Abstract
Infections with the diarrheagenic protozoan pathogen Giardia lamblia are most commonly treated with metronidazole (Mz). Treatment failures with Mz occur in 10 to 20% of cases and Mz resistance develops in the laboratory, yet clinically, Mz-resistant (Mzr) G. lamblia has rarely been isolated from patients. To understand why clinical Mzr isolates are rare, we questioned whether Mz resistance entails fitness costs to the parasite. Our studies employed several newly generated and established isogenic Mzr cell lines with stable, high-level resistance to Mz and significant cross-resistance to tinidazole, nitazoxanide, and furazolidone. Oral infection of suckling mice revealed that three of five Mzr cell lines could not establish infection, while two Mzr cell lines infected pups, albeit with reduced efficiencies. Failure to colonize resulted from a diminished capacity of the parasite to attach to the intestinal mucosa in vivo and to epithelial cells and plastic surfaces in vitro. The attachment defect was related to impaired glucose metabolism, since the noninfectious Mzr lines consumed less glucose, and glucose promoted ATP-independent parasite attachment in the parental lines. Thus, resistance of Giardia to Mz is accompanied by a glucose metabolism-related attachment defect that can interfere with colonization of the host. Because glucose-metabolizing pathways are important for activation of the prodrug Mz, it follows that a fitness trade-off exists between diminished Mz activation and reduced infectivity, which may explain the observed paucity of clinical Mzr isolates of Giardia. However, the data also caution that some forms of Mz resistance do not markedly interfere with in vivo infectivity.
INTRODUCTION
Giardia lamblia (“G. intestinalis” or “G. duodenalis”) is one of the most common protozoan causes of diarrheal disease in humans worldwide, infecting hundreds of millions of people every year. The WHO has included giardiasis in its Neglected Diseases Initiative (31). Giardia is acquired by ingestion of highly infectious cysts from contaminated water or food, i.e., by fecal-oral transmission. Disease symptoms develop in about half of infected persons and are typically transient, but a significant fraction of infected individuals remain symptomatic for extended periods (43). Chronic giardiasis can lead to severe malabsorption and weight loss and may contribute to increased mortality in the context of underlying immune deficiency (1).
Upon ingestion, Giardia excysts in the proximal small intestine, the primary site of colonization in the host. Adhesion of trophozoites to the intestinal epithelium is crucial to both the initial colonization and the maintenance of infection, since parasites that do not attach or cannot move in the flow of intestinal fluid are expelled (17). The ability of the parasite to establish a niche within the intestinal tract and interact with the mucosal immune system is probably crucial to disease pathogenesis, since classical virulence factors, such as enterotoxins, have not been identified (1, 33).
Metronidazole (Mz) and a related 5-nitroimidazole (NI), tinidazole, are the most commonly used drugs in the treatment of giardiasis. Mz, which is active against a variety of anaerobic protozoa and bacteria, enters the cell as a prodrug by passive diffusion and is activated in either the cytoplasm or in specific organelles in different microbes (40). The mechanism of action of Mz in anaerobic microbes requires reduction of the 5-nitro group of the imidazole ring by ferredoxin and perhaps other microbial electron donors, leading to formation of toxic radicals that damage and inactivate critical molecules. Ferredoxin itself is reduced by the membrane-localized enzyme pyruvate:ferredoxin oxidoreductase (PFOR) (37). Giardia lacks mitochondria and, under physiological conditions, its PFOR decarboxylates pyruvate and donates electrons to ferredoxin, which in turn reduces other components in the electron transport chain and leads to ATP generation. Organisms that have mitochondrial oxidative phosphorylation are insensitive to 5-NI drugs.
Despite the general efficacy of 5-NI drugs, treatment failures in giardiasis occur in up to 20% of cases (40). Moreover, chronic cases in immunodeficient and normal individuals can be refractile to drug treatment (27). Mz resistance in Giardia develops in the laboratory (36), and murine challenge studies have shown a correlation between higher Mz doses required for parasite clearance and prior treatment failure in patients (23). The mechanisms of Mz resistance appear to be diverse. In some but not all Mz-resistant (Mzr) Giardia cell lines, decreases in PFOR levels and activity of ferredoxin are involved (2, 8, 24, 37). Downregulation of PFOR most probably affects the glycolytic metabolism in Mzr Giardia, but that has not been determined conclusively. Other mechanisms of resistance most probably exist in Giardia (11). Despite the potential for Mz resistance and treatment failures, isolation of clinical Mzr isolates of G. lamblia is uncommon (28). By comparison, Mzr isolates of Trichomonas vaginalis, another protozoan parasite that is often treated with Mz, are well documented (40).
The goal of this study was to define possible reasons for the apparently rare occurrence of clinical Mz resistance in G. lamblia. Specifically, we tested the hypothesis that Mzr entails significant fitness costs to the parasite. Using newly generated and already established syngeneic pairs of Mzr and Mz-sensitive (Mzs) Giardia cell lines, we demonstrate that Mzr can compromise attachment and infectivity of the parasite. Thus, a relative trade-off exists between Mzr and infectious fitness in Giardia that may impact the likelihood of Mzr development under clinical conditions.
MATERIALS AND METHODS
G. lamblia isolates and culture.
The following G. lamblia isolates were used: WB (ATCC 50803), BRIS/83/HEPU/106 (106), and BRIS/83/HEPU/713 (713) (36) and their respective isogenic Mzr cell lines, 106-2ID10 and 713-M3 (5). The C17-resistant cell line 106-C17A, which acquired Mz resistance as a result of being exposed to the 5-NI compound C17, was derived from the 106 isolate (11). Two new Mzr cell lines, WB-M1 and WB-M2, were generated from WB by UV-induced mutagenesis and selection on Mz. Briefly, 106 trophozoites in 10 ml of phosphate-buffered saline (PBS) were added to a 10-cm cell culture dish and exposed to 1,200 J of UV light in a Stratalinker UV cross-linker. Trophozoites were chilled on ice, collected, centrifuged, resuspended in TYI-S-33 medium, and cultured for 24 to 48 h without antibiotics to allow recovery. Subsequently, trophozoites were kept in the continuous presence of 50 μM Mz. After an initial 4- to 8-week period of poor growth, Mzr cells began to emerge in the cultures and were developed into stable cell lines. Furthermore, we kept WB-M1 for ∼4 months in growth medium without Mz to produce a revertant cell line, WB-M1NR, which was no longer resistant to Mz. All cell lines were grown in TYI-S-33 medium supplemented with 10% bovine calf serum and 1 mg/ml bovine bile (Giardia growth medium) under anaerobic conditions (AnaeroPack system; Remel). The Mzr cell lines were routinely maintained in 50 μM Mz but were grown without Mz for 2 to 3 days before experiments.
Drug susceptibility assays.
Drug stocks (10 mM in dimethyl sulfoxide) were diluted in PBS to 75 μM, and 1:3 serial dilutions were made in 40 μl Giardia growth medium in 96-well microtiter plates. A 10-μl volume of 2 × 105 Mzs trophozoites/ml or 6 ×105 Mzr trophozoites/ml was added to the plates to yield 2,000 or 6,000 cells, respectively, in 50 μl (total volume) per well. Final drug concentrations ranged from 25 μM to 0.1 nM. Diluted solvent alone was used as a control. Cultures were incubated for 2 days at 37°C under anaerobic conditions, and cell growth and viability were determined with a luminescence-based ATP assay (41). BacTiter-Glo microbial cell viability assay reagent (Promega) was added to the cultures (50 μl/well), and luminescence was assayed in a microplate reader. The 50% effective concentration (EC50), the drug concentration that inhibited G. lamblia growth by 50% compared to parallel cultures without added drugs, was determined by extrapolation of the concentration-response curves.
