in monkeys [44] and has been studied in clinical trials for lupus nephritis and kidney transplantation (Phase II) [201] |
|
| |||||
| OX40 (CD134) | Activated T cells [15] | OX40L (CD252) |
Professional APCs, can be induced on activated B cells, mature DCs, plasmacytoid DCs and macrophages [15] |
Promotes T-cell proliferation, differentiation, survival, cytokine production and effector cell function of T cells [15] OX40–OX40L interactions additionally modulate the differentiation and activity of regulatory T cells (both suppress and promote proliferation of these cells) [15] |
Anti-OX40 and -OX40L mAb agonists have shown benefits in cancer. Anti-OX40 mAb was studied in clinical trials (Phase I/II) [49-51,201]. There is an onging clinical study of human mAb OX40L for treatment of asthma (Phase II) [201] Neutralizing anti-OX40L ameliorated the severity of disease in experimental autoimmune encephalomyelitis [52], colitis [53], arthritis [54], asthma [55] and diabetes [56] in vivo |
|
| |||||
| GITR | Naive T cells and Treg It is upregulated on effector T cells [57] and on tissue infiltrating macrophages |
GITRL | B cells, macrophages, DCs, endothelial cells thymic precursors and activated T cells [57] |
Suppresses function of Treg [58] Activates effector T cells and NK cells [58] Induces inflammatory activation of macrophage [57] |
Anti-GITR mAb agonist induced antitumor immunity [59,60] Phase I clinical study is being performed for treatment of malignant melanoma [201] GITR fusion protein binding to GITRL prolonged allograft survival and showed potential anti-inflammatory properties in animal studies [61,62] Neutralizing anti-GITRL mAb protected the progression of diabetes [63] |
|
| |||||
| CD27 | Naive T cells, B cells, NK cells, memory and antigen-exposed T cells [64,65] |
CD70 | Activated T cells, B cells, macrophages, and activated DCs [64,65] |
Stimulates T-cell activation, expansion and survival [66] Promotes B-cell activation and production of antibodies [64] |
Anti-CD70 mAb agonist exhibited antitumor immunity in in vivo studies [67,68] Neutralizing anti-CD70 mAb improved disease severity in inflammatory and autoimmune disease [69,70] |
|
| |||||
| LIGHT (TNFSF14) |
Activated T cells and immature DCs [65,71] |
HVEM (this is the receptor) |
T cells, B cells, DCs, macrophages and NK cells [65,72] |
Promotes T-cell proliferation, cytokine production, and activation of NF-κB [8] |
LTβR-Ig fusion protein has been shown to ameliorate trinitrobenzene sulfonic acid-induced colitis and prolonged survival of GVHD mice [73] Anti-HVEM mAb profoundly ameliorated GVHD in mice [74] HVEM-Ig fusion protein plus cyclosporine A prolonged allograft survival in mice [75] |
|
| |||||
| 4–1BB (CD137) |
Activated T cells and NK cells [76-78] |
4–1BBL | Activated DCs, B cells and macrophages [76-78] |
Provides costimulation of T-cell activation and promotes T-cell survival [76-78] |
Anti-4–1BB mAb agonist has shown to have anti-tumor immunity in vivo [79]. It is now being studied in Phase II clinical trials [201] Neutralizing anti-4–1BBL mAb attenuated acute rejection in rat liver transplantation [80] |
|
| |||||
| VLA-4 | T cells, B cells, monocytes, NK cells, eosinophils, neutrophils and hematopoietic cells [78] |
VCAM-1 (CD106) and fibronectin |
Endothelial cells [78] | Regulates adult hematopoiesis [78] Promotes the adhesion of lymphocytes, monocytes, eosinophils and basophils [8] |
Natalizumab (anti-VLA-4 antagonist) was approved by FDA for the treatment of relapsing forms of multiple sclerosis [81] |
|
| |||||
| LFA-1 | Leukocytes [82] | ICAM-1 (CD54) and ICAM-2 (CD102) |
Vascular endothelium, macrophages and lymphocytes [8] |
Mediates adhesive interaction between T cells and APCs in the immunological synapse and mediates leukocyte trafficking during inflammation [82] |
Efalizumab (anti-LFA-1 mAb) was approved to use in psoriasis but withdrawn due to an increased risk of PML [83] Anti-ICAM-1 mAb was studied in Phase III clinical trials for Crohn’s disease (alicaforsen) [84,201]and ischemic stroke (enlimomab) [85] None of them were continued in the post-marketing phase |
|
| |||||
| Mac-1 | Leukocytes [82] | ICAM-1 (CD54) |
Vascular endothelium, macrophages, and lymphocytes [8] |
Mediates leukocyte trafficking during inflammation and contributes to leukocyte activation in immune responses [82] |
Anti-Mac-1 showed no effect in graft survival [86] |
APC: Antigen-presenting cell; BTLA: B- and T-lymphocyte attenuator; DC: Dendritic cell; GVHD: Graft-versus-host disease; HVEM: Herpes virus entry mediator; ICOS: Inducible costimulator; LFA: Leukocyte function-associated antigen; mAb: Monoclonal antibody; PD: Programmed death; PML: Progressive multifocal myeloencephalopathy; RA: Rheumatoid arthritis.
Costimulatory molecules of the immunoglobulin superfamily
CD28–B7 costimulatory pathway
The interaction of CD28 on T cells and B7 family on APCs provides a crucial positive costimulatory signal for T-cell activation. CD28 is a disulfide-linked homodimeric-transmembrane member of the immunoglobulin superfamily and is constitutively expressed on CD4+ T cells and CD8+ T cells. The ligands for CD28, B7-1 (CD80) and B7-2 (CD86), are constitutively expressed on dendritic cells (DCs) and induced on activated macrophages and activated B cells. CD80 and CD86 are upregulated during maturation of DCs [87]. Binding of CD28 and their ligands promotes proliferation of activated T cells and differentiation of naive T cells into T helper cells and enhances the production of antibodies by B cells [8,88].
CTLA-4–B7 costimulatory pathway
Cytotoxic T-lymphocyte antigen 4 (CTLA-4 or CD152) is structurally homologous to CD28. CTLA-4 is expressed on T-cell membranes and binds to the same two ligands as CD28 (B7-1 and B7-2) with higher affinity. CTLA-4 is upregulated and activated after binding of CD28 and B7 ligands and T-cell activation. CTLA-4 is undetectable on resting T cells, whereas CD28 is expressed on both resting and activated cells [2,20]. CTLA-4 has similar structure to CD28, but their functions are different. The CD28–B7 interaction delivers a positive costimulatory signal to T cells, whereas the CTLA-4–B7 interaction delivers an inhibitory signal to T cells resulting in the attenuation of T-cell activation and proliferation. Due to the higher binding affinity to B7, the upregulated CTLA-4 competes for the B7-1 or B7-2 ligands to turn off T-cell activation and hence regulate lymphocyte homeostasis [88].
Therapeutics targeting CD28-mediated costimulation
Abatacept is a fusion protein composed of the extracellular binding domain of CTLA-4 fused to an Fc domain of human IgG. It was developed to block the binding of CD28 to B7 [88]. Abatacept was approved for the treatment of moderate to severe rheumatoid arthritis (RA) in the case of inadequate response to anti-TNFα therapy. A recent report, however, has shown that RA patients respond poorly to abatacept [89]. Furthermore, abatacept was shown to lack efficacy in organ transplantation in non-human primates, which was suggested to be due to the lower affinity of abatacept to CD86 compared with CD80 [8]. For this reason, belatacept was developed. Belatacept is a second-generation CTLA-4-Ig, differing from abatacept only by two amino acids leading to a superior binding to CD80 (twofold increase) and CD86 (fourfold increase) compared with abatacept [89]. Belatacept provided more of the potent immunosuppressive properties required for transplantation [20]. Belatacept is intended to provide extended graft survival and is currently continuing through clinical trials.
PD-1–PDL costimulatory pathway
Programmed death-1 (PD-1 or CD279) is an inhibitory costimulatory molecule [90]. It is a member of the immunoglobulin superfamily expressed on activated T cells, B cells, monocytes, and macrophages [91]. PD-1 binds PDL-1 (B7-H1) and PDL-2 (B7-DC). PDL-1 is expressed on B cells, DCs, and T cells including Treg. PDL-2 expression is limited to DCs and macrophages. Ligation of PD-1 on lymphocytes delivers a negative costimulatory signal to T cells and plays a critical role in the regulation of peripheral tolerance [92-95]. Engagement of PD-1 by its ligands, PDL-1 or PDL-2, blocks TCR signaling and transduces a signal that inhibits T- and B-cell proliferation, cytokine production and cytotoxicity of CD8+ T cells [91,95].
Therapeutic targeting of PD-1–PDL interactions
An in vitro study has shown that PDL-1-Ig stimulated PD-1, thus inhibiting CD4+ and CD8+ T-cell proliferation [8]. PDL-1-Ig has been shown to ameliorate collagen-induced arthritis (CIA) in the CIA mouse model as evaluated by clinical score, histology and reduced production of inflammatory cytokines. These results suggested a beneficial effect of PDL-1-Ig in this arthritis model via an anti-inflammatory action and inhibition of cell proliferation [23]. PDL-1-Ig prolonged corneal allograft survival in mice by augmented ligation of the PD-1 negative costimulatory molecule [25].
