Abstract
For enterococcal implant-associated infections, the optimal treatment regimen has not been defined. We investigated the activity of daptomycin, vancomycin, and gentamicin (and their combinations) against Enterococcus faecalis in vitro and in a foreign-body infection model. Antimicrobial activity was investigated by time-kill and growth-related heat production studies (microcalorimetry) as well as with a guinea pig model using subcutaneously implanted cages. Infection was established by percutaneous injection of E. faecalis in the cage. Antibiotic treatment for 4 days was started 3 h after infection. Cages were removed 5 days after end of treatment to determine the cure rate. The MIC, the minimal bactericidal concentration (MBC) in the logarithmic phase, and the MBC in the stationary phase were 1.25, 5, and >20 μg/ml for daptomycin, 1, >64, and >64 μg/ml for vancomycin, and 16, 32, and 4 μg/ml for gentamicin, respectively. In vitro, gentamicin at subinhibitory concentrations improved the activity against E. faecalis when combined with daptomycin or vancomycin in the logarithmic and stationary phases. In the animal model, daptomycin cured 25%, vancomycin 17%, and gentamicin 50% of infected cages. In combination with gentamicin, the cure rate for daptomycin increased to 55% and that of vancomycin increased to 33%. In conclusion, daptomycin was more active than vancomycin against adherent E. faecalis, and its activity was further improved by the addition of gentamicin. Despite a short duration of infection (3 h), the cure rates did not exceed 55%, highlighting the difficulty of eradicating E. faecalis from implants already in the early stage of implant-associated infection.
INTRODUCTION
Implant-associated infections are caused by microorganisms growing attached on the device surface as biofilms (12, 31). The biofilm mode of growth represents a survival strategy through which microorganisms can attach and better resist antibiotics and the host defense system. In a biofilm, microorganisms can be up to 1,000-fold more resistant to antimicrobials than their planktonic counterparts (27). Therefore, infections in the presence of an implant are persistent and difficult to eradicate. Often, removal of all foreign-body material is performed, and it is replaced after several weeks, if needed. This approach causes considerable soft tissue damage and bone stock loss and requires repeated surgical interventions and a prolonged hospital stay (40). Therefore, new treatment options using antibiotics with increased activity against biofilms are being investigated, potentially allowing successful eradication of implant-associated infections without device removal.
Enterococci are increasingly recognized as the cause of health care-acquired infections, especially in immunocompromised hosts, such as critically ill patients and recipients of intravascular and extravascular implants (20, 25). Enterococci cause 3 to 10% of periprosthetic joint infections, and the treatment is challenging due to high failure rates and common relapses (22, 40). Reasons for the common treatment failure of enterococcal implant-associated infections are not entirely clear. They may include the low killing rate of enterococci by β-lactam antibiotics and glycopeptides, antimicrobial tolerance, persistence in host cells (potentially mediated by the enterococcal surface proteins), and unavailability of potent antibiofilm agents, such as rifampin, which is active against staphylococcal biofilms (15, 26, 40). In addition, enterococci may acquire genes encoding resistance against β-lactams, glycopeptides, aminoglycosides, and oxazolidinones, which further complicates the treatment of enterococcal infections (17, 23, 38).
Daptomycin may be a treatment option for enterococcal biofilms due to its mechanism of action, particularly its rapid killing and concentration-dependent activity (9). We investigated the activity of daptomycin and vancomycin, alone and in combination with gentamicin, against planktonic and adherent Enterococcus faecalis in vitro and in a foreign-body infection model using guinea pigs. This model was previously used to evaluate treatment regimens of implant-associated infections caused by staphylococci and Escherichia coli (1, 5, 16, 30, 36, 39) but has not yet been employed for enterococcal infections.
(Parts of the results of the present study were presented at the 19th European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], Helsinki, Finland, 16 to 19 September 2009, and at the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], Boston, MA, 12 to 15 September 2010.)
MATERIALS AND METHODS
Study organism.
All experiments were performed with E. faecalis strain ATCC 19433. The strain has been shown to form biofilms (7). Bacteria were stored at −70°C by using a cryovial bead preservation system (Microbank; Pro-Lab Diagnostics, Richmond Hill, Ontario, Canada). To prepare the inoculum, one bead was spread on a blood agar plate and incubated at 37°C. One colony was resuspended in 5 ml of tryptic soy broth (TSB) and incubated at 37°C. Overnight cultures were adjusted to a turbidity of McFarland 0.5 (corresponding to ∼5 × 107 CFU/ml). The exact inoculum was determined by CFU counting. For the infection inoculum, the overnight culture of E. faecalis was washed twice with sterile pyrogen-free 0.9% saline.
Antimicrobial agents.
