Abstract
Voriconazole is approved for treating invasive fungal infections. We examined voriconazole exposure-response relationships for patients from nine published clinical trials. The relationship between the mean voriconazole plasma concentration (Cavg) and clinical response and between the free Cavg/MIC ratio versus the clinical response were explored using logistic regression. The impact of covariates on response was also assessed. Monte Carlo simulation was used to estimate the relationship between the trough concentration/MIC ratio and the probability of response. The covariates individually related to response were as follows: study (P < 0.001), therapy (primary/salvage, P < 0.001), primary diagnosis (P < 0.001), race (P = 0.004), baseline bilirubin (P < 0.001), baseline alkaline phosphatase (P = 0.014), and pathogen (yeast/mold, P < 0.001). The Cavg for 72% of the patients was 0.5 to 5.0 μg/ml, with the maximum response rate (74%) at 3.0 to 4.0 μg/ml. The Cavg showed a nonlinear relationship to response (P < 0.003), with a lower probability at the extremes. For patients with Cavg < 0.5 μg/ml, the response rate was 57%. The lowest response rate (56%) was seen with a Cavg ≥ 5.0 μg/ml (18% of patients) and was associated with significantly lower mold infection responses compared to yeasts (P < 0.001) but not with voriconazole toxicity. Higher free Cavg/MIC ratios were associated with a progressively higher probability of response. Monte Carlo simulation suggested that a trough/MIC ratio of 2 to 5 is associated with a near-maximal probability of response. The probability of response is lower at the extremes of Cavg. Patients with higher free Cavg/MIC ratios have a higher probability of clinical response. A trough/MIC ratio of 2 to 5 can be used as a target for therapeutic drug monitoring.
INTRODUCTION
Therapeutic drug monitoring (TDM) is advocated increasingly for antifungal agents (13, 36) and is potentially an important tool to optimize the therapeutic outcome of patients with invasive fungal infections (3, 15). However, before TDM can be used widely, it is first necessary to establish the relationships between drug exposure and both clinical efficacy and toxicity. This information can then be used to guide optimal antifungal regimens for individual patients. Unfortunately, the determination of these exposure-response relationships in patients with invasive fungal infections is frequently confounded by multiple factors that have an impact upon clinical outcome.
Voriconazole is a triazole with broad spectrum antifungal activity and is currently considered the first line agent for the treatment of invasive aspergillosis (27, 42). The relationship between mean voriconazole plasma concentrations and drug-related toxicity was described by Tan et al. in 2006 (39). That study, in over 1,000 patients, suggested a relationship between mean voriconazole plasma concentrations and the probability of visual adverse events, as well as with elevated liver function tests. However, the relationship between voriconazole concentrations in plasma and clinical response has only been investigated in relatively small studies (9, 18, 23, 24, 25, 37). Despite a variety of methodological approaches being used, there do appear to be clinically important exposure-response relationships. Voriconazole TDM may improve clinical efficacy (18, 23, 24, 37), but this has not been formally demonstrated. Furthermore, the optimal target concentration range for this antifungal compound is not known.
Laboratory animal models of disseminated Candida albicans infection suggest that the pharmacokinetic-pharmacodynamic index that optimally links drug exposure with outcome for voriconazole and other triazole antifungal agents is the AUC/MIC ratio (2). Furthermore, a free AUC/MIC value of ∼25 is associated with half-maximal antifungal effect in murine models of disseminated candidiasis (3). Unfortunately, there is a relative paucity of high-quality population pharmacokinetic data from patients with invasive fungal infections (5). This makes assessment of a correlation between triazole concentrations and efficacy difficult. For voriconazole this situation is further complicated by nonlinear pharmacokinetics in adults, but not in pediatric patients aged 2 to 11 years (43), and high inter-individual pharmacokinetic variability (30). The most important factor accounting for inter-individual variability is the CYP2C19 genotype (17). However, voriconazole plasma concentrations, like those of other triazoles, may also be affected by various drug-drug interactions (5, 6, 20). In addition, the impact of underlying condition on the pharmacokinetics of voriconazole has only been described in a small number of hematopoietic cell transplant recipients, where the pharmacokinetics appear to be similar to healthy volunteers (7).
We explore here the relationship between plasma voriconazole concentrations and clinical response in a population of 825 patients from nine, previously published clinical trials. We initially explore the relationship between mean plasma concentrations (Cavg) and clinical efficacy and then describe the impact of the voriconazole MICs on drug exposure-response relationships. Logistic regression modeling enables the potential effect of a number of covariates on the drug exposure-response relationships to be examined. Finally, we use Monte Carlo simulation to estimate the relationship between the trough concentration/MIC ratio and the clinical response, thereby providing further insight into drug exposure targets for TDM.
MATERIALS AND METHODS
Sources of data.