ICOS–ICOSL costimulatory pathway
Inducible costimulator (ICOS) is also a member of the immunoglobulin superfamily of costimulatory molecules. ICOS is induced after TCR engagement upon T-cell activation. ICOSL (B7H) is constitutively expressed on DCs, monocytes, macrophages, B cells, endothelial cells, fibroblasts, and renal epithelial cells [32]. ICOS has a similar sequence to CD28 and CTLA-4. Unlike other costimulatory molecules such as CTLA-4, ICOS can be upregulated in the absence of a CD28–B7 interaction [6]. ICOS promotes T-cell differentiation and cytokine production [8,96]. ICOS can stimulate both Th1 and Th2 effector cytokine production, especially IL-4 and -10 [8,96]. Since ICOS signaling does not sustain IL-2 production, it does not stimulate T-cell clonal expansion [97,98].
Therapeutic targeting of ICOS–ICOSL interactions
The ICOS–ICOSL costimulatory pathway is crucial for T-cell activation, differentiation and effector function. Anti-ICOS mAb treatment resulted in significant inhibition of graft-versushost disease (GVHD) and reduced the number of Ag-specific T cells that reject the bone marrow graft [36]. The blockade of ICOS–ICOSL was effective in prolonging cardiac and liver allograft survival [8]. ICOS blockade also prevented development of spontaneous disease in prediabetic non-obese diabetic (NOD) mice [99]. Transient blockade of ICOS/ICOSL profoundly reduced the severity of glucose-6 phosphate isomerase (G6PI)-induced arthritis [33]. These observations suggested that ICOS targeting may be a beneficial therapeutic scheme for allograft transplantation and autoimmune diseases [96].
BTLA–HVEM costimulatory pathway
B- and T-lymphocyte attenuator (BTLA or CD272) is an immunoglobulin-like molecule belonging to the immunoglobulin superfamily. BTLA is not expressed by naive T cells, but it is induced during activation and remains expressed on Th1 but not on Th2 cells [39]. It is also constitutively expressed at high levels on B cells. BTLA was identified as an inhibitory receptor with structural and functional similarities to CTLA-4 and PD-1 [100]. The engagement of BTLA and herpes virus entry mediator (HVEM), which is a member of the TNF receptor (TNFR) superfamily, delivers an inhibitory signal. BTLA interaction with HVEM impairs T-cell activation resulting in decreased T-cell proliferation and cytokine production.
Therapeutic targeting of BTLA–HVEM interactions
Blockade of BTLA–HVEM via anti-BTLA mAb in combination with CTLA-4-Ig can prolong cardiac allograft survival [41]. In a different study, a combination therapy of anti-BTLA mAb and CTLA-4-Ig induced donor-specific tolerance in a murine model of islet allograft [42].
Costimulatory molecules of the TNF–TNFR family
CD40–CD40L costimulatory pathway
CD40 is constitutively expressed on B cells, DCs, monocytes, platelets, and macrophages, and can be induced on non-hemato poietic cells such as endothelial cells and fibroblats in the presence of inflammation [43]. Ligation of CD40 and its subsequent signaling leads to activation of B cells, as well as activation and maturation of DCs, priming of helper and cytotoxic T cells, and propagation of a variety of inflammatory reactions [44]. The ligand for CD40 (CD40L or CD154), a member of the TNF family, is expressed on activated T cells, after stimulation by TCR–MHC complex engagement and signaling from CD28-B7 [8]. Blockade of the CD40–CD40L pathway alone or together with the CD28–B7 pathway can lead to long-term allograft survival in animal transplantation models and can inhibit the development of autoimmune diseases [44].
Therapeutic targeting of CD40–CD40L interactions
Blocking CD40–CD40L was examined by using an anti-CD40L mAb, which has shown promise in both rodent and non-human primate models [9]. Anti-CD40L mAb can prevent acute allograft rejection and promote allograft and xenograft survival in rodents and non-human primates [8]. Although treatment with anti-CD40L mAb promoted long-term allograft survival in experimental transplantation, clinical trials in autoimmune diseases and transplantation were halted due to unanticipated thromboembolic complications. This complication may be explained by the high expression of CD40L on platelets; however, observed stabilization of arterial thrombi were thought not to involve CD40 molecules [9]. An anti-CD40 mAb has also been developed and was shown to block binding of CD154 and to inhibit CD154-induced B-cell proliferation in vitro. Anti-CD40 mAbs promoted moderate prolongation of graft survival when used alone. When combined with belatacept, anti-CD40 mAbs synergized the effect in prolonging graft survival in non-human primate models of renal and islet transplantation [9].
OX40–OX40L costimulatory pathway
OX40 (CD134) is predominantly expressed on activated T cells, but not naive CD4+, CD8+ T cells, and memory T cells [15]. Following activation, OX40L is induced on activated B cells and mature DCs, endothelial cells and T cells. It is not expressed on resting APC. Expression of OX40 is delayed and decreased in the absence of CD28. OX40-positive T cells were found at the site of inflammation in experimental allergic encephalomyelitis (EAE), GVHD and RA [101]. Targeting the OX40–OX40L interaction with anti-OX40L mAb can delay disease progression in EAE, colitis, arthritis, asthma and diabetes in mice [52-56].
GITR–GITRL costimulatory pathway
Glucocorticoid-induced TNFR-related gene (GITR or TNFRSF18) is a transmembrane protein of the TNFR superfamily. GITR is preferentially expressed by Treg and is also upregulated in conventional effector T cells upon activation [61]. GITR ligand (GITRL) is mainly expressed on B cells, macrophages, DCs, endothelial cells, and activated T cells, but not on resting T cells [57,61]. Binding of GITRL to its receptor is involved in the development of autoimmune or inflammatory responses due to costimulation of effector T cells, inhibition of Treg cells, and activation of macrophages [58]. GITR fusion protein binding to GITRL prolonged allograft survival and showed potential anti-inflammatory properties in animal studies [61,62]. Neutralizing anti-GITRL mAb protected against the progression of diabetes in mice [63].
Molecules of the integrin family
LFA-1 and ICAM-1 belong to the integrin family and immonuglobulin superfamily, respectively. Although not traditionally considered a ‘costimulatory’ signal, they mediate strong interaction of leukocytes to cells bearing the receptor (e.g., APC), and enhance T-cell activation signaling [102]. Binding of LFA-1 and ICAM-1 stabilizes the interaction of TCR– and MHC–peptide complexes in IS.
Therapeutic targeting of ICAM-1/LFA-1 interactions
The effect of anti-ICAM-1 mAb on preventing rejection was reported in a rat transplantation model [103]. Intravenous administration of anti-ICAM-1 mAb alone could not prevent acute lung allograft rejection; however, combination with cyclosporine significantly reduced infiltration of leukocytes into the allograft [103]. Anti-ICAM-1 mAbs were studied in clinical trials for Crohn’s disease (alicaforsen) and ischemic stroke (enlimomab) [84,85]. None of these were commercialized.
Anti-LFA-1 mAb (efalizumab) has been shown to be effective in reducing the severity of chronic psoriasis without lymphocyte depletion and prevented rejection in kidney transplantation [104,105]. Recently, efalizumab was withdrawn from clinical use due to concerns about the development of progressive multifocal myeloencephalopathy (PML) in several patients receiving the drug as treatment for psoriasis [106]. In other studies, Posselt et al. described a novel immunosuppressive regimen using efalizumab, which permitted long-term islet allograft survival while reducing the need for corticosteroids and calcineurin inhibitors (CNI) [107].
Costimulatory mediators & orientation in the IS
The IS is a junction between immune cells in which a variety of receptors and adhesion molecules interact with their ligands on the opposing cell surface [108]. One such junction occurs between T cells and APCs. The IS includes specific binding between MHC class II with ‘loaded’ peptide and the opposing TCR that recognizes the presented antigen [108]. Pairs of many of the aforementioned costimulatory receptors and their ligands also accumulate at the center of the IS while clusters of LFA-1 and ICAM-1 and other receptor–ligand pairs are localized to the peripheral ring of the IS [109,110]. T-cell activation requires sustained conjugate formation between T cells and APCs because the number of peptide–MHC complexes can be very low and because the interaction between the TCR and its ligand has low affinity [111-113]. In the IS, the ligands and their receptors are arranged in an orderly manner, increasing the probability that multiple ligands will bind simultaneously with multiple receptors to enhance binding specificity, affinity and avidity and to prolong the duration of signaling [114]. Reports suggest that, T-cell activation which takes place during a 24–48 h interval [115] is mediated by the longevity of stabilized LFA-1–ICAM-1 binding [114]. Antigen presentation is amplified and sustained by translocation of TCR and MHC complexes to the center of the IS [110,112,114].
Many of the proteins participating in the IS have become the focus of therapeutic interventions (Table 1). The surface proteins located within the IS on T cells include T-cell receptors, costimulatory molecules CD2, CD28, CD40L, LFA-1, very late antigen (VLA)-4, ICAM-1, ICAM-3 and various cytokine and chemokine receptors [116]. Proteins on APCs include CD80, CD86, CD40, ICAM-1, ICAM-3 and other surface and cytoplasmic proteins [117]. The structure of the mature IS was described as a ‘bull’s eye’ with three distinct regions [109]. The central region of the IS, called the central supramolecular activation cluster (cSMAC), contains TCR complexes interacting with peptide-MHC complexes as well as interactions of costimulatory molecules such as CD2, CD28 and CD40 [109,110,114,116,118,119]. The intermediate ring of the bull’s eye, called the peripheral supramolecular activation cluster (pSMAC), contains complexes of integrin LFA-1 binding to ICAM-1 and the cytoskeletal protein talin, among others [110,118]. The outermost region is the distal supramolecular activation cluster (dSMAC), contains CD45 and CD43 [109,119].
