Abstract
The targeted inhibition of cyst but not trophic development by anidulafungin, caspofungin, and micafungin on Pneumocystis murina and Pneumocystis carinii in rodent models of Pneumocystis carinii pneumonia (PCP) was recently reported by us (M. T. Cushion et al., PLoS One 5:e8524, 2010). To better understand the effects of echinocandins on P. carinii, the same three compounds were evaluated in standard suspension and biofilm cultures supplemented with various concentrations of sera using the measurement of ATP as the indicator. In suspension cultures with 1 and 5% serum, anidulafungin was the most active compound but 10 and 20% serum abrogated the efficacy of all three echinocandins. Established biofilm cultures that included both the nonadherent and adherent phases were more resistant to micafungin than caspofungin regardless of serum concentration, while anidulafungin had significant activity at 1 and 5% serum concentrations. Nascent biofilms were mostly affected by anidulafungin in 1 and 5% serum, but none of the compounds showed significant activity in 20% serum. We show for the first time that (i) echinocandins differ in their abilities to deplete the ATP of Pneumocystis in biofilms and in suspension cultures, (ii) this variability mostly reflected the reported efficacies in animal models of infection, and (iii) high serum levels decreased the anti-Pneumocystis activities of the echinocandins in both in vitro systems.
INTRODUCTION
Treatment of Pneumocystis carinii pneumonia (PCP) remains a clinical challenge due to evolving mutations in the targets of standard anti-Pneumocystis compounds, including trimethoprim-sulfamethoxazole and atovaquone, and the toxicity associated with standard therapies (11). However, few drugs are in the discovery pipeline due to elimination of research programs supported within the pharmaceutical industry and shifting priorities of the National Institutes of Health. Identification of potential anti-Pneumocystis drugs is critical, as the numbers of infected patients expand into other niches besides the frankly immunocompromised host. Pneumocystis pneumonia is increasingly being reported as a cause of morbidity and mortality in patients with rheumatoid arthritis or other chronic conditions requiring anti-tumor necrosis factor alpha (anti-TNF-α) therapies (18, 19). Colonization with P. jirovecii is associated with a poorer outcome and more severe disease in patients with chronic obstructive pulmonary disease than in those without (23).
Standard antifungal therapies such as the azole family and amphotericin B are not effective against PCP, possibly due to the lack of ergosterol biosynthesis by these fungi (16). Anidulafungin, caspofungin, and micafungin are new antifungals that target a different biosynthetic process in fungi, glucan synthesis, and have been approved to treat infections with some Candida and Aspergillus species (28). While anecdotal reports suggest efficacy against PCP with the use of one or another of these drugs alone or in combination with standard anti-PCP agents, others did not report such effects (2, 15, 35, 37). We systematically evaluated the therapeutic effects of all three of these echinocandins in the mouse model of PCP (9) and found that while the cyst form was significantly depleted, large numbers of the trophic forms remained after 3 weeks of therapy. This modulation of the life cycle had not been reported for other fungal species and likely was due to the presence of glucan in the cyst form, while glucan is nonexistent or at very low levels in the trophic forms. Moreover, anidulafungin and caspofungin were more effective than micafungin in reducing cyst counts.
Our laboratory has been involved in preclinical drug discovery for anti-PCP agents for over 20 years. A pipeline was established that begins with an in vitro screening phase that uses a standard suspension culture method and includes activity ranking by determination of the 50% inhibitory concentration (IC50), toxicity testing, and prioritization, which ultimately feeds into the second phase of in vivo evaluation in rodent models of PCP (11). The pipeline has a predictive power that is similar to that of other standard in vitro systems featuring suspension cultures (6), but with the recent establishment of a biofilm system for P. carinii and Pneumocystis murina, there is now the unique opportunity to evaluate the activities of compounds directly on Pneumocystis biofilms in vitro. The inhibitory properties of compounds have been reported to vary (sometimes significantly) between standard suspension cultures and biofilms used for antifungal efficacy, as well as their abilities to predict clinical efficacy (3, 32–34, 36). The layers of Pneumocystis organisms found in the mammalian alveoli are redolent of biofilm stratification (8), and thus the biofilm system adds an important facet to the in vitro assessment of candidate anti-Pneumocystis compounds. The effects of serum levels were also examined because of conflicting reports of the enhancement or decrease of antifungal activities with echinocandins in media with human or animal sera (4, 26, 38).
MATERIALS AND METHODS
Pneumocystis carinii.
P. carinii was obtained from male CD rats (Charles River, Portage, MI). To provide P. carinii for these assays, animals were housed at the Cincinnati Veteran's Affairs Veterinary Medical Unit under barrier conditions. Their immune system suppression was induced by weekly injections of 20 mg/kg methylprednisolone (Depo-Medrol; Pfizer Pharmacia, New York, NY). After 2 weeks of suppression, rats were inoculated by intratracheal or intranasal instillation of 2 ×107 cryopreserved P. carinii organisms by nucleus count.