Daptomycin for injection was supplied by Novartis Pharma Schweiz AG (Bern, Switzerland). A stock solution of 50 mg/ml was prepared in sterile and pyrogen-free 0.9% saline. Vancomycin was delivered by Teva Pharma AG (Aesch, Switzerland) as 10 mg of powder in ampoules. The stock solution of 50 mg/ml was prepared in sterile and pyrogen-free 0.9% saline. Gentamicin was purchased from Essex Chemie AG (Lucerne, Switzerland) as a 40-mg/ml solution.
Antimicrobial susceptibility.
The MICs and minimal bactericidal concentrations (MBCs) for daptomycin, vancomycin, and gentamicin were determined by the broth macrodilution method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (10). A standard inoculum of ∼3 × 105 CFU/ml was used. Serial 2-fold dilutions of the antimicrobials were prepared in cation-adjusted Mueller-Hinton broth (CAMHB) for the logarithmic growth phase. The MIC was defined as the lowest concentration of antibiotic that completely inhibited visible growth. The MBClog was defined as the lowest antimicrobial concentration which killed ≥99.9% of the initial bacterial count (i.e., ≥3 log10 CFU/ml) in 24 h (10). For stationary-growth-phase bacteria, MBCstat was determined by using phosphate-buffered saline (PBS) supplemented with 0.1% TSB (34). Growth media for daptomycin studies were supplemented with 50 mg/liter Ca2+. All experiments were performed in triplicate.
Time-kill studies.
The activities of daptomycin, vancomycin, and gentamicin were investigated by time-kill studies under logarithmic- and stationary-growth-phase conditions. The initial inoculum was 5 × 105 CFU/ml. Medium inoculated with bacteria only served as the growth control. CAMHB was used for the logarithmic growth phase, and PBS supplemented with 0.1% TSB was used for the stationary growth phase. Growth media for daptomycin studies were supplemented with 50 mg/liter Ca2+. Tubes were incubated under static conditions at 37°C, and aliquots were taken after 0, 2, 4, 6, 8, and 24 h. To avoid antibiotic carry-over, samples were washed with sterile 0.9% saline and appropriate dilutions were spread on Muller-Hinton agar. The experiments were performed in triplicate, and means and standard deviations were plotted in a semilogarithmic graph. A bactericidal effect was defined as a ≥3-log10 (≥99.9%) reduction of the initial bacterial count in 24 h.
Microcalorimetry.
To test the growth inhibition by antimicrobials and their combinations, measurements of bacterial heat production were performed with a 48-channel batch calorimeter (thermal activity monitor, model 3102 TAM III; TA Instruments, New Castle, DE). Microcalorimetry is a highly sensitive and accurate method for measurement of heat production generated by microbial growth. The effect of antibiotics on bacterial growth can be evaluated by the delay of heat flow and reduction of the heat flow peak compared to results with the growth control without the presence of antibiotics (2, 3, 6, 28, 29, 33). Calorimeter ampoules with 3 ml of CAMHB supplemented with Ca2+ containing daptomycin, vancomycin, or gentamicin at 0.125× MIC, 0.25× MIC, 0.5× MIC, and 1× MIC were inoculated with 3 × 105 CFU/ml. In combination studies, daptomycin and vancomycin at 0.25× MIC were combined with gentamicin in concentrations of 0.125× MIC, 0.25× MIC, and 0.5× MIC. The heat flow at 37°C was measured for 24 h. The results were plotted as heat flow over time.
Animal model.
Male albino guinea pigs (Charles River, Sulzfeld, Germany) were kept in the Animal Facility of the University Hospital Lausanne, Lausanne, Switzerland, and the experiments were performed according to the regulations of Swiss veterinary law. Guinea pigs were weighed daily to monitor their well-being during the experiment. Short-term studies (4-day treatment) were performed in a foreign-body cage model for guinea pigs developed by Zimmerli et al. (41). Four sterile polytetrafluorethylene (Teflon) cages with 130 regularly spaced perforations 1 mm in diameter (Angst-Pfister AG, Zürich, Switzerland) were subcutaneously implanted in flanks of the guinea pigs (450 to 550 g) under aseptic conditions. After complete wound healing (approximately 2 weeks after implantation), the sterility of the cages was confirmed by culturing aspirated cage fluid on blood agar plates. The bacterial inoculation was performed only in initially sterile cages.
Pharmacokinetic studies.
Pharmacokinetic studies for daptomycin and vancomycin were performed in previous studies (5, 16). For gentamicin pharmacokinetic studies, cage fluid was aspirated from noninfected animals over 24 h (2, 4, 6, 10, and 24 h) following intraperitoneal administration of a single dose of gentamicin at 10 mg/kg. Three guinea pigs were used; i.e., 12 cages. At each time point, 150-μl aliquots of cage fluid were aspirated from two cages from each animal (i.e., six replicates per time point). The collected fluid was centrifuged (2,100 × g for 7 min) at 4°C and the supernatant was stored at −20°C until further analysis. The means ± standard deviations (SD) of the peak concentration (Cmax), the time to reach Cmax (Tmax), and the trough concentration at 24 h after dosing (Cmin) were determined.
