Abstract
Surgical site infection (SSI) is a common and preventable complication of surgery, but the relative importance of individual measures recommended by guidelines has not been determined. Elective general surgical, neurological, and orthopedic procedures requiring antibiotic prophylaxis from a 3-month period were retrospectively studied to determine concordance with SSI prevention guidelines and to identify factors which predicted the development of SSIs. A total of 216 surgeries were reviewed, with 18 SSIs (8.3%). A mean of 1.4 antibiotic prophylaxis errors per surgery were identified, with correct antibiotic type identified for 64%, antibiotic timing for 83%, supplemental antibiotic dosing for 34%, and antibiotic duration of less than 24 h for 44%. Normothermia was present in 79% of surgeries, and normoglycemia was present in 17%. Univariate analysis of the SSI rate identified four significant factors. Antibiotic prophylaxis for less than 24 h postoperatively (odds ratio [OR], 0.213; 95% confidence interval [95% CI]0.060 to 0.757) and neurosurgery (OR, 0.118; 95% CI, 0.15 to 0.903) correlated with a reduced risk of SSI. The mean number of prophylaxis errors (OR, 1.6; 95% CI, 1.02 to 2.4) and a duration of surgical drainage for more than 3 days (OR, 2.679; 95% CI, 1.009 to 7.113) predicted SSI. By multivariate analysis, errors in individual antibiotic prophylaxis measures were not significantly associated with SSI; however, the presence of more than two errors was significant (OR, 4.030; 95% CI, 1.018 to 15.96). A strong correlation was identified between the degree of concordance to SSI prevention guidelines and the SSI rate (P = 0.001, Mantel-Haenszel linear-by-linear association chi-square test).
INTRODUCTION
National and international surveillance programs (9, 33) and numerous guidelines (1–3, 11, 19, 24, 26) have been developed to prevent surgical site infection (SSI), the most common nosocomial infection in postsurgery patients. These guidelines emphasize the role of appropriate evidence-based antibiotic prophylaxis, hair removal by clipping as needed (31), avoidance of hypothermia (except in cardiac surgery) (16), and normoglycemia for diabetic patients (17) to reduce SSI rates.
A recent pilot project at our institution prior to the current study demonstrated that implementation of such guidelines can be successful locally, but compliance elsewhere has been shown to be poor (4, 18). Furthermore, there is a high prevalence of multidrug-resistant bacteria in Singapore hospitals, and inappropriate antibiotic use, including for SSI prophylaxis, can aggravate the selective pressure for antimicrobial resistance (15).
We evaluated SSI prevention practiced in our hospital to assess concordance with published international evidence-based SSI prevention guidelines and the effects of failure to comply with subsequent SSI rates.
MATERIALS AND METHODS
Study cohort.
Elective inpatient general surgical, neurosurgical, and orthopedic cases recommended to receive antibiotic prophylaxis by the Scottish Intercollegiate Guidelines Network (SIGN) (26) were retrospectively identified from operating lists. The study period was 3 months, from 1 January to 31 March 2008, at Tan Tock Seng Hospital, Singapore, a 1,400-bed university teaching hospital.
Case records from wards, operating theaters, and clinics as well as nationwide polyclinic and emergency department electronic medical records were obtained and reviewed. Details collated include demographics, medical history, surgery details, and American Society of Anesthesiologists (ASA) scores. Antibiotic choice, dosage, timing of administration and supplementation, time of surgical incision, and completion were also recorded. Duration of antibiotic treatment was estimated from first preoperative dose to time of last administration. Presence of postoperative surgical drains, dates of surgical drain removal, and discharge from hospital were noted. SSIs within 30 days were identified if documented explicitly or inferable from records of clinical symptoms and signs, laboratory evaluation, and treatment. These were classified as superficial incisional, deep incisional, or organ/space, as defined by National Healthcare Safety Network (NHSN) criteria (14). All positive microbiological cultures from surgical and other sites collected postoperatively were collected to identify possible perioperative acquisition of multidrug-resistant organisms (MDROs). MDROs were defined as having resistance to at least three antibiotic classes, including penicillins, cephalosporins, carbapenems, aminoglycosides, fluoroquinolones, tetracyclines, and folate antagonists (10).