Lungs from moribund animals (about 8 wk postinoculation) were removed after IACUC-approved euthanasia, homogenized, and filtered, and erythrocytes were lysed with aqueous ammonium chloride as previously described (12). Nuclei were enumerated by microscopic analysis of Hema-3-stained slides (Fisher Scientific, Kalamazoo, MI). Aliquots of P. carinii were cryopreserved in RPMI 1640 (Invitrogen-Gibco, Grand Island, NY) containing 10% calf serum (Atlanta Biologicals, Lawrenceville, GA) and 7.5% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) and stored in liquid nitrogen.
Two separate isolates of cryopreserved P. carinii were used for the in vitro assays. Each was derived from a single immunosuppressed rat and vetted for use by assessment of the ATP content and lack of microbial contamination by plating on Sabouraud's dextrose agar and nutrient agar plates (11). The cryovials were rapidly thawed at 37°C and suspended in RPMI 1640 containing 1, 5, 10, or 20% calf serum (Atlanta Biologicals). Experimental groups were treated with pharmaceutical-grade anidulafungin (Eraxis; Pfizer, New York, NY), caspofungin (Cancidas; Merck & Co., Whitehouse Station, NJ), or micafungin (Mycamine; Astellas Pharma US, Deerfield, IL). Drugs to be tested were prepared in sterile water according to package directions and subsequently diluted in RPMI 1640-based medium. Each drug concentration was assayed in triplicate wells in at least two different suspension assays using the two different batches of P. carinii. Media without drug, with P. carinii, and with 10 μg of ampicillin/ml (Fisher Scientific, Fair Lawn, NJ) served as negative controls; pentamidine isethionate (Sigma-Aldrich) at 1 μg/ml served as the positive drug activity control.
Standard suspension cultures.
P. carinii nuclei at 5 × 107 were inoculated into wells of Costar 3548 multiwell plates in a 0.25-ml volume (Fisher Scientific). Media, experimental compounds, and control compounds were added at a 2-fold concentration in 0.25 ml of medium. Plates were incubated at 5% CO2, 37°C. At 6, 24, 48, and 72 h, 50-μl samples were transferred to opaque white plates (USA Scientific, Ocala, FL) and assessed for ATP content using ATPlite-M (Perkin-Elmer, Waltham, MA) (6).
Biofilms.
P. carinii biofilms were prepared as previously reported (8). Briefly, 5 × 107 P. carinii nuclei were inoculated in a volume of 400 μl onto 12-mm Millicell-CM membrane inserts (Millipore, County Cork, Ireland). A volume of 0.5 ml medium was added to the well outside the membrane prior to inoculation. In established biofilm assays, cultures were incubated for 7 days prior to drug exposure at 5% CO2, 37°C, in 20% calf serum-supplemented medium. At day 0, medium was aspirated from the well, allowing inserts to drain. After a second aspiration, medium containing the appropriate serum concentration and test compounds was added to the insert.
For assays of nascent biofilms, P. carinii and compounds were added simultaneously to inserts in medium with the appropriate serum content.
Medium was refreshed every 48 h by aspirating liquid from the outer well and adding fresh medium with test compounds to the insert. At the appropriate time points, duplicate inserts were gently agitated and the P. carinii organisms that were suspended in the supernatants of the biofilms, termed the “nonadherent” phase, were harvested by pipette and transferred to microcentrifuge tubes. Phosphate-buffered saline (PBS) was then added to the inserts, which were manually scraped with a modified transfer pipette to loosen P. carinii organisms adherent to the membrane, termed the “adherent” phase. Adherent insert contents were placed into microcentrifuge tubes. Cells from both the nonadherent and adherent phasess were pelleted at 10,000 rpm, for 1 min, the supernatant was aspirated, and the cells were suspended in 50 μl PBS for measurement of the ATP content. Two biofilm studies were performed with similar results. One experiment is presented as representative of the two studies.
ATP assay.
The in vitro ATP bioluminescent assay to evaluate the efficacy of compounds against P. carinii was conducted as previously described (6, 7, 17). The linear range of the ATP assay is 1 μM to 100 fM (∼20,000,000 to 2,000 relative light units [RLU]). Samples removed from suspension or biofilm cultures were lysed and placed in opaque white plates, and the ATP content was measured with a luciferin-luciferase kit (ATPlite, Perkin-Elmer, Inc.), for light emission at 562 nm with a FluoSTAR Optima plate reader (BMG Labtechnologies, Inc.). A quench control to evaluate effects on the enzyme-substrate reaction was run for every drug tested, and no inhibition was observed with any compound. In this article, we use the following terms and definitions to describe the effects of echinocandins on P. carinii: “viability” refers to a measure of cell activity reflected in the ATP content of a population of P. carinii; “efficacy” is the ability of a compound to produce a decrease in ATP content; “resistance” is the degree of unresponsiveness to a drug as reflected by the ATP content compared to that of the untreated control; “susceptibility” is the degree to which the ATP content was reduced compared to that of the untreated control; and “activity” is a measure of the drug response as reflected in the ATP content.
Data analysis.