Animal models of G. lamblia infection.
Adult C57BL/6 mice were obtained from The Jackson Laboratory and bred at UCSD. For infection of suckling mice (5 to 7 days old), G. lamblia trophozoites were grown to mid-logarithmic phase and administered by oral gavage at 107/mouse in a 50-μl volume in Giardia growth medium. At different times after inoculation, the entire small intestine was removed, opened longitudinally, placed into 2 to 5 ml PBS, and cooled on ice for 10 min. After vigorous shaking to detach trophozoites, live trophozoites were counted in a hemocytometer. In studies to determine the localization of trophozoites along the orad-caudad axis of the intestinal tract, the small intestine was divided into three equal-sized segments, and the cecum and colon were collected. Trophozoites were enumerated separately in each segment. Control mice were given 50 μl of a suspension of 105 fluorescent polystyrene beads (10-μm diameter; Fluoresbrite YG carboxylate microspheres; Polysciences, Inc.), and the number of beads in the small intestine was determined by fluorescence microscopy. Adult Mongolian gerbils (Meriones unguiculatus) were obtained from Charles River Laboratories. Gerbils were fasted for 3 h and inoculated with 107 trophozoites in 200 μl of Giardia growth medium, and trophozoite numbers were determined as described for mice. All animal studies were reviewed and approved by the UCSD Institutional Animal Care and Use Committee.
G. lamblia axenization.
Five days after oral infection of suckling mice with 107 G. lamblia trophozoites, the small intestine was removed, cut into 1-mm pieces, placed into 1 ml cold PBS, and shaken vigorously to detach trophozoites. Intestinal contents were passed through a cell strainer (40-μm pore size), and 0.5 ml of the filtrate was injected intraperitoneally into adult C57BL/6 mice. After 24 h, mice were euthanized and intraperitoneal content was removed by injecting 0.5 ml of cold PBS into the peritoneal cavity with a syringe, followed by withdrawal of the fluid into the same syringe. The withdrawn fluid was mixed with Giardia growth medium supplemented with ciprofloxacin and cefixime and incubated at 37°C. After 4 to 6 h of incubation, the growth medium containing nonattached cells was removed and replaced with fresh growth medium supplemented with antibiotics. Attached G. lamblia trophozoites were allowed to grow for 3 to 7 days without exposure to Mz, after which cells were tested for Mz susceptibility.
In vitro attachment assays.
For assessment of adherence to a plastic surface, trophozoites in culture tubes were chilled on ice until detached, counted, added to 15-ml plastic tubes at ∼1 × 107 trophozoites/tube in fresh prewarmed Giardia growth medium, and incubated at 37°C under anaerobic conditions. At different times, culture supernatants were removed and fresh growth medium was added to the remaining attached cells. The tubes containing the attached cells were then placed on ice for 10 min and trophozoites were counted. The percentage of attached cells was determined relative to the total number of trophozoites initially added to the tubes.
For assessment of attachment to epithelial cells, Caco-2 human intestinal epithelial cells (ATCC HTB-37) were used. Cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, penicillin, and streptomycin. For experiments, Caco-2 cells were seeded into 12-well plates and grown over several days to 100% confluence. Giardia trophozoites were washed in PBS and resuspended in DMEM with 10% fetal bovine serum. Medium was aspirated from the cultures, and monolayers were gently washed with prewarmed DMEM to remove debris. Trophozoites were then added to the Caco-2 monolayers at 106/well in a final volume of 1 ml/well, and cultures were incubated for up to 2 h at 37°C in 95% air–5% CO2. Nonattached trophozoites were removed by gently rinsing the monolayers three times with prewarmed DMEM. The remaining attached trophozoites were detached by placing plates on ice for 10 min and then counted. The percentage of trophozoites attached to Caco-2 cells was calculated relative to the total number of trophozoites added to the cultures.
Glucose consumption assays.
Harvested trophozoites were washed twice with cold PBS and resuspended at 3 × 107 trophozoites/ml in glucose-free RPMI medium with 1% fetal calf serum, with or without 5 mM added glucose. Cells were plated into 24-well tissue culture plates (1 ml/well) and immediately placed into an anaerobic chamber. Anaerobic conditions were generated within 5 min by repeatedly pumping the air from the chamber and replacing it with 100% nitrogen gas. The chamber was placed in an incubator at 37°C for 5 h, and glucose levels were measured with a quantitative strip test (Accu-Check Advantage; Roche).
Morphological studies.
To determine trophozoite morphology, WB and WB-M1 trophozoites were plated on glass-bottom plates (no. 1.5 coverglass; MatTek) and imaged live under a phase-contrast microscope at 60× magnification (Perkin-Elmer UltraView Vox spinning disc confocal microscope). Immunofluorescence staining for α-tubulin was performed as described previously on methanol-fixed and demembranized cells (9, 21).
RNA extraction and real-time PCR.
Mzr lines were grown without Mz for 2 to 3 days before harvesting. Cells were plated at 1 × 105 to 3 × 105/ml in tissue culture plates and grown under anaerobic conditions at 37°C overnight. Trophozoites were detached by chilling on ice and pelleted by centrifugation. Total RNA was extracted using an RNeasy kit (Qiagen). Contaminating genomic DNA was removed by DNase I digestion. The reverse transcription reaction was performed with Moloney murine leukemia virus reverse transcriptase and hexadeoxynucleotide random primers (Promega). Real-time PCR was performed using Power Sybr green (Applied Biosystems) in a StepOnePlus PCR system (Applied Biosystems). Data were analyzed using the comparative cycle threshold method. The following primers (based on the genome sequence GL50803) were used to amplify the different genes, using tubulin for standardization: TrxR (ORF 9827), TAA GTG CGT TAC CTC GGT CTC CAT (sense) and AAA TAC GCC ATC CAC GGA AGT CCT C (antisense); ferredoxin nitroreductase 2 (ORF 22677), TGC TGT GGC TTC TTC TCA GGA ACT (sense) and ATG TCG ACA CCC TCC TCA AGG TTT (antisense); PFOR1 (ORF 114609), TTC CTC GAA GAT CAA GTT CCG CGT (sense) and TGC CCT GGG TGA ACT GAA GAG AAT (antisense); ferredoxin (35), CCA TCA AGC TCA CTG CCT CCA AGT (sense) and GGG AAG CTC GAA GAC AGA GAC GG (antisense); α-tubulin (ORF 103676), CAA GTA CAT GGC GTG CTG CAT GAT (sense) and TAG TTG ATG CCG ACC TTG AAG CCT (antisense).
Statistical analysis.
Trophozoite counts were log10 transformed, and means and standard errors of the means (SEM) were calculated from the log values. Samples without detectable trophozoites were assigned a log value equivalent to half of the detection limit of the assay (103 trophozoites/intestine). Differences between groups were compared by paired or unpaired t test or Mann-Whitney test, as appropriate, with P values of <0.05 considered significant.
RESULTS
Development of new Mzr cell lines of G. lamblia.