Surgical site infection prevention guidelines.
Recently published SSI prevention guidelines were obtained and summarized (see the supplemental material). Minimum expected standards were synthesized from these guidelines and adapted for local practice.
Antibiotic prophylaxis standards include (i) monotherapy with first- or second-generation cephalosporin for orthopedic surgery and neurosurgery and metronidazole plus first- or second-generation cephalosporin for colorectal surgery, (ii) antibiotics given within the hour before skin incision or 60 to 120 min before for ciprofloxacin and vancomycin, (iii) supplemental dosing given during surgery if operating time is longer than the expected therapeutic window of the prophylactic antibiotic or if there is major blood loss (more than 1.5 liters), and (iv) prophylactic antibiotic discontinued within 24 h after completion of surgery.
For the first standard, vancomycin is considered an acceptable prophylactic antibiotic due to the high prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in Singapore hospitals, even if there is no β-lactam allergy or known MRSA colonization. Antibiotic dosages and redosing frequencies were adopted from the literature (2).
Homeostasis standards include the following. (i) Hair removal is minimized, and clippers are used if necessary (31). (ii) Body temperature is maintained at ≥36°C perioperatively and for at least 6 h after surgery (16). (iii) Normoglycemia is maintained for 48 h postoperatively in diabetic patients by a fasting serum glucose level of ≥45 mg/dl and ≤200 mg/dl (17).
Additional measures, such as preoperative patient hygiene and MRSA active surveillance and decolonization were considered beyond the scope of this study.
Analysis of concordance.
To estimate the degree of concordance with antibiotic guidelines, each patient was assessed with the four antibiotic prophylaxis standards listed above. Contravention to each standard was considered one “error,” resulting in a maximum number of 3 or 4 errors per patient (depending on the need for dose supplementation). The number of errors was correlated with SSI rates and the presence of MDROs. Univariate and multivariate analyses were performed to compare those who did and did not develop SSI. To identify trends associated with antibiotic prophylaxis error rates, Mantel-Haenszel linear-by-linear association chi-square test was used. Statistical calculations were performed using PASW Statistics (release 18.0).
Ethics.
Approval for the study was obtained from the chairman of the Medical Board at Tan Tock Seng Hospital as a patient safety and quality improvement project.
RESULTS
Baseline data.
In total, 222 patients were identified. For two patients, antibiotic prophylaxis was not documented, and timings for four patients were unverifiable. These were excluded from this analysis (Table 1).
Table 1.
Summary of patient characteristics, antibiotic prophylaxis, and outcomes for each surgical discipline
| Characteristica | Value for: |
|||
|---|---|---|---|---|
| Colorectal surgery (n = 50) | Neurosurgery (n = 67) | Orthopedic surgery (n = 99) | Summary (n = 216) | |
| Median patient age (range) in yr | 63 (29–95) | 49 (15–85) | 61 (19–84) | 60 (15–95) |
| Male | 28 (56) | 40 (60) | 38 (38) | 106 (49) |
| ASA score of ≥3b | 16 (32) | 25 (37) | 23 (23) | 64 (30) |
| Type of surgery (no. of patients) | Colectomy (22), anterior resection (19), others (8) | Craniotomy (26), spinal surgery (16), aneurysm repair (7) | Knee replacement (61), spinal surgery (25), arthroplasty (13) | |