The IC50s for suspension cultures were calculated using linear regression of the percent decrease in ATP content of a compound versus the log drug concentrations (GraphPad Software version 2 for Science; GraphPad, San Diego, CA). Based on the IC50s, each agent was classified by using an activity scale of five rankings ranging from “highly active” (compounds with an IC50 of <0.010 μg/ml), “very marked” (IC50s of 0.011 to 0.099 μg/ml), “marked” (IC50s from 0.10 to 0.99 μg/ml), “moderate” (IC50s from 1.0 to 9.99 μg/ml), “slight” (IC50s from 10.0 to 49.9 μg/ml), and “none” (i.e., inactive; IC50s of ≥50 μg/ml) (11). For evaluation of biofilms, the numbers of RLU from each echinocandin treatment and untreated control well within each serum concentration were analyzed for significance using one-way analysis of variance (ANOVA) and Tukey's multiple-comparison test (GraphPad) and subsequently expressed as the percent decrease in ATP of treated groups relative to that in untreated groups in the established or nascent biofilms.
RESULTS
Effect of serum concentrations on P. carinii in suspension cultures and biofilms.
Our laboratory routinely assesses candidate anti-Pneumocystis compounds in a standard suspension culture of RPMI 1640 supplemented with 20% calf serum and other added components (11). Because of reports of the interference or enhancing effects of serum sources and concentrations on the efficacy of echinocandins in other in vitro fungal systems (4, 26, 38), we sought to evaluate the effects of serum on the activities of three echinocandins against P. carinii in two very different in vitro systems, suspension and biofilm cultures. Both systems were chosen for assay since a growing number of reports have shown that antifungal efficacy is also dependent upon the nature of the in vitro system, with biofilms being generally more resistant than suspension cultures (25, 27). To begin these studies, the effects of serum concentrations alone on the ATP levels of P. carinii in standard suspension cultures (Fig. 1) and in our recently reported biofilm system (8) (Fig. 2) were first evaluated.
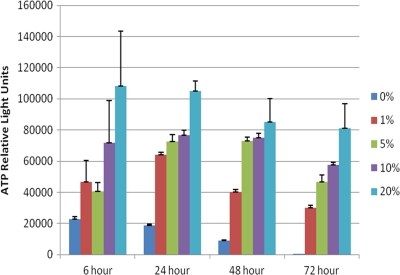
Fig. 1.
Effects of calf serum concentrations on P. carinii in suspension cultures. The cultures were set up as described in Materials and Methods. Briefly, 5 × 107 P. carinii nuclei were inoculated into triplicate wells containing medium and serum at the different concentrations. Wells were sampled over time, and ATP levels were measured using a bioluminescent, ATP-driven assay. Serum concentrations follow the color legend to the right of the figure. The differences in values for the following serum concentrations were statistically significant: (i) for 6 h, 0% versus 20%, P < 0.01; 1% versus 20%, P < 0.05; 5% versus 20%, P < 0.05; (ii) for 24 h, 0% versus all other concentrations, P < 0.001; 1% versus 10%, P < 0.05; 1% versus 20%, P < 0.001; 5% versus 20%, P < 0.001; 10% versus 20%, P < 0.001; (iii) for 48 h, 0% versus all other concentrations, P < 0.01 (versus 1%), P < 0.001 (all other concentrations); 1% versus 5%, P < 0.01; 1% versus 10 and 20%, P < 0.001; (iv) for 72 h, 0% versus 1%, P < 0.01; 0% versus 5, 10, and 20%, P < 0.001; 1% versus 10%, P < 0.01; 1% versus 20%, P < 0.001; 5% versus 20%, P < 0.01; 10% versus 20%, P < 0.05.
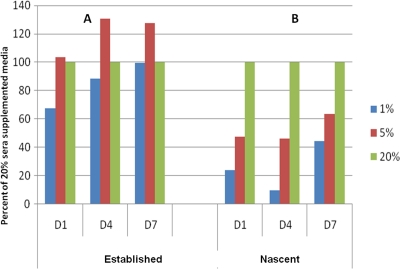
Fig. 2.
Effects of calf serum concentrations on established and newly inoculated P. carinii biofilms. Serum concentrations follow the color legend to the right of the figure. Data are expressed as the percentage of ATP from biofilms established with 20% serum for 7 days (control) and then switched to 1 and 5% sera (A) or as the percentage of viability of P. carinii inoculated with 1 and 5% serum versus 20% serum (B). (A) No significant differences among the biofilm ATP levels. (B) The differences in values between the following serum concentrations were significant: for day 1, 1% versus 20%, P < 0.001; 5% versus 20%, P < 0.01; for day 4, 1% versus 20%, P < 0.001; 5% versus 20%, P < 0.001; for day 7, 1% versus 20%, P > 0.05; 5% versus 20%, P > 0.05.