To investigate the functional consequences of Mz resistance in G. lamblia yet avoid idiosyncratic conclusions, it was important to employ several independently derived cell lines with stable and significant resistance. Although clinical isolates can show increased Mz resistance upon infection in animal models (23), none of these has been axenized for in vitro studies. Furthermore, even if such axenic lines existed, isogenic Mzs controls would not be available, so genetic differences between isolates that are not related to Mz resistance could confound the studies. Therefore, we utilized isogenic pairs of Mzs parental isolates and their laboratory-produced Mzr derivative cell lines. Two parental isolates, 713 and 106, had previously been used to develop the isogenic Mzr lines 713-M3, 106-2ID10, and 106-C17A (11), respectively. In addition, we developed two new Mzr lines from a cloned line of the G. lamblia genome strain, WB. These new lines, WB-M1 and WB-M2, were established by brief exposure of trophozoites to sublethal doses of UV light (to promote mutagenesis) and subsequent prolonged selection in the presence of Mz. The level of Mz resistance achieved in the new lines was marked, since the effective Mz concentrations that killed half of the trophozoites (EC50) was >10-fold higher than in the parental isolate (Table 1), making them close to peak serum Mz concentrations (50 to 70 μM) achieved by oral administration of recommended doses (39). Concentrations of the drug in the intestinal lumen have not been determined, but they are likely to be significantly lower than in serum (39), which suggests that the newly developed Mzr lines would exhibit clinically relevant Mz resistance in vivo. Moreover, Mz resistance was apparently stable in the new cell lines, since retesting for Mz susceptibility after several days in culture without Mz selection pressure yielded similar EC50s, and Mz resistance survived several cycles of freezing and thawing of the lines. Nonetheless, continued growth of one of the Mzr cell lines, WB-M1, in Mz-free medium over several months led to reversion of the Mz resistance phenotype, since these cells (termed WB-M1NR, for “M1-no longer resistant”) had an EC50 similar to the parental Mzs WB cells (Table 1). Because the revertant line had not been subjected to additional mutagenesis, we reasoned that it would be a good control for phenotypic consequences specifically related to Mz resistance. We note, however, that we have no further information on the nature of the reversion events. Genetic or epigenetic mechanisms, or both, may be involved, but the current lack of a true understanding of Mz resistance in Giardia makes it difficult to distinguish between these possibilities.
Table 1.
Antimicrobial sensitivities of G. lamblia lines used in the study
| G. lamblia line | EC50 (ratio)a |
|||||
|---|---|---|---|---|---|---|
| Metronidazole | Tinidazole | Ornidazole | Nitazoxanide | Furazolidone | Quinacrine | |
| WB | 2.9 ± 0.2 (1.0) | 0.5 ± 0.3 (1.0) | 0.8 ± 0.3 (1.0) | 2.8 ± 0.9 (1.0) | 1.1 ± 0.4 (1.0) | 0.17 ± 0.02 (1.0) |
| WB-M1 | 34.7 ± 4.6 (11.9) | 3.5 ± 0.9 (6.5) | 6.0 ± 1.2 (7.3) | 22.0 ± 3.6 (7.9) | 2.3 ± 0.9 (2.2) | 0.16 ± 0.08 (0.9) |
| WB-M1NR | 3.4 ± 0.5 (1.2) | 0.6 ± 0.1 (1.1) | 1.0 ± 0.1 (1.2) | 1.6 ± 0.4 (0.6) | 0.5 ± 0.1 (0.5) | 0.14 ± 0.01 (0.8) |
| WB-M2 | 80.4 ± 7.5 (27.5) | 19.6 ± 3.8 (36.3) | 29.4 ± 7.3 (35.9) | 37.1 ± 9.1 (13.5) | 3.3 ± 0.5 (3.1) | 0.16 ± 0.02 (0.9) |
| 106 | 3.6 ± 0.6 (1.0) | 1.1 ± 0.3 (1.0) | 1.3 ± 0.2 (1.0) | 5.1 ± 1.5 (1.0) | 0.7 ± 0.1 (1.0) | 0.28 ± 0.07 (1.0) |
| 106-2ID10 | 78.4 ± 7.7 (21.6) | 24.1 ± 3.4 (22.0) | 40.6 ± 6.6 (30.3) | 28.2 ± 6.7 (5.5) | 3.5 ± 0.9 (4.9) | 0.17 ± 0.00 (0.6) |
| 106-C17A | 61.0 ± 8.7 (16.8) | 20.5 ± 3.2 (18.7) | 24.5 ± 3.8 (18.3) | 16.0 ± 3.4 (3.6) | 6.0 ± 1.9 (8.6) | 0.17 ± 0.03 (0.6) |
| 713 | 2.0 ± 0.3 (1.0) | 1.6 ± 0.3 (1.0) | 1.8 ± 0.2 (1.0) | 2.8 ± 0.4 (1.0) | 0.4 ± 0.1 (1.0) | 0.18 ± 0.01 (1.0) |
| 713-M3 | 50.2 ± 3.6 (24.9) | 7.1 ± 0.7 (4.6) | 10.1 ± 1.7 (5.5) | 21.8 ± 1.6 (7.9) | 3.0 ± 0.7 (8.0) | 0.18 ± 0.03 (1.0) |
Activity of the indicated antimicrobials against G. lamblia was determined as the EC50, the compound concentration (in μM) that inhibited growth of the parasite by 50% over the assay period. Data are means ± SEM of the results of 3 to 4 separate experiments. Ratios are relative to the mean EC50 of the respective parental isolate for each antimicrobial drug.
To characterize the antimicrobial susceptibility of the Mzr Giardia cell lines more broadly, we tested their sensitivities to other clinically utilized 5-NI drugs, tinidazole and ornidazole, as well as to two other nitro-bearing antimicrobials, nitazoxanide and furazolidone. As a control, we tested a structurally unrelated antigiardial drug, quinacrine, with a different mechanism of action. The Mzr cell lines exhibited an overall similar degree of resistance to tinidazole and ornidazole compared to Mz, although the 713-M3 line showed only a 4- to 6-fold increase in resistance to these two 5-NI drugs compared to 25-fold for Mz (Table 1). The Mzr cell lines were also cross-resistant to nitazoxanide and furazolidone, albeit to a lesser extent than to the 5-NI drugs. In contrast, the revertant cell line, WB-M1NR, was not resistant to any of the tested nitro drugs (Table 1), which demonstrates that the loss of 5-NI drug resistance in this cell line was apparently phenotypically complete. Furthermore, no resistance against quinacrine was observed in any of the Mzr cell lines, indicating that antimicrobial resistance in these cell lines was not universal but limited to nitro-bearing antimicrobial drugs.
Differential expression in Mzr lines of genes associated with 5-NI drug reduction.