Determination of drug concentrations.
Gentamicin concentrations in cage fluid were determined by an agar plate diffusion bioassay with Bacillus subtilis strain ATCC 6051 as the indicator organism. Mueller-Hinton agar (Difco, BD, Le Pont de Claix, France) was suspended in sterile pyrogen-free water, and the mixture was autoclaved. The medium was then cooled to 45°C, inoculated with the overnight culture of the indicator organism (100 μl per 165 ml of medium), and poured into large assay plates (30 cm by 30 cm). Calibration curves were plotted for the assay plate, and the regression-fitting equation was extrapolated. The standard solutions were prepared in 0.9% NaCl by preparing 2-fold serial dilutions. One hundred microliters of the cage fluid samples and duplicates of the gentamicin standard solutions were spotted into holes punched in the assay plates, and the plates were incubated overnight at room temperature, followed by incubation at 37°C for 24 h. The diameter of the inhibition zone was measured with calipers. The bioassay detection limit corresponded to the gentamicin 0.5× MIC of the indicator organism (i.e., 0.5 μg/ml).
Antimicrobial treatment.
Cages were infected by percutaneous inoculation of 200 μl of E. faecalis containing 3 × 104 CFU. The exact infection inoculum was determined by colony counts. Three hours after infection, quantitative cultures of aspirated cage fluid were performed, followed by the start of antimicrobial treatment. In each of the following five treatment regimens, three animals were randomized, each having 4 cages (i.e., 12 cages per treatment regimen): control group (no antibiotic treatment); daptomycin, 40 mg/kg of body weight; daptomycin, 40 mg/kg plus gentamicin 10 mg/kg; vancomycin, 15 mg/kg; vancomycin, 15 mg/kg plus gentamicin 10 mg/kg; and gentamicin, 10 mg/kg. All antimicrobial agents were given intraperitoneally twice daily, except daptomycin, which was given once daily. The duration of treatment was 4 days.
Antimicrobial efficacy on planktonic and adherent E. faecalis.
Cage fluid was aspirated before the start of treatment, during treatment (immediately before the application of the last dose), and 5 days after completion of treatment. The bacterial counts were expressed as log10 CFU/ml of cage fluid. The quantification limit for bacteria in the cage fluid was set to 200 CFU/ml (>10 CFU in 50 μl of a 10-fold dilution). The clearance rate (as a percentage) was defined as the number of cage fluid cultures without growth of E. faecalis divided by the total number of cage fluid cultures in the individual treatment group. To determine the antimicrobial efficacy against adherent bacteria, animals were sacrificed 5 days after completion of treatment, and cages were explanted under aseptic conditions and incubated in 5 ml TSB. After a 48-h incubation of the cages in TSB, aliquots of 100 μl were spread on a blood agar plate and incubated at 37°C for additional 48 h. The treatment efficacy against adherent bacteria was expressed as the cure rate (as a percentage), defined as the number of cages without E. faecalis growth divided by the total number of cages in the individual treatment group.
Statistical calculations.
Comparisons for continuous variables were performed by the Mann-Whitney U test. For all tests, differences were considered significant when P values were <0.05. The graphs in the figures were plotted with Prism (version 5.0a) software (GraphPad Software, La Jolla, CA).
RESULTS
In vitro antimicrobial susceptibility.
Table 1 summarizes the in vitro susceptibility of E. faecalis. Daptomycin and vancomycin had low MIC values (1.25 and 1 μg/ml, respectively) but did not kill the bacteria in the stationary growth phase. Daptomycin showed bactericidal activity in the logarithmic phase (MBClog, 5 μg/ml), and gentamicin was killing E. faecalis in both the logarithmic (MBClog, 32 μg/ml) and the stationary (MBCstat, 4 μg/ml) growth phases.
Table 1.
In vitro susceptibility of E. faecalis strain ATCC 19433 against daptomycin, vancomycin, and gentamicin as determined by broth macrodilutiona
| Antimicrobial | MIC (μg/ml) | MBClog (μg/ml) | MBCstat (μg/ml) |
|---|---|---|---|
| Daptomycin | 1.25 | 5 | >20 |
| Vancomycin | 1 | >64 | >64 |
| Gentamicin | 16 | 32 | 4 |
MBClog, minimal bactericidal concentration in the logarithmic growth phase; MBCstat, minimal bactericidal concentration in the stationary growth phase. The results are mean values from triplicate measurements.
Time-kill studies.