It was clear that P. carinii in the standard suspension cultures required a serum supplement, and the optimal concentration was 20% (vol/vol) serum (Fig. 1). Serum-free cultures did not recover from cryopreservation and lost 99.5% of the RLU by day 3 compared to P. carinii in cultures with 20% serum. Serum-free cultures were not used in subsequent studies. Supplements of 5 to 10% retained viability for 48 h, but the ATP levels dropped significantly at 72 h. The ATP content of P. carinii in medium with a 20% calf serum supplement did not significantly change over 72 h in suspension culture. Higher levels of sera were found to be deleterious to the viability of P. carinii in previous studies (10) and were not evaluated here.
The effect of serum concentration on P. carinii biofilm viability was evaluated using both “nascent” biofilms and “established” biofilms. Nascent biofilms were defined as those resulting from coinoculation of membrane inserts with P. carinii and drug, serum, or both, while “established” biofilms were those that were cultured on inserts for 7 days in 20% serum-supplemented medium, after which the medium was changed to the test serum concentrations and subsequently sampled. As illustrated in Fig. 2, established biofilm cultures were less sensitive to changes in serum concentration (Fig. 2A) than nascent biofilms with 5% and 1% serum-supplemented media (Fig. 2B). (Data are expressed as the percentage of RLU of the cultures supplemented with 20% serum [“control”] in each panel). There were no significant differences between the biofilms established in 20% serum supplements after they were switched to 1 and 5% serum concentrations at any of the time points. Conversely, nascent biofilms initiated with 1 and 5% serum concentrations had statistically lower ATP values than those initiated with a 20% serum concentration, at days 1 and 4, and failed to produce robust biofilms (Fig. 2B). Adequate serum concentration was crucial to P. carinii viability when the organisms were in the process of attachment to the membrane inserts, but this effect lessened after 7 days in culture.
Effect of serum concentrations on echinocandin efficacy in suspension cultures of P. carinii.
Increasing serum concentration decreased the ATP inhibitory activities of anidulafungin, caspofungin, and micafungin against P. carinii in suspension cultures. Shown in Table 1 are the concentrations of the echinocandins that decreased the ATP levels by 50% compared with those of the untreated organisms in the respective serum concentrations (IC50). P. carinii organisms were exposed to 100, 10, and 1 μg/ml anidulafungin, caspofungin, and micafungin in media containing 1 to 20% calf serum. In 1% and 5% serum supplements (the minimal concentrations needed to sustain temporary viability) (Fig. 1), the anti-P. carinii activity of anidulafungin clearly increased over time. For example, in 1% serum-supplemented medium, the inhibitory activity increased from an IC50 of 10.6 μg/ml at 6 h (considered slight activity) to 0.15 μg/ml at 72 h (considered marked inhibitory activity). At 10% and 20% sera, the ability to decrease ATP levels by anidulafungin was completely inhibited (no activity), even at the highest concentration tested (100 μg/ml). Caspofungin had a less-robust anti-P. carinii effect and was also blocked by increasing serum concentrations, although moderate inhibitory activity was observed at 1 and 5% serum concentrations after 48 h of exposure. Micafungin treatment decreased ATP levels at a “moderate” level of activity in 5% serum concentration at three time points (8.5, 9.9, and 2.5 μg/ml), had moderate and marked activity in 1% serum concentration at 48 and 72 h postexposure (0.8 and 3.7 μg/ml), and showed slight activity in 10 and 20% serum-supplemented media after 72 h of exposure (20.7 and 47.64 μg/ml); this was the only echinocandin to show any activity at the higher serum levels.
Table 1.
IC50s of echinocandin-treated suspension cultures of P. carinii in 1 to 20% serum concentrations
| Echinocandin and time of measurement | IC50 (μg/ml) (activity rankinga) at serum concn of: |
|||
|---|---|---|---|---|
| 1% | 5% | 10% | 20% | |
| Anidulafungin | ||||
| 6 h | 10.6 (S) | 18.2 (S) | >100 (N) | >100 (N) |
| 24 h | 2.6 (Mo) | 3.0 (Mo) | >100 (N) | >100 (N) |
| 48 h | 0.2 (Ma) | 0.4 (Ma) | >100 (N) | >100 (N) |
| 72 h | 0.15 (Ma) | 0.4 (Ma) | >100 (N) | >100 (N) |
| Caspofungin | ||||
| 6 h | >100 (N) | 20.6 (S) | >100 (N) | >100 (N) |
| 24 h | 9.4 (Mo) | 24.2 (S) | >100 (N) | >100 (N) |
| 48 h | 2.9 (Mo) | 7.6 (Mo) | >100 (N) | 62.8 (N) |
| 72 h | 3.3 (Mo) | 4.0 (Mo) | >100 (N) | 83.8 (N) |
| Micafungin | ||||
| 6 h | >100 (N) | 8.5 (Mo) | >100 (N) | >100 (N) |
| 24 h | >100 (N) | 27.4 (S) | >100 (N) | >100 (N) |
| 48 h | 0.8 (Ma) | 9.9 (Mo) | >100 (N) | >100 (N) |
| 72 h | 3.7 (Mo) | 2.5 (Mo) | 20.7 (S) | 47.64 (S) |
Based on the activity ranking system described in Materials and Methods. Ma, marked; Mo, moderate; S, slight; N, none.