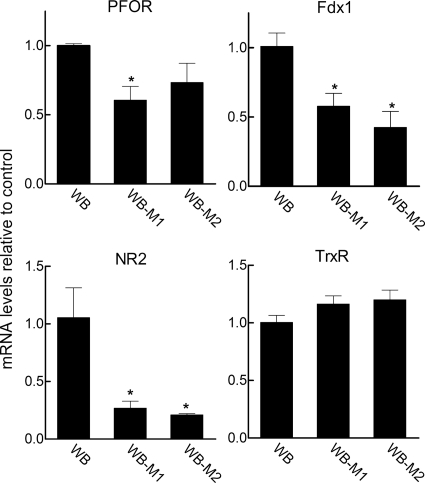
To further characterize the new Mzr lines, we assessed the expression levels of genes associated with Mz activation and resistance and compared them with those reported for the previously published Mzr lines. PFOR and ferredoxin have a key role in nitro drug activation and are decreased in certain Mzr lines, including 106-2ID10, but not others (22, 25). Consistent with this variability, PFOR expression levels differed between the two new Mzr lines, since WB-M1 had a significant, albeit modest, decrease, while WB-M2 had no significant change (Fig. 1). In contrast, ferredoxin (Fdx1) was significantly decreased in both Mzr lines. Furthermore, an alternative nitro drug activation enzyme, nitroreductase-2 (25, 26), was also markedly downregulated in both lines, whereas another enzyme recently found to reduce and activate nitro drugs, thioredoxin reductase (22), was not changed in either of the two lines (Fig. 1). These data on the new Mzr lines, along with results from other Mzr lines (22, 26), indicate that expression patterns of nitro drug activation-related genes can vary between different lines and isolates, confirming that the mechanisms of Mz resistance are likely to be diverse and complex.
Fig. 1.
Differential expression in new Mzr lines of genes associated with reduction of 5-NI drugs. The indicated cells were examined by real-time PCR for mRNA levels of PFOR, ferredoxin 1 (Fdx1), nitroreductase 2 (NR2), and thioredoxin reductase (TrxR). Expression levels are shown relative to those in the parental WB cells (means + SEM; n = 3 to 4 per group). *, P < 0.05 (t test) relative to WB.
In vivo infectivity of Mzr G. lamblia cell lines.
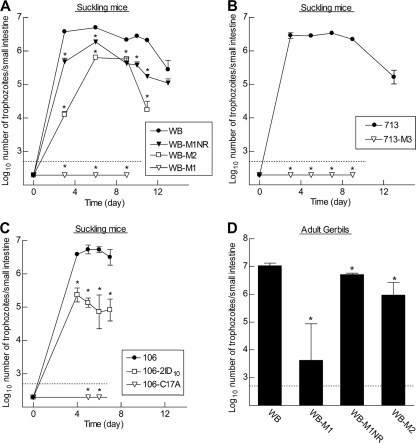
The three parental G. lamblia cell lines used in this study belong to genetic assemblage A of Giardia. To test their infectivity in an animal model, we employed the suckling mouse model of giardiasis, as this model, in contrast to adult mice, is permissive to a wide range of assemblage A (and B) isolates (4). Suckling mice (3 to 7 days old) were infected by oral gavage with 107 trophozoites, and parasite numbers in the small intestine were determined at various times postinoculation. All three parental isolates colonized suckling mice effectively, with maximal trophozoite loads of 2 × 106 to 6 × 106 per mouse at 4 to 9 days after inoculation (Fig. 2A to C). In contrast, only two of the five Mzr cell lines infected suckling mice (Fig. 2A and C). The two infectious Mzr cell lines were derived from different parental lines (WB and 106), suggesting that the particular genetic background did not determine infectivity. Interestingly, the two infectious Mzr cell lines showed significantly lower peak parasite loads than their Mzs parental cells and an apparently shorter duration of infection. For example, the Mzr line WB-M2 had an 8-fold-lower peak trophozoite count than the parental cell line, WB, on day 6, and the infection was nearly cleared by day 11, whereas ∼2 ×106 trophozoites of WB were still present in the small intestine at that time (Fig. 2A). To further explore whether the lack of infectivity in some G. lamblia cell lines was related to changes associated directly with Mz resistance or to incidental phenotypic changes, we employed the revertant cell line WB-M1NR. Its immediate parental cell line, WB-M1, could not infect suckling mice, yet the revertant regained the ability to infect (Fig. 2A). Although the peak infectious load was 5-fold lower than the original parental WB, the revertant produced a more robust infection than any of the Mzr cell lines. These data strongly suggest that an inverse relationship exists between Mz resistance and infectivity in vivo.
Fig. 2.
Infectivity of Mzr G. lamblia cell lines in different animal models. Suckling mice (A to C) and adult gerbils (D) were infected orally with 107 trophozoites of the indicated G. lamblia isolates and lines. Trophozoite numbers were determined in the small intestine at different times (A to C) or after 10 days (D) and are expressed as means ± SEM of the log10 values of 3 to 6 mice or 4 to 6 gerbils for each data point. The dotted horizontal line depicts the detection limit of the assay. *, P < 0.05 (t test [A to C] or Mann-Whitney test [D]) relative to the respective parental cell line at the same time point.
To confirm the key findings from suckling mice, we extended our studies to a gerbil model of giardiasis, as these animals can be reliably infected as adults with a range of different G. lamblia isolates. Consistent with this, gerbils were effectively colonized by the parental line, WB, at the time of peak infection (10 days). By comparison, one of the tested Mzr lines, WB-M2, infected gerbils at 2- to 6-fold-lower levels than WB, while the other Mzr line, WB-M1, barely infected gerbils at all, with >50-fold-lower trophozoite numbers than in the parental cells (Fig. 2D). The revertant line, WB-M1NR, had regained infectivity over WB-M1 but remained less infectious than WB. These results show that the infectious phenotypes in gerbils were similar (albeit less severe) to those in suckling mice, further supporting the notion that Mz resistance compromises in vivo infectivity.
Stability of Mz resistance in Giardia in vivo.
Given that the Mzr cell line G. lamblia WB-M1 was not infectious in suckling mice and barely infected adult gerbils, whereas its revertant, WB-M1NR, had regained infectivity, the question arose whether the more infectious Mzr cell lines might have become infective because they had lost their Mz resistance phenotype in vivo. To address this question, we infected suckling mice with the parental Mzs isolate 106 and its Mzr derivative, 106-2ID10, and we recovered trophozoites after 5 days from the small intestine for subsequent antimicrobial sensitivity testing. The parasites needed to be axenic for these tests, but we encountered problems in axenizing them using typical protocols with various antibiotic combinations (15). Instead, we developed a new method of axenization by passaging crudely filtered luminal washes containing trophozoites through the peritoneal cavity of adult mice. After 24 h, the peritoneal cavity was washed out under sterile conditions and the surviving parasites were expanded in vitro in regular Giardia growth medium supplemented with standard antibiotics. This procedure reproducibly yielded axenic parasites for all the tested lines. Antimicrobial sensitivity assays of the parental Mzs cell line, 106, and the Mzr line, 106-2ID10, revealed no differences in the EC50 for Mz before and after 5 days of infection (the EC50 before and after infection was, respectively, 3.2 μM and 3.9 μM for the 106 line and 126 μM and 100 μM for the 106-2ID10 line). Thus, Mz resistance was stable during infection, suggesting that the observed infectivity of those Mzr lines that could infect animals was not due to reversion of their Mzr phenotype.
Diminished retention of Mzr G. lamblia in the small intestine.