After T-cell and APC contact, the binding of TCR to peptide–MHC complexes leads to the formation of small TCR microclusters thought to contain about 100 TCR/ MHC each, distributed in the periphery of the T cell–APC interface (dSMAC) [120,121]. The increased density of TCR and MHC by forming microclusters facilitates the engagement of this low affinity pair. Segregated microclusters of LFA are also formed in dSMAC [122]. TCR and LFA-1 microclusters then translocate to pSMAC [119]. After T cells begin to contract and have unstructured morphology, TCR microclusters migrate towards the center region of the contact area (cSMAC), while integrin microclusters accumulate in a surrounding area (pSMAC) [110,111,119,122-124]. Once at the center, TCR microclusters assemble to form larger clusters and a stable cSMAC to sustain the activation signal [125]. Similarly, MHC and ICAM-1 microclusters are also formed at the contact interface on the APC. cSMAC is surrounded by pSMAC containing adhesion molecules such as LFA-1 and ICAM-1. ICAM-1 on APCs forms oligomers that bind to LFA-1 clusters on T cells [116,126]. The interactions of integrins and their ligands play a critical role in the formation of tight and prolonged contacts between T cells and APCs. A stable IS cannot be formed in the absence of integrins [116].
In addition to the formation of TCR clusters, clustering of costimulatory molecules and their ligands is also required for T-cell activation to occur. The costimulatory molecules CD28 and CTLA-4 have been reported to accumulate in the cSMAC [112]. The accumulation of CD28–B7 pairs enhanced T-cell response by modifying T-cell signaling and by stabilizing the IS [109]. Other costimulatory receptor–ligand pairs, including PD-1–PDL-1/PDL-2, ICOS–ICOSL and CD40–CD40L were also reported to localize to the cSMAC [109]. CD28 and CTLA-4 are expressed as homodimers on the T-cell surface with monovalent and bivalent binding capacities, respectively. B7-1 is also present on the surface as a homodimer, while B7-2 is monomeric [120]. A homodimeric CTLA-4 molecule can bind two B7-1 molecules at low concentration of CTLA-4. At high concentration, CTLA-4 can form a linear array of B7-1–CTLA-4 multimers containing 7–18 receptor–ligand pairs [127]. At low concentration, one CD28 molecule can bind two molecules of B7-1. At high concentration, two molecules of CD28 can bind a dimer of B7-1 without forming a multimeric array [127]. As a result of multimerization, CTLA-4 has 10–100-fold higher binding avidity for B7 ligands than CD28 [120].
Strategies & design of multivalent, targeted therapeutics
Multivalent receptor–ligand interactions regulate many important biological responses. Pairs of receptors and ligands often orientate in arrays to elicit a cellular response [128]. For example, the IS requires sustained conjugate formation between T cells and APCs mediated by a large member of ‘clustered’ binding events. Receptor clustering and arrangement into long-range orderly arrays enhances binding and prolongs the duration of antigen recognition and simultaneously provides important signals leading to an inflammatory or regulatory response. Multivalent ligand strategies have been shown to increase avidity of ligands as expected [129]. In addition, multivalent conjugates can potentially offer novel autoimmune interventions by improving ligand-binding specificity, by providing costimulatory or regulatory signals, by triggering receptor-mediated endocytosis, or by inducing unique cell responses [130-133].
In the immunological system, multimerization of ligands and receptors plays an important role in orchestrating the localization and function of immune cells. Binding of cell-surface proteins with their ligands on leukocytes is necessary for signal transduction inducing immunological function and regulation. The binding of antigen to TCR can either activate or inhibit T cells depending on antigen array size, ligand valency and concentration [134]. For example, multivalent MHC complexes (MHC–Ig dimers) demonstrated stable binding to antigen-specific T cells with high avidity for cognate T-cell receptors [135]. In addition, a scaffold of multivalent single-chain T-cell receptor fusion protein complexes exhibited enhanced antigen binding and biological activity due to an increase in avidity between ligand and polymeric carrier [136].
In addition to T-cell receptor signaling, multivalent interaction of costimulatory molecules also regulate binding avidity and specificity to their receptors and T-cell and B-cell responses [137]. Monomeric costimulatory molecules such as CD80/86 on APCs and CD28 or CTLA-4 on T cells bind their ligands/receptors with low affinity and dissociate rapidly. Costimulatory molecules, therefore, require high avidity binding by clustering or ‘oligomerization’ of ligands and receptors. It has been reported that CTLA-4 requires dimerization for high avidity binding and oligomerization was required for CD80/86 to bind CTLA-4 with high avidity [138]. Conjugation of CTLA-4 Ig to liposomes improved the efficiency of the targeting and blocking of B7 costimulatory molecules by increasing the binding avidity through a multivalent ligand effect and by increasing the concentration of CTLA-4-Ig at the target site [139].
Other examples of multivalency involving cell-adhesion molecules are also prevalent in the literature. The interaction of monomeric LFA-3 and CD2 was reported to have low binding affinity, whereas multimeric LFA-3 bound CD2 with much higher avidity [140]. Another report showed that multimerization of RGD led to an increase in binding affinity for the αVβ3 integrin receptor and substantially enhanced specificity for αVβ3 integrin in vivo when compared to the more promiscuous monomeric RGD [141]. In a different study, a scaffold of RGD was created using dendrimers to target αvβ3 integrin, which also showed higher receptor selectivity compared with monovalent RGD [141]. Compared with monomeric CD200-Fc fusion proteins, a pentamer of CD200 with cartilage matrix protein yielded a higher avidity and more specific binding to CD200 receptor on myeloid cells such as macrophages [142].
High-affinity binding and selectivity are important properties of targeted therapeutics [101,143,144]. Targeted therapeutics often use specific types of membrane-bound protein including surface antigens or cell-surface receptors as targets. Tools for targeting specific cellular populations have been widely explored by using various targeting ligands including antibodies, aptamers, peptides and small molecules [145-148]. This strategy can result in an increased therapeutic efficacy and reduced side effects and toxicity [149]. The ideal targeted therapeutic should have low affinity for the targeted receptor expressed in normal cells, but high affinity to receptors on diseased or activated cells [101]. Therapeutics with a high affinity may actually show poor specificity if the target receptors are expressed in both diseased and healthy cells, even if the differential receptor expression (diseased vs healthy) is high [101].
As an example, multivalent therapeutics and drug-delivery systems have been reported to differentially target integrins in cancer models, which may provide insight into approaches in autoimmune diseases. One active targeting method for cancer treatment focuses on αvβ3 integrin, which mediates cell–cell attachment and cellular adhesion to extracellular matrix. An example of a more selective targeting strategy was the use of an αvβ3 integrin ligand conjugated to an anti-galactose antibody. The antibody portion interacted weakly with monovalent oligosaccharide but bound with higher affinity to multivalent galactose epitopes. Binding of the αvβ3 integrin ligand to integrin on cancer cell surface resulted in triggering complement-mediated cell lysis. Only cancer cells with high levels of αvβ3 integrin were killed. This study implicated multivalent interactions to distinguish cells with different levels of αvβ3 integrin to induce differential killing of cancer cells [150].
In many examples of multivalent therapeutics, the specificity of targeting was improved by transitioning from the low affinity of a single, free ligand to multivalent ligand presentation on a single construct (higher avidity). Multivalent ligands may be attained synthetically or by attaching to a carrier such as dendrimers, quantum dots, polymers, microparticles or nanoparticles. The resulting array should allow receptor access to the ligand (i.e., proper flexibility and spacing) to facilitate binding to multiple receptors simultaneously. When properly designed, multimeric ligands usually exhibit substantially stronger binding compared with their corresponding monomers [151]. Studies have shown enhanced binding avidities of multivalent therapeutics on 1 to 9 orders of magnitude when compared with the ligand alone [143]. Several investigators have also designed multivalent therapeutics displaying different types of ligands to achieve even more selective targeting [151]. These strategies are now emerging in the form of multivalent therapeutics targeting costimulation or adhesion of immune cells.
The importance of multivalency: a case study of LFA-1 & ICAM-1
LFA-1 mediates multiple events in the immune system, such as leukocyte trafficking, transmigration and formation of the IS. As mentioned, binding of LFA-1 to ICAM-1 is needed for the initiation and stabilization of T cell–APC conjugation. As discussed earlier, the affinity of integrins are often regulated by the alteration of integrin conformation, whereas avidity regulation is mediated by changes in cell-surface receptor diffusivity or density that alter the number of adhesive bonds [152]. The ligands of LFA-1 are members of the immunoglobulin superfamily known as ICAMs expressed on APCs, endothelial cells and epithelial cells, and are inducible by inflammatory cytokines.
LFA-1 on resting leukocytes circulating in the blood is normally inactive (i.e., a non-ligand-binding conformation). The conformational change of LFA-1 from a low- to high-affinity state can be activated by the binding of other receptors on leukocytes, such as chemokine receptors, antigen-specific TCRs or B-cell receptors (BCRs) [153]. The high-affinity state of LFA-1 mediates its ability to bind ICAMs. There are three different LFA-1 conformations that occur at different stages of activation [153]. The low affinity conformation of LFA-1 is a bent form where the LFA-1 I domain is bent and close to the cell membrane. The extended form with a closed binding domain has intermediate affinity and the extended form in which the ligand-binding I domain is opened to bind domain 1 of ICAM-1 with the highest affinity [82,154]. The interaction of intermediate affinity LFA-1 with ICAM-1 induces the high-affinity conformation that stabilizes adhesion.