Effect of serum concentrations on echinocandin efficacy in nascent and established P. carinii biofilms.
Maturity of the biofilm, as well as serum concentration, affected the ATP levels of P. carinii biofilms during echinocandin treatment (Table 2). These data are expressed as the percent reduction in ATP (% inhibition) of biofilms treated with 100 μg/ml echinocandin versus that of untreated controls and not as IC50s, since biofilms cannot be continuously sampled over time as in the suspension culture system and would require hundreds of insert cultures to determine the IC50, which would be cost prohibitive. As shown in Table 2, P. carinii organisms were exposed to anidulafungin, caspofungin, or micafungin in media containing 1, 5, and 20% serum concentrations at the time of inoculation onto the insert (nascent biofilms) or after 7 days of incubation in standard 20% serum-supplemented medium (established biofilms).
Table 2.
Percent reduction in ATP of P. carinii biofilms exposed to 100 μg/ml anidulafungin, caspofungin, and micafungin in 1%, 5%, and 20% serum concentrations
| Biofilm and day of exposure | % ATP reduction in biofims in indicated serum concna |
|||||
|---|---|---|---|---|---|---|
| Nascent |
Established |
|||||
| 1% | 5% | 20% | 1% | 5% | 20% | |
| Anidulafungin | ||||||
| 1 | 98.3 | 55.1 | 4.5 | 9.5 | 6.2 | 0 |
| 4 | 98.8 | 96.7 | 0 | 98.4 | 64.2 | 0 |
| 7 | 97.1 | 97.8 | 0 | 97.7 | 94.6 | 0 |
| Caspofungin | ||||||
| 1 | 28.6 | 19.4 | 27.6 | 21.9 | 27.1 | 20.9 |
| 4 | 85.7 | 9.2 | 6.4 | 0 | 9.4 | 14.1 |
| 7 | 96.4 | 13.9 | 0 | 48.9 | 46.2 | 33.5 |
| Micafungin | ||||||
| 1 | 22.9 | 29.8 | 23.3 | 3.2 | 21.9 | 17.0 |
| 4 | 3.6 | 62.1 | 29.2 | 0 | 18.8 | 0 |
| 7 | 32.6 | 0 | 0 | 0 | 0 | 0 |
The ATP levels of P. carinii in each serum concentration in the echinocandin-treated groups were compared to those in the same concentration without the addition of the echinocandin to achieve the percent reduction. The following results reached significance (P < 0.05) after analysis by one-way ANOVA and Tukey's multiple-comparison test. For nascent biofilms, for day 1 with 1% serum, anidulafungin versus caspofungin, anidulafungin versus micafungin; for day 1 with 5% serum, anidulafungin versus caspofungin; for day 4 with 1% serum, anidulafungin versus micafungin, caspofungin versus micafungin; for day 4 with 5% serum, anidulafungin versus caspofungin; for day 4 with 20% serum, anidulfungin versus micafungin; for day 7 with 1% serum, anidulfungin versus micafungin, caspofungin versus micafungin; for day 7 with 5% serum, anidulafungin versus caspofungin, anidulafungin versus micafungin, caspofungin versus micafungin. For established biofilms, for day 1, no significant differences at any serum level; for day 4 with 1% serum, anidulafungin versus micafungin, anidulafungin versus caspofungin; for day 4 with 5% serum, anidulafungin versus caspofungin; anidulafungin versus micafungin; for day 7 with 1% serum, anidulfungin versus caspofungin, anidulafungin versus micafungin, caspofungin versus micafungin; for day 7 with 5% serum, anidulafungin versus caspofungin, anidulafungin versus micafungin, caspofungin versus micafungin.
In nascent biofilms, anidulafungin prevented biofilm formation in 1% and 5% serum concentrations, as evidenced by an almost complete loss of ATP at all time points except at day 1 in 5% serum, at which point there was a 55% loss. However, at the standard 20% serum supplement, anidulafungin was not effective in sustaining the reduction in ATP levels over the sampling period. This general trend in loss of ATP at the lower serum concentrations was apparent in the established biofilms as well but required at least a day of exposure. As in the nascent biofilms, there was a loss of anidulafungin activity in the established biofilms in 20% serum.
Caspofungin had less activity than anidulafungin against P. carinii in established or nascent biofilms at the 5 and 20% serum concentrations, with a slight to moderate effect at the 1% serum level. Micafungin was the least effective treatment in either biofilm system. Generally, the established biofilms were less perturbed by the addition of any of the echinocandins than those that were not yet formed, and anidulafungin was the most efficacious of the three echinocandins tested.
Response of nonadherent and adherent P. carinii organisms in biofilms to serum and echinocandins.