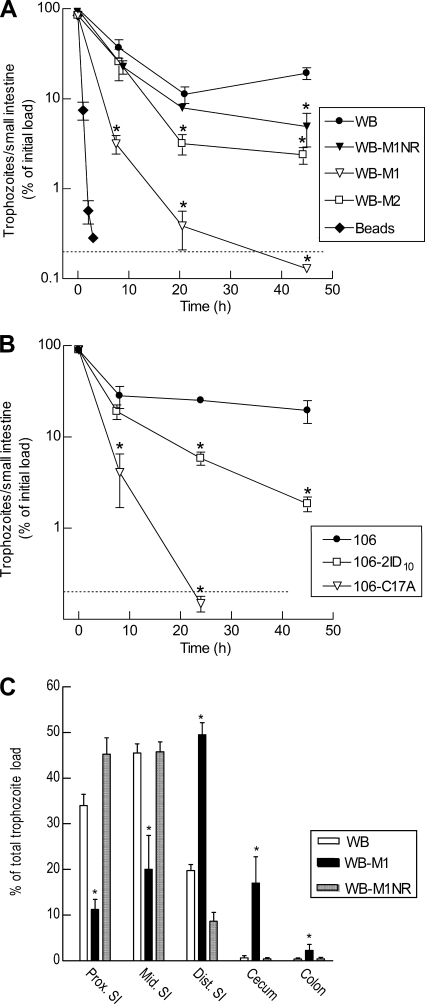
To begin to explore the reasons for the loss of infectivity in the Mzr cell lines, we examined the kinetics of parasite colonization early after infection, as adequate initial adherence of inoculated trophozoites to the intestinal wall is a prerequisite for successful infection of the host. Polystyrene beads (10-μm diameter), used as a control for passive transport along the intestine, had almost completely (>99%) passed out of the small intestine of suckling mice within 2 h after oral administration (Fig. 3A). In contrast, 30 to 50% of the inoculum of the parental Mzs cells, WB, remained in the small intestine after 8 h (Fig. 3A), suggesting that a substantial proportion of the cells, in contrast to latex beads, had actively adhered to the intestinal wall. Trophozoite numbers reached a nadir by 21 h with ∼10% of the inoculum but began to increase again after 45 h, presumably because proliferation was outpacing any losses due to the luminal bulk flow at that time. In contrast, the noninfectious Mzr line, WB-M1, had 10-fold-lower trophozoite numbers than the parental Mzs cells at 8 h and barely detectable numbers after 21 h and beyond. By comparison, the revertant line of WB-M1, WB-M1NR, was not significantly different from the parental Mzs cells over the first 21 h, although it had lower trophozoite numbers after 45 h (Fig. 3A). The other Mzr derivative of WB, WB-M2, which was infectious in mice and gerbils, displayed an intermediate phenotype, with 15 to 30% of the inoculum at 8 h and 2 to 5% at 21 h retained in the small intestine (Fig. 3A). Similar results were obtained for the 106-derived Mzr lines, as 20 to 30% of the inoculum of the parental cell line, 106, was present in the intestine after 24 h, whereas only 4 to 7% of the Mzr cell line, 106-2ID10, and only 0.1 to 0.2% of the other Mzr line, 106-C17A, were observed at that time (Fig. 3B). These data indicate that the noninfectious Mzr lines had a diminished ability, compared to infectious Mzr lines and the parental Mzs lines, to be retained in the small intestine early after inoculation (8 to 24 h), a time when any possible differences in parasite proliferation could not account for the observed differences in trophozoite numbers.
Fig. 3.
Retention and distribution of Mzr and Mzs G. lamblia in the small intestine. (A and B) Suckling mice were inoculated with 107 trophozoites of the indicated G. lamblia isolates and lines, or with polystyrene beads, and trophozoites and beads were enumerated in the small intestine at the designated times. Data are means ± SEM of the percentages of the initial inoculum (3 to 6 animals per data point). *, P < 0.05 relative to the respective parental line. (C) Distribution of Giardia along the orad-caudad axis was determined for three small intestine (SI) segments (proximal, mid, and distal), the cecum, and colon of suckling mice 8 h after inoculation with 107 trophozoites. Counts are expressed as means ± SEM of the percentage relative to the total counts in the entire gastrointestinal tract in each animal (n = 3 to 4 per group). *, P < 0.05 (t test) relative to the parental cell line for each segment.
The early decrease in parasite numbers after infection could result from attenuated attachment to the mucosa. To address this issue, we determined the localization of trophozoites along the orad-caudad axis of the small intestine shortly after infection, reasoning that reduced attachment would lead to a caudad shift of the infection. After 8 h of infection, we divided the small intestine into three equal-sized segments and also collected cecum and colon, enumerated viable trophozoites separately in all five gut segments, and expressed the numbers as percentages of the total parasite load in the entire intestinal tract (Fig. 3C). For the parental Mzs cells, WB, and the revertant cell line, WB-M1NR, >75% of the trophozoites were observed in the first two-thirds of the small intestine and <5% were found in the cecum and colon. In contrast, <30% of trophozoites of the noninfectious Mzr cell line, WB-M1, were present in the first two intestinal segments, while ∼50% were localized in the third segment and ∼20% of viable trophozoites were found in the cecum and colon (Fig. 3C). Taken together, these results indicate that the noninfectious Mzr cell line exhibited a diminished capacity to attach to the intestinal mucosa after inoculation.
Impaired attachment of Mzr G. lamblia in vitro.
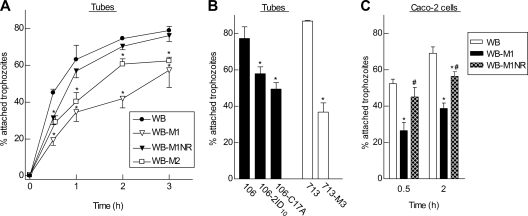
To investigate parasite attachment under better-controlled conditions, we tested the ability of the different Mzs and Mzr cells to attach to a plastic surface in standard Giardia growth medium. Suspensions of trophozoites were added to plastic tubes, and the percentage of attached cells compared to total cells was determined after different times. Attachment increased rapidly over the first 1 h and plateaued at 60 to 80% by 2 to 3 h for the parental Mzs cells, WB (Fig. 4A). The kinetics and extent of attachment of the noninfectious Mzr cell line, WB-M1, differed, showing a marked delay over the first 2 h and lower overall attachment by 3 h. Attachment of the revertant cell line WB-M1NR was only slightly, and not significantly, lower than that observed in the parental line over the 3-h period. The infectious Mzr cell line WB-M2 had an intermediate attachment phenotype, with significantly less attachment than the parental cells but more than the noninfectious cells, WB-M1 (Fig. 4A). Similar observations were made for the Mzr cell lines derived from 106 and 713, as the noninfectious Mzr cell lines showed consistently less attachment than the corresponding parental Mzs cells (Fig. 4B).
Fig. 4.
In vitro attachment of different G. lamblia lines. Different G. lamblia isolates and lines were added to fresh culture tubes and incubated for the indicated times (A) or for 2 h (B) or were coincubated for 0.5 and 2 h with confluent monolayers of Caco-2 cells (C). Numbers of attached and nonattached trophozoites were determined separately and were used to calculate total numbers in the cultures. Data are means ± SEM of the percentage of attached relative to total trophozoites in each group from 3 to 5 separate experiments. *, P < 0.05 (t test) relative to each parental line; #, P < 0.05 (t test) relative to WB-M1 in panel C.
In an additional attachment assay that mimicked the in vivo situation more closely, we added trophozoites to monolayers of Caco-2 human intestinal epithelial cells. Here again, the parental Mzs cell line, WB, exhibited 65 to 75% attachment after 2 h, while the noninfectious Mzr derivative, WB-M1, was significantly attachment impaired, whereas the infectious revertant line, WB-M1NR, had a much less pronounced attachment defect (Fig. 4C). Based on these studies, it is clear that the noninfectious Mzr cell lines exhibited significant defects of attachment to both inert and biological surfaces.