| Median length of surgery (range) in min | 155 (35–440) | 185 (50–690) | 175 (60–655) | 170 (35–690) |
| Drains | ||||
| No. of patients | 13 (26) | 39 (58) | 76 (77) | 128 (59) |
| Median duration in situ (range) in days | 3 (1–32) | 1 (1–59) | 3 (1–5) | 3 (1–59) |
| Prophylactic antibiotic (no. of patients) | Ceftriaxone + metronidazole (37), cefazolin + metronidazole (6), ciprofloxacin + metronidazole (5), others (2) | Cefazolin (60), vancomycin (4), others (3) | Cefazolin (61), cefazolin + gentamicin/ciprofloxacin (30), vancomycin +/− gentamicin (8) | |
| Antibiotics | ||||
| No. given for ≤24 h | 23 (46) | 58 (87) | 15 (15) | 96 (44) |
| Median (range) in days | 1 (0–8) | 1 (0–32) | 3 (0–9) | 2 (0–32) |
| Appropriate choice/dose | 6 (12) | 64 (96) | 68 (69) | 138 (64) |
| Timed correctly | 41 (82) | 54 (81) | 85 (86) | 180 (83) |
| Supplemented correctly | 0/2 (0) | 8/23 (35) | 27/77 (35) | 35/102 (34) |
| No. with normoglycemia (≤200mg/dl)/no. of diabetic patients | 0/8 (0) | 4/13 (31) | 3/20 (15) | 7/41 (17) |
| Normothermia (≥36.0°C) | 33 (66) | 47 (70) | 91 (92) | 171 (79) |
| No. of SSIs | 6 (12) | 1 (1.5) | 11 (11.1) | 18 (8.3) |
| Median hospital stay (range) in days | 8 (1–42) | 11 (1–97) | 6 (1–91) | 7 (1–97) |
| No. of positive cultures | 6 | 13 | 3 | 22 |
| Multidrug-resistant organisms | 5 (83) | 6 (46) | 2 (67) | 13 (59) |
Number (percentage in parentheses) shown unless otherwise specified.
ASA, American Society of Anesthesiologists.
Cefazolin (1 g) intravenously used alone or in combination was the main prophylactic antibiotic in 157 (73%) operations. Appropriate surgical antibiotic prophylaxis selection was recorded in 64% of cases. The majority (83%) of antibiotics were appropriately administered in the hour before incision, of which 122 were given within 30 min. However, only 2/20 (10%) of vancomycin or ciprofloxacin doses were given 60 to 120 min prior to incision. All antibiotic dosages were given as recommended. The need for perioperative redosing was uncommon, as the majority of antibiotics were expected to maintain therapeutic tissue levels throughout surgery. Supplemental doses were given to 91 patients, with 47 (52%) of these administered less than 120 min after the initial dose.
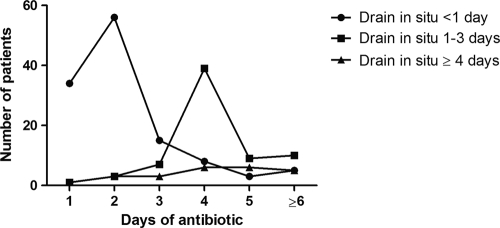
For 96 patients (44%), antibiotic prophylaxis was stopped in the operating theater or within 24 h. For 91 patients (42%), prophylaxis was continued for 3 days or more. The reasons for continuation were not documented but correlated with the presence of postoperative drains. A total of 90 of the 121 patients (74%) with either no drain or a drain in situ for less than 24 h received antibiotic prophylaxis for less than 24 h. In contrast, for patients with a drain in situ for more than 1 day, only 10 of 95 (11%) received prophylaxis for less than 24 h (P < 0.0001, chi-square test) (Fig. 1).
Fig. 1.
Postoperative drainage for more than 24 h was significantly associated with antibiotic prophylaxis for more than 24 h (P < 0.0001, chi-square test).
The hair removal technique was documented for only 14 patients (12/14 [86%] by clipping). Perioperative temperature recordings were not available. Postoperative records were analyzed instead with normothermia maintained in 179 patients (79%). Postoperative normoglycemia was maintained for only 17% of the 41 patients with diabetes mellitus.
Surgical site infections.