In Table 2, the two compartments of a P. carinii biofilm were combined as a means to compare the overall effects of the serum concentrations and the echinocandins on nascent biofilms and established biofilms. When the ATP levels of the nonadherent and adherent P. carinii populations in the same study were analyzed individually, anidulafungin was shown to have similar inhibitory effects for the two populations (see Tables S1 and S2 in the supplemental material). With 20% serum concentrations, both phases were mostly resistant to this echinocandin, with the exception of the results on day 1 (nascent, nonadherent) and day 7 (established, nonadherent), which showed moderate to slight activities. Thus, serum concentrations exerted a pronounced effect on the inhibitory capacity of anidulafungin in both compartments of the nascent and established biofilms, with lower concentrations of serum providing higher efficacy; in other words, higher serum concentrations blocked the inhibitory effects of anidulafungin.
Generally, the efficacy of caspofungin on the nonadherent phase increased over time of exposure at the 5 and 1% serum concentrations in the nascent and established biofilms. While caspofungin had a pronounced effect on the ATP levels of adherent P. carinii in 1% serum in nascent biofilms (see Table S2 in the supplemental material), this effect was abrogated at 5 or 20% serum concentrations. Interestingly, the ATP pools of the adherent populations in the established biofilms were affected moderately over time at all serum concentrations (see Table S2 in the supplemental material).
As for the total biofilm data shown in Table 2, micafungin was the least effective of the three echinocandins in either the nascent or established biofilms. However, it exerted more of an inhibitory effect on the ATP in the nonadherent populations in the nascent biofilms than in the established biofilms, although at a 20% serum concentration, this inhibitory effect was overcome by 7 days of exposure.
DISCUSSION
Many drugs have reduced efficacy against fungi within biofilms (1, 13, 21, 29, 31), and microbes within biofilms are less susceptible to host immune responses (20). Biofilm formation is an especially important problem with pathogenic fungi, and recommendations for in vitro screening of antifungal compounds have now begun to include biofilms assays (1, 30, 31), since there is a noticeable lack of correlation between the standard CLSI (Clinical and Laboratory Standards Institute) (5) recommendations for screening suspension cultures and patient outcomes (30). We recently reported a method for the establishment of biofilms by the rodent-derived P. murina (from mouse) and P. carinii (from rats) (8). The establishment of such a system was undertaken due to our observation that the structure of Pneumocystis organisms within the lung alveoli resembles the layers of a fungal biofilm. We sought such a system to better understand the nonresponsiveness of many clinical infections of Pneumocystis carinii pneumonia (PCP) to standard antipneumocystis therapies as well as to explore potential strategies used by these fungi to colonize individuals with intact immune systems. Our data suggest that biofilm formation in the mammalian lung is a potential mechanism by which these fungi might resist therapeutic interventions.
Our laboratory typically uses a suspension culture to screen candidate drugs that is similar to broth microdilution methods adopted for antifungal susceptibility determinations (11). A direct comparison of the suspension culture and biofilm assays is not possible, due to the difference in results expressed in of IC50 (suspension) and percent inhibition (biofilms) and the single dose of 100 μg/ml used for the biofilm assays. However, all of the echinocandins in the suspension assay provided moderate to marked activity after 72 h in 1% or 5% serum concentrations. Generally, ATP levels were reduced over time at these serum concentrations, with anidulafungin achieving an IC50 of <2 μg/ml after 48 to 72 h of exposure in 1 and 5% serum concentrations. This value is in line with echinocandin activity that is considered efficacious in other fungal assay systems (28). As with the P. carinii biofilms and other fungal culture systems, a pronounced abrogation of effect was observed with higher concentrations of (10 and 20%) serum additives. We did not observe any paradoxical effects, as reported for Candida albicans (22) (data not shown), but did convincingly show that the efficacy of anidulafungin in this assay was higher at lower serum concentrations than the efficacies of caspofungin and micafungin.
The efficacies of the echinocandins against P. carinii in the suspension cultures were somewhat surprising, since P. carinii organisms in the suspension culture are primarily trophic forms, which would have been predicted to be less susceptible to the echinocandins since they contain little or no glucan, and in our previous animal studies, viable trophic forms remained after echinocandin treatment (9). The in vitro susceptibility reported here may be due to other, lesser-known mechanisms of action of these compounds or the artificiality of suspension cultures, as previously noted (5, 32).
Anidulafungin was the only echinocandin that could consistently reduce viability by 50% or more in nascent or established biofilms in 1% or 5% serum concentrations. The established biofilms were generally more resistant to micafungin than caspofungin at all three serum concentrations, with slightly more efficacy in the newly forming (nascent) biofilms, especially caspofungin at a 1% serum concentration. Increased serum concentrations of 10% (data not shown) or 20% totally abrogated the efficacy of anidulafungin. This abrogation of activity with addition of sera has also been reported for Candida glabrata using the microdilution methodology (38). In another study of 16 Candida isolates and 8 Aspergillus isolates, addition of 50% human serum using variants of the microdilution method resulted in consistently elevated MICs for anidulafungin and micafungin (26). The inhibitory effect of serum has been attributed to protein binding of the compounds and subsequent inhibition of effect (26), but there is no general consensus regarding the mechanism.