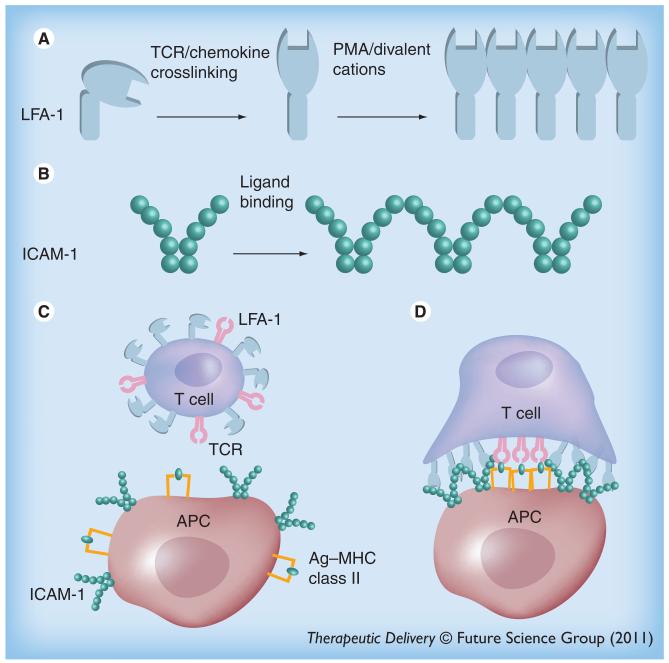
The dynamic adhesion of LFA-1 is also controlled by its avidity resulting from clustering of LFA-1 [153]. Microclusters of LFA-1 on T cells are formed after initial multivalent binding of LFA-1 to ICAM-1 [72]. ICAM-1 is the primary ligand of LFA-1, expressed on both T cells and APCs. Some have suggested that ICAM-1 on APC may act as a costimulatory receptor that facilitates the immune response [126]. Oligomerization and clustering of ICAM-1 increases ligand binding avidity by allowing the ‘rebinding’ of receptors and ligands after dissociation, since monomeric adhesion molecules tend to have relatively fast dissociation rates [126]. ICAM-1 molecules organize into non-covalently linked homodimers via interactions between domain 4 of ICAM-1 (D4-D4) on the cell surface. Two homodimers bind via D1, resulting in W-shaped tetramers (Figure 1). These tetramers can form linear arrays between the T cell and APC. This linear structure may bend and close to form a peripheral ring in the IS facilitating the adhesion between APC and T cells. The adhesion ring may be composed of thousands of LFA-1/ICAM-1 pairs [126].
Figure 1. Multivalency in immune cell signaling.
(A) The linear array of LFA-1 when triggered with PMA or divalent cations. (B) ICAM-1 forms a tetramer when binding to a cluster of LFA-1. (C) Non-ligand binding conformation of LFA-1 on the surface of resting T cells and the dimeric form of ICAM-1 on APC. (D) Simultaneous binding of multimeric LFA-1 to an array of ICAM-1. LFA-1 conformation has been changed into an active form to bind to ICAM-1. APC: Antigen-presenting cell; PMA: Phorbol 12-myristate 13-acetate; TCR: T-cell receptor.
LFA-1 and ICAM-1 possess characteristics that can be leveraged for multivalent therapeutics. As mentioned, LFA-1 and ICAM-1 on resting leukocytes, APC, endothelial and epithelial cells are normally inactive (i.e., a non-ligand binding conformation). Once triggered (e.g., by binding of TCR to MHC class II complex), molecules like LFA-1 change conformation to enhance binding to ICAM-1 [126]. Second, LFA-1 and ICAM-1 are upregulated on activated T cells and on endothelial cells in inflammatory diseases or autoimmune diseases. Third, LFA-1 and ICAM-1 ligand binding is controlled by their avidities resulting from clustering [126,153]. This last effect has been directly observed in a study to prevent human rhinovirus infection. Trivalent and tetravalent anti-ICAM-1 mAbs decreased the dissociation rate constant of these multivalent antibodies compared with the monomeric form and dramatically improved efficacy to protect cells from rhinovirus infection via an increase in binding avidity to ICAM-1, which is a major human rhinovirus receptor [155].
Multivalent ligands conjugated to nanoparticles have been found to offer several features that were distinct from monomeric ligands in solution. First, this type of multivalent targeting strategy has been proven effective when targeting a variety of different types of receptors that cluster [156]. The binding of LFA-1 and ICAM-1 is also regulated by binding avidity resulting from receptor oligomerization [157,158]. This LFA-1 and ICAM-1 clustering during activation was expected to enhance interaction with multivalent ligands. Indeed, nanoparticles displaying ligands (antibodies or peptides) were found to differentially target LFA-1 or ICAM-1 on activated T cells or APC populations [159-161].
Besides enhancing binding avidity and selectivity, multivalent therapeutics may also facilitate internalization of the construct via receptor-mediated endocytosis. For example, targeting LFA-1 and ICAM-1 internalization was triggered by the multivalent engagement of ligands [162]. The conjugation of multiple LFA-1 and ICAM-1 ligands on nanoparticles mediated intracellular delivery. This may offer more potent or more sustained inhibition of receptor signaling by internalization of surface receptors. Also, intracellular drug delivery enables targeted delivery of immune therapeutics to cells expressing these receptors. As one may expect, the valency of ligand can also be optimized to adjust binding, internalization, or cellular response [156,163].
Since the oligomeric states of LFA-1 and ICAM-1 molecules contribute to their ability to regulate T-cell responses, it was hypothesized that these nanoparticles may also interfere with signaling at the IS between DCs and T cells. In vitro studies of co-cultures of DCs with T cells showed that nanoparticles targeting ICAM-1 or LFA-1 were significantly better inhibitors of synapse formation compared with similar concentrations of free ligands [164]. Furthermore, the targeted nanoparticles drammatically shifted the response of these cells as evidenced by a significant shift in cytokine expression profiles and T-cell proliferation. Multivalent therapeutics, therefore, provide a unique, emerging opportunity to develop new interventions in immune signaling and to shape the immune response.
Future perspective
In autoimmune diseases and immune therapy in general, current interventions often lead to unwanted side effects such as general suppression of the immune system or, at the other extreme, inflammatory cytokine storms. Drugs targeting costimulatory molecules play an important role in directing T-cell activation. The roles of these proteins and the latest therapeutic approaches applied to autoimmune diseases have been reviewed in this article. In addition, the contributions of these proteins as mediators of costimulation or adhesion in the IS were reviewed. Multivalent therapeutic agents were proposed as a logical strategy to intervene in orchestrated signaling events between cells. Evidence from the literature suggests that multivalent ligands can contribute to an increase in ligand-targeting specificity and that these constructs may offer unique strategies towards novel autoimmune therapies.
There are a great number of membrane-bound proteins on immune cells that participate in multivalent signaling events. Many research groups have successfully designed and synthesized multivalent therapeutics using small molecules, peptides, mAbs or aptamers to increase binding affinity, avidity and selectivity to receptors. In addition, drug-delivery systems such as nanoparticles, polymers, dendrimers, and the like, are being explored as multivalent constructs and carriers of drugs. Such systems serve as a platform with readily adjustable binding specificity, stability and, perhaps, efficacy. Finally, multivalent presentation of ligands can induce unique cellular responses not observed from the monomer, such as triggered, receptor-mediated endocytosis.
Multimeric ligands seem to provide a great opportunity as a promising option for a specific treatment of autoimmune diseases. Further development of a multivalent ligand delivery system would have a great impact on autoimmune disease therapy. Future studies may focus more on discovery and targeting costimulatory/adhesion molecules which are proteins, oligosaccharides and carbohydrates that require a clustered state of the ligands. In addition to the therapeutic agents, diagnostic agents may be developed to be used in diagnostic applications of autoimmune diseases. A variety of chemistry methods for conjugation of multimeric ligands may be more widely investigated to provide options for coupling ligand to the multimeric scaffold.
Key Terms.
Antigen: A substance that is recognized by an antibody.
T cell: A type of white blood cell that plays a role in cell-mediated immunity. Some T cells (e.g., cytotoxic) protect the body from infection by identifying and destroying infected cells. Other T cells (e.g., regulatory) can dampen an inflammatory immune response.
Cytokine: A peptide, protein, or glycoprotein that acts as a signaling molecule, regulates the extent and duration of immune response and mediates intercellular communication.
Integrin: A heterodimeric transmembrane glycoprotein that mediates cellular attachment to other cells or extracellular matrix. Integrin also plays a role in cell signaling.
Monoclonal antibody: A class of antibodies generated by a single clone of immune cells. Monoclonal antibody binds specifically to a particular epitope.
Antigen-presenting cell: A cell that presents antigen to T cells and sends out signals to induce a response to the antigen.
Costimulatory molecules: Membrane-bound or secreted protein that is required for signal transduction leading to T-cell activation.
Executive summary.
-
■
Autoimmune disease results from the breakdown of immune tolerance and failure of the immune system to distinguish between self and non-self antigens, hence producing autoreactive antibodies or autoreactive T cells.
-
■
T-cell activation requires two signals, the interaction of the T-cell receptor (TCR) on T cells and antigenic peptide–MHC complex on antigen-presenting cells (APCs; signal 1) and a costimulatory signal (signal 2), to promote T-cell proliferation, cytokine secretion and effector function.
-
■
Monoclonal antibodies and fusion proteins have been developed as agonists or antagonists against costimulatory molecules for the treatment of autoimmune diseases.
-
■
T-cell activation requires sustained conjugate formation between T cells and APCs and communication through the TCR and peptide–MHC complexes.
-
■
Multivalent interaction of costimulatory molecules regulates binding avidity and specificity to their receptors. The multivalent interaction modulates T -cell and B-cell responses.
-
■
In the immunological synapse, the ligands and their receptors are arranged in an orderly manner, increasing the probability that multiple ligands will bind simultaneously with multiple receptors to enhance binding specificity, affinity and avidity and to prolong the duration of signaling.
-
■
The potential of multivalent therapeutics targeting large, organized interfaces (e.g., the immunological synapse) has been reviewed.