Role of glucose metabolism in attachment defect of Mzr G. lamblia.
Giardia trophozoites attach to the host epithelium and other surfaces by the ventral disc (10, 19), a process that is affected by several factors, probably including negative pressure produced by the beating of the ventral flagella (18). To determine whether structural features may be involved in diminished attachment of Mzr G. lamblia, we examined by immunofluorescence staining the abundance and distribution of a key cytoskeletal protein, α-tubulin, in the Mzr lines. No morphological differences between the isogenic Mzs and Mzr cell lines were apparent in the cytoskeleton or the four readily visible pairs of flagella (data not shown). Furthermore, high-speed time-lapse imaging revealed no obvious differences in the beating frequency or amplitude of the flagella between Mzr and Mzs cells.
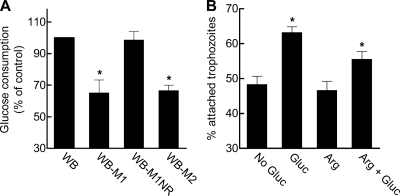
As major structural differences were not observed, we hypothesized that functional processes may account for the observed adhesion defect. Mz resistance in Giardia is probably mediated by several mechanisms, one of which is downregulation of the metabolic enzyme PFOR (8, 40), which is critical for ATP generation from glucose and plays a key role in the reductive activation of Mz. We therefore investigated glucose consumption in Mzs and Mzr parasites. The noninfectious Mzr line WB-M1 consumed glucose at a significantly reduced rate compared to the parental Mzs cells, WB, or the revertant, WB-M1NR (Fig. 5A). Similarly, the noninfectious Mzr line 713-M3 showed a 23% decrease in glucose utilization relative to its parental Mzs line, 713.
Fig. 5.
Role of glucose in attachment of G. lamblia. (A) Trophozoites in medium containing 5 mM glucose were plated into 24-well tissue culture plates and incubated under anaerobic conditions, and levels of glucose remaining in the medium were measured after a 5-h incubation period. Glucose consumption is expressed as the mean + SEM percent relative to consumption of the parental cell line WB (n = 3 to 4 experiments). The average glucose consumption of WB cells was 21 nmol/106 cells/h. *, P < 0.05 (paired t test) relative to the parental cell line. (B) Attachment of G. lamblia WB to plastic tubes was assessed 1.5 h after incubation in RPMI medium containing cysteine, bile, fetal calf serum, and 5 mM glucose (Gluc) and/or 5 mM arginine (Arg). Data are means + SEM percentages of attached cells relative to total cells in the culture (n = 3 experiments). *, P < 0.05 (t test) relative to the percentage of attached cells in medium without glucose or added arginine (No Gluc).
The apparent correlation between infectivity and glucose consumption prompted us to explore the role of glucose in parasite attachment. WB trophozoites were tested in an attachment assay with and without glucose in the medium. The ability of trophozoites to attach to plastic was significantly increased by addition of physiological concentrations (5 mM) of glucose (Fig. 5B). Because glucose metabolism leads to ATP generation, we questioned whether the lack of glucose may have led to the loss of cellular ATP and thus the ability for normal attachment, a presumably energy-consuming process (e.g., beating of the ventral flagella). However, this was not the case, since ATP levels were similar in glucose-starved and glucose-fed cells (13 ± 2 versus 12 ± 3 nmol ATP per 105 trophozoites in the absence or presence of 5 mM glucose, respectively, after 3 h in culture). Furthermore, addition of arginine, which serves as a major energy source for Giardia (12), did not increase attachment in the absence or presence of glucose (Fig. 5B), further underlining that glucose metabolism has a unique role in parasite attachment that extends beyond energy generation.
DISCUSSION
Development of antibiotic resistance is always a threat in the treatment of microbial infections. However, epidemiological studies have shown that resistance to different antimicrobial agents may develop unevenly in different microbes. In the case of 5-NI, resistance can be widespread or relatively uncommon. For example, up to 10 to 30% of clinical isolates of Helicobacter pylori and T. vaginalis show clinically relevant resistance to Mz, which has been commonly used against these pathogens (20, 40). In contrast, clinical Mz resistance has not been observed in Entamoeba histolytica, and laboratory-acquired resistance can only be induced at low levels in this parasite (28, 40). Currently, Giardia holds an intermediate position in this spectrum, with at least one report of Mz resistance of Giardia strains from patients who had failed Mz therapy (23) and strong evidence for marked laboratory-acquired Mz resistance, as shown here and in prior work (26, 38). Yet, Mz resistance has so far not been identified as a major clinical problem in the treatment of giardiasis. Our studies can provide an explanation for the relative rarity of Mz resistance in Giardia, since we found in several independently derived lines that resistance is associated with a significant fitness cost, as shown by attenuated infectivity in animal models. This fitness cost manifests itself as a reduced capacity of trophozoites to attach to inert and living surfaces. Together, these results suggest that the apparent rarity of clinical Mz resistance in Giardia despite the widespread use of Mz in the treatment of giardiasis can be explained, at least partly, by loss of parasite attachment and thereby infectivity.
The metabolism of Mzr Giardia is altered in important ways, since prior studies had shown that several lines of Mzr Giardia proliferate more slowly in vitro than their isogenic Mzs counterparts (11). For example, resistance to 5-NI drugs has been associated with decreased expression and activity of a key enzyme, PFOR, involved in the final steps of ATP generation from glucose (8, 11, 25). Indeed, we found that several Mzr lines consumed less glucose than their parental Mzs lines and, importantly, glucose promoted parasite attachment, thus providing a possible explanation for the attachment defect observed in Mzr lines. To our surprise, however, the attachment functions in the presence of glucose appeared to be independent of ATP generation, raising questions about the underlying reasons. Several mechanisms have been proposed to explain attachment of trophozoites to inert surfaces and the intestinal mucosa, including a suction force beneath the adhesive disc generated by the beating of ventral flagella (16, 18), a combination of mechanical and hydrodynamic forces (34), and binding mediated by lectin surface molecules on Giardia (14). The relative importance of these mechanisms is not understood, nor is the role that glucose utilization may play in them, but an intriguing possibility could be that glycosylation of proteins critical for attachment may be impacted (30).
Beyond glucose-dependent metabolic processes, other factors may play a role in altered adherence of Mzr trophozoites. For example, specific variable surface proteins (VSPs) can affect the ability of Giardia isolates to establish infection in mice (42). Consistent with this, we observed in preliminary studies that expression of several VSPs differs in the Mzr lines compared to the parental Mzs cells, although the data did not suggest any obvious correlations between specific VSPs and infectivity in vivo or attachment capability in vitro. Nonetheless, comprehensive analysis of the impact of Mz resistance on the expressed VSP repertoire may reveal new insights into the functions of specific VSPs in parasite attachment.