The heightened efficacy of anidulafungin over that of caspofungin and micafungin was also observed in the rodent animal models of Pneumocystis pneumonia when cyst numbers were enumerated, suggesting that there may differences in metabolism by the animals or targeting and/or entry of these compounds into the organisms. The variable responses of different fungi to the different echinocandins have been noted for the dematiaceous fungi and among Candida species (14), supporting our lack of understanding of differences in the mechanisms of action among these β-1,3-d-glucan inhibitors.
Studies of caspofungin on the different phases of Aspergillus fumigatus biofilms showed more activity against the actively growing phases than against the mature, multicellular structures (24), which our studies of Pneumocystis appear to support with the greater effects on the nonadherent cells than on the adherent biofilm cells; these findings are similar to the enhanced effects against the suspension culture organisms. The apparent efficacy of the echinocandins on the nonadherent cell populations within the nascent biofilms may be explained by the inhibition of glucan which is necessary to establish the biofilm structure. In our previous study, the β-1,3-d-glucan content of forming P. carinii biofilms increased from about 90 to 120 pg/ml over 8 days, although once the biofilms are established, there may be less reliance on the glucan for structural purposes. Biofilms are never formed in suspension cultures, and this could explain the enhanced efficacy of the echinocandins in that system.
In summary, there are several concepts that emerged from these studies regarding the effects of the antifungal drugs anidulafungin, caspofungin, and micafungin on P. carinii in two different in vitro assay systems with various concentrations of calf serum: (i) P. carinii organisms were more susceptible to the echinocandins in the suspension assay than in either of the biofilm systems; (ii) newly forming (nascent) biofilms were more susceptible than established biofilms; (iii) the populations in the nonadherent phase of the biofilms were generally more susceptible to echinocandin activity than the adherent populations; (iv) higher serum concentrations abrogated the efficacy of the echinocandins, especially anidulafungin, in suspension or biofilm assay systems; and (v) exposure to anidulafungin consistently reduced the ATP levels better than exposure to caspofungin or micafungin in either assay system.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health (NIAID R01 AI076104) and the Department of Veterans Affairs. We also recognize the support of the NIH (through contract mechanism N01-A1-25647) for development of the in vitro methods used for the screening of candidate anti-Pneumocystis agents.
We acknowledge the advice of Chris Lambros, Program Officer, NIAID.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 25 July 2011.
REFERENCES
- 1. Bachmann S. P., et al. 2002. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 46:3591–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beltz K., et al. 2006. Combined trimethoprim and caspofungin treatment for severe Pneumocystis jiroveci pneumonia in a five year old boy with acute lymphoblastic leukemia. Klin. Padiatr. 218:177–179 [DOI] [PubMed] [Google Scholar]
- 3. Chandra J., et al. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chiller T., Farrokhshad K., Brummer E., Stevens D. A. 2000. Influence of human sera on the in vitro activity of the echinocandin caspofungin (MK-0991) against Aspergillus fumigatus. Antimicrob. Agents Chemother. 44:3302–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 3rd ed M27-A3 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Cushion M. T., Chen F., Kloepfer N. 1997. A cytotoxicity assay for evaluation of candidate anti-Pneumocystis carinii agents. Antimicrob. Agents Chemother. 41:379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cushion M. T., Collins M., Hazra B., Kaneshiro E. S. 2000. Effects of atovaquone and diospyrin-based drugs on the cellular ATP of Pneumocystis carinii f. sp. carinii. Antimicrob. Agents Chemother. 44:713–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cushion M. T., Collins M. S., Linke M. J. 2009. Biofilm formation by Pneumocystis spp. Eukaryot. Cell 8:197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cushion M. T., et al. 2010. Echinocandin treatment of Pneumocystis pneumonia in rodent models depletes cysts leaving significant trophic burdens that cannot transmit the infection. PLoS One 5(1):e8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cushion M. T., Ruffolo J. J., Linke M. J., Walzer P. D. 1985. Pneumocystis carinii: growth variables and estimates in the A549 and WI-38 VA13 human cell lines. Exp. Parasitol. 60:43–54 [DOI] [PubMed] [Google Scholar]
- 11. Cushion M. T., Walzer P. D. 2009. Preclinical drug discovery for new anti-Pneumocystis compounds. Curr. Med. Chem. 16:2514–2530 [DOI] [PubMed] [Google Scholar]