Acknowledgments
The authors have received funds from NIAID/NIH (R01-AI-063002, R016443–06 and R56AI-063002).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■■ of considerable interest
- 1.Wraith DC. Therapeutic peptide vaccines for treatment of autoimmune diseases. Immunol. Lett. 2009;122(2):134–136. doi: 10.1016/j.imlet.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kindt TJ, Goldsby RA, Osborne BA, Kuby J. Kuby Immunology. WH Freeman; NY, USA: 2007. [Google Scholar]
- 3.Wraith DC, Goldman M, Lambert PH. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003;362(9396):1659–1666. doi: 10.1016/S0140-6736(03)14802-7. [DOI] [PubMed] [Google Scholar]
- 4.Larche M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat. Med. 2005;11(4 Suppl.):S69–S76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- 5.Balague C, Kunkel SL, Godessart N. Understanding autoimmune disease: new targets for drug discovery. Drug Discov. Today. 2009;14(19-20):926–934. doi: 10.1016/j.drudis.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Stuart RW, Racke MK. Targeting T cell costimulation in autoimmune disease. Exp. Opin. Ther. Targets. 2002;6(3):275–289. doi: 10.1517/14728222.6.3.275. [DOI] [PubMed] [Google Scholar]
- 7.Bucks CM, Katsikis PD. New insights into classical costimulation of CD8+ T cell responses. Adv. Exp. Med. Biol. 2009;633:91–111. doi: 10.1007/978-0-387-79311-5_9. [DOI] [PubMed] [Google Scholar]
- 8.Ma A, Zhang L, Wang X, Chen H. New look at therapeutic strategies for blocking costimulatory signal in experimental and pre-clinical transplantation. Curr. Drug Saf. 2009;4(2):155–166. doi: 10.2174/157488609788172991. ■■ Describes the role of costimulatory pathway and therapeutic strategies for costimulatory blockade in experimental and preclinical transplantation.
- 9.Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol. Rev. 2009;229(1):294–306. doi: 10.1111/j.1600-065X.2009.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldmann M, Steinman L. Design of effective immunotherapy for human autoimmunity. Nature. 2005;435(7042):612–619. doi: 10.1038/nature03727. [DOI] [PubMed] [Google Scholar]
- 11.Woerly G, Lacy P, Younes AB, et al. Human eosinophils express and release IL-13 following CD28-dependent activation. J. Leukoc. Biol. 2002;72(4):769–779. [PubMed] [Google Scholar]
- 12.Woerly G, Roger N, Loiseau S, Dombrowicz D, Capron A, Capron M. Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes. J. Exp. Med. 1999;190(4):487–495. doi: 10.1084/jem.190.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dilioglou S, Cruse JM, Lewis RE. Function of CD80 and CD86 on monocyte- and stem cell-derived dendritic cells. Exp. Mol. Pathol. 2003;75(3):217–227. doi: 10.1016/s0014-4800(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 14.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat. Rev. Immunol. 2001;1(3):220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 15.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu. Rev. Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb AB, Kang S, Linden KG, et al. Evaluation of safety and clinical activity of multiple doses of the anti-CD80 monoclonal antibody, galiximab, in patients with moderate to severe plaque psoriasis. Clin. Immunol. 2004;111(1):28–37. doi: 10.1016/j.clim.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Gottlieb AB, Lebwohl M, Totoritis MC, et al. Clinical and histologic response to single-dose treatment of moderate to severe psoriasis with an anti-CD80 monoclonal antibody. J. Am. Acad. Dermatol. 2002;47(5):692–700. doi: 10.1067/mjd.2002.124698. [DOI] [PubMed] [Google Scholar]
- 18.Mease P, Genovese MC, Gladstein G, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, Phase II trial. Arthritis Rheum. 2011;63(4):939–948. doi: 10.1002/art.30176. [DOI] [PubMed] [Google Scholar]
- 19.Sakthivel P. Bench to bedside of CTLA-4: a novel immuno-therapeutic agent for inflammatory disorders. Recent Pat. Inflamm. Allergy Drug Discov. 2009;3(2):84–95. doi: 10.2174/187221309788489805. [DOI] [PubMed] [Google Scholar]
- 20.Rangel EB. Belatacept in clinical and experimental transplantation – progress and promise. Drugs Today (Barc) 2010;46(4):235–242. doi: 10.1358/dot.2010.46.4.1446426. [DOI] [PubMed] [Google Scholar]
- 21.Rostaing L, Massari P, Garcia VD, et al. Switching from calcineurin inhibitor-based regimens to a belatacept-based regimen in renal transplant recipients: a randomized Phase II study. Clin. J. Am. Soc. Nephrol. 2011;6(2):430–439. doi: 10.2215/CJN.05840710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard JP, Friedberg JW, Younes A, et al. A Phase I/II study of galiximab (an anti-CD80 monoclonal antibody) in combination with rituximab for relapsed or refractory, follicular lymphoma. Ann. Oncol. 2007;18(7):1216–1223. doi: 10.1093/annonc/mdm114. [DOI] [PubMed] [Google Scholar]
- 23.Wang G, Hu P, Yang J, Shen G, Wu X. The effects of PDL-Ig on collagen-induced arthritis. Rheumatol. In. 2011;31(4):513–519. doi: 10.1007/s00296-009-1249-0. [DOI] [PubMed] [Google Scholar]
- 24.Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann. NY Acad. Sci. 2011;1217:45–59. doi: 10.1111/j.1749-6632.2010.05919.x. [DOI] [PubMed] [Google Scholar]
- 25.Watson MP, George AJ, Larkin DF. Differential effects of costimulatory pathway modulation on corneal allograft survival. Invest. Ophthalmol. Vis. Sci. 2006;47(8):3417–3422. doi: 10.1167/iovs.05-1597. [DOI] [PubMed] [Google Scholar]
- 26.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benson DM, Jr, Bakan CE, Mishra A, et al. The PD-1/PDL-1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116(13):2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaehler KC, Piel S, Livingstone E, Schilling B, Hauschild A, Schadendorf D. Update on immunologic therapy with anti-CTLA-4 antibodies in melanoma: identification of clinical and biological response patterns, immune-related adverse events, and their management. Semin. Oncol. 2010;37(5):485–498. doi: 10.1053/j.seminoncol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 29.O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm Phase II study. Ann. Oncol. 2010;21(8):1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 30.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, Phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 31.Kirkwood JM, Lorigan P, Hersey P, et al. Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin. Cancer Res. 2010;16(3):1042–1048. doi: 10.1158/1078-0432.CCR-09-2033. [DOI] [PubMed] [Google Scholar]
- 32.Yong PF, Salzer U, Grimbacher B. The role of costimulation in antibody deficiencies: ICOS and common variable immunodeficiency. Immunol. Rev. 2009;229(1):101–113. doi: 10.1111/j.1600-065X.2009.00764.x. [DOI] [PubMed] [Google Scholar]
- 33.Frey O, Meisel J, Hutloff A, et al. Inducible costimulator (ICOS) blockade inhibits accumulation of polyfunctional T helper 1/T helper 17 cells and mitigates autoimmune arthritis. Ann. Rheum. Dis. 2010;69(8):1495–1501. doi: 10.1136/ard.2009.119164. [DOI] [PubMed] [Google Scholar]
- 34.Fujimura J, Takeda K, Kaduka Y, et al. Contribution of B7RP-1/ICOS co-stimulation to lethal acute GVHD. Pediatr. Transplant. 2010;14(4):540–548. doi: 10.1111/j.1399-3046.2009.01279.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang QW, Rabant M, Schenk A, Valujskikh A. ICOS-dependent and -independent functions of memory CD4 T cells in allograft rejection. Am. J. Transplant. 2008;8(3):497–506. doi: 10.1111/j.1600-6143.2007.02096.x. [DOI] [PubMed] [Google Scholar]
- 36.Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, et al. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM) Blood. 2005;105(8):3372–3380. doi: 10.1182/blood-2004-10-3869. [DOI] [PubMed] [Google Scholar]
- 37.Pan XC, Guo L, Deng YB, et al. Further study of anti-ICOS immunotherapy for rat cardiac allograft rejection. Surg. Today. 2008;38(9):815–825. doi: 10.1007/s00595-007-3734-y. [DOI] [PubMed] [Google Scholar]
- 38.Murphy KM, Nelson CA, Sedy JR. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat. Rev. Immunol. 2006;6(9):671–681. doi: 10.1038/nri1917. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe N, Gavrieli M, Sedy JR, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 2003;4(7):670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 40.Sedy JR, Gavrieli M, Potter KG, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat. Immunol. 2005;6(1):90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 41.Truong W, Plester JC, Hancock WW, et al. Negative and positive co-signaling with anti-BTLA (PJ196) and CTLA-4-Ig prolongs islet allograft survival. Transplantation. 2007;84(10):1368–1372. doi: 10.1097/01.tp.0000289995.70390.20. [DOI] [PubMed] [Google Scholar]