The Mzr Giardia lines employed in this study showed almost complete cross-resistance to other 5-NI drugs, whereas resistance to a different nitro drug, nitazoxanide, was less pronounced. Conversely, primary resistance of Giardia to nitazoxanide was reported to lead to significant but less complete resistance to Mz (26). Similar 5-NI drug cross-resistance was observed in T. vaginalis (6, 44), while Mzr H. pylori remained sensitive to other 5-NI compounds and to nitazoxanide (32, 38). These data suggest that the mechanisms of 5-NI drug resistance overlap in different microbes, but they are not the same for different structural classes of nitro drugs. Specific mechanisms of 5-NI drug resistance are beginning to be understood in protozoa (28), although it is likely that other mechanisms remain to be discovered, as also suggested by the differential expression of several genes whose products can reduce and activate 5-NI drugs, as well as other genes coding for proteins involved in stress responses, in the present and in prior studies (25, 26). However, in many clinical situations of treatment failure with Mz, at present it is unlikely that a specific drug resistance mechanism can be identified or the consequences for cross-resistance predicted. Our data suggest that it would be advisable under those circumstances to employ the most structurally different nitro drugs in secondary treatment regimens, as that would reduce the risk of clinically relevant cross-resistance to Mz (45). Of course, use of entirely unrelated antimicrobial agents with different microbial targets, such as quinacrine, would be even less likely to result in problems with cross-resistance, as none was observed in any of the tested Mzr lines of Giardia.
Our studies required axenization of Giardia after recovery of trophozoites from infected animals, as only axenized lines can be tested reliably in antimicrobial assays. After testing common axenization protocols using antibiotic cocktails (15, 29) without success, we discovered that inoculation of trophozoites into the peritoneal cavity of mice, followed by expansion of the survivors in cell culture, allowed reliable axenization. This new method presumably relies on innate murine immune defenses to eradicate contaminating bacteria, but not Giardia, in the inocula. It must be acknowledged that Giardia is not completely resistant to innate defenses, as it can activate complement (13), and trophozoite numbers decreased markedly after in vivo passage. Yet, sufficient trophozoites could escape over the 24-h passage period in vivo to allow expansion of the population upon outgrowth with standard antibiotics in vitro. Interestingly, Giardia trophozoites have been described in the peritoneal fluid of patients with abdominal trauma, underlining that live parasites can survive innate immune defenses (3). Another approach for axenizing Giardia trophozoites has employed disinfection of concentrated cysts and subsequent excystation to trophozoites (7). This method requires a fairly large number of cysts and depends on sometimes unreliable excystation in vitro. It may be possible to infect animal models with such cyst preparations and recover trophozoites for further processing. Our data suggest that a combination of different methods, including short-term passage of trophozoites through the peritoneal cavity of mice and expansion in growth medium with suitable antibiotics, may be an efficient and reliable strategy for axenizing diverse Giardia lines. Use of this strategy in clinical settings could be an important step toward investigating and understanding drug resistance mechanisms of this parasite.
The present study and others (26, 38) have shown that Giardia can readily acquire laboratory-induced resistance to Mz, which can have fitness costs to the parasite in the form of attenuated infectivity. However, it must be noted that the five Giardia lines studied here may not represent all possible phenotypes of Mz resistance, so we cannot exclude that Mz resistance can occur without attenuation of infectivity. Furthermore, several Mzr lines could infect suckling mice and adult gerbils (albeit at lower levels), and Mz resistance was reported in 4 of 11 isolates from infected patients who had failed Mz therapy (23). Thus, viewed from the perspective of drug resistance rather than biological fitness, our data provide strong new support for the notion that clinically relevant Mz resistance can and does exist in Giardia. In this regard, Giardia resembles other microbial pathogens, such as T. vaginalis and H. pylori, and the findings caution that the indiscriminate use of nitro-bearing antimicrobial drugs may result in selection of fitter and hence more prevalent clinically Mzr isolates of Giardia in the future.
ACKNOWLEDGMENTS
We are grateful to Yolanda Andersen and Lucia Hall for expert technical support.
This work was supported by NIH U01 cooperative research agreement AI75527, NIH grant DK35108, and the UCSD Digestive Diseases Research Development Center (DK80506).
Footnotes
Published ahead of print on 8 August 2011.
REFERENCES
- 1. Ankarklev J., Jerlstrom-Hultqvist J., Ringqvist E., Troell K., Svard S. G. 2010. Behind the smile: cell biology and disease mechanisms of Giardia species. Nat. Rev. Microbiol. 8:413–422 [DOI] [PubMed] [Google Scholar]
- 2. Arguello-Garcia R., Cruz-Soto M., Romero-Montoya L., Ortega-Pierres G. 2009. In vitro resistance to 5-nitroimidazoles and benzimidazoles in Giardia duodenalis: variability and variation in gene expression. Infect. Genet. Evol. 9:1057–1064 [DOI] [PubMed] [Google Scholar]
- 3. Bloch T., Davis T. E., Jr., Schwenk G. R., Jr 1987. Giardia lamblia in peritoneal fluid. Acta Cytol. 31:783–784 [PubMed] [Google Scholar]
- 4. Boreham P. F., Phillips R. E., Shepherd R. W. 1986. The activity of drugs against Giardia intestinalis in neonatal mice. J. Antimicrob. Chemother. 18:393–398 [DOI] [PubMed] [Google Scholar]
- 5. Boreham P. F., Phillips R. E., Shepherd R. W. 1988. Altered uptake of metronidazole in vitro by stocks of Giardia intestinalis with different drug sensitivities. Trans. R. Soc. Trop. Med. Hyg. 82:104–106 [DOI] [PubMed] [Google Scholar]
- 6. Crowell A. L., Sanders-Lewis K. A., Secor W. E. 2003. In vitro metronidazole and tinidazole activities against metronidazole-resistant strains of Trichomonas vaginalis. Antimicrob. Agents Chemother. 47:1407–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cruz A., et al. 2003. Isolation, excystation and axenization of Giardia lamblia isolates: in vitro susceptibility to metronidazole and albendazole. J. Antimicrob. Chemother. 51:1017–1020 [DOI] [PubMed] [Google Scholar]
- 8. Dan M., Wang A. L., Wang C. C. 2000. Inhibition of pyruvate-ferredoxin oxidoreductase gene expression in Giardia lamblia by a virus-mediated hammerhead ribozyme. Mol. Microbiol. 36:447–456 [DOI] [PubMed] [Google Scholar]
- 9. Davids B. J., Williams S., Lauwaet T., Palanca T., Gillin F. D. 2008. Giardia lamblia aurora kinase: a regulator of mitosis in a binucleate parasite. Int. J. Parasitol. 38:353–369 [DOI] [PubMed] [Google Scholar]
- 10. Dawson S. C. 2010. An insider's guide to the microtubule cytoskeleton of Giardia. Cell. Microbiol. 12:588–598 [DOI] [PubMed] [Google Scholar]