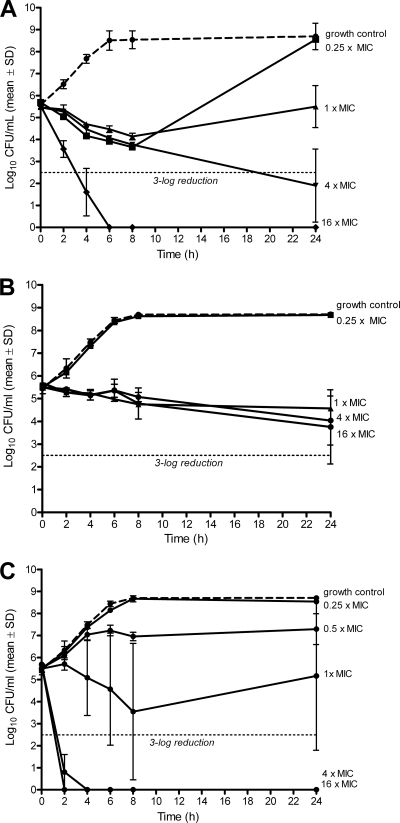
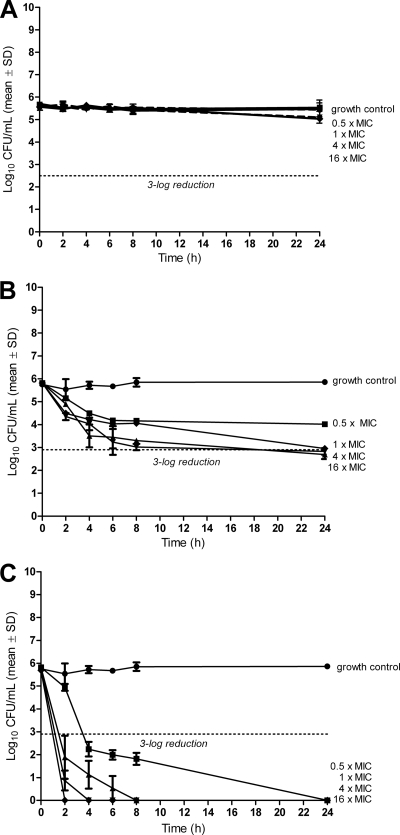
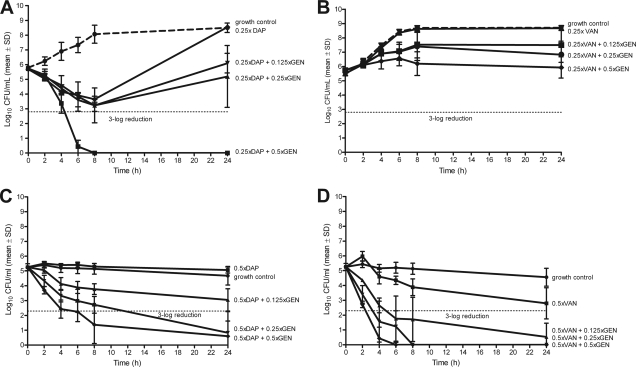
Figure 1 demonstrates killing of bacteria in the logarithmic growth phase. Daptomycin and gentamicin (Fig. 1A and C) show a dose-dependent activity at concentrations from 0.25× to 16× MIC, both being bactericidal (i.e., 3-log reduction) at 4× MIC. Vancomycin (Fig. 1B), on the other hand, showed no bactericidal effect at concentrations of up to 16× MIC. Figure 2 shows the antimicrobial activity against bacteria in the stationary phase. Daptomycin showed no bactericidal effect at up to 16× MIC (Fig. 2A), whereas vancomycin reduced bacterial counts by approximately 3-log at 1× to 16× MIC (Fig. 2B). Gentamicin was the most active antimicrobial agent, being bactericidal at 0.5× MIC (Fig. 2C). When combining daptomycin at 0.25× MIC with gentamicin at different subinhibitory concentrations, the antimicrobial activity was increased against E. faecalis in the logarithmic phase (Fig. 3A). This additive effect was less pronounced when combining vancomycin and gentamicin (Fig. 3B). In the stationary phase, gentamicin improved the killing of E. faecalis by daptomycin and vancomycin (Fig. 3C and D), with a time-kill curve resembling that of gentamicin alone.
Fig. 1.
Time-kill studies for daptomycin (A), vancomycin (B), and gentamicin (C) in logarithmic growth phase. Values represent means and standard deviations (SD) of triplicate measurements. The limit of detection was 200 CFU/ml. The horizontal dashed line represents the reduction of 3 log10 CFU/ml of the initial inoculum. The dashed line represents the growth control.
Fig. 2.
Time-kill studies for daptomycin (A), vancomycin (B), and gentamicin (C) in stationary growth phase. Values represent means and standard deviations (SD) of triplicate measurements. The limit of detection was 200 CFU/ml. The horizontal dashed line represents the reduction of 3 log10 CFU/ml of the initial inoculum.
Fig. 3.
Time-kill curves of E. faecalis in logarithmic (A and B) and stationary (C and D) growth phase with daptomycin (DAP; A and C) and vancomycin (VAN; B and D) combined with gentamicin (GEN) at different concentrations. Values represent means and standard deviations (SD) of triplicate measurements. The horizontal dashed line represents the reduction of 3 log10 CFU/ml of the initial inoculum.
In vitro activity on E. faecalis evaluated by microcalorimetry.