- 42.Truong W, Plester JC, Hancock WW, et al. Combined coinhibitory and costimulatory modulation with anti-BTLA and CTLA-4-Ig facilitates tolerance in murine islet allografts. Am. J. Transplant. 2007;7(12):2663–2674. doi: 10.1111/j.1600-6143.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- 43.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009;229(1):152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Law CL, Grewal IS. Therapeutic interventions targeting CD40L (CD154) and CD40: the opportunities and challenges. Adv. Exp. Med. Biol. 2009;647:8–36. doi: 10.1007/978-0-387-89520-8_2. [DOI] [PubMed] [Google Scholar]
- 45.Hussein M, Berenson JR, Niesvizky R, et al. A Phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma. Haematologica. 2010;95(5):845–848. doi: 10.3324/haematol.2009.008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Advani R, Forero-Torres A, Furman RR, et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin’s lymphoma. J. Clin. Oncol. 2009;27(26):4371–4377. doi: 10.1200/JCO.2008.21.3017. [DOI] [PubMed] [Google Scholar]
- 47.Khubchandani S, Czuczman MS, Hernandez-Ilizaliturri FJ. Dacetuzumab, a humanized mAb against CD40 for the treatment of hematological malignancies. Curr. Opin. Investig. Drugs. 2009;10(6):579–587. [PubMed] [Google Scholar]
- 48.Furman RR, Forero-Torres A, Shustov A, Drachman JG. A Phase I study of dacetuzumab (SGN-40, a humanized anti-CD40 monoclonal antibody) in patients with chronic lymphocytic leukemia. Leuk. Lymphoma. 2010;51(2):228–235. doi: 10.3109/10428190903440946. [DOI] [PubMed] [Google Scholar]
- 49.Redmond WL, Gough MJ, Charbonneau B, Ratliff TL, Weinberg AD. Defects in the acquisition of CD8 T-cell effector function after priming with tumor or soluble antigen can be overcome by the addition of an OX40 agonist. J. Immunol. 2007;179(11):7244–7253. doi: 10.4049/jimmunol.179.11.7244. [DOI] [PubMed] [Google Scholar]
- 50.Weinberg AD. The role of OX40 (CD134) in T-cell memory generation. Adv. Exp. Med. Biol. 2010;684:57–68. doi: 10.1007/978-1-4419-6451-9_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadun RE, Hsu WE, Zhang N, et al. Fc-mOX40L fusion protein produces complete remission and enhanced survival in 2 murine tumor models. J. Immunother. 2008;31(3):235–245. doi: 10.1097/CJI.0b013e31816a88e0. [DOI] [PubMed] [Google Scholar]
- 52.Nohara C, Akiba H, Nakajima A, et al. Amelioration of experimental autoimmune encephalomyelitis with anti-OX40 ligand monoclonal antibody: a critical role for OX40 ligand in migration, but not development, of pathogenic T cells. J. Immunol. 2001;166(3):2108–2115. doi: 10.4049/jimmunol.166.3.2108. [DOI] [PubMed] [Google Scholar]
- 53.Totsuka T, Kanai T, Uraushihara K, et al. Therapeutic effect of anti-OX40L and anti-TNF-α mAbs in a murine model of chronic colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284(4):G595–603. doi: 10.1152/ajpgi.00450.2002. [DOI] [PubMed] [Google Scholar]
- 54.Yoshioka T, Nakajima A, Akiba H, et al. Contribution of OX40/OX40 ligand interaction to the pathogenesis of rheumatoid arthritis. Eur. J. Immunol. 2000;30(10):2815–2823. doi: 10.1002/1521-4141(200010)30:10<2815::AID-IMMU2815>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 55.Huang L, Ji W, Zhou WF, Shi Q, Chen XY, Hu YM. Effects of costimulatory pathway OX40/OX40L on the pathogenesis of allergic asthma in mice. Zhonghua Er Ke Za Zhi. 2006;44(6):455–458. [PubMed] [Google Scholar]
- 56.Pakala SV, Bansal-Pakala P, Halteman BS, Croft M. Prevention of diabetes in NOD mice at a late stage by targeting OX40/OX40 ligand interactions. Eur. J. Immunol. 2004;34(11):3039–3046. doi: 10.1002/eji.200425141. [DOI] [PubMed] [Google Scholar]
- 57.Hwang H, Lee S, Lee WH, Lee HJ, Suk K. Stimulation of glucocorticoid-induced tumor necrosis factor receptor family-related protein ligand (GITRL) induces inflammatory activation of microglia in culture. J. Neurosci. Res. 2010;88(10):2188–2196. doi: 10.1002/jnr.22378. [DOI] [PubMed] [Google Scholar]
- 58.Nocentini G, Riccardi C. GITR: a modulator of immune response and inflammation. Adv. Exp. Med. Biol. 2009;647:156–173. doi: 10.1007/978-0-387-89520-8_11. [DOI] [PubMed] [Google Scholar]
- 59.Zhou P, Qiu J, L’Italien L, et al. Mature B cells are critical to T-cell-mediated tumor immunity induced by an agonist anti-GITR monoclonal antibody. J. Immunother. 2010;33(8):789–797. doi: 10.1097/CJI.0b013e3181ee6ba9. [DOI] [PubMed] [Google Scholar]
- 60.Cote AL, Zhang P, O’Sullivan JA, et al. Stimulation of the glucocorticoid-induced TNF receptor family-related receptor on CD8 T cells induces protective and high-avidity T-cell responses to tumor-specific antigens. J. Immunol. 2010;186(1):275–283. doi: 10.4049/jimmunol.1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim JI, Sonawane SB, Lee MK, et al. Blockade of GITR–GITRL interaction maintains Treg function to prolong allograft survival. Eur. J. Immunol. 2010;40(5):1369–1374. doi: 10.1002/eji.200940046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nocentini G, Cuzzocrea S, Genovese T, et al. Glucocorticoid-induced tumor necrosis factor receptor-related (GITR)-Fc fusion protein inhibits GITR triggering and protects from the inflammatory response after spinal cord injury. Mol. Pharmacol. 2008;73(6):1610–1621. doi: 10.1124/mol.107.044354. [DOI] [PubMed] [Google Scholar]
- 63.You S, Poulton L, Cobbold S, et al. Key role of the GITR/GITRLigand pathway in the development of murine autoimmune diabetes: a potential therapeutic target. PLoS One. 2009;4(11):e7848. doi: 10.1371/journal.pone.0007848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boursalian TE, McEarchern JA, Law CL, Grewal IS. Targeting CD70 for human therapeutic use. Adv. Exp. Med. Biol. 2009;647:108–119. doi: 10.1007/978-0-387-89520-8_7. [DOI] [PubMed] [Google Scholar]
- 65.Agarwal A, Newell KA. The role of positive costimulatory molecules in transplantation and tolerance. Curr. Opin. Organ Transplant. 2008;13(4):366–372. doi: 10.1097/MOT.0b013e328306115b. [DOI] [PubMed] [Google Scholar]
- 66.Denoeud J, Moser M. Role of CD27/CD70 pathway of activation in immunity and tolerance. J. Leukoc. Biol. 2010;89(2):195–203. doi: 10.1189/jlb.0610351. [DOI] [PubMed] [Google Scholar]
- 67.Roberts DJ, Franklin NA, Kingeter LM, et al. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8+ T cells. J. Immunother. 2010;33(8):769–779. doi: 10.1097/CJI.0b013e3181ee238f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McEarchern JA, Oflazoglu E, Francisco L, et al. Engineered anti-CD70 antibody with multiple effector functions exhibits in vitro and in vivo antitumor activities. Blood. 2007;109(3):1185–1192. doi: 10.1182/blood-2006-07-034017. [DOI] [PubMed] [Google Scholar]
- 69.Oflazoglu E, Boursalian TE, Zeng W, et al. Blocking of CD27-CD70 pathway by anti-CD70 antibody ameliorates joint disease in murine collagen-induced arthritis. J. Immunol. 2009;183(6):3770–3777. doi: 10.4049/jimmunol.0901637. [DOI] [PubMed] [Google Scholar]
- 70.Yanagisawa S, Takeichi N, Kaneyama T, et al. Effects of anti-CD70 mAb on Theiler’s murine encephalomyelitis virus-induced demyelinating disease. Brain Res. 2010;1317:236–245. doi: 10.1016/j.brainres.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 71.Wang J, Fu YX. The role of LIGHT in T cell-mediated immunity. Immunol. Res. 2004;30(2):201–214. doi: 10.1385/IR:30:2:201. [DOI] [PubMed] [Google Scholar]
- 72.Wang H, McCann FE, Gordan JD, et al. ADAP-SLP-76 binding differentially regulates supramolecular activation cluster (SMAC) formation relative to T cell-APC conjugation. J. Exp. Med. 2004;200(8):1063–1074. doi: 10.1084/jem.20040780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu Q, Fu YX, Sontheimer RD. Blockade of lymphotoxin signaling inhibits the clinical expression of murine graft-versus-host skin disease. J. Immunol. 2004;172(3):1630–1636. doi: 10.4049/jimmunol.172.3.1630. [DOI] [PubMed] [Google Scholar]
- 74.Xu Y, Flies AS, Flies DB, et al. Selective targeting of the LIGHT–HVEM costimulatory system for the treatment of graft-versus-host disease. Blood. 2007;109(9):4097–4104. doi: 10.1182/blood-2006-09-047332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ye Q, Fraser CC, Gao W, et al. Modulation of LIGHT–HVEM costimulation prolongs cardiac allograft survival. J. Exp. Med. 2002;195(6):795–800. doi: 10.1084/jem.20012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melero I, Murillo O, Dubrot J, Hervas-Stubbs S, Perez-Gracia JL. Multi-layered action mechanisms of CD137 (4–1BB)-targeted immunotherapies. Trends Pharmacol. Sci. 2008;29(8):383–390. doi: 10.1016/j.tips.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 77.Lynch DH. The promise of 4–1BB (CD137)-mediated immunomodulation and the immunotherapy of cancer. Immunol. Rev. 2008;222:277–286. doi: 10.1111/j.1600-065X.2008.00621.x. [DOI] [PubMed] [Google Scholar]
- 78.Imai Y, Shimaoka M, Kurokawa M. Essential roles of VLA-4 in the hematopoietic system. Int. J. Hematol. 2010;91(4):569–575. doi: 10.1007/s12185-010-0555-3. [DOI] [PubMed] [Google Scholar]