- 11. Dunn L. A., et al. 2010. A new-generation 5-nitroimidazole can induce highly metronidazole-resistant Giardia lamblia in vitro. Int. J. Antimicrob. Agents 36:37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edwards M. R., Schofield P. J., O'Sullivan W. J., Costello M. 1992. Arginine metabolism during culture of Giardia intestinalis. Mol. Biochem. Parasitol. 53:97–103 [DOI] [PubMed] [Google Scholar]
- 13. Evans-Osses I., Ansa-Addo E. A., Inal J. M., Ramirez M. I. 2010. Involvement of lectin pathway activation in the complement killing of Giardia intestinalis. Biochem. Biophys. Res. Commun. 395:382–386 [DOI] [PubMed] [Google Scholar]
- 14. Farthing M. J., Pereira M. E., Keusch G. T. 1986. Description and characterization of a surface lectin from Giardia lamblia. Infect. Immun. 51:661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gordts B., et al. 1985. Evaluation of a new method for routine in vitro cultivation of Giardia lamblia from human duodenal fluid. J. Clin. Microbiol. 22:702–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hansen W. R., Tulyathan O., Dawson S. C., Cande W. Z., Fletcher D. A. 2006. Giardia lamblia attachment force is insensitive to surface treatments. Eukaryot. Cell 5:781–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hernandez-Sanchez J., Linan R. F., Salinas-Tobon Mdel R., Ortega-Pierres G. 2008. Giardia duodenalis: adhesion-deficient clones have reduced ability to establish infection in Mongolian gerbils. Exp. Parasitol. 119:364–372 [DOI] [PubMed] [Google Scholar]
- 18. Holberton D. V. 1974. Attachment of Giardia: a hydrodynamic model based on flagellar activity. J. Exp. Biol. 60:207–221 [DOI] [PubMed] [Google Scholar]
- 19. Jenkins M. C., et al. 2009. Antibodies to the ventral disc protein delta-giardin prevent in vitro binding of Giardia lamblia trophozoites. J. Parasitol. 95:895–899 [DOI] [PubMed] [Google Scholar]
- 20. Kariuki S., Hart C. A. 2001. Global aspects of antimicrobial-resistant enteric bacteria. Curr. Opin. Infect. Dis. 14:579–586 [DOI] [PubMed] [Google Scholar]
- 21. Lauwaet T., et al. 2007. Protein phosphatase 2A plays a crucial role in Giardia lamblia differentiation. Mol. Biochem. Parasitol. 152:80–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leitsch D., et al. 2011. Pyruvate:ferredoxin oxidoreductase and thioredoxin reductase are involved in 5-nitroimidazole activation while flavin metabolism is linked to 5-nitroimidazole resistance in Giardia lamblia. J. Antimicrob. Chemother. 66:1756–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lemee V., et al. 2000. Metronidazole and albendazole susceptibility of 11 clinical isolates of Giardia duodenalis from France. J. Antimicrob. Chemother. 46:819–821 [DOI] [PubMed] [Google Scholar]
- 24. Liu S. M., Brown D. M., O'Donoghue P., Upcroft P., Upcroft J. A. 2000. Ferredoxin involvement in metronidazole resistance of Giardia duodenalis. Mol. Biochem. Parasitol. 108:137–140 [DOI] [PubMed] [Google Scholar]
- 25. Muller J., Ley S., Felger I., Hemphill A., Muller N. 2008. Identification of differentially expressed genes in a Giardia lamblia WB C6 clone resistant to nitazoxanide and metronidazole. J. Antimicrob. Chemother. 62:72–82 [DOI] [PubMed] [Google Scholar]
- 26. Muller J., Sterk M., Hemphill A., Muller N. 2007. Characterization of Giardia lamblia WB C6 clones resistant to nitazoxanide and to metronidazole. J. Antimicrob. Chemother. 60:280–287 [DOI] [PubMed] [Google Scholar]
- 27. Nash T. E., et al. 2001. Treatment of patients with refractory giardiasis. Clin. Infect. Dis. 33:22–28 [DOI] [PubMed] [Google Scholar]
- 28. Pal D., et al. 2009. Giardia, Entamoeba, and Trichomonas enzymes activate metronidazole (nitroreductases) and inactivate metronidazole (nitroimidazole reductases). Antimicrob. Agents Chemother. 53:458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park S. J., Yong T. S., Yang H. W., Lee D. H., Lee K. 1999. Axenic cultivation and characterization of Giardia lamblia isolated from humans in Korea. Korean J. Parasitol. 37:121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ratner D. M., et al. 2008. Changes in the N-glycome, glycoproteins with Asn-linked glycans, of Giardia lamblia with differentiation from trophozoites to cysts. Eukaryot. Cell 7:1930–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Savioli L., Smith H., Thompson A. 2006. Giardia and Cryptosporidium join the “Neglected Diseases Initiative.” Trends Parasitol. 22:203–208 [DOI] [PubMed] [Google Scholar]
- 32. Sisson G., et al. 2002. Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob. Agents Chemother. 46:2116–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith P. D., Gillin F. D., Spira W. M., Nash T. E. 1982. Chronic giardiasis: studies on drug sensitivity, toxin production, and host immune response. Gastroenterology 83:797–803 [PubMed] [Google Scholar]
- 34. Sousa M. C., Goncalves C. A., Bairos V. A., Poiares-Da-Silva J. 2001. Adherence of Giardia lamblia trophozoites to Int-407 human intestinal cells. Clin. Diagn. Lab. Immunol. 8:258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Townson S. M., Hanson G. R., Upcroft J. A., Upcroft P. 1994. A purified ferredoxin from Giardia duodenalis. Eur. J. Biochem. 220:439–446 [DOI] [PubMed] [Google Scholar]
- 36. Townson S. M., Laqua H., Upcroft P., Boreham P. F., Upcroft J. A. 1992. Induction of metronidazole and furazolidone resistance in Giardia. Trans. R. Soc. Trop. Med. Hyg. 86:521–522 [DOI] [PubMed] [Google Scholar]
- 37. Townson S. M., Upcroft J. A., Upcroft P. 1996. Characterisation and purification of pyruvate:ferredoxin oxidoreductase from Giardia duodenalis. Mol. Biochem. Parasitol. 79:183–193 [DOI] [PubMed] [Google Scholar]
- 38. Upcroft J. A., Campbell R. W., Benakli K., Upcroft P., Vanelle P. 1999. Efficacy of new 5-nitroimidazoles against metronidazole-susceptible and -resistant Giardia, Trichomonas, and Entamoeba spp. Antimicrob. Agents Chemother. 43:73–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Upcroft J. A., Upcroft P. 1993. Drug resistance and Giardia. Parasitol. Today 9:187–190 [DOI] [PubMed] [Google Scholar]
- 40. Upcroft P., Upcroft J. A. 2001. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol. Rev. 14:150–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Valdez C. A., et al. 2009. Synthesis and electrochemistry of 2-ethenyl and 2-ethanyl derivatives of 5-nitroimidazole and antimicrobial activity against Giardia lamblia. J. Med. Chem. 52:4038–4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. von Allmen N., Bienz M., Hemphill A., Muller N. 2004. Experimental infections of neonatal mice with cysts of Giardia lamblia clone GS/M-83-H7 are associated with an antigenic reset of the parasite. Infect. Immun. 72:4763–4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wensaas K. A., Langeland N., Rortveit G. 2010. Post-infectious gastrointestinal symptoms after acute giardiasis. A 1-year follow-up in general practice. Fam. Pract. 27:255–259 [DOI] [PubMed] [Google Scholar]
- 44. Wright J. M., et al. 2010. Susceptibility in vitro of clinically metronidazole-resistant Trichomonas vaginalis to nitazoxanide, toyocamycin, and 2-fluoro-2′-deoxyadenosine. Parasitol. Res. 107:847–853 [DOI] [PubMed] [Google Scholar]
- 45. Wright J. M., Dunn L. A., Upcroft P., Upcroft J. A. 2003. Efficacy of antigiardial drugs. Expert Opin. Drug Saf. 2:529–541 [DOI] [PubMed] [Google Scholar]