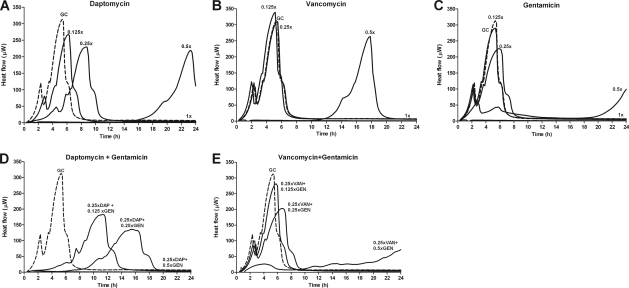
The effect of daptomycin, vancomycin, and gentamicin at subinhibitory concentrations and 1× MIC on E. faecalis growth-related heat production was investigated using a batch calorimeter. The drugs were tested alone (Fig. 4A to C) and in combination with gentamicin (Fig. 4D and E) at subinhibitory concentrations. Since bacteria in the stationary phase do not produce detectable heat, the experiment was performed only with logarithmic phase bacteria. A fixed concentration of daptomycin and vancomycin at 0.25× MIC was combined with gentamicin at 0.125×, 0.25×, and 0.5× MIC.
Fig. 4.
Calorimetric measurements of heat produced by E. faecalis during 24 h exposed to different concentration of daptomycin (DAP; A), vancomycin (VAN; B), gentamicin (GEN; C) and their combinations (D and E). All drugs were measured at concentrations of 0.125× MIC, 0.25× MIC, 0.5× MIC and 1× MIC. The combinations were tested with fixed concentrations of daptomycin and vancomycin (0.25× MIC) with three concentrations of gentamicin (0.125×, 0.25×, and 0.5× MIC). GC, growth control (dashed line).
The growth control had a maximal heat flow peak of 315 μW at 5.6 h of incubation. The activity of the used antibiotics resulted in decreased and delayed peaks of the heat flow curves. At 1× MIC, heat production was completely inhibited for 24 h by all antibiotics. Daptomycin alone was more active in the suppression of growth-related heat production of E. faecalis than vancomycin and gentamicin alone using the same concentrations. Whereas a delay in the heat flow peak was observed for daptomycin already at 0.125× MIC (Fig. 4A), the inhibitory effect of vancomycin (Fig. 4B) and gentamicin (Fig. 4C) was present only at 0.5× MIC.
The addition of gentamicin at a low concentration (0.125× MIC) enhanced the antienterococcal activity of both daptomycin and vancomycin (Fig. 4 D and E), whereas gentamicin alone showed a minimal antimicrobial effect at this concentration (Fig. 4C). The combination of daptomycin and gentamicin was more active in the suppression of growth-related heat production than the combination of vancomycin and gentamicin using the same fractional concentrations of the MIC.
Pharmacokinetics of gentamicin in the cage fluid.
After an intraperitoneal application of gentamicin (10 mg/kg), a Cmax (mean ± SD) of 6.2 ± 1.6 μg/ml was reached after 2 h (Tmax). The concentration of gentamicin in the cage fluid 24 h after application (Cmin) was 0 μg/ml.
In vivo activity on planktonic E. faecalis.
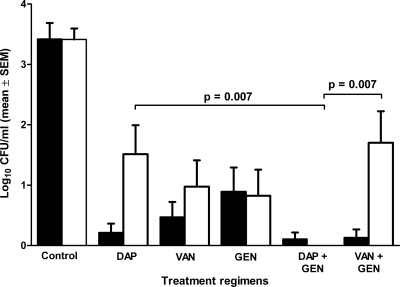
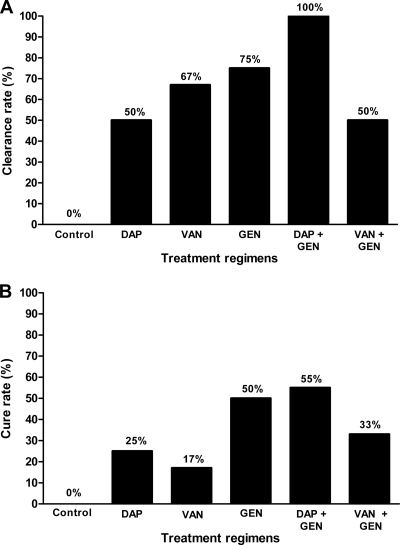
Figure 5 shows the bacterial load in the cage fluid throughout the experiment. After 3 h of infection, before the start of treatment, the bacterial load in the aspirated cage fluid was 3.5 log10. No spontaneous clearance of planktonic bacteria from the cage fluid in the untreated group was observed, and the bacterial load remained unchanged during the experiment (∼3.5 log10). Five days after completion of therapy, the planktonic bacteria in the cage fluid had decreased by 1.9 log10 with daptomycin, by 2.5 log10 with vancomycin, and by 2.6 log10 with gentamicin. The combination of gentamicin and daptomycin was the most efficient regimen in killing planktonic E. faecalis, with complete clearance of the cage fluid (Fig. 6A). When a combination of gentamicin with vancomycin was used, planktonic bacteria decreased by 1.8 log10 and clearance of planktonic bacteria was achieved in 50% of cage fluid samples.