- 79.Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin. Oncol. 2010;37(5):508–516. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 80.Qin L, Guan HG, Zhou XJ, Yin J, Lan J, Qian HX. Blockade of 4–1BB/4–1BB ligand interactions prevents acute rejection in rat liver transplantation. Chin. Med. J. (Engl.) 2010;123(2):212–215. [PubMed] [Google Scholar]
- 81.Coyle PK. The role of natalizumab in the treatment of multiple sclerosis. Am. J. Manag. Care. 2010;16(6 Suppl.):S164–S170. [PubMed] [Google Scholar]
- 82.Evans R, Patzak I, Svensson L, et al. Integrins in immunity. J. Cell Sci. 2009;122(Pt 2):215–225. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- 83.Stengel FM, Petri V, Campbell GA, et al. Control of moderate-to-severe plaque psoriasis with efalizumab: 24-week, open-label, Phase IIIb/IV Latin American study results. Arch. Drug Inf. 2009;2(4):71–78. doi: 10.1111/j.1753-5174.2009.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yacyshyn B, Chey WY, Wedel MK, Yu RZ, Paul D, Chuang E. A randomized, double-masked, placebo-controlled study of alicaforsen, an antisense inhibitor of intercellular adhesion molecule 1, for the treatment of subjects with active Crohn’s disease. Clin. Gastroenterol. Hepatol. 2007;5(2):215–220. doi: 10.1016/j.cgh.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 85.del Zoppo GJ. Acute anti-inflammatory approaches to ischemic stroke. Ann. NY Acad. Sci. 2010;1207:143–148. doi: 10.1111/j.1749-6632.2010.05761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paul LC, Davidoff A, Benediktsson H, Issekutz T. Anti-integrin (LFA-1, VLA-4, and Mac-1) antibody treatment and acute cardiac graft rejection in the rat. Transpl. Int. 1996;9(4):420–425. doi: 10.1007/BF00335706. [DOI] [PubMed] [Google Scholar]
- 87.Larsen CP, Ritchie SC, Hendrix R, et al. Regulation of immunostimulatory function and costimulatory molecule (B7-1 and B7-2) expression on murine dendritic cells. J. Immunol. 1994;152(11):5208–5219. [PubMed] [Google Scholar]
- 88.Vincenti F. Costimulation blockade in autoimmunity and transplantation. J. Allergy Clin. Immunol. 2008;121(2):299–306. doi: 10.1016/j.jaci.2008.01.002. quiz 307–298. [DOI] [PubMed] [Google Scholar]
- 89.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA-4-Ig with potent immunosuppressive properties. Am. J. Transplant. 2005;5(3):443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 90.Ansari MJ, Salama AD, Chitnis T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 2003;198(1):63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Folkl A, Bienzle D. Structure and function of programmed death (PD) molecules. Vet. Immunol. Immunopathol. 2010;134(1–2):33–38. doi: 10.1016/j.vetimm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 92.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996;8(5):765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 94.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Latchman Y, Wood CR, Chernova T, et al. PDL-2 is a second ligand for PD-1 and inhibits T-cell activation. Nat. Immunol. 2001;2(3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 96.Nurieva RI, Liu X, Dong C. Yin-Yang of costimulation: crucial controls of immune tolerance and function. Immunol. Rev. 2009;229(1):88–100. doi: 10.1111/j.1600-065X.2009.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sharpe A. Costimulation and regulation of autoimmunity and tolerance. J. Pediatr. Gastroenterol. Nutr. 2005;40(Suppl. 1):S20–S21. doi: 10.1097/00005176-200504001-00011. [DOI] [PubMed] [Google Scholar]
- 98.Dong C, Juedes AE, Temann UA, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409(6816):97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 99.Ansari MJ, Fiorina P, Dada S, et al. Role of ICOS pathway in autoimmune and alloimmune responses in NOD mice. Clin. Immunol. 2008;126(2):140–147. doi: 10.1016/j.clim.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 100.Derre L, Rivals JP, Jandus C, et al. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J. Clin. Invest. 2010;120(1):157–167. doi: 10.1172/JCI40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simnick AJ, Valencia CA, Liu R, Chilkoti A. Morphing low-affinity ligands into high-avidity nanoparticles by thermally triggered self-assembly of a genetically encoded polymer. ACS Nano. 2010;4(4):2217–2227. doi: 10.1021/nn901732h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Salmaso C, Olive D, Pesce G, Bagnasco M. Costimulatory molecules and autoimmune thyroid diseases. Autoimmunity. 2002;35(3):159–167. doi: 10.1080/08916930290013441. [DOI] [PubMed] [Google Scholar]
- 103.Brandt M, Grotkop C, Steinmann J, Steinhoff G. Prevention of lung allograft rejection by combined treatment with adhesion molecule antibodies and cyclosporine. Interact. Cardiovasc. Thorac. Surg. 2003;2(4):509–513. doi: 10.1016/S1569-9293(03)00131-2. [DOI] [PubMed] [Google Scholar]
- 104.Dedrick RL, Garovoy M. Anti-adhesion antibodies efalizumab, a humanized anti-CD11a monoclonal antibody. Transpl. Immunol. 2002;9:181–186. doi: 10.1016/s0966-3274(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 105.Vincenti F, Pescovitz M, Rajagopalan PR, et al. A Phase I/II randomized open-label multicenter trial of efalizumab, a humanized anti-CD11a, anti-LFA-1 in renal transplantation. Am. J. Transplant. 2007;7:1770–1777. doi: 10.1111/j.1600-6143.2007.01845.x. [DOI] [PubMed] [Google Scholar]
- 106.Oberholzer J, Kinzer K, Fiorina P. The anti-LFA-1 trial in islet transplantation. Am. J. Transplant. 2010;10(8):1725–1726. doi: 10.1111/j.1600-6143.2010.03184.x. [DOI] [PubMed] [Google Scholar]
- 107.Posselt AM, Bellin MD, Tavakol M, et al. Islet transplantation in Type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am. J. Transplant. 2010;10(8):1870–1880. doi: 10.1111/j.1600-6143.2010.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dustin ML, Tseng SY, Varma R, Campi G. T cell-dendritic cell immunological synapses. Curr. Opin. Immunol. 2006;18(4):512–516. doi: 10.1016/j.coi.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 109.Yokosuka T, Saito T. Dynamic regulation of T-cell costimulation through TCR-CD28 microclusters. Immunol. Rev. 2009;229(1):27–40. doi: 10.1111/j.1600-065X.2009.00779.x. ■■ Describes the dynamic feature of microclusters of T-cell receptor and costimulatory molecules that regulates T-cell activation.
- 110.Saito T, Yokosuka T, Hashimoto-Tane A. Dynamic regulation of T-cell activation and co-stimulation through TCR-microclusters. FEBS Lett. 2010;584(24):4865–4871. doi: 10.1016/j.febslet.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 111.Wulfing C, Davis MM. A receptor/cytoskeletal movement triggered by costimulation during T-cell activation. Science. 1998;282(5397):2266–2269. doi: 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- 112.Saito T, Yokosuka T. Immunological synapse and microclusters: the site for recognition and activation of T cells. Curr. Opin. Immunol. 2006;18(3):305–313. doi: 10.1016/j.coi.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 113.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375(6527):148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 114.Bromley SK, Burack WR, Johnson KG, et al. The immunological synapse. Annu. Rev. Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 115.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427(6970):154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 116.Rodriguez-Fernandez JL, Riol-Blanco L, Delgado-Martin C. What is the function of the dendritic cell side of the immunological synapse? Sci. Signal. 2010;3(105):re2. doi: 10.1126/scisignal.3105re2. [DOI] [PubMed] [Google Scholar]
- 117.Grakoui A, Bromley SK, Sumen C, et al. The immunological synapse: a molecular machine controlling T-cell activation. Science. 1999;285(5425):221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 118.Jo EK, Wang H, Rudd CE. An essential role for SKAP-55 in LFA-1 clustering on T cells that cannot be substituted by SKAP-55R. J. Exp. Med. 2005;201(11):1733–1739. doi: 10.1084/jem.20042577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dustin ML. Modular design of immunological synapses and kinapses. Cold Spring Harb. Perspect. Biol. 2009;1(1):a002873. doi: 10.1101/cshperspect.a002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Teft WA, Madrenas J. The immunological synapse as a novel therapeutic target. Curr. Opin. Investig. Drugs. 2006;7(11):1008–1013. [PubMed] [Google Scholar]
- 121.Hartman NC, Nye JA, Groves JT. Cluster size regulates protein sorting in the immunological synapse. Proc. Natl Acad. Sci. USA. 2009;106(31):12729–12734. doi: 10.1073/pnas.0902621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dustin ML, Chakraborty AK, Shaw AS. Understanding the structure and function of the immunological synapse. Cold Spring Harb. Perspect. Biol. 2010;2(10):a002311. doi: 10.1101/cshperspect.a002311. ■■ Describes the function of immunological synapse and the structure of immunological synapse (cSMAC, pSMAC and dSMAC) and molecules that are accumulated in each region.