The data are from nine primary or salvage therapy clinical studies totaling 1,091 patients, with 1,007 having both a clinical outcome and measured voriconazole concentrations in plasma. These phase II and III clinical trials were completed before year 2000, and the results have all been published (Table 1). The modified intent to treat the population from these studies included 896 patients. To be included in our primary analyses, these patients had to have one of the following prespecified, investigator-determined, outcomes at the end of therapy: a complete, partial, stable, or failed response to therapy (34) or to have died (any causality) or to have discontinued therapy due to an adverse events or laboratory abnormality (any causality). Of the 896 patients, 71 (7.9%) had one of the following outcomes—protocol violation (n = 16), withdrew consent (n = 16), other (n = 15), or lost to follow-up (n = 24)—and were thus excluded, giving a final population of 825 patients for primary analysis.
Table 1.
Clinical studies included in the efficacy versus voriconazole plasma concentration analysis
| Indicationa | No. of patients (% success) | Voriconazole doseb | Maximum duration of therapy (wks) | Reference |
|---|---|---|---|---|
| Chronic IFIs in non-neutropenic patients | 52 (60) | p.o., 200 mg q12h* | 24 | 32 |
| Invasive aspergillosis | 110 (63) | i.v., 6 mg/kg ×2 q12h → 3 mg/kg q12h; p.o., 200 mg q12h* | 24 | 9 |
| Esophageal candidiasis in AIDS | 185 (76) | p.o., 200 mg q12h | 6 | 1 |
| Invasive aspergillosisa | 166 (63) | i.v., 6 mg/kg ×2 q12h → 4 mg/kg q12h; p.o., 200 mg q12h* | 12 | 14 |
| Empirical therapyc | 13 (46) | i.v., 6 mg/kg ×2 q12h → 3 mg/kg q12h; p.o., 200 mg q12h* | 12 | 44 |
| Invasive fungal infectionsd | 313 (51) | i.v., 6 mg/kg ×2 q12h → 4 mg/kg q12h; p.o., 200 mg q12h* | 12 | 26 |
| Non-neutropenic candidemia | 252 (75) | i.v., 6 mg/kg ×2 q12h → 4 mg/kg q12h; p.o., 200 mg q12h* | 8 | 21 |
Two separate studies.
*, Dose escalation allowed. p.o., peroral; i.v., intravenous; q12h, every 12 h; ×2, administered twice.
Only patients with data review committee-determined baseline infections were included.
Salvage therapy, intolerance, no approved therapy.
Organism and MIC.
Invasive fungal isolates were identified to the species level using standard phenotypic techniques. Voriconazole MICs were obtained for fungi isolated at the start of therapy from 404 of the 825 (49%) patients in six of the clinical studies (Table 1) (1, 14, 21, 26). These MICs were measured at two reference laboratories according to Clinical and Laboratory Standards Institute methodology (M27-A2 and M38-A for yeasts and molds, respectively, with 48-h MIC readings) and have been published elsewhere (12, 19).
Voriconazole concentrations in plasma.
Voriconazole concentrations were measured at a central reference laboratory using a well-validated, high-performance liquid chromatography assay (38). Voriconazole exhibits plasma protein binding at ca. 60% in humans (31). This value was used to estimate the mean unbound plasma fraction for each patient infected with a fungal isolate for which an MIC had been determined.
Drug exposure, pharmacokinetics, and efficacy.
Plasma samples were taken at various times during the voriconazole dosing interval. Differences in clinical protocol design (including the voriconazole intravenous [i.v.]/peroral [p.o.] dosing regimen and the duration of therapy) led to considerable variation in the number and timing of plasma samples obtained from each patient. Furthermore, these samples were not necessarily obtained at optimally informative times, and there were relatively few samples for which there was reliable information on the time of collection relative to the time of drug administration. In addition, the CYP2C19 genotype was not available for any patient. Consequently, these data could not be used to develop a population pharmacokinetic model. Nevertheless, phase I studies suggest that there is low intra-individual variation in plasma concentrations relative to inter-individual variability (22, 29, 30), thus allowing the mean plasma concentration per patient (Cavg) to be used as a measure of drug exposure.
Data analysis.
The relationship between Cavg and efficacy was investigated by logistic regression using the logarithm of an individual's mean plasma concentration and the binary outcome measure (i.e., success or failure). A range of covariates (listed in Table 4) that could have an impact upon the probability of obtaining a clinical response to voriconazole were also investigated. In addition to graphical displays using splines, quadratic functions of linear terms were used to explore nonlinearity. There is no supposition of the exact form of the nonlinear relationships. Data analysis used SAS/STAT software (33).
Table 4.
Simple relationship of covariates with efficacy in logistic modeling for all 825 patients and/or 404 patients from the MIC data set
| Factor | Pa | Category, % success |
|---|---|---|
| Therapy | <0.001 | Salvage, 55%; primary, 72% |
| Infection type | <0.001 | Mold, 60%; yeast, 76% |
| Primary diagnosis | <0.001 | Aspergillosis, 60%; candidiasis, 77%; other, 55% |
| Race | 0.004 | Caucasian, 66%; other, 78% |
| Gender | 0.080 | Female, 65%; male, 71% |
| Region* | 0.016 | Americas, 67%; Europe, 74%; Asia/Oceania, 84%; Africa, 87% |
| Study | <0.001 | See Table 1 for full population |
| Site of infection* | <0.001 | 54 to 87% |
| Underlying condition* | <0.001 | 57 to 86% |
| Baseline variantsb | ||
| Wt (kg) | 0.637 | 55, 69.4%; 74, 68.5% |
| Age (yr) | 0.075 | 34, 70.9%; 57, 66.8% |
| Total bilirubin/ULNc | <0.001 | 0.34, 74.4%; 0.92, 69.6% |
| Alkaline phosphatase/ULN | 0.033 | 0.66, 71.5%; 1.45, 69.1% |
| ALT/ULN | 0.097 | 0.42, 70.8%; 1.24, 70.0% |
| AST/ULN | 0.296 | 0.47, 70.4%; 1.21, 69.8% |
| Creatinine/ULN | 0.132 | 0.57, 71.1%; 0.93, 69.1% |
Determined by logistic regression, except as noted. *, Determined using the MIC data set only.
Baseline variant values represent the percent predicted success and are presented as “25th percentile; 75th percentile”.
Upper level of normal.
Subset analysis of the impact of MICs on clinical outcome.
The relationship between the Cavg free plasma concentration/MIC ratio and clinical response was also investigated using logistic regression, as described above. Three additional covariates only available for this particular subset of patients were included in the modeling: geographic location, underlying condition, and site of infection (Table 4).
Relationship between Cavg and trough concentration.
To provide a more clinically tractable measure of drug exposure, Monte Carlo simulation was used to estimate the relationship between Cavg and trough voriconazole concentrations (40). The population pharmacokinetic model used in this analysis was fitted to data from healthy volunteers and patients with invasive aspergillosis. The structural model included the parameters Vmax and Km (the maximum rate of enzyme activity and the voriconazole concentration at which enzyme activity is half-maximal, respectively) to enable the nonlinear pharmacokinetics to be estimated (W. Hope, unpublished data). The mean population parameter values and their associated variances were inserted into subroutine PRIOR of ADAPT 5. A 5,000-patient simulation was performed.
The simulation module in ADAPT 5 was used to calculate the AUC at the end of the first week of i.v. therapy after the administration of 6 mg/kg every 12 h (q12h) i.v. on day 1, followed by 4 mg/kg i.v. administered q12h thereafter (8). The average concentration was calculated by dividing the AUC0-12 by the dosing interval (i.e., 12 h). The Cavg free fraction was obtained by multiplying this value by 0.4. The probability of clinical response was determined from the logistic regression parameters that described the relationship between the Cavg/MIC ratio and the probability of clinical response. The corresponding trough concentration at the end of the first week of therapy in each of the simulated patients was determined. The trough/MIC ratio was determined for each patient by using MIC values of 0.125 to 64 mg/liter. To further estimate the overall likelihood of a given outcome across the simulated population, an expectation across the distribution was calculated by using an approach described elsewhere (10, 11). The expectation for the trough/MIC ratio and probability of clinical response was calculated in the following manner. The distribution of trough/MIC ratio and the probabilities of successful outcomes for the 5,000 simulated patients were described by using a histogram with 20 subgroups. The midpoint value for each subgroup was multiplied by the fraction of simulated patients in each respective subgroup (e.g., if the subgroup contained 1,000 patients with trough/MIC values of 100 to 150, the midpoint value of 125 is multiplied by 1,000/total population; this process was repeated across the entire distribution). The overall estimate for the simulated population was then determined by summation of each of these products.
RESULTS
Patient population.
There were 825 patients with a recorded clinical response and voriconazole plasma level measurements used in the primary analysis. The individual clinical studies from which they came, the voriconazole regimens and the overall response rates of the studies are summarized in Table 1. These 825 patients had an investigator-determined overall clinical response rate of 68.8%.
The patients were from approximately 200 centers in over 25 countries; 546 (66%) were male, and 650 (79%) were Caucasian. The age range was 12 to 90 years (median, 44 years), and the weight range was 26 to 132 kg (median, 64 kg). Seventy-two patients (7.6%) weighed ≥90 kg. Approximately 15% of patients with yeast infections, but 72% of patients with mold infections, were receiving voriconazole treatment as salvage therapy or because of intolerance to a licensed antifungal agent (26).
Organism and MIC.
Yeast isolates (100% Candida spp.) were cultured predominantly from patients with non-neutropenic candidemia or AIDS, while the molds (79% Aspergillus spp.) were cultured mostly from hematopoietic cell transplant or solid organ transplant recipients and patients with hematological malignancy. MICs ranged from 0.0039 to ≥16.00 μg/ml, but the MIC50 and MIC90 values for the 259 yeasts and 109 molds were 0.03 and 1.0 μg/ml and 0.25 and 0.5 μg/ml, respectively.
Voriconazole concentrations in plasma.
The 825 patients contributed a total of 3,052 plasma samples (median, 3; range,1 to 24 samples per patient). Their mean concentrations ranged from <0.01 to 15.8 μg/ml (median, 2.4 μg/ml). At least one plasma sample containing <0.1 μg of voriconazole/ml was detected in 128 patients (16%), but only 16 patients (1.9%) had a Cavg of <0.1 μg/ml, while 87 patients (11%) had a Cavg of <0.5 μg/ml (Table 2).
Table 2.
Investigator-determined success at end of therapy for 825 patients categorized by mean plasma voriconazole concentration interval
| Mean plasma concn (μg/ml) | No. of patients (% success)a |
||
|---|---|---|---|
| Total, n = 825 (69) | Yeast infected, n = 432 (77) | Mold infected, n = 388 (60) | |
| <0.5 | 87 (57) | 52 (63) | 34 (47) |
| 0.5–<1.0 | 75 (71) | 34 (82) | 40 (60) |
| 1.0–<1.5 | 94 (71) | 38 (84) | 56 (63) |
| 1.5–<2.0 | 100 (74) | 47 (87) | 53 (62) |
| 2.0–<3.0 | 151 (75) | 70 (80) | 80 (70) |
| 3.0–<4.0 | 100 (78) | 53 (81) | 47 (74) |
| 4.0–<5.0 | 71 (70) | 46 (85) | 25 (44) |
| 5.0–<6.0 | 47 (60) | 24 (71) | 23 (48) |
| 6.0–<8.0 | 55 (51) | 37 (54) | 17 (47) |
| 8.0–<10.0 | 26 (62) | 18 (78) | 8 (25) |
| ≥10.0 | 19 (58) | 13 (85) | 5 (0) |
A total of five patients were not confirmed to have either yeast or mold infection.
Description of the relationship between Cavg and clinical efficacy.
The clinical response rate, expressed as a function of mean plasma concentration interval for all 825 patients, is summarized in Table 2. The clinical response rate for all patients with Cavg values between 0.5 and 5.0 μg/ml ranged from 70 to 78% (mean, 74%). This concentration range also included 72% (591/825) of the patient population. The clinical success rates associated with the various concentration intervals for patients infected with yeasts and molds were 77% and 60%, respectively (Table 2). However, the maximum clinical response for yeasts or molds (81% or 74%, respectively) was achieved at Cavg levels of 3.0 to 4.0 μg/ml. Patients infected with yeasts had higher response rates than those infected with molds across the entire range of voriconazole concentration intervals used in this analysis. Mold infections were also associated with a significantly lower clinical response rate compared to yeast infections (P < 0.001) for patients with plasma concentrations of ≥5.0 μg/ml (Table 2).
There were 87 (11%) patients with a Cavg of <0.5 μg/ml. These patients had a lower response rate (57%) compared to patients with a Cavg of 0.5 to <5.0 μg/ml (Table 2). However, the lowest response rate (56%) occurred in the 147 (18%) patients, where the Cavg was ≥5.0 μg/ml. Dosage escalation, for any reason, occurred in only 4.8% of these patients (7/147; four successes and three failures). The outcomes for the 147 patients with a Cavg of ≥5.0 μg/ml are summarized in Table 3.
Table 3.
Outcomes of 147 patients with mean plasma concentrations of >5.0 μg/ml
| Patient category | No. (%) of patientsa |
||
|---|---|---|---|
| All (n = 147) | Yeast infected (n = 92) | Mold infected (n = 53) | |
| Outcome | |||
| Therapy success | 83 (56) | 62 (67) | 21 (40) |
| Therapy failure | 64* (44) | 30 (33) | 32 (60) |
| Reasons for therapy failure | |||
| Insufficient efficacy | 14 (22) | 6 (26) | 8 (22) |
| Died (all causes) | 32* (50) | 14 (36) | 17 (56) |
| Discontinued due to adverse event or laboratory abnormality | 18* (28) | 10 (23) | 7 (20) |
| Total | 64 | 30 | 32 |
| Adverse event/laboratory abnormality due to voriconazole | 4 (6) | 0 (0) | 4 (13) |
*, Includes (two) patients not confirmed to have yeast or mold infection.
Just over half (83/147 [56%]) of these patients completed therapy successfully, while the remaining 64 (44%) had stable or failed outcomes. These 64 patients included 36 receiving primary therapy and 28 receiving salvage therapy. There were 32/64 (50%) patients who died due to their underlying disease or fungal infection, while the remainder discontinued therapy (lack of efficacy, adverse events, or laboratory abnormalities, Table 3). In only 4/18 (22%) patients who discontinued therapy due to an adverse event or laboratory abnormality was this discontinuation ascribed, by the investigator, to voriconazole (1 elevated transaminases, 1 elevated bilirubin, 1 elevated alkaline phosphatase, and 1 hypoglycemia). None of the 18 patients discontinued therapy due to central nervous system adverse events of any causality.
Mean plasma concentration and efficacy.
The simple response rate was significantly better for patients with the following characteristics: patients in primary therapy not salvage, patients infected with yeasts and not molds, patients with candidiasis not aspergillosis, and patients with lower baseline bilirubin and alkaline phosphatase levels (Table 4). There were also differences between categories of race, regions, studies, sites of infection, and underlying conditions. Clearly, some of these variables are likely to be confounded. In contrast, patient gender, age, weight, and baseline alanine aminotransferase (ALT), aspartate transaminase (AST), or creatinine levels were not related to clinical outcome.
Logistic modeling for the 825 patients revealed a significant, nonlinear relationship (as exemplified by a quadratic polynomial term, P < 0.003) between mean voriconazole plasma concentrations and clinical response. The parametric polynomial model does not describe the nonparametric spline fit well where data are sparse (Fig. 1), but it does confirm that a more complex model than a simply linear one is required. The inclusion of covariates (from Table 4) in this nonlinear model did not change the significance of the curved relationship between the Cavg and response, although some had additional explanatory power (infection type, study, and baseline bilirubin). Furthermore, the relationship was not affected by the removal of a large study of esophageal candidiasis in AIDS patients (n = 154) that contained the highest response rates (Table 1) and may conceivably have obfuscated any underlying relationship for the invasive infections.
Fig. 1.
Binomial data and quadratic logistic fit for investigator outcome versus the mean voriconazole plasma concentration for 825 patients. ○, Data points separated as clinical success or clinical failure; - — -, spline (moving average); ——, line of predicted fit; - - -, upper and lower 95% confidence intervals. The curvature was significant at P < 0.003.
Mean free plasma concentration/MIC ratio and efficacy.
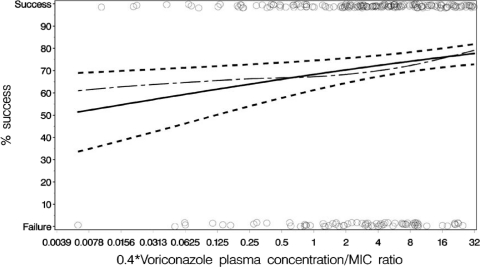
The impact of the MIC on the drug exposure-response relationships was explored by using the free Cavg/MIC ratio as the independent variable in a logistic regression model. The Cavg-versus-response relationship for the 404 patients in whom an MIC was available was also nonlinear, with a shape similar to that shown in Fig. 1, and was not affected by the inclusion of covariates in Table 4. Importantly, however, the relationship between Cavg/MIC ratio-versus-response was best described by using a linear term (parameter estimates for the final model are shown in Fig. 2), reflecting the fact that higher Cavg/MIC ratio values were associated with progressively better clinical responses. Of note, this relationship was only statistically significant when the data from yeasts and molds were combined. The relationships of the free Cavg ratio versus response for yeast (295 patients) and mold (109 patients) when examined separately were not statistically significant (P = 0.368 and 0.172 for yeasts and molds, respectively).
Fig. 2.
Binomial data and linear logistic fit for investigator outcome versus the mean voriconazole free drug/MIC ratio for 404 patients. ○, Data points separated as clinical success or clinical failure. Curves: - — -, spline; ——, line of predicted fit; - - -, upper and lower 95% confidence intervals. The slope was significant at P = 0.005. Note that the free ratio is 0.4 × the ratio. Calculation: logit (P) = (0.766 + 0.139) × loge free ratio, where P is the probability of response.
Relationship between trough/MIC ratio and clinical response.
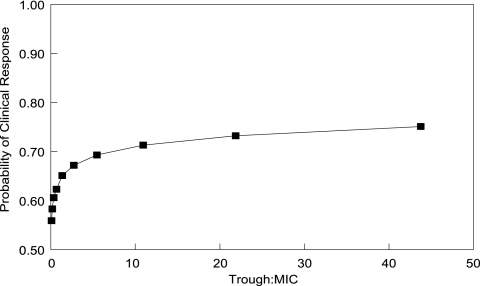
The population parameter values and their dispersions were readily recapitulated in the Monte Carlo simulation. The administration of the currently recommended i.v. regimen resulted in a median trough concentration ± the standard deviation of 2.48 ± 6.44 μg/ml. The estimated relationship between the trough/MIC ratio and the probability of clinical response is shown in Fig. 3. Although the probability of a clinical response progressively increased with a higher trough concentration/MIC ratio, the probability of a successful outcome was near maximal with trough/MIC values of ca. 2 to 3.
Fig. 3.
Relationship between the estimated trough concentration/MIC ratio and the probability of clinical response.
DISCUSSION
The relationship between both mean plasma voriconazole concentrations and mean concentration/MIC ratios with efficacy were analyzed for patients from nine phase II and III clinical studies. These studies used the currently recommended voriconazole clinical dose regimens for adults, which was also used in the Monte Carlo simulations. The median Cavg of 2.37 μg/ml for these 825 patients falls well within the range of steady-state voriconazole mean concentrations achieved after multiple i.v. and p.o. dosing in healthy volunteers or in hematopoietic cell transplant recipients (7, 28). This is also the largest patient population used to date to examine the voriconazole drug exposure and clinical response relationship and provides useful insights into potential drug exposure targets for TDM.
There is a significantly lower rate of clinical response at the extremes of drug exposure. Although the poorer clinical response in patients with lower drug exposures is intuitively obvious and consistent with other studies (16, 20, 23), the lower rate of clinical response at higher concentrations is more difficult to understand. The extent of curvature, depicted in Fig. 1, did not change with the incorporation of any of the covariates (including primary or salvage therapy and yeast or mold pathogen) or with the removal of the majority of noninvasive yeast infections (esophageal candidiasis study) from the analysis. This suggests that none of these factors have a simple impact upon Cavg response relationships. Dose escalation was reported for only 4.8% of the 147 patients with mean plasma levels of ≥5.0 μg/ml and so cannot account for the vast majority of these concentrations. Furthermore, there is no evidence that the lower response rate seen at these high mean concentrations is related to voriconazole toxicity, suggesting that other unidentified factors (or combinations of factors) may be important. Patients with mold infections did exhibit a significantly worse response than those with yeast infections at mean plasma levels of ≥5.0 μg/ml, but the reason for this is not clear. Potentially the severity of illness of these patients not only led to worse responses but also impaired their ability to clear voriconazole.
One factor that clearly has a powerful impact upon exposure response relationships is the MIC. The use of Cavg/MIC ratio as the independent variable yields a linear relationship, reflecting the fact that patients with higher free Cavg/MIC values are significantly more likely to respond to voriconazole therapy. This finding also suggests that the MIC conveys important information that can be used to optimize voriconazole therapy at the bedside. Interestingly, the free Cavg/MIC ratio that is associated with a higher probability of clinical response is largely concordant with pharmacodynamic targets derived from experimental models of disseminated candidiasis (2, 4). A free voriconazole AUC/MIC ratio of ∼24 for various Candida albicans strains is associated with half-maximal antifungal effect and is consistent with other members of the triazole antifungal class (4). A free AUC/MIC ratio of 24 is comparable to a free Cavg/MIC ratio of ∼1, which is associated with a clinical response of ∼65% in this analysis (Fig. 2).
One problem with using Cavg as a measure of drug exposure is that it is cumbersome in a clinical context. Although a trough concentration is not necessarily optimally precise, it is more clinically tractable. Monte Carlo simulation provides an estimate of the relationship between total voriconazole trough concentration/MIC ratio and the clinical response. Figure 3 suggests that a progressively higher trough/MIC ratio is associated with a progressively higher probability of clinical response. The probability of clinical response is near maximal with a trough/MIC ratio that is somewhere between ca. 2 and 5. This finding can help guide therapy for patients when the MIC of the invading pathogen is known and enables prompt dosage escalation or a change in therapy if this target cannot be achieved. In circumstances in which the MIC is not known, an MIC90 of 1.0 μg/ml for both Candida and Aspergillus spp. for the isolates in the present study could be used to determine a drug exposure target for TDM. It should be noted that the Monte Carlo simulation is based only on i.v. dosing at 4 mg/kg (after day 1). Voriconazole levels following p.o. dosing (200 mg p.o. is approximately equivalent to 3.5 mg/kg) are somewhat lower than after i.v. dosing at 4 mg/kg. This may account for the median trough levels in the simulation being very similar to the measured median Cavg levels for the 825 patients (see the dose regimens of the various studies in Table 1).
There are several limitations to our study. (i) Combination of patient types and a wide range of pathogens have been included. Exposure response relationships may vary significantly between different types of patient and various fungal genera or even species. (ii) Patients with a wide variety of predisposing conditions have been included. The natural history and clinical response is likely to vary considerably between these groups, although underlying conditions and fungal genus were included as cofactors examined in the analysis. (iii) A relatively crude measure of drug exposure was used. Ideally, each patient would have had high-quality pharmacokinetic data collected, enabling robust estimates of drug exposure and therefore maximizing the chance of identifying clinically relevant exposure response relationships (35, 36, 41).
In conclusion, our findings are unique not only due to the size of this study but also because all of the patients were from formal clinical trials, which may help reduce interpatient variability and enhance the uniformity of efficacy and safety reporting, as well as the voriconazole level measurement. Furthermore, we emphasize here the importance of considering the MIC, when it is available, together with the plasma concentration, in predicting the therapeutic response for patients receiving voriconazole.
ACKNOWLEDGMENTS
The pharmacokinetic source data were collected as part of clinical studies sponsored by Pfizer, Inc. The analyses presented here were funded by Pfizer, Inc. W.W.H. is funded by a National Institute of Health Research Clinician Scientist Award. Pfizer, Inc., had no decision-making role in the design, execution, analysis or reporting of this research.
P.F.T. was an employee of and is currently a consultant to Pfizer. He received an honorarium from Pfizer in connection with the development of the manuscript. H.H.P. was a statistical consultant to Pfizer and received an honorarium from Pfizer in connection with the development of this manuscript. W.W.H. has served on advisory boards and has received speaker fees and research funding from Pfizer, Inc.
Footnotes
Published ahead of print on 18 July 2011.
REFERENCES
- 1. Ally R., et al. 2001. A randomized, double-blind, double-dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patients. Clin. Infect. Dis. 33:1447–1454 [DOI] [PubMed] [Google Scholar]
- 2. Andes D. 2004. Antifungal pharmacokinetics and pharmacodynamics: understanding the implications for antifungal drug resistance. Drug Resist. Updates 7:185–194 [DOI] [PubMed] [Google Scholar]
- 3. Andes D. 2003. Pharmacokinetics and pharmacodynamics in the development of antifungal compounds. Curr. Opin. Invest. Drugs 4:991–998 [PubMed] [Google Scholar]
- 4. Andes D., Marchillo K., Stamstad T., Conklin R. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:3165–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andes D., Pascual A., Marchetti O. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob. Agents Chemother. 53:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bruggemann R., et al. 2009. Reviews of anti-infective agents: clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin. Infect. Dis. 48:1441–1458 [DOI] [PubMed] [Google Scholar]
- 7. Bruggemann R. J., et al. 2010. Pharmacokinetics and safety of 14 days intravenous voriconazole in allogeneic hematopoietic stem cell transplant recipients. J. Antimicrob. Chemother. 65:107–113 [DOI] [PubMed] [Google Scholar]
- 8. D'Argenio D., Schumitzky A. 1997. ADAPT II: a program for simulation, identification, and optimal experimental design, user manual. Biomedical Simulations Resource, University of Southern California, Los Angeles, CA [Google Scholar]
- 9. Denning D. W., et al. 2002. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 34:563–571 [DOI] [PubMed] [Google Scholar]
- 10. Drusano G. A. L. 2009. Editorial commentary: how many steps along the path is too far? Clin. Infect. Dis. 50:3. [DOI] [PubMed] [Google Scholar]
- 11. Drusano G. L., et al. 2007. Back to the future: using aminoglycosides again and how to dose them optimally. Clin. Infect. Dis. 45:753–760 [DOI] [PubMed] [Google Scholar]
- 12. Espinel-Ingroff A., Johnson E., Hockey H., Troke P. 2008. Activities of voriconazole, itraconazole, and amphotericin B in vitro against 590 moulds from 323 patients in the voriconazole phase III clinical studies. J. Antimicrob. Chemother. 61:616–620 [DOI] [PubMed] [Google Scholar]
- 13. Goodwin M. L., Drew R. H. 2008. Antifungal serum concentration monitoring: an update. J. Antimicrob. Chemother. 61:17–25 [DOI] [PubMed] [Google Scholar]
- 14. Herbrecht R., et al. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408–415 [DOI] [PubMed] [Google Scholar]
- 15. Hope W. W., Drusano G. L. 2009. Antifungal pharmacokinetics and pharmacodynamics: bridging from the bench to bedside. Clin. Microbiol. Infect. 15:602–612 [DOI] [PubMed] [Google Scholar]
- 16. Howard A., Hoffman J., Sheth A. 2008. Clinical application of voriconazole concentrations in the treatment of invasive aspergillosis. Ann. Pharmacother. 42:1859–1864 [DOI] [PubMed] [Google Scholar]
- 17. Ikeda Y., et al. 2004. Pharmacokinetics of voriconazole and cytochrome P450 2C19 genetic status. Clin. Pharmacol. Ther. 75:587–588 [DOI] [PubMed] [Google Scholar]
- 18. Imhof A., Schaer D. J., Schanz U., Schwarz U. 2006. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring. Swiss Med. Wkly. 136:739–742 [DOI] [PubMed] [Google Scholar]
- 19. Johnson E., Espinel-Ingroff A., Szekely A., Hockey H., Troke P. 2008. Activity of voriconazole, itraconazole, fluconazole, and amphotericin B in vitro against 1763 yeasts from 472 patients in the voriconazole phase III clinical studies. Int. J. Antimicrob. Agents 32:511–514 [DOI] [PubMed] [Google Scholar]
- 20. Johnston A. 2003. The pharmacokinetics of voriconazole. Br. J. Clin. Pharmacol. 56:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kullberg B. J., et al. 2005. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet 366:1435–1442 [DOI] [PubMed] [Google Scholar]
- 22. Liu P., et al. 2007. Steady-state pharmacokinetic and safety profiles of voriconazole and ritonavir in healthy male subjects. Antimicrob. Agents Chemother. 51:3617–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miyakis S., van Hal S. J., Ray J., Marriott D. 2009. Voriconazole concentrations and outcome of invasive fungal infections. Clin. Microbiol. Infect. 16:927–933 [DOI] [PubMed] [Google Scholar]
- 24. Neely M., Rushing T., Kovacs A., Jelliffe R., Hoffman J. 2010. Voriconazole pharmacokinetics and pharmacodynamics in children. Clin. Infect. Dis. 50:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pascual A., et al. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201–211 [DOI] [PubMed] [Google Scholar]
- 26. Perfect J. R., et al. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122–1131 [DOI] [PubMed] [Google Scholar]
- 27. Prentice A. G., et al. 2008. Guidelines on the management of invasive fungal infection during therapy for haematological malignancy. British Committee for Standards in Haematology, London, United Kingdom [Google Scholar]
- 28. Purkins L., et al. 2002. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob. Agents Chemother. 46:2546–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Purkins L., Wood N., Greenhalgh K., Allen M. J., Oliver S. D. 2003. Voriconazole, a novel wide-spectrum triazole: oral pharmacokinetics and safety. Br. J. Clin. Pharmacol. 56:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Purkins L., et al. 2003. The pharmacokinetics and safety of intravenous voriconazole: a novel wide-spectrum antifungal agent. Br. J. Clin. Pharmacol. 56:2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roffey S. J., et al. 2003. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab. Dispos. 31:731–741 [DOI] [PubMed] [Google Scholar]
- 32. Sambatakou H., Dupont B., Lode H., Denning D. W. 2006. Voriconazole treatment for subacute invasive and chronic pulmonary aspergillosis. Am. J. Med. 119:527e17–527e24 [DOI] [PubMed] [Google Scholar]
- 33. SAS Institute 2003. SAS/STAT users guide, version 8.2. SAS Institute, Inc., Cary, NC [Google Scholar]
- 34. Segal B. H., et al. 2008. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer Consensus criteria. Clin. Infect. Dis. 47:674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seibel N., et al. 2005. Safety, tolerability, and pharmacokinetics of micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob. Agents Chemother. 49:3317–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith J., Andes D. 2008. Therapeutic drug monitoring of antifungals: pharmacokinetic and pharmacodynamic considerations. Ther. Drug Monit. 30:167–172 [DOI] [PubMed] [Google Scholar]
- 37. Smith J., et al. 2006. Voriconazole therapeutic drug monitoring. Antimicrob. Agents Chemother. 50:1570–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stopher D. A., Gage R. 1997. Determination of a new antifungal agent, voriconazole, by multidimensional high-performance liquid chromatography with direct plasma injection onto a size-exclusion column. J. Chromatogr. B Biomed. Sci. Appl. 691:441–448 [DOI] [PubMed] [Google Scholar]
- 39. Tan K., Brayshaw N., Tomaszewski K., Troke P., Wood N. 2006. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J. Clin. Pharmacol. 46:235–243 [DOI] [PubMed] [Google Scholar]
- 40. U.S. Food and Drug Administration 2001. Voriconazole briefing document. FDA Antiviral Drugs Advisory Committee, Rockville, MD: http://www.fda.gov/ohrms/dockets/AC/01/briefing/3792b2_01_Pfizer.pdf [Google Scholar]
- 41. Vandewoude K., et al. 1997. Concentrations in plasma and safety of 7 days of intravenous itraconazole followed by 2 weeks of oral itraconazole solution in patients in intensive care units. Antimicrob. Agents Chemother. 41:2714–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walsh T., et al. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327–360 [DOI] [PubMed] [Google Scholar]
- 43. Walsh T. J., et al. 2004. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob. Agents Chemother. 48:2166–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walsh T. J., et al. 2002. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N. Engl. J. Med. 346:225–234 [DOI] [PubMed] [Google Scholar]