Abstract
Cyclopropavir (CPV) is active against human cytomegalovirus (CMV), as well as both variants of human herpesvirus 6 and human herpesvirus 8. The mechanism of action of CPV against CMV is similar to that of ganciclovir (GCV) in that it is phosphorylated initially by the CMV UL97 kinase, resulting in inhibition of viral DNA synthesis. Resistance to CPV maps to the UL97 kinase but is associated primarily with H520Q mutations and thus retains good antiviral activity against most GCV-resistant isolates. An examination of CMV-infected cultures treated with CPV revealed unusual cell morphology typically associated with the absence of UL97 kinase activity. A surrogate assay for UL97 kinase activity confirmed that CPV inhibited the activity of this enzyme and that its action was similar to the inhibition seen with maribavir (MBV) in this assay. Combination studies using real-time PCR indicated that, like MBV, CPV also antagonized the efficacy of GCV and were consistent with the observed inhibition of the UL97 kinase. Deep sequencing of CPV-resistant laboratory isolates identified a frameshift mutation in UL27, presumably to compensate for a loss of UL97 enzymatic activity. We conclude that the mechanism of action of CPV against CMV is complex and involves both the inhibition of DNA synthesis and the inhibition of the normal activity of the UL97 kinase.
INTRODUCTION
Human cytomegalovirus (CMV) infections are a significant problem in immunocompromised individuals, including transplant recipients and those infected with HIV (38). Infection with CMV also appears to drive events that lead to graft rejection after renal transplantation and accelerated atherosclerosis in heart transplant patients (15, 38). Both preemptive and prophylactic therapy with ganciclovir (GCV) appears to provide some clinical benefit (37), yet resistance to this drug occurs frequently in this population and appears to be related to levels of viral replication that occur notwithstanding GCV therapy (12, 15). Although foscarnet (PFA) and cidofovir (CDV) are available to treat resistant infections, their associated renal toxicity limits their utility (1, 21). This virus also infects up to 1% of all newborns and is the leading cause of brain damage and sensorineural hearing loss in the United States (23). Congenital infections associated with primary infection are associated with more severe sequelae, but prior maternal immunity is only partially protective (4) and does not completely protect neonates from hearing loss (36). Further investigation demonstrated that minor degrees of central nervous system damage also occur in up to 8% of congenitally infected but otherwise normal-appearing newborn infants (24). More recent studies indicated that GCV treatment of neonates with congenital CMV infection prevented further hearing deterioration, yet most had significant neutropenia during the 6-week course of therapy (18). Costs associated with hearing loss due to CMV infection exceed $2 billion annually in the United States (24); thus, better therapies to treat these infections may result in improved health and lower cost. Long-term therapy required to treat CMV infections in immunocompromised patients, coupled with the development of resistance (13), necessitates the continued development of new antiviral agents for the treatment of CMV infections that are more effective and active against resistant strains of the virus (1, 2, 15).
Methylenecyclopropane analogs have been shown to be active against a number of human herpesviruses (HHVs). These include the (Z)- and (E)-[2-fluoro-2-(hydroxymethyl)cyclopropylidene]methylpurines and -pyrimidines, which exhibit activity against CMV, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus (EBV) (41–43). Additionally, some (Z)- and (E)-9-([(2-fluoromethyl-2-hydroxymethyl)-cyclopropylidene]methyl)adenine and -guanine analogs were modestly effective against CMV (20). These results were extended to include (Z)- and (E)-2-(1,2-dihydroxyethyl)methylenecyclopropane analogues of 2′-deoxyadenosine and 2′-deoxyguanosine, some of which were active against CMV, murine CMV, and EBV (40). The lead compound cyclopropavir (CPV; ZSM-I-62) was evaluated against all of the HHVs, and we reported previously that it was active in vitro against CMV, HHV-6A, HHV-6B, and HHV-8 (17). It was also effective in vivo in two animal models of human CMV infection (16).
This compound appears to require the UL97 kinase for its antiviral activity since a recombinant virus that does not express this enzyme was more than 20-fold resistant to the antiviral activity of CPV (17). It also appears to exert its antiviral effects through the inhibition of DNA synthesis since its inhibition of the accumulation of viral DNA closely parallels its antiviral activity (17). Enzymatic studies confirmed that CPV was a better substrate for the kinase than GCV (11) and that the CPV monophosphate could be phosphorylated to the triphosphate metabolite by GMP kinase (10). Further studies showed that CPV phosphonate analogs also had antiviral activity against CMV and retained activity against GCV-resistant isolates with mutations in the UL97 kinase, as expected for these monophosphate analogs (22). Interestingly, CPV retained antiviral activity against a panel of 9 GCV-resistant clinical isolates, with the concentrations required to inhibit viral replication by 50% (EC50s) all below 10 μM (17). Recently, CPV-resistant isolates were selected using a recombinant CMV with a mutation in the DNA polymerase exonuclease domain, and the M460I, H520Q, and C603R mutations, which conferred resistance to this compound, were identified in the UL97 kinase (7). Among GCV-resistant clinical isolates, the M460I and H520Q mutations are observed at a frequency of <10% and were not represented in the initial panel of 9 GCV-resistant clinical isolates initially used to evaluate CPV (17).
The goal of the present studies was to investigate the mechanism of action of CPV to better understand how it inhibits the replication of CMV. The compound was used to select two independent isolates that were resistant to CPV, and population sequencing indicated that both had the H520Q mutation in the UL97 kinase but no mutations were observed in the DNA polymerase. The complete genomes of the two independently selected resistant isolates were also subjected to deep sequencing to identify any additional mutations that might impart resistance to the compound. This analysis identified subpopulations that had mutations near the active site of the UL97 kinase and also had mutations in the UL27 open reading frame, presumably to compensate for the loss of UL97 kinase activity. A surrogate assay for UL97 kinase activity indicated that CPV inhibited the activity of this enzyme and that its activity was similar to that of maribavir (MBV). The consequences of this inhibition were evaluated in combined efficacy studies that revealed robust antagonism with combinations of CPV and GCV and were consistent with the inhibition of UL97 kinase activity. These data suggest that the mechanism of action of CPV is complex and that it is phosphorylated by the UL97 protein kinase but also interferes with the normal function of this enzyme.
MATERIALS AND METHODS
Cells and viruses.
Primary human foreskin fibroblast (HFF) cells were prepared as described previously (39). HEL299 cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA). The cells were routinely passaged in minimal essential medium with 10% fetal bovine serum, l-glutamine, penicillin, and gentamicin and used below passage 10. Primary renal proximal tubule epithelial cells (RPTEC) were purchased from Lonza Biosciences (Walkersville, MD) and passaged according to the vendor's recommendations. Isolates of CMV resistant to GCV were obtained from Karen Biron (GlaxoSmithKline, Research Triangle Park, NC) and have been described previously (3).