- 123.Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 2000;1(1):23–29. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- 124.Krummel M, Wulfing C, Sumen C, Davis MM. Thirty-six views of T-cell recognition. Philos. Trans. R Soc. Lond. B Biol. Sci. 2000;355(1400):1071–1076. doi: 10.1098/rstb.2000.0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25(1):117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lebedeva T, Dustin ML, Sykulev Y. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr. Opin. Immunol. 2005;17(3):251–258. doi: 10.1016/j.coi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 127.Bhatia S, Edidin M, Almo SC, Nathenson SG. B7-1 and B7-2: similar costimulatory ligands with different biochemical, oligomeric and signaling properties. Immunol. Lett. 2006;104(1-2):70–75. doi: 10.1016/j.imlet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 128.Kiessling LL, Strong LE. Synthetic multivalent ligands as probes of signal transduction. Angew. Chem. Int. Ed. Engl. 2006;45:2348–2368. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ev CMR. Targeting drugs to combinations of receptors: a modeling analysis of potential specificity. Ann. Biomed. Eng. 2005;33:1113–1124. doi: 10.1007/s10439-005-5779-1. [DOI] [PubMed] [Google Scholar]
- 130.Johnson RN, Kopecek J. Synthesis and evaluation of multivalent branched HPMA copolymer-Fab’ conjugates targeted to the B-cell antigen CD20. Bioconjug. Chem. 2009;20:129–137. doi: 10.1021/bc800351m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Puffer EB, Hollenbeck JJ, Kink JA, Kiessling LL. Activating B cell signaling with defined multivalent ligands. ACS Chem. Biol. 2007;2:252–262. doi: 10.1021/cb600489g. [DOI] [PubMed] [Google Scholar]
- 132.Muro S, Qiu W, Leferovich J, Cui X, Berk E, Muzykantov VR. Endothelial targeting of high-affinity multivalent polymer nanocarriers directed to intercellular adhesion molecule 1. J. Pharmacol. Exp. Ther. 2006;317:1161–1169. doi: 10.1124/jpet.105.098970. [DOI] [PubMed] [Google Scholar]
- 133.Muro S, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J. Cell Sci. 2003;116:1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 134.Symer DE, Diamond DJ, Dintzis HM. Inhibition or activation of human T cell receptor transfectants is controlled by defined, soluble antigen arrays. J. Exp. Med. 1992;176:1421–1430. doi: 10.1084/jem.176.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schneck JP, O’Herrin SM, Greten TF. Monitoring antigen-specific T cells using MHC-Ig dimers. Curr. Protoc. Immunol. 2001;17(17):12. doi: 10.1002/0471142735.im1702s35. [DOI] [PubMed] [Google Scholar]
- 136.Wong RL, Zhu X, You L, et al. Interleukin-15:interleukin-15 receptor {α} scaffold for creation of multivalent targeted immune molecules. Protein Eng. Des. Sel. 2010;24(4):373–383. doi: 10.1093/protein/gzq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mallikaratchy PR, Gardner JR, Kuryavyi V, et al. A multivalent DNA aptamer specific for the B-cell receptor on human lymphoma and leukemia. Nucleic Acids Res. 2010;39(6):2458–2469. doi: 10.1093/nar/gkq996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Greene JL, Emswiler J, Peach R, Bajorath J, Cosand W, Linsley PS. Covalent dimerization of CD28/CTLA-4 and oligomerization of CD80/CD86 regulate T cell costimulatory interactions. J. Biol. Chem. 1996;43:26762–26771. doi: 10.1074/jbc.271.43.26762. [DOI] [PubMed] [Google Scholar]
- 139.Park CG, Lee KM, Szot GL, Bluestone JA, Lee KD. Targeting and blocking B7 costimulatory molecules on antigen-presenting cells using CTLA-4-Ig-conjugated liposomes: in vitro characterization and in vivo factors affecting biodistribution. Pharm. Res. 2003;20:1239–1248. doi: 10.1023/a:1025057216492. [DOI] [PubMed] [Google Scholar]
- 140.Dustin ML, Springer TA. Correlation of CD2 binding and functional properties of multimeric and monomeric lymphocyte function-associated antigen 3. J. Exp. Med. 1989;169:503–517. doi: 10.1084/jem.169.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jin ZH, Waki A, Akaji K, Coll JL, Saga T, Fujibayashi Y. Effect of multimerization of a linear Arg-Gly-Asp peptide on integrin binding affinity and specificity. Biol. Pharm. Bull. 2010;33:370–378. doi: 10.1248/bpb.33.370. [DOI] [PubMed] [Google Scholar]
- 142.Voulgaraki D, Letarte M, Foster-Cuevas M, Brown MH, Barclay AN. Multivalent recombinant proteins for probing functions of leucocyte surface proteins such as the CD200 receptor. Immunology. 2005;115:337–346. doi: 10.1111/j.1365-2567.2005.02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hong S, Leroueil PR, Majoros IJ, Orr BG, Baker JR, Jr, Banaszak Holl MM. The binding avidity of a nanoparticle-based multivalent targeted drug delivery platform. Chem. Biol. 2007;14(1):107–115. doi: 10.1016/j.chembiol.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 144.Stukel JM, Li RC, Maynard HD, Caplan MR. Two-step synthesis of multivalent cancer-targeting constructs. Biomacromolecules. 2010;11(1):160–167. doi: 10.1021/bm9010276. [DOI] [PubMed] [Google Scholar]
- 145.Farokhzad OC, Cheng J, Teply BA, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl Acad. Sci. USA. 2006;103(16):6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Johnson RN, Kopeckova P, Kopecek J. Synthesis and evaluation of multivalent branched HPMA copolymer-Fab’ conjugates targeted to the B-cell antigen CD20. Bioconjug. Chem. 2009;20(1):129–137. doi: 10.1021/bc800351m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meng H, Chen JY, Mi L, et al. Conjugates of folic acids with BSA-coated quantum dots for cancer cell targeting and imaging by single-photon and two-photon excitation. J. Biol. Inorg. Chem. 2011;16(1):117–123. doi: 10.1007/s00775-010-0709-z. [DOI] [PubMed] [Google Scholar]
- 148.Han HD, Mangala LS, Lee JW, et al. Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clin. Cancer Res. 2010;16(15):3910–3922. doi: 10.1158/1078-0432.CCR-10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 150.Carlson CB, Mowery P, Owen RM, Dykhuizen EC, Kiessling LL. Selective tumor cell targeting using low-affinity, multivalent interactions. ACS Chem. Biol. 2007;2(2):119–127. doi: 10.1021/cb6003788. [DOI] [PubMed] [Google Scholar]
- 151.Shewmake TA, Solis FJ, Gillies RJ, Caplan MR. Effects of linker length and flexibility on multivalent targeting. Biomacromolecules. 2008;9(11):3057–3064. doi: 10.1021/bm800529b. [DOI] [PubMed] [Google Scholar]
- 152.Kim M, Carman CV, Yang W, Salas A, Springer TA. The primacy of affinity over clustering in regulation of adhesiveness of the integrin {α}L{β}2. J. Cell Biol. 2004;167(6):1241–1253. doi: 10.1083/jcb.200404160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hogg N, Laschinger M, Shimaoka M, Springer TA. Therapeutic antagonists and conformational regulation of integrin function. Nat. Rev. Drug Discov. 2003;2(9):703–716. doi: 10.1038/nrd1174. [DOI] [PubMed] [Google Scholar]
- 154.Shimaoka M, Springer TA. Therapeutic antagonists and conformational regulation of integrin function. Nat. Rev. Drug Discov. 2003;2(9):703–716. doi: 10.1038/nrd1174. [DOI] [PubMed] [Google Scholar]
- 155.Charles CH, Kohlstaedt LA, Morantte IG, et al. Prevention of human rhinovirus infection by multivalent fab molecules directed against ICAM-1. Antimicrob. Agents Chemother. 2003;47:1503–1508. doi: 10.1128/AAC.47.5.1503-1508.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gestwicki JE, Strong LE, Oetjen KA, Kiessling LL. Influencing receptor–ligand binding mechanisms with multivalent ligand architecture. J. Am. Chem. Soc. 2002;124:14922–14933. doi: 10.1021/ja027184x. ■■ Describes the effect of molecular feature of multivalent ligands on receptor inhibition and receptor clustering.
- 157.Lebedeva T, Dustin ML, Sykulev Y. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr. Opin. Immunol. 2005;17:251–258. doi: 10.1016/j.coi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 158.Hogg N, Bates PA, Stanley P, Randi AM. The sticking point: how integrins bind to their ligands. Trends Cell Biol. 1994;4:379–382. doi: 10.1016/0962-8924(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 159.Chittasupho C, Krise JP, Siahaan TJ, Berkland C. cIBR effectively targets nanoparticles to LFA-1 on acute lymphoblastic T cells. Mol. Pharm. 2010;7:146–155. doi: 10.1021/mp900185u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang N, Duangrat C, Siahaan TJ, Berkland C. PLGA nanoparticle–peptide conjugate effectively targets intercellular cell-adhesion molecule-1. Bioconjug. Chem. 2008;19:145–152. doi: 10.1021/bc700227z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chittasupho C, Baoum A, Yakovleva T, Siahaan TJ, Berkland CJ. ICAM-1 targeting of doxorubicin-loaded PLGA nanoparticles to lung epithelial cells. Eur. J. Pharm. Sci. 2009;37:141–150. doi: 10.1016/j.ejps.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Muro S, Gajewski C, Koval M, Muzykantov VR. ICAM-1 recycling in endothelial cells: a novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood. 2005;105(2):650–658. doi: 10.1182/blood-2004-05-1714. [DOI] [PubMed] [Google Scholar]
- 163.Fakhari A, Baoum A, Siahaan TJ, Le KB, Berkland C. Controlling ligand surface density optimizes nanoparticle binding to ICAM-1. J. Pharm. Sci. 2011;100(3):1045–1056. doi: 10.1002/jps.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chittasupho C, Shannon L, Siahaan TJ, Vines CM, Berkland C. Nanoparticles targeting dendritic cell surface molecules effectively block T cell conjugation and shift response. ACS Nano. 2011;5(3):1693–1702. doi: 10.1021/nn102159g. [DOI] [PMC free article] [PubMed] [Google Scholar]
■ Website
- 201. ClinicalTrials.org [Accessed 23 April 2011]; ClinicalTrials.org www.clinicaltrials.org.