A total of 18 SSIs developed in 18 patients (8.3%). Three were deep incisional SSIs, and three were organ space SSIs requiring surgical revision, with no mortalities. The remainders were superficial incisional SSIs. A microbiological diagnosis was available for only 5 cases. Isolates included 1 methicillin-sensitive Staphylococcus aureus, 2 Enterobacteriaceae, and 2 Pseudomonas aeruginosa isolates.
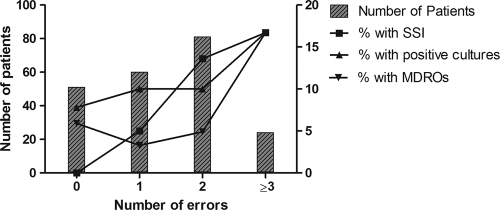
A mean of 1.4 errors in antibiotic prophylaxis per patient was observed, with a significant positive correlation between the number of antibiotic errors and SSI rates (P = 0.001, Mantel-Haenszel linear-by-linear association chi-square test) (Fig. 2). No SSIs were recorded for the 51 patients (24%) with no prophylaxis errors. Positive microbial cultures (P = 0.344) and identification of MDROs (P = 0.201) were not associated with significantly higher error rates (Mantel-Haenszel linear-by-linear association chi-square test).
Fig. 2.
Rates of surgical site infection (SSI) significantly correlated with antibiotic prophylaxis errors (P = 0.001, Mantel-Haenszel linear-by-linear association chi-square test) but not with positive cultures or acquisition of multidrug-resistant organisms (MDROs).
Univariate and multivariate analyses.
Neurosurgery (odds ratio [OR], 0.12; 95% confidence interval [95% CI], 0.15 to 0.90) and antibiotic prophylaxis for <24 h (OR, 0.21; 95% CI, 0.06 to 0.76) were associated with significantly fewer SSIs by univariate analysis. The number of antibiotic errors (OR, 1.57; 95% CI, 1.02 to 2.40) and surgical drainage for 3 days or longer (OR, 2.68; 95% CI, 1.01 to 7.11) were associated with more SSIs (Table 2). There were no significant differences in antibiotic choice between the two groups. By multivariate analysis, no individual risk factors were associated with SSI, but the composite measure of more than two antibiotic prophylaxis errors was significant (OR, 4.03; 95% CI, 1.02 to 15.96) (Table 3).
Table 2.
Multivariate and univariate analyses of clinical and antibiotic prophylaxis variables associated with SSIs
| Variable | Analysis |
|||||
|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
|||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 1.010 | 0.978–1.043 | 0.529 | |||
| Male | 0.490 | 0.177–1.357 | 0.170 | 0.506 | 0.168–1.522 | 0.275 |
| General surgery | 1.740 | 0.621–4.930 | 0.290 | |||
| Neurosurgery | 0.118 | 0.15–0.903 | 0.040 | 0.135 | 0.015–1.208 | 0.073 |
| Orthopedic surgery | 1.964 | 0.731–5.277 | 0.181 | 0.461 | 0.122–1.737 | 0.252 |
| ASA score of >3 | 1.522 | 0.481–4.815 | 0.475 | |||
| Duration of drain, <24 h | 2.130 | 0.794–5.732 | 0.133 | |||
| Duration of drain, 3 days or more | 2.679 | 1.009–7.113 | 0.048 | 1.725 | 0.507–5.862 | 0.383 |
| Appropriate antibiotic type | 0.535 | 0.203–1.410 | 0.206 | 0.856 | 0.270–3.381 | 0.944 |
| Appropriate antibiotic timing | 0.675 | 0.209–2.182 | 0.511 | |||
| Appropriate supplemental dose | 0.414 | 0.157–1.096 | 0.076 | 0.458 | 0.130–1.612 | 0.224 |
| Antibiotic, <24 h | 0.213 | 0.060–0.757 | 0.017 | 0.372 | 0.079–1.748 | 0.210 |
| Duration of antibiotic | 1.080 | 0.963–1.212 | 0.188 | |||
| Normothermia | 2.154 | 0.476–9.740 | 0.319 | |||
| Normoglycemia | 1.542 | 0.338–7.036 | 0.576 | |||
Table 3.