Fig. 5.
Bacterial load of planktonic bacteria in cage fluid during treatment (black bars) and 5 days after treatment (white bars). Values are means ± standard errors of the means (SEM). DAP, daptomycin; VAN, vancomycin; GEN, gentamicin.
Fig. 6.
Clearance rate of planktonic bacteria in cage fluid (A) and cure rate of adherent bacteria from explanted cages (B). DAP, daptomycin; VAN, vancomycin; GEN, gentamicin. The numbers above the columns indicate the clearance and cure rate, respectively.
In vivo activity on adherent E. faecalis.
Figure 6B shows the cure rates of different antibiotic regimes against adherent bacteria. Without antibiotics (control animals), the cure rate was 0%. Daptomycin, vancomycin, and gentamicin as monotherapy eradicated the adherent bacteria in 25%, 17%, and 50% of cages, respectively. In combination with gentamicin, the cure rate increased to 55% (daptomycin) and 33% (vancomycin).
DISCUSSION
The management of implant-associated infections is challenging, and clinical experience is limited, especially with retention of the infected device (40). Therefore, we compared the in vitro and in vivo activities of daptomycin and vancomycin, alone and in combination with gentamicin, against planktonic and adherent E. faecalis. The chosen E. faecalis strain is representative of the current epidemiological situation, with susceptibility to lipopeptides and glycopeptides, without demonstrating high-level resistance to gentamicin (MIC, 16 μg/ml). However, the prevalence of multiresistant enterococcal strains may increase in the future. Therefore, daptomycin was chosen as one of the treatment regimens. Daptomycin is a promising agent due to its mechanism of action, particularly its rapid killing and concentration-dependent activity (9). According to CLSI (11) and EUCAST (http://www.eucast.org/) guidelines, the majority of current clinical isolates of enterococci are susceptible to daptomycin (MIC, ≤4 μg/ml). Moreover, daptomycin is not associated with cross-resistance to other antimicrobials and is also active against most resistant isolates, including vancomycin-resistant enterococci (VRE) (9). However, the risk of emergence of daptomycin resistance during treatment of enterococcal infections is of concern. The genetic basis for daptomycin resistance in enterococci was recently reviewed (21).
The in vitro experiments using time-kill studies and measurement of growth-related bacterial heat production (microcalorimetry) showed an improved antibacterial activity of daptomycin and vancomycin when in combination with gentamicin. This effect was observed in the logarithmic phase, whereas in the stationary phase, the killing effect of gentamicin combination represented mostly the pronounced killing effect of gentamicin alone. Based on these in vitro results, gentamicin was included in the treatment combination regimens in the experimental studies in the guinea pig model. The animal model was adapted from the previously described setting (1, 16, 30, 39) by reducing the infection inoculum to 3 × 104 CFU/cage and shortening the duration of infection to 3 h. Despite the fact that we have not demonstrated the formation of biofilm on explanted cages, we assume that adherent bacteria in biofilm are responsible for frequent treatment failure. With the 24-h duration of infection, no cure of infected cages was observed (data not shown). Under adapted experimental conditions (using a short infection duration), cure rates did not exceed 55%. This highlights the propensity of E. faecalis to rapidly cause persistent infections, which are difficult to eradicate despite a low MIC of all tested antimicrobial drugs. In staphylococcal infection, cure rates were significantly improved by combination with rifampin, which acts against biofilms (1, 16, 31, 32, 35, 42). In addition, combination regimens may prevent the emergence of resistant strains.
In our animal studies, the injected dose of daptomycin in guinea pigs (40 mg/kg) corresponds to a dose of 8 mg/kg in humans (4, 13, 37), which is higher than that currently recommended for staphylococcal infection in clinical practice (19). The most efficient regimen for killing planktonic E. faecalis was the combination of daptomycin and gentamicin, followed by gentamicin alone, as observed in the in vitro experiments. When evaluating the activity against adherent bacteria, daptomycin with gentamicin showed the highest cure rate (55%), whereas vancomycin with gentamicin showed a lower cure rate (33%). Gentamicin alone was able to eradicate the biofilm in 50% of the cages. However, gentamicin monotherapy is not recommended in clinical use because of the risk of emergence of resistance. This was shown in a study by Lefort et al. (18), in which gentamicin alone showed a significant reduction of the number of E. faecalis bacteria in the vegetation in a rabbit model of endocarditis but selected gentamicin-resistant mutants.