Compounds and plasmids.
CPV was provided by Microbiotix Inc. (Worcester, MA). CDV was provided by Mick Hitchcock (Gilead Sciences, Inc., Foster City, CA). MBV was obtained from John Drach (University of Michigan). Plasmids expressing the pp65-GFP (green fluorescent protein) fusion protein, the UL97 kinase, and the inactive UL97 kinase (K355M) were described previously (34). The plasmid expressing a V5-tagged version of the UL97 kinase (pMP307) was subjected to QuikChange mutagenesis to generate a V356G mutation (pMP565). The entire open reading frame was sequenced, and only the V356G mutation was observed.
Cytotoxicity assays.
Cytotoxicity was evaluated in primary HFF, HEL299 cells, and RPTEC. Neutral Red uptake assays were conducted by methods published previously (32). Briefly, cells were seeded in 96-well plates at a density of 2 × 104 per well. Cell monolayers were then incubated for 7 days with a series of compound solutions prepared using 3-fold serial dilutions in cell culture medium, with the highest concentration being 300 μM. Growth medium was then aspirated, 200 μl of a 0.066% Neutral Red stain solution in phosphate-buffered saline was added, and the mixture was incubated for 1 h. The remaining dye was washed from the monolayers, and the dye internalized by the cells was solubilized in a solution containing 50% ethanol and 1% glacial acetic acid. The plates were mixed for 15 min on a rotating shaker, and the optical densities at 550 nm were determined with a microplate reader. Concentrations of compounds sufficient to reduce cell viability by 50% (CC50s) were interpolated from experimental data. Cytotoxicity was also evaluated using CellTiter-Glo assays (Promega, Madison, WI) by methods we reported previously (28). Monolayers of cells in 96-well plates were prepared and exposed to a series of compound concentrations for 7 days as described above. The plates were then incubated at ambient temperature for 30 min, after which 50 μl of CellTiter-Glo reagent was added to each well and the plates were mixed for 2 min on an orbital shaker. The plates were then incubated for an additional 10 min at room temperature, and luminescence was quantified on a luminometer. Evaluation of cytotoxicity in proliferating cells was done by counting viable cells in a Coulter counter by methods reported previously (30). Briefly, cells were seeded in 6-well plates at a concentration of 2.5 × 104 cells per well. After 24 h, the medium was aspirated and drug was added to duplicate wells. The concentrations used started at 300 μM and were serially diluted 1:5. The plates were incubated for 72 h at 37°C, after which the cells were dispersed with trypsin and counted in a Coulter counter. The CC50s of compounds were interpolated from the experimental data.
Selection of CPV-resistant isolates.
The AD169 strain of CMV was subjected to selective pressure with CPV to select isolates that were resistant to its antiviral activity. Selection for resistant isolates was initiated at 1 μM, which is close to the EC50, and the concentration was doubled at 4 subsequent passages of the virus such that the final stocks were propagated in 16 μM CPV. Two separate cultures were selected in parallel so that the mutations could be compared.
Characterization of drug-resistant isolates.
The UL97 and UL54 open reading frames were amplified by PCR using a high-fidelity enzyme mix (Roche Applied Sciences, Indianapolis, IN). Both strands of the PCR products were then sequenced by standard methods in the University of Alabama at Birmingham DNA Sequencing Core facility.
Whole-genome sequencing.
The genomes of CMV strain AD169varATCC and the two isolates resistant to CPV, CPVR1 and CPVR2, were sequenced with the Illumina GAIIx Genome Analyzer on a paired-end, multiplexed sequencing run according to the manufacturer's instructions (Illumina, San Diego, CA). For each isolate, 0.2 to 5 μg double-stranded DNA in a volume of 200 μl was sheared using a 96-well plate with the SonicMAN (Matrical Bioscience, Spokane, WA) to a size range of 200 to 1,000 bp, with the majority of material at 600 bp. The enzymatic processing (end repair, phosphorylation, A tailing, and adaptor ligation) of the DNA followed the guidelines described in the Illumina protocol. The enzymes for processing were obtained from New England BioLabs (Ipswich, MA), and the oligonucleotides and adaptors were obtained from Illumina. After ligation of the adaptors, the DNA was run on a 2% agarose gel for 2 h, after which a gel slice containing 500- to 600-bp fragments of each DNA sample was isolated and purified using the QIAquick Gel Extraction kit (Qiagen, Valencia, CA). Individual libraries were quantified by quantitative PCR on an ABI 7900HT (Life Technologies Corporation, Carlsbad, CA) in triplicate at two concentrations, 1:1,000 and 1:2,000, using the Kapa Library Quantification Kit (Kapa Biosystems, Woburn, MA). Libraries were pooled and run in a single lane on a 50-bp paired-end run. At the end of the run, images were processed using the Solexa Data Analysis Pipeline 0.2.2.6. Sequence read data from the Illumina GAII were aligned to an annotated GenBank reference for each gene, UL97 or UL54, individually and across an entire genome using the Lasergene SeqMan NGen v.2.2 software (DNASTAR, Madison, WI). Percentages of mixed bases were determined using the depth of coverage and base composition at each position in the Lasergene SeqMan software.
Evaluation of combination chemotherapy with GCV.
The activity of compound combinations was evaluated using in vitro antiviral assays to assess combined efficacy using an experimental approach similar to that described previously (33). Briefly, 96-well plates with monolayers of HFF cells were prepared and a checkerboard matrix of dilutions of CPV and other compounds was prepared using a BioMek 2000. Four replicate plates were infected with the AD169 strain of CMV at a multiplicity of infection of 0.01 PFU/cell. At 7 days after infection, total DNA from each well was prepared using the Wizard SV 96 genomic DNA purification system (Promega, Madison, WI). Viral loads were quantified by real-time PCR using primers 5′-AGG TCT TCA AGG AAC TCA GCA AGA-3′ and 5′-CGG CAA TCG GTT TGT TGT AAA-3′ with the probe 5′-(6FAM) CCG TCA GCC ATT CTC TCG GC TAMRA-3′ and the plasmid pMP217 to provide absolute quantification. Results from combination studies were evaluated using a modified version of MacSynergy II (http://medicine.uab.edu/Peds/54568/infectious/79968/). This software was adapted to calculate statistically significant synergy using the genome copy number and is similar to methods we reported previously (26). The use of the genome copy number provided a greater dynamic range than traditional methods and facilitated the identification of synergistic interactions over a much wider range of compound concentrations. Two replicate plates remained uninfected but were exposed to the same combination of drug concentrations, and cell viability was evaluated using CellTiter-Glo (Promega). Cell viability was also evaluated by these methods to reveal any unexpected exacerbation of cytotoxicity.
Inhibition of UL97 kinase activity.