Multivariate and univariate analyses of clinical and antibiotic prophylaxis composite variables associated with surgical site infections
| Antibiotic administration error | Analysisa |
|||||
|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
|||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Mean | 1.566 | 1.024–2.396 | 0.039 | 1.683 | 0.927–3.053 | 0.087 |
| ≥2 | 14.755 | 1.926–113.0 | 0.010 | 4.030 | 1.018–15.96 | 0.047 |
| ≥3 | 1.202 | 0.408–3.544 | 0.739 | |||
Results for baseline characteristics included (not shown) did not significantly change compared with those obtained using the original model.
DISCUSSION
The use of surgical antibiotic prophylaxis to prevent surgical site infection (SSI) can be described using a simple model: achieve adequate antibiotic tissue concentrations at the site and time of incision, which is effective against the expected contaminating organisms, and maintain this for the duration of the procedure (or at least until wound closure) (8).
Clinical studies and meta-analyses provided evidence for each measure in this model (20). A consistent relationship between timing of antimicrobial administration preoperatively and SSI risk has been shown by Classen et al. and Steinberg et al. (6, 29). Prophylaxis that is “stopped too early” (i.e., not supplemented perioperatively when indicated) was described as a significant risk factor by Miliani et al. and Zanetti et al. (23, 34). Subtherapeutic serum antibiotic concentrations at the end of surgery correlated with an increased SSI risk (35).
In addition, many studies illustrated a lack of protective benefit from broad-spectrum antibiotic use versus appropriate narrow-spectrum antibiotics (28, 32).
Continuing antibiotic prophylaxis for more than 24 h postoperatively also offers no additional protective benefit. A meta-analysis of randomized surgical antibiotic prophylaxis studies by McDonald et al. found no benefit of multiple doses versus a single dose or more than 24 h versus equal or less than 24 h of surgical antibiotic prophylaxis (22). In fact, prolonged surgical antibiotic prophylaxis has been reported as harmful. De Chiara et al. found an increased risk of SSIs (7), and Harbarth et al. reported an increased risk of MDROs (12).
Implementing these measures is not simple, however. In a baseline U.S. study, selection of the right antibiotic was averaged at 90%, the right timing of antibiotic administration within 60 min of skin incision was at 80%, and cessation of surgical antibiotic prophylaxis within 24 h was at 67.2% (5). In our study, we found the right antibiotic selection in 64% of cases, the right timing in 83%, and surgical antibiotic prophylaxis not exceeding 24 h in 44%. As in the United States, the worst-performing indicator in our study was continuation of surgical antibiotic prophylaxis beyond the first 24 h.
To improve outcomes, the Surgical Care Improvement Project (SCIP) has been developed in the United States. This provides clear, standardized protocols with a reporting system for SSIs and has successfully reduced error rates, health care cost, and infections (13, 21). In a large survey of patients at U.S. hospitals, an “all-or-none” adherence to the SCIP bundle was significantly associated with a lower rate of SSIs. Individual measures did not reach statistical significance (30). Our study also identified an all-or-none effect. Full concordance equated with no SSIs, but one or more errors led to an SSI rate of 18/165 (11%). By using individual patient data, we can also illustrate the interaction of each preventative measure, showing how the degree of concordance correlates with the SSI rate. This may reflect a compounding of errors in an inverse of Reason's Swiss cheese model. Effective prevention of SSIs relies on lining up and guiding a patient through the holes (25). Missing one hole increases the chance of missing the next and an SSI developing. For example, broad-spectrum antibiotics with adequate antistaphylococcal and antistreptococcal activity may not significantly increase the risk of SSI when administered as a single dose but can be detrimental when continued for an extended postoperative period. Ceftriaxone was used in 74% of our general surgery cases.