- 12. Cushion M. T., et al. 2006. In vitro selection and in vivo efficacy of piperazine- and alkanediamide-linked bisbenzamidines against Pneumocystis pneumonia in mice. Antimicrob. Agents Chemother. 50:2337–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Bonaventura G., et al. 2006. Biofilm formation by the emerging fungal pathogen Trichosporon asahii: development, architecture, and antifungal resistance. Antimicrob. Agents Chemother. 50:3269–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Espinel-Ingroff A. 2003. In vitro antifungal activities of anidulafungin and micafungin, licensed agents and the investigational triazole posaconazole as determined by NCCLS methods for 12,052 fungal isolates: review of the literature. Rev. Iberoam. Micol. 20:121–136 [PubMed] [Google Scholar]
- 15. Hof H., Schnulle P. 2008. Pneumocystis jiroveci pneumonia in a patient with Wegener's granulomatosis treated efficiently with caspofungin. Mycoses 51(Suppl. 1):65–67 [DOI] [PubMed] [Google Scholar]
- 16. Joffrion T. M., Cushion M. T. 2010. Sterol biosynthesis and sterol uptake in the fungal pathogen Pneumocystis carinii. FEMS Microbiol. Lett. 311:1–9 [DOI] [PubMed] [Google Scholar]
- 17. Kaneshiro E. S., Collins M. S., Cushion M. T. 2000. Inhibitors of sterol biosynthesis and amphotericin B reduce the viability of Pneumocystis carinii f. sp. carinii. Antimicrob. Agents Chemother. 44:1630–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaur N., Mahl T. C. 2004. Pneumocystis carinii pneumonia with oral candidiasis after infliximab therapy for Crohn's disease. Dig. Dis. Sci. 49:1458–1460 [DOI] [PubMed] [Google Scholar]
- 19. Kaur N., Mahl T. C. 2007. Pneumocystis jiroveci (carinii) pneumonia after infliximab therapy: a review of 84 cases. Dig. Dis. Sci. 52:1481–1484 [DOI] [PubMed] [Google Scholar]
- 20. Martinez L. R., Casadevall A. 2006. Cryptococcus neoformans cells in biofilms are less susceptible than planktonic cells to antimicrobial molecules produced by the innate immune system. Infect. Immun. 74:6118–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martinez L. R., Casadevall A. 2006. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 50:1021–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miceli M. H., Bernardo S. M., Lee S. A. 2009. In vitro analysis of the occurrence of a paradoxical effect with different echinocandins and Candida albicans biofilms. Int. J. Antimicrob. Agents 34:500–502 [DOI] [PubMed] [Google Scholar]
- 23. Morris A., et al. 2004. Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. Am. J. Respir. Crit. Care Med. 170:408–413 [DOI] [PubMed] [Google Scholar]
- 24. Mowat E., et al. 2008. Phase-dependent antifungal activity against Aspergillus fumigatus developing multicellular filamentous biofilms. J. Antimicrob. Chemother. 62:1281–1284 [DOI] [PubMed] [Google Scholar]
- 25. Nett J. E., Guite K. M., Ringeisen A., Holoyda K. A., Andes D. R. 2008. Reduced biocide susceptibility in Candida albicans biofilms. Antimicrob. Agents Chemother. 52:3411–3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Odabasi Z., Paetznick V., Rex J. H., Ostrosky-Zeichner L. 2007. Effects of serum on in vitro susceptibility testing of echinocandins. Antimicrob. Agents Chemother. 51:4214–4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pierce C. G., et al. 2008. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 3:1494–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pound M. W., Townsend M. L., Drew R. H. 2010. Echinocandin pharmacodynamics: review and clinical implications. J. Antimicrob. Chemother. 65:1108–1118 [DOI] [PubMed] [Google Scholar]
- 29. Ramage G., Saville S. P., Thomas D. P., Lopez-Ribot J. L. 2005. Candida biofilms: an update. Eukaryot. Cell 4:633–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramage G., Vande W. K., Wickes B. L., Lopez-Ribot J. L. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramage G., Vandewalle K., Bachmann S. P., Wickes B. L., Lopez-Ribot J. L. 2002. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob. Agents Chemother. 46:3634–3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rex J. H., et al. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard, 2nd ed M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 33. Shuford J. A., Piper K. E., Steckelberg J. M., Patel R. 2007. In vitro biofilm characterization and activity of antifungal agents alone and in combination against sessile and planktonic clinical Candida albicans isolates. Diagn. Microbiol. Infect. Dis. 57:277–281 [DOI] [PubMed] [Google Scholar]
- 34. Tobudic S., Kratzer C., Lassnigg A., Graninger W., Presterl E. 2010. In vitro activity of antifungal combinations against Candida albicans biofilms. J. Antimicrob. Chemother. 65:271–274 [DOI] [PubMed] [Google Scholar]
- 35. Utili R., et al. 2007. Efficacy of caspofungin addition to trimethoprim-sulfamethoxazole treatment for severe pneumocystis pneumonia in solid organ transplant recipients. Transplantation 84:685–688 [DOI] [PubMed] [Google Scholar]
- 36. Vediyappan G., Rossignol T., d'Enfert C. 2010. Interaction of Candida albicans biofilms with antifungals: transcriptional response and binding of antifungals to beta-glucans. Antimicrob. Agents Chemother. 54:2096–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Waters L., Nelson M. 2007. The use of caspofungin in HIV-infected individuals. Expert. Opin. Invest. Drugs. 16:899–908 [DOI] [PubMed] [Google Scholar]
- 38. Wiederhold N. P., et al. 2007. In vivo efficacy of anidulafungin and caspofungin against Candida glabrata and association with in vitro potency in the presence of sera. Antimicrob. Agents Chemother. 51:1616–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.