The activity of gentamicin combination against enterococcal biofilms was investigated in several laboratory and clinical studies. In vitro enterococcal biofilms were inhibited by high concentrations of ampicillin, vancomycin, and linezolid. In combination with gentamicin, these agents significantly reduced the MIC, MBC, and minimal biofilm inhibition concentration (MBIC) against several tested enterococcal isolates (24). Furthermore, in a rabbit endocarditis model, improved activity of daptomycin was observed against Enterococcus faecalis when combined with gentamicin (8). However, in a retrospective clinical study, including enterococcal prosthetic joint infections, no benefit for the treatment outcome of infections of the addition of gentamicin to aminopenicillins or glycopeptides was observed (14). Whether daptomycin in combination with gentamicin is superior to other antimicrobial regimens against adherent enterococci, including vancomycin-resistant enterococci (VRE), and whether the infected implants can be retained with this combination, needs to be investigated in future clinical studies. The optimal regimen against strains with a high level of resistance to gentamicin remains to be determined.
ACKNOWLEDGMENTS
This study was supported by the Swiss National Science Foundation (K-32K1_120531) and by an educational grant from Novartis Pharma AG, Basel, Switzerland.
We thank Alain Bizzini for critical reviews of the manuscript, Anne-Katrin John for practical advice, and Zarko Rajacic for laboratory assistance.
Footnotes
Published ahead of print on 1 August 2011.
REFERENCES
- 1. Baldoni D., Haschke M., Rajacic Z., Zimmerli W., Trampuz A. 2009. Linezolid alone or combined with rifampin against methicillin-resistant Staphylococcus aureus in experimental foreign-body infection. Antimicrob. Agents Chemother. 53:1142–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baldoni D., Hermann H., Frei R., Trampuz A., Steinhuber A. 2009. Performance of microcalorimetry for early detection of methicillin resistance in clinical isolates of Staphylococcus aureus. J. Clin. Microbiol. 47:774–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baldoni D., Steinhuber A., Zimmerli W., Trampuz A. 2010. In vitro activity of gallium maltolate against Staphylococci in logarithmic, stationary, and biofilm growth phases: comparison of conventional and calorimetric susceptibility testing methods. Antimicrob. Agents Chemother. 54:157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benvenuto M., Benziger D. P., Yankelev S., Vigliani G. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blaser J., Vergeres P., Widmer A. F., Zimmerli W. 1995. In vivo verification of in vitro model of antibiotic treatment of device-related infection. Antimicrob. Agents Chemother. 39:1134–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braissant O., Wirz D., Gopfert B., Daniels A. U. 2010. Use of isothermal microcalorimetry to monitor microbial activities. FEMS Microbiol. Lett. 303:1–8 [DOI] [PubMed] [Google Scholar]
- 7. Bridier A., Dubois-Brissonnet F., Boubetra A., Thomas V., Briandet R. 2010. The biofilm architecture of sixty opportunistic pathogens deciphered using a high throughput CLSM method. J. Microbiol. Methods 82:64–70 [DOI] [PubMed] [Google Scholar]
- 8. Bush L. M., Boscia J. A., Kaye D. 1988. Daptomycin (LY146032) treatment of experimental enterococcal endocarditis. Antimicrob. Agents Chemother. 32:877–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Canton R., Ruiz-Garbajosa P., Chaves R. L., Johnson A. P. 2010. A potential role for daptomycin in enterococcal infections: what is the evidence? J. Antimicrob. Chemother. 65:1126–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clinical and Laboratory Standards Institute 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline, M26-A. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 11. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing: eighteenth informational supplement, M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 12. Costerton J. W., Stewart P. S., Greenberg E. P. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 13. Dvorchik B. H., Brazier D., DeBruin M. F., Arbeit R. D. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El Helou O. C., et al. 2008. Outcome of enterococcal prosthetic joint infection: is combination systemic therapy superior to monotherapy? Clin. Infect. Dis. 47:903–909 [DOI] [PubMed] [Google Scholar]
- 15. Fabretti F., Huebner J. 2005. Implant infections due to enterococci: role of capsular polysaccharides and biofilm. Int. J. Artif. Organs 28:1079–1090 [DOI] [PubMed] [Google Scholar]
- 16. John A. K., et al. 2009. Efficacy of daptomycin in implant-associated infection due to methicillin-resistant Staphylococcus aureus: importance of combination with rifampin. Antimicrob. Agents Chemother. 53:2719–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kristich C. J., Wells C. L., Dunny G. M. 2007. A eukaryotic-type Ser/Thr kinase in Enterococcus faecalis mediates antimicrobial resistance and intestinal persistence. Proc. Natl. Acad. Sci. U. S. A. 104:3508–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lefort A., et al. 2000. Bactericidal activity of gentamicin against Enterococcus faecalis in vitro and in vivo. Antimicrob. Agents Chemother. 44:2077–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu C., et al. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:e18–e55 [DOI] [PubMed] [Google Scholar]