Perception of SSI risk may affect prescribing habits. In our study, use of postoperative surgical drains correlated strongly with the duration of antibiotic prophylaxis. Even though prolonged drainage has been associated with bacterial colonization and SSIs, continued antibiotic prophylaxis is not recommended in favor of judicious removal (27). A nonsignificant trend toward prophylaxis errors and higher rates of infections (as reflected by positive culture specimens) and isolation of MDROs was observed, but it is difficult to determine significance given the hospital's environment and microbiological epidemiology.
It is possible that a higher rate of errors is a marker for less attention paid to “holistic” SSI prevention, such as maintaining glucose control and hair clipping. The lack of documentation prevented assessment of the impact that these measures had on the SSI rate.
The design of this study has several limitations. Our SSI prevention standards were developed and applied retrospectively. They are likely to be appropriate for the local context given the consistency of recommendations across guidelines. When variations existed—for example, the timing of perioperative antibiotic supplementation—the most permissive ones were chosen. The quality of our data relied on accurate recording by hospital staff and may obscure treatment decisions if explanations were not documented. It is possible that the exceptionally high rate of SSIs reflects oversensitive diagnosis.
Surgical disciplines were combined, as a similar relationship is expected between SSI rate and antibiotic prophylaxis error, but this masks the expected SSI rates for different procedures. When considered separately, neurosurgery had low rates of errors and SSIs. Orthopedic and colorectal surgeries had poorer compliance and higher rates of SSIs. No significant difference between disciplines, however, was identified by multivariate analysis. Only one SSI was observed in neurosurgical patients, but a consistently higher rate of SSI within each department with increasing number of errors was observed.
In summary, we describe adherence to SSI prevention guidelines and a graded relationship between the inappropriate use of antibiotic prophylaxis and an increasing incidence of SSI. We look forward to introducing a structured SCIP into our hospital.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge Angela Chow's assistance with statistical analysis and Nicole Lim's proofreading.
There are no potential conflicts of interest for any author.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 8 August 2011.
REFERENCES
- 1. Anderson D. J., et al. 2008. Strategies to prevent surgical site infections in acute care hospitals. Infect. Control Hosp. Epidemiol. 29(Suppl. 1):S51–S61 [DOI] [PubMed] [Google Scholar]
- 2. Anonymous 2006. Antimicrobial prophylaxis for surgery. Treat. Guidel. Med. Lett. 4:83–88 [PubMed] [Google Scholar]
- 3. Bratzler D. W., Houck P. M. 2004. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin. Infect. Dis. 38:1706–1715 [DOI] [PubMed] [Google Scholar]
- 4. Bratzler D. W., et al. 2005. Use of antimicrobial prophylaxis for major surgery: baseline results from the National Surgical Infection Prevention Project. Arch. Surg. 140:174–182 [DOI] [PubMed] [Google Scholar]
- 5. Bratzler D. W., Hunt D. R. 2006. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin. Infect. Dis. 43:322–330 [DOI] [PubMed] [Google Scholar]
- 6. Classen D. C., et al. 1992. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N. Engl. J. Med. 326:281–286 [DOI] [PubMed] [Google Scholar]
- 7. De Chiara S., et al. 2010. Prolongation of antibiotic prophylaxis after clean and clean-contaminated surgery and surgical site infection. Minerva Anestesiol. 76:413–419 [PubMed] [Google Scholar]
- 8. Dellinger E. P. 2007. Prophylactic antibiotics: administration and timing before operation are more important than administration after operation. Clin. Infect. Dis. 44:928–930 [DOI] [PubMed] [Google Scholar]
- 9. Edwards J. R., et al. 2008. National Healthcare Safety Network (NHSN) report, data summary for 2006 through 2007, issued November 2008. Am. J. Infect. Control 36:609–626 [DOI] [PubMed] [Google Scholar]
- 10. Falagas M. E., Karageorgopoulos D. E. 2008. Pandrug resistance (PDR), extensive drug resistance (XDR), and multidrug resistance (MDR) among Gram-negative bacilli: need for international harmonization in terminology. Clin. Infect. Dis. 46:1121–1122 (Author's reply, 46:1122.