Inhibition of UL97 kinase activity was determined in a surrogate assay using COS7 cells transfected with plasmids expressing UL97 kinase (pMP92) and a reporter plasmid expressing a pp65-GFP fusion (pMP111) (27, 34). Transfected cells expressing UL97 kinase inhibit the formation of nuclear aggresomes induced by the coexpression of a pp65-GFP fusion. Either the genetic inactivation of the UL97 kinase with a K355M mutation or the pharmacologic inhibition of its enzymatic activity reduces the capacity of the enzyme to prevent aggresome formation. Expression of the UL97 kinase was confirmed using a V5 monoclonal antibody (Invitrogen, Carlsbad, CA) and a Texas Red-conjugated secondary antibody (Southern Biotech, Birmingham, AL). Aggresome formation was evaluated under a fluorescence microscope, and the proportion of cells with aggresomes was determined by counting at least 10 separate fields. In all experiments, the compounds were used at a final concentration of 15 μM so that results could be compared directly.
RESULTS
Cytotoxicity of CPV in primary human cells.
Previous reports indicated that CPV was more active against CMV than GCV was, with EC50s improved by 4- to 6-fold (7, 17). The cytotoxicity of CPV was also reported to be similar to that of GCV in quiescent cells by a Neutral Red assay but slightly higher than that of GCV in proliferating HFF cells, which is predictive of toxicity in animal models, in our experience, and is a more sensitive indicator of cytotoxicity (17). The cytotoxicity of this compound was further characterized prior to detailed mechanistic analyses to ensure that the effects observed were unrelated to potential toxicities. Cell monolayers in 96-well plates were exposed to CPV, GCV, CDV, and acyclovir (ACV), and cell viability was determined by Neutral Red uptake and CellTiter-Glo assays (Table 1). In HFF cells, CPV appeared to be more toxic than the other compounds. In HEL299 cells, the toxicity of CPV appeared to be comparable to that of CDV but was greater than that of GCV in Neutral Red uptake assays. In RPTEC, CDV was more toxic than either GCV or CPV. These data were similar to those we reported previously and indicated that CPV was not particularly toxic in quiescent cells.
Table 1.
Cytotoxicity in primary human cells using Neutral Red, CellTiter-Glo, and proliferation assays
| Assay and cell type | CC50s (μM)a |
||||
|---|---|---|---|---|---|
| CPV | GCV | CDV | ACV | FIAUc | |
| Neutral Red uptake | |||||
| HFF | >298 ± 2.9b | >300, >300 | >300, >300 | >300, >300 | |
| HEL299 | 242, 199 | >300, >300 | 231, 189 | >300, >300 | |
| RPTEC | >300, >300 | >300, >300 | 281, 210 | >300, >300 | |
| CellTiter-Glo | |||||
| HFF | >251 ± 83b | >300, >300 | >300, >300 | >300, >300 | |
| HEL299 | >300, >300 | >300, >300 | >300, >300 | >300, >300 | |
| RPTEC | >300, >300 | >300, >300 | 273, 242 | >300, >300 | |
| Cell proliferation | |||||
| HFF | 82 ± 16b | 272, 230b | 150, 159 | 9.2 ± 1.3 | |
| HEL299 | 288, 262 | 292, >300 | 174, 121 | 9.9, 34 | |
| RPTEC | 132, 146 | >300, >300 | 199, 122 | 21, 29 | |
The values shown are results of two independent experiments, unless indicated otherwise.
Shown is the average of four independent experiments with the standard deviation values.
The positive control in the cell proliferation assay was FIAU rather than ACV.
In cell proliferation assays, HFF cells in 6-well plates were exposed to the compounds and fialuridine (FIAU) was used as a positive control. In these studies, CPV had a CC50 of 82 μM, which is equivalent to the value of 70 μM we reported previously (17). This value was approximately 3-fold lower than that of GCV and 2-fold lower than that of CDV in the same cell type. This was not observed in HEL299 fibroblasts, where the CC50s of CPV were only marginally lower than those of GCV but greater than those of CDV. In RPTEC, the CC50s of CPV were about 2-fold lower than those of GCV and similar to those of CDV. These data suggest that CPV is more toxic than GCV in proliferating cells and is comparable to CDV. All of the data, taken together, suggest that CPV would be well tolerated in animal models and is consistent with in vivo studies reported previously (16). Although CPV appears to be slightly more toxic than GCV (approximately 3-fold more toxic to HFF cells in proliferation assays), it also exhibits greater efficacy than GCV, such that its selective index is superior to that of GCV.
Selection of CPV-resistant isolates.
To identify mutations that impart resistance to CPV, the AD169 strain of CMV was subjected to selection with increasing concentrations of CPV during 5 serial passages. Resistant viruses isolated from two separate series of flasks were plaque purified, and the independently selected isolates CPVR1 and CPVR2 were highly resistant to CPV. The UL97 and UL54 open reading frames from both isolates were sequenced. In both cultures, the H520Q mutation was observed in the inferred amino acid sequence of the UL97 kinase in most of the plaque isolates sequenced. No polymorphisms in the DNA polymerase open reading frame were detected in any of the 12 plaque isolates sequenced. The EC50 for CPVR2 was 20-fold higher than that for the parental virus and was the same as that reported previously for a recombinant with the H520Q mutation (7). Thus, the H520Q mutation is sufficient to confer resistance to CPV and also to GCV. Our previous report characterizing the efficacy of CPV against a panel of 9 drug-resistant CMV isolates noted only a single isolate that exhibited 4-fold or greater resistance to CPV (17). This was interesting, since the M460I, H520Q, and C603R mutations in the UL97 kinase that are associated with CPV resistance are also present in some clinical isolates resistant to GCV (7). A subsequent genotypic analysis of this panel of resistant isolates revealed that none carried any of these three mutations (Table 2). Although 2 isolates had the M460V mutation, it did not appear to have a significant impact on their susceptibility to CPV, and this is also consistent with results from the recent work of Chou and Bowlin (7). These data confirmed that the UL97 kinase is important for the activation of CPV and suggest that the profile of point mutations conferring resistance to the compound is distinct from that of isolates typically obtained from GCV-resistant infections.
Table 2.
Activity of CPV against GCV- and PFA-resistant isolates of CMVa
| Isolate | UL97 polymorphism(s)a | UL54 polymorphism(s)a | Avg EC50 (μM)b ± SD |
||
|---|---|---|---|---|---|
| CPV | GCV | CDV | |||
| AD169 | None | None | 1.3 ± 0.7 | 5.5 ± 3.5 | 0.7 ± 0.5 |
| CPVR1 | H520Q | None | 44 | 55 | 0.19 |
| CPVR2 | H520Q | None | 27 ± 1.4 | 43 ± 25 | 0.17 |
| C8914-6 | I244V, L595F | G347D, L501F, S655L, N685S, L890F, N898D, N1147F | 9.9 ± 3.8 | 232 ± 125 | 3.2 ± 3.5 |
| 37-1-1 | N68D, S108N, I244V, M460V | P682L, S655L, N865S, ins 885T, A884S | 2.7 ± 0.1 | 26 ± 11 | 0.7 ± 0.2 |
| 1-4-4 | T17K, N68D, L126Q, I244V, Q449K, M460V | P682L, S655L, N865S, A844S, ins 885T | 1.5 ± 0.7 | 68 ± 34 | 2.1 |
| 9-1-4 | N68D, L89Q, I244V, L595S, V665I | S655L, N685S, A885T, N898D, N116H, A1122T | 0.3 | 18 | 0.4 |
| 759RD100 | del 580–581 and 590–593, A582E, L583A, R566L | A987G | 4.5 ± 3.4 | 175 ± 127 | 1.8 ± 1.0 |
| GDGRK17 | del 590–593 | None | 2.4 | 7.1 | 1.6 ± 0.3 |
| GDGRP53 | None | A987G | 0.8 ± 0.6 | 47 ± 35 | 3.5 ± 3.5 |
| VR4760R | None | Not sequenced | 1.6 ± 0.1 | >227 ± 153c | 0.6 ± 0.4 |
| VR4955R | None | P628L. S655L, T700A, N685S, ins 885T, A884S, E1089G, K1056R | 1.7 ± 0.03 | 170 ± 153c | 0.5 ± 0.4 |
Deep-sequencing results.
To define other genotypic characteristics of the 2 CPV-resistant cultures prior to plaque purification, whole-genome deep sequencing was performed. The goals were to identify low-frequency mutations in UL97 and UL54 and to identify other genes that may have an impact on CMV resistance to CPV. The parental virus used to derive the resistant isolates (AD169varATCC) was also sequenced for comparison. Multiplexed sequencing techniques allowed for an average depth of coverage of greater than 100 reads for both CPVR1 and CPVR2 and an average depth of 200 reads for parental strain AD169.
Compared to the AD169 reference strain (GenBank accession number X17403), analysis of UL97 confirmed that the H520Q mutation was the major allele present in both CPV-resistant isolates and was consistent with the population sequencing results. This mutation was the result of selective pressure by CPV, since it was not observed in the parental virus. Additional low-frequency UL97 polymorphisms of unknown consequence in CPVR1 included V60G (10%) and V356G (4.7%). However, V60G and V356G polymorphisms were also found at very low levels (2.6% and 2.7%, respectively) in parental strain AD169, so the significance of the increased mutation rates in the CPV-resistant viruses is unclear. In UL54, deep sequencing revealed a G333E polymorphism at a level of 7% in CPVR2 and an A449P polymorphism in both CPVR1 (7.6%) and CPVR2 (5%). Neither of these changes was represented in the parental virus sequence. One low-frequency UL54 polymorphism in the parental virus, D652G (1.3%), was found at an increased, yet still relatively low, level in CPVR1 (7.5%). Due to the baseline presence of this polymorphism in the parental virus, the significance of the modest increase in its rate in CPVR1 is not certain. An analysis of other open reading frames in the genomes identified a single nucleotide deletion in UL27 that resulted in a frameshift following codon 520 and a premature stop codon following amino acid 553. The frequency of this polymorphism was 23% in CPV2, and it was never observed in the parental virus. These data, taken together, were intriguing because the V356G mutation is adjacent to the essential lysine (K355) and is near the active site that is often mutated in isolates resistant to MBV, and mutations in UL27 are also frequently observed in MBV-resistant isolates (5).
CPV inhibits the UL97 kinase.
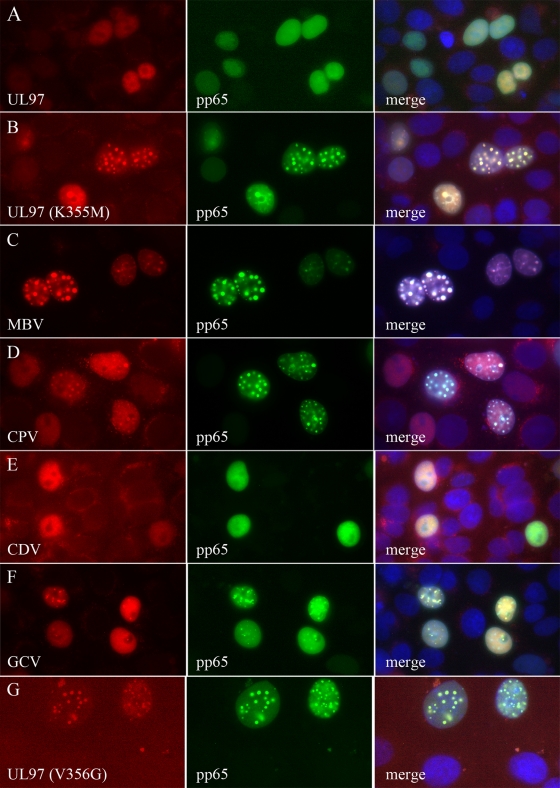
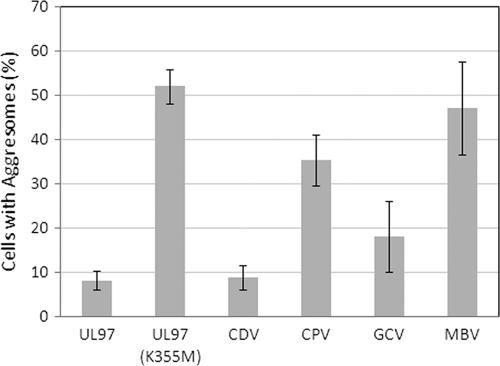
During the selection of CPV-resistant isolates, we noted that infected cells exhibited the distinctive morphology characteristic of infected cells in the absence of UL97 kinase (29). These data, taken together with the observed polymorphisms in UL97 and UL27, suggested that CPV might be inhibiting the activity of the UL97 kinase. To test this hypothesis, we evaluated CPV in a surrogate assay of UL97 kinase activity reported previously (14, 27, 34). In COS7 cells, transient expression of the UL97 kinase inhibited the formation of nuclear aggresomes induced by the pp65-GFP fusion (Fig. 1A). Either the genetic inactivation of UL97 kinase activity with a K355M mutation (Fig. 1B) or inhibition of its enzymatic activity with MBV (Fig. 1C) prevented the kinase from inhibiting the formation of aggresomes. The addition of 15 μM CPV also efficiently induced the formation of aggresomes (Fig. 1D), whereas the CDV negative control did not inhibit the kinase (Fig. 1E). GCV was also included as a second negative control, and it also failed to inhibit kinase activity in this assay (Fig. 1F). Although some condensation of pp65-GFP was observed in the nuclei of cells treated with this drug, it did not form the large round inclusions characteristic of aggresomes. When the proportion of cells containing aggresomes was evaluated in three separate experiments, only MBV and CPV significantly increased their formation (P < 0.001) (Fig. 2). It remains possible that GCV also inhibits the function of the kinase at concentrations higher than 15 μM, but the effects were not statistically significant in these studies. These data confirmed that CPV inhibits the UL97 kinase in this assay and that its activity is similar to that of MBV.
Fig. 1.
Inhibition of UL97 kinase activity by CPV. Transient expression of a pp65-GFP fusion in COS7 cells (green staining in the middle column) induces the formation of nuclear aggresomes, and coexpression of the CMV UL97 kinase (red staining in the first column) can inhibit their formation in a kinase-dependent manner (compare row A with the catalytically inactive UL97 mutant in row B). Pharmacologic inhibition of the UL97 kinase by 15 μM MBV also inhibits the ability of the kinase to prevent aggresome formation (row C) and leads to recruitment of the kinase to aggresomes (merged image in the last column with blue 4′,6-diamidino-2-phenylindole [DAPI] staining). Treatment with 15 μM CPV also inhibits the UL97 kinase and induces its recruitment into aggresomes (row D), whereas neither CDV nor GCV exhibits this activity at a concentration of 15 μM (rows E and F, respectively). UL97 with the V3656G mutation shows an inability to prevent aggresome formation (row G).
Fig. 2.
CPV increases the proportion of cells with aggresomes. COS7 cells coexpressing pp65-GFP and the UL97 kinase were treated with the compounds shown at a concentration of 15 μM. The number of cotransfected cells containing aggresomes was evaluated in three separate experiments, and the average proportion of cells is shown with the standard deviation of the data.
The aggresome inhibition assay was also used to evaluate the effect of the V356G polymorphism that was identified in the deep-sequencing experiments. The coexpression of a V5-tagged version of UL97 with the V356 mutation together with the pp65-GFP fusion failed to inhibit the formation of aggresomes (Fig. 1G). Thus, both the V356G and K355M mutations appear to abrogate UL97 kinase activity, and this is not surprising, given their proximity.
CPV antagonizes the antiviral activity of GCV.
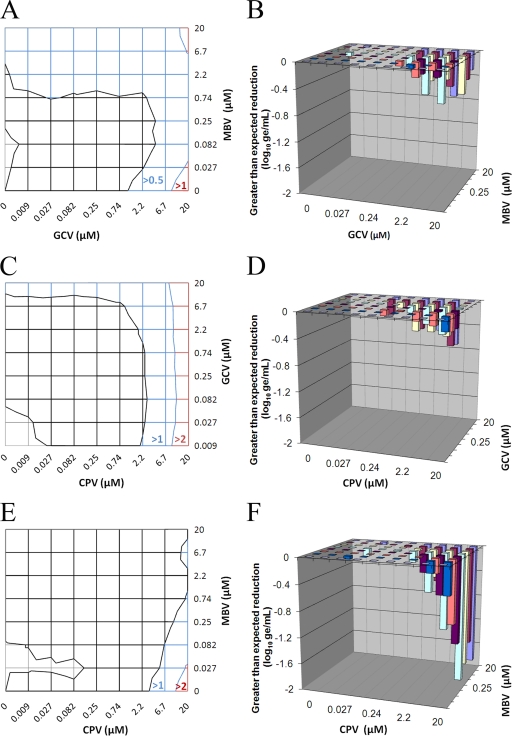
The inhibition of UL97 kinase activity by CPV was interesting and is significant because this activity is required for the initial phosphorylation of GCV. To investigate this further, the combined efficacy of CPV with GCV was evaluated by methods published previously to quantify statistically significant drug interactions (31, 33). These studies used the combination of MBV and GCV as a control because MBV was reported previously to antagonize the antiviral activity of GCV through the inhibition of the UL97 kinase (8). If CPV also inhibits UL97 kinase activity, it would be predicted to antagonize the antiviral activity of GCV. The control combination of MBV and GCV was evaluated, and the data are presented in Fig. 3A and B. The contour plot in Fig. 3A represents the reduction in the number of genome equivalents (GE) in response to GCV and MBV shown at the concentrations where they occur. The intersection of the blue region and the black region is designated by a line of equal effectiveness that shows all of the combinations of concentrations that reduced the genome copy number by the level indicated (0.5 log10 GE). This line is shifted away from the origin and indicates that increased concentrations of the compounds in combination are required to attain the same reduction in the genome copy number obtained with each compound used individually. Statistically significant antagonism (less-than-expected efficacy) was quantified and plotted in perspective with respect to GCV and MBV concentrations (Fig. 3B). This analysis revealed a substantial region of antagonism, as indicated by columns representing less-than-expected genome copy number reductions. The volume of antagonism from two independent experiments was −7.3 μM2log10 GE, indicating that the reduction in the total genome copy number was less than expected by 7.3 log10 GE at 95% confidence (Table 3). These data represent a conservative estimate of antagonism and illustrate that the effect was reproducible. In contrast, another control combination of CPV and PFA was slightly synergistic. The combined efficacy of CPV and GCV was also evaluated by methods described above, and CPV also appeared to antagonize the antiviral activity of GCV. The contour plot of genome copy number reductions obtained with all of the combinations of concentrations indicated that the combination of CPV and GCV exhibited lower-than-expected efficacy, as indicated by the lines of equal efficacy that shifted away from the origin of the plot (Fig. 3C). The synergy plot of the interactions also identified regions of significant antagonism (Fig. 3D), with a volume of −5.3 μM2log10 GE at 95% confidence in two independent experiments (Table 3). Thus, the antagonism of GCV activity by CPV was similar in magnitude to that induced by MBV and is consistent with the observed inhibition of UL97 kinase activity by CPV.
Fig. 3.
Combined efficacy against CMV as shown by real-time PCR. Infected HFF cells were treated with the combinations of compound concentrations shown, and genome copy numbers were determined by real-time PCR. (A) The matrix represents the combined GCV and MBV concentrations indicated on the axes, with the resulting reductions in the number of GE represented by the blue and red regions indicating >0.5 and >1 log10 GE, respectively. (B) A plot of antagonism shows less-than-expected inhibition of viral replication (log10 GE) at the combinations of compound concentrations indicated on the two horizontal axes; these data confirm that inhibition of the UL97 kinase by MBV antagonizes the antiviral activity of GCV. (C) Combinations of CPV and GCV were also evaluated by the same approach as in panel A, with genome copy number reductions of >1 and >2 log10 GE represented by blue and red, respectively. (D) CPV also appeared to antagonize the antiviral activity of GCV when evaluated by the method used for panel B. Since the UL97 kinase is also required for the antiviral activity of CPV, the effect of MBV on its antiviral activity was also evaluated. (E) Reduction of genome copy numbers with CPV and MBV combinations was observed, with blue and red regions representing >1 and >2 log10 GE, respectively. (F) As expected, MBV also antagonized the antiviral activity of CPV with an intensity greater than that observed for the other two combinations. The small areas depicted in gray in panels A, C, and E represent the few instances where the genome copy number is slightly greater than that of the virus control; this is due to the normal variability of the assay.
Table 3.
Combined efficacy of CPV and other inhibitors of CMV replication
| Drug combination | Vol of synergy or antagonism (μM2log10 GE) |
|
|---|---|---|
| Avga | Value at 95% confidenceb | |
| CPV-PFA | 5 ± 0.57 | 0.8 ± 0.15 |
| CPV-GCV | −9.2 ± 0.99 | −5.3 ± 1.9 |
| MBV-GCV | −12 ± 0.57 | −7.3 ± 0.49 |
| MBV-CPV | −27 ± 3.8 | −19 ± 1.9 |
Shown are average volumes from two independent experiments, with values of >0 indicating synergy and values of <0 indicating antagonism.
Values shown at 95% confidence represent conservative estimates of synergy or antagonism obtained by using the lower bound of the 95% confidence interval to calculate the volumes rather than the average of the experimental data.
Since both GCV and CPV require an initial phosphorylation event by the UL97 kinase, MBV should also antagonize the antiviral activity of CPV. An evaluation of this combination revealed that, as predicted, MBV antagonized the antiviral activity of CPV. As with the other two combinations, the lines of equal efficacy shifted away from the origin (Fig. 3E). The synergy plot was much more informative and indicated that the same concentrations of MBV that antagonized GCV also antagonized CPV (Fig. 3F). However, the average volume of antagonism from two experiments was −19 μM2log10 GE at 95% confidence, which is also significantly greater than that obtained with the other two combinations. The increased level of antagonism exhibited by this combination might reflect the reduced phosphorylation of CPV in the presence of MBV, as well as the competition for binding sites on the enzyme.
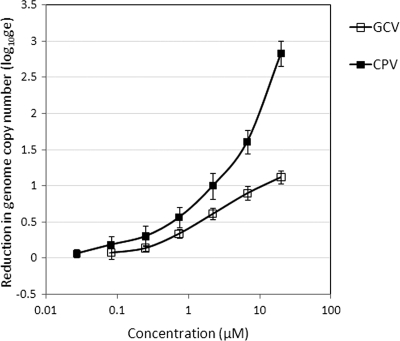
Studies evaluating the combined efficacy of CPV and GCV necessarily included the dose-response curves for both compounds. The data were highly reproducible and were interesting because the genome copy number reductions in response to CPV were greater than those in response to GCV (Fig. 4). The concentrations required to reduce the genome copy number by 10-fold (EC90 values) were 12.7 and 2.2 μM for GCV and CPV, respectively, and represent a 5-fold improvement in efficacy.
Fig. 4.
Inhibition of CMV replication by CPV and GCV. Dose-response curves for both CPV and GCV were obtained in combination studies. The curves shown represent the average data from three separate experiments and the standard deviations.
DISCUSSION
Due to the continued emergence of GCV-resistant CMV infections in immunocompromised patient populations, as well as the toxicity-related limitations of the alternate therapies PFA and CDV, other therapeutic options are required. Data generated in the preclinical development of CPV indicated that its antiviral activity was greater than that of GCV, and CPV had a superior selective or therapeutic index, as determined by plaque reduction assays and Neutral Red cytotoxicity assays in HFF cells (17). The real-time PCR studies presented here confirmed that CPV was more than 5 times as potent as GCV and agree well with previous reports (7, 17). When these values are used to calculate a selective index using toxicity data from the most sensitive cells (HFF) and the most sensitive cytotoxicity assay (cell proliferation), the selective index for CPV is 37, compared to 20 for GCV. These data, taken together, confirm results we reported previously.
Results presented here also confirmed that the resistance profile of CPV was distinct from that of GCV, such that it will retain antiviral activity against most GCV-resistant and PFA-resistant CMV isolates (17). An analysis of the 2 CMV isolates described here revealed the H520Q mutation that was previously reported (7). The effects of the M460I and H520Q mutations first characterized by Chou and Bowlin (7) are reductions in CPV susceptibility of 12- and 20-fold, respectively, and are in good agreement with the results presented here. Neither of the 2 CPV-resistant isolates exhibited any significant UL54 mutations.
We further investigated mutations in the two populations of viruses resistant to CPV using Next Gen sequencing methods to sequence the isolate populations and also to identify low-level polymorphisms that arise in response to the selective pressure of CPV. This analysis confirmed the H520Q mutation identified by population sequencing but did not detect the emergence of any subpopulations of other known mutations that impart resistance to GCV. Interestingly, the selective pressure from CPV appeared to increase the frequency of the V356G polymorphism from 2.7 to 4.7%. This particular polymorphism has also been observed at a frequency of about 5% of the genomic population in clinical specimens from individuals treated with GCV and likely arose in response to its selective pressure, since it was not observed prior to therapy (data not presented). Like the kinase-null K355M mutation, the V356G mutation also failed to inhibit aggresome formation, and we conclude that this mutation compromises the enzymatic activity of the UL97 kinase. Thus, this mutation would also be expected to confer resistance to both GCV and CPV, but this needs to be confirmed in a recombinant phenotyping experiment. The selection for the mutation at codon 356 was interesting because it corresponds to the region of known MBV resistance mutations (codons 353, 397, 409, and 411). Further analysis of the isolates via deep sequencing yielded subpopulations of 3 mutations of unknown significance (G333E, A449P, and D652G) in the DNA polymerase, and G333E and A449P were not represented in the parental virus. None of these mutations occur in regions associated with antiviral resistance. While polymorphisms in the DNA polymerase are expected to confer resistance to CPV, the selection of additional drug-resistant isolates will likely be required to identify those associated with resistance.
The emergence of a UL27 frameshift mutation in 23% of the population of CPVR2 following codon 520 during CPV exposure was unexpected but is consistent with other experiments that suggest that CPV inhibits UL97 kinase activity. Compensatory UL27 mutations have been previously reported to arise in the absence of UL97 kinase function (6). When UL97 kinase activity is inhibited by MBV, the resulting UL27 mutations appear to compensate for the loss of this activity and impart low-level resistance to the drug. The selective pressure of CPV also appeared to select for the inactivation of UL27 and indicated that this may be a characteristic of all inhibitors of the UL97 kinase. A recent report found that the UL27 gene product promoted the proteasome-dependent degradation of Tip60 (35). When UL27 is mutated, the presence of Tip60 leads to reduced levels of p21Waf/CIP1 that are insufficient to reduce cyclin-dependent kinases (CDKs). The CDKs can then substitute for some of the activities of the UL97 kinase, including the hyperphosphorylation of retinoblastoma protein, and can compensate for the inhibition of the UL97 kinase. Thus, the selection of a frameshift mutation in the UL27 open reading frame in cells treated with CPV is a good indication that UL97 kinase activity is compromised and suggests that mutations in this open reading frame might also have some small impact on CPV susceptibility.
Characteristic changes in the morphology of infected cells treated with CPV also suggested that it was inhibiting the UL97 kinase. The formation of large inclusion bodies in the nuclei of infected cells was first noted in the context of a recombinant virus with a large deletion in the UL97 open reading frame (29). This characteristic formed the basis of a surrogate assay for UL97 kinase activity (27, 34) and was used here to evaluate the inhibition of UL97 kinase activity by CPV. Cells treated with CPV resembled those treated with MBV and CPV clearly inhibited the ability of the kinase to inhibit aggresome formation. So, although the activity of the kinase is required for the phosphorylation of CPV, it also appears to inhibit the normal function of the enzyme.
There are a number of potential explanations for this observation. The phosphorylation of CPV appears to be much more efficient than that of GCV, and it may simply compete with natural substrates for binding sites on the kinase, as suggested in a previous report (11). That report also demonstrated that MBV is a competitive inhibitor of CPV phosphorylation, which suggests mutually exclusive binding of these compounds. Since MBV inhibits the kinase through an interaction with the ATP binding site, it is possible that CPV might bind close to this site as well. A previous report also showed that the UL97 kinase can phosphorylate CPV directly and preferentially yields the (+)-CPV-monophosphate (11) that is the preferred substrate of GMP kinase (19). It is also possible that one of the two enantiomers of CPV-monophosphate might actually inhibit the activity of the kinase by dissociating slowly from the active site of the enzyme or by competing with natural substrates. Additional studies are required to define the mechanism, but CPV clearly inhibits the activity of the UL97 kinase and its triphosphate metabolite inhibits the DNA polymerase. Both activities likely contribute to the antiviral activity of the compound, and this complex mechanism of action is consistent with the impressive efficacy of this inhibitor.
Inhibition of UL97 kinase activity by CPV is also significant because a previous report suggested that the inhibition of the UL97 kinase by MBV antagonized the antiviral activity of GCV, presumably by reducing its phosphorylation by this kinase (8). The experimental approach described here allowed us to quantify the antagonism of CPV and GCV and confirmed that its antagonism was both significant and similar in magnitude to that of MBV and GCV (Table 3). While it is possible that GCV and CPV compete for phosphorylation by the UL97 kinase, it would not be expected to result in the robust antagonism observed with this combination. It is difficult to assess the significance of the quantity of antagonism determined with our experimental approach because there are no examples of such data that predicted a reduced efficacy of drug combinations against CMV (or any other herpesvirus) in clinical trials. However, in a previous study, we evaluated the efficacy of combinations of antiviral drugs against influenza virus infection by methods very similar to those reported here and noted that zanamivir antagonized the efficacy of oseltamivir with a volume of −197 μg2% against A/California/10/2009 (25). This value is equivalent to −19.7 ± 6 μg2log10% (26; J. T. Nguyen, personal communication) and can be compared directly to results presented here (−9.2 ± 0.99 and −12 ± 0.57 for the combinations of CPV-GCV and MBV-GCV, respectively). This is significant because a subsequent clinical trial demonstrated that the combination of zanamivir and oseltamivir was demonstrably inferior to oseltamivir alone, as evaluated by the time to resolution of illness (P = 0.015) and the symptom score at the end of treatment (P = 0.0006) (9). Thus, the magnitude of antagonism presented here might have the potential to influence efficacy in the clinic.
Results reported here support the further development of CPV for the therapy of CMV infections. Its activity against CMV is better than that of GCV and yields an improved therapeutic index. We propose that CPV's inhibition of UL97 kinase activity and its inhibition of the DNA polymerase act in concert to inhibit viral replication and that both factors contribute to its potency. Clinical studies are required to assess its tolerability and evaluate its efficacy as a therapy for CMV infections.
ACKNOWLEDGMENTS
These studies were funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contracts N01-AI-30049 and HHSN2722011000010C.
Footnotes
Published ahead of print on 25 July 2011.
REFERENCES
- 1. Andrei G., De Clercq E., Snoeck R. 2009. Drug targets in cytomegalovirus infection. Infect. Disord. Drug Targets 9:201–222 [DOI] [PubMed] [Google Scholar]
- 2. Biron K. K. 2006. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 71:154–163 [DOI] [PubMed] [Google Scholar]
- 3. Biron K. K., et al. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boppana S. B., Fowler K. B., Britt W. J., Stagno S., Pass R. F. 1999. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 104:55–60 [DOI] [PubMed] [Google Scholar]
- 5. Chou S. 2008. Cytomegalovirus UL97 mutations in the era of ganciclovir and maribavir. Rev. Med. Virol. 18:233–246 [DOI] [PubMed] [Google Scholar]
- 6. Chou S. 2009. Diverse cytomegalovirus UL27 mutations adapt to loss of viral UL97 kinase activity under maribavir. Antimicrob. Agents Chemother. 53:81–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chou S., Bowlin T. L. 2011. Cytomegalovirus UL97 mutations affecting cyclopropavir and ganciclovir susceptibility. Antimicrob. Agents Chemother. 55:382–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chou S., Marousek G. I. 2006. Maribavir antagonizes the antiviral action of ganciclovir on human cytomegalovirus. Antimicrob. Agents Chemother. 50:3470–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duval X., et al. 2010. Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med. 7:e1000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gentry B. G., Gentry S. N., Jackson T. L., Zemlicka J., Drach J. C. 2011. Phosphorylation of antiviral and endogenous nucleotides to di- and triphosphates by GMP kinase. Biochem. Pharmacol. 81:43–49 [DOI] [PubMed] [Google Scholar]
- 11. Gentry B. G., Kamil J. P., Coen D. M., Zemlicka J., Drach J. C. 2010. Stereoselective phosphorylation of cyclopropavir by pUL97 and competitive inhibition by maribavir. Antimicrob. Agents Chemother. 54:3093–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gilbert C., Bestman-Smith J., Boivin G. 2002. Resistance of herpesviruses to antiviral drugs: clinical impacts and molecular mechanisms. Drug Resist. Updat. 5:88–114 [DOI] [PubMed] [Google Scholar]
- 13. Gilbert C., Boivin G. 2005. Human cytomegalovirus resistance to antiviral drugs. Antimicrob. Agents Chemother. 49:873–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gill R. B., Frederick S. L., Hartline C. B., Chou S., Prichard M. N. 2009. Conserved retinoblastoma protein-binding motif in human cytomegalovirus UL97 kinase minimally impacts viral replication but affects susceptibility to maribavir. Virol. J. 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Griffiths P. D. 2001. Cytomegalovirus therapy: current constraints and future opportunities. Curr. Opin. Infect. Dis. 14:765–768 [DOI] [PubMed] [Google Scholar]
- 16. Kern E. R., et al. 2004. Oral activity of a methylenecyclopropane analog, cyclopropavir, in animal models for cytomegalovirus infections. Antimicrob. Agents Chemother. 48:4745–4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kern E. R., et al. 2005. In vitro activity and mechanism of action of methylenecyclopropane analogs of nucleosides against herpesvirus replication. Antimicrob. Agents Chemother. 49:1039–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kimberlin D. W., et al. 2003. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J. Pediatr. 143:16–25 [DOI] [PubMed] [Google Scholar]
- 19. Li C., Gentry B. G., Drach J. C., Zemlicka J. 2009. Synthesis and enantioselectivity of cyclopropavir phosphates for cellular GMP kinase. Nucleosides Nucleotides Nucleic Acids 28:795–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li C., Prichard M. N., Korba B. E., Drach J. C., Zemlicka J. 2008. Fluorinated methylenecyclopropane analogues of nucleosides. Synthesis and antiviral activity of (Z)- and (E)-9-{[(2-fluoromethyl-2-hydroxymethyl)-cyclopropylidene]methyl}adenine and -guanine. Bioorg. Med. Chem. 16:2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lurain N. S., Chou S. 2010. Antiviral drug resistance of human cytomegalovirus. Clin. Microbiol. Rev. 23:689–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mhaske S. B., Ksebati B., Prichard M. N., Drach J. C., Zemlicka J. 2009. Phosphonate analogues of cyclopropavir phosphates and their E-isomers. Synthesis and antiviral activity. Bioorg. Med. Chem. 17:3892–3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morton C. C., Nance W. E. 2006. Newborn hearing screening—a silent revolution. N. Engl. J. Med. 354:2151–2164 [DOI] [PubMed] [Google Scholar]
- 24. Nassetta L., Kimberlin D., Whitley R. 2009. Treatment of congenital cytomegalovirus infection: implications for future therapeutic strategies. J. Antimicrob. Chemother. 63:862–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nguyen J. T., et al. 2010. Triple combination of amantadine, ribavirin, and oseltamivir is highly active and synergistic against drug resistant influenza virus strains in vitro. PLoS One 5:e9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nguyen J. T., et al. 2009. Triple combination of oseltamivir, amantadine, and ribavirin displays synergistic activity against multiple influenza virus strains in vitro. Antimicrob. Agents Chemother. 53:4115–4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prichard M. N., Britt W. J., Daily S. L., Hartline C. B., Kern E. R. 2005. Human cytomegalovirus UL97 kinase is required for the normal intranuclear distribution of pp65 and virion morphogenesis. J. Virol. 79:15494–15502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prichard M. N., Daily S. L., Jefferson G. M., Perry A. L., Kern E. R. 2007. A rapid DNA hybridization assay for the evaluation of antiviral compounds against Epstein-Barr virus. J. Virol. Methods 144:86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prichard M. N., et al. 1999. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J. Virol. 73:5663–5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prichard M. N., Keith K. A., Quenelle D. C., Kern E. R. 2006. Activity and mechanism of action of N-methanocarbathymidine against herpesvirus and orthopoxvirus infections. Antimicrob. Agents Chemother. 50:1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prichard M. N., Kern E. R., Hartline C. B., Lanier R., Quenelle D. C. 25 July 2011, posting date CMX001 potentiates the efficacy of acyclovir in herpes simplex virus infections. Antimicrob. Agents Chemother. [Epub ahead of print.] doi:10.1128/AAC.00545-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prichard M. N., et al. 2009. Inhibition of herpesvirus replication by 5-substituted 4′-thiopyrimidine nucleosides. Antimicrob. Agents Chemother. 53:5251–5258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prichard M. N., Shipman C., Jr 1990. A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 14:181–205 [DOI] [PubMed] [Google Scholar]
- 34. Prichard M. N., et al. 2008. Human cytomegalovirus UL97 kinase activity is required for the hyperphosphorylation of retinoblastoma protein and inhibits the formation of nuclear aggresomes. J. Virol. 82:5054–5067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reitsma J. M., et al. 2011. Antiviral inhibition targeting the HCMV kinase pUL97 requires pUL27-dependent degradation of Tip60 acetyltransferase and cell-cycle arrest. Cell Host Microbe 9:103–114 [DOI] [PubMed] [Google Scholar]
- 36. Ross S. A., et al. 2006. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J. Pediatr. 148:332–336 [DOI] [PubMed] [Google Scholar]
- 37. Strippoli G. F., Hodson E. M., Jones C., Craig J. C. 2006. Preemptive treatment for cytomegalovirus viremia to prevent cytomegalovirus disease in solid organ transplant recipients. Transplantation 81:139–145 [DOI] [PubMed] [Google Scholar]
- 38. Torres-Madriz G., Boucher H. W. 2008. Immunocompromised hosts: perspectives in the treatment and prophylaxis of cytomegalovirus disease in solid-organ transplant recipients. Clin. Infect. Dis. 47:702–711 [DOI] [PubMed] [Google Scholar]
- 39. Williams-Aziz S. L., et al. 2005. Comparative activities of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro. Antimicrob. Agents Chemother. 49:3724–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou S., Drach J. C., Prichard M. N., Zemlicka J. 2009. (Z)- and (E)-2-(1,2-dihydroxyethyl)methylenecyclopropane analogues of 2′-deoxyadenosine and 2′-deoxyguanosine. Synthesis of all stereoisomers, absolute configuration, and antiviral activity. J. Med. Chem. 52:3397–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou S., et al. 2004. (Z)- and (E)-[2-Fluoro-2-(hydroxymethyl)cyclopropylidene]methylpurines and -pyrimidines, a new class of methylenecyclopropane analogues of nucleosides: synthesis and antiviral activity. J. Med. Chem. 47:6964–6972 [DOI] [PubMed] [Google Scholar]
- 42. Zhou S., Zemlicka J. 2007. Synthesis of 2,2,3-tris(hydroxymethyl)methylenecyclopropane analogues of nucleosides. Nucleosides Nucleotides Nucleic Acids 26:391–402 [DOI] [PubMed] [Google Scholar]
- 43. Zhou S., Zemlicka J., Kern E. R., Drach J. C. 2007. Fluoroanalogues of anti-cytomegalovirus agent cyclopropavir: synthesis and antiviral activity of (E)- and (Z)-9-{[2,2-bis(hydroxymethyl)-3-fluorocyclopropylidene]methyl}-adenines and guanines. Nucleosides Nucleotides Nucleic Acids 26:231–243 [DOI] [PubMed] [Google Scholar]