- 20. Mohamed J. A., Huang D. B. 2007. Biofilm formation by enterococci. J. Med. Microbiol. 56:1581–1588 [DOI] [PubMed] [Google Scholar]
- 21. Palmer K. L., Daniel A., Hardy C., Silverman J., Gilmore M. S. 2011. Genetic basis for daptomycin resistance in enterococci. Antimicrob. Agents Chemother. 55:3345–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phillips J. E., Crane T. P., Noy M., Elliott T. S., Grimer R. J. 2006. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J. Bone Joint Surg. Br. 88:943–948 [DOI] [PubMed] [Google Scholar]
- 23. Sandoe J. A., et al. 2002. Enterococcal intravascular catheter-related bloodstream infection: management and outcome of 61 consecutive cases. J. Antimicrob. Chemother. 50:577–582 [DOI] [PubMed] [Google Scholar]
- 24. Sandoe J. A., Wysome J., West A. P., Heritage J., Wilcox M. H. 2006. Measurement of ampicillin, vancomycin, linezolid and gentamicin activity against enterococcal biofilms. J. Antimicrob. Chemother. 57:767–770 [DOI] [PubMed] [Google Scholar]
- 25. Sava I. G., Heikens E., Huebner J. 2010. Pathogenesis and immunity in enterococcal infections. Clin. Microbiol. Infect. 16:533–540 [DOI] [PubMed] [Google Scholar]
- 26. Sava I. G., et al. 2010. Enterococcal surface protein contributes to persistence in the host but is not a target of opsonic and protective antibodies in Enterococcus faecium infection. J. Med. Microbiol. 59:1001–1004 [DOI] [PubMed] [Google Scholar]
- 27. Stewart P. S., Costerton J. W. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138 [DOI] [PubMed] [Google Scholar]
- 28. Trampuz A., Salzmann S., Antheaume J., Daniels A. U. 2007. Microcalorimetry: a novel method for detection of microbial contamination in platelet products. Transfusion 47:1643–1650 [DOI] [PubMed] [Google Scholar]
- 29. Trampuz A., Steinhuber A., Wittwer M., Leib S. L. 2007. Rapid diagnosis of experimental meningitis by bacterial heat production in cerebrospinal fluid. BMC Infect. Dis. 7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trampuz A., et al. 2007. Efficacy of a novel rifamycin derivative, ABI-0043, against Staphylococcus aureus in an experimental model of foreign-body infection. Antimicrob. Agents Chemother. 51:2540–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trampuz A., Widmer A. F. 2006. Infections associated with orthopedic implants. Curr. Opin. Infect. Dis. 19:349–356 [DOI] [PubMed] [Google Scholar]
- 32. Vergidis P., et al. 2011. Treatment with linezolid or vancomycin in combination with rifampin is effective in an animal model of methicillin-resistant Staphylococcus aureus foreign body osteomyelitis. Antimicrob. Agents Chemother. 55:1182–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. von Ah U., Wirz D., Daniels A. U. 2009. Isothermal micro calorimetry—a new method for MIC determinations: results for 12 antibiotics and reference strains of E. coli and S. aureus. BMC Microbiol. 9:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Widmer A. F., Frei R., Rajacic Z., Zimmerli W. 1990. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J. Infect. Dis. 162:96–102 [DOI] [PubMed] [Google Scholar]
- 35. Widmer A. F., Gaechter A., Ochsner P. E., Zimmerli W. 1992. Antimicrobial treatment of orthopedic implant-related infections with rifampin combinations. Clin. Infect. Dis. 14:1251–1253 [DOI] [PubMed] [Google Scholar]
- 36. Widmer A. F., Wiestner A., Frei R., Zimmerli W. 1991. Killing of nongrowing and adherent Escherichia coli determines drug efficacy in device-related infections. Antimicrob. Agents Chemother. 35:741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wise R., Gee T., Andrews J. M., Dvorchik B., Marshall G. 2002. Pharmacokinetics and inflammatory fluid penetration of intravenous daptomycin in volunteers. Antimicrob. Agents Chemother. 46:31–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Woodford N., et al. 2002. Detection of oxazolidinone-resistant Enterococcus faecalis and Enterococcus faecium strains by real-time PCR and PCR-restriction fragment length polymorphism analysis. J. Clin. Microbiol. 40:4298–4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zimmerli W., Frei R., Widmer A. F., Rajacic Z. 1994. Microbiological tests to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus. J. Antimicrob. Chemother. 33:959–967 [DOI] [PubMed] [Google Scholar]
- 40. Zimmerli W., Trampuz A., Ochsner P. E. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645–1654 [DOI] [PubMed] [Google Scholar]
- 41. Zimmerli W., Waldvogel F. A., Vaudaux P., Nydegger U. E. 1982. Pathogenesis of foreign body infection: description and characteristics of an animal model. J. Infect. Dis. 146:487–497 [DOI] [PubMed] [Google Scholar]
- 42. Zimmerli W., Widmer A. F., Blatter M., Frei R., Ochsner P. E., et al. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. JAMA 279:1537–1541 [DOI] [PubMed] [Google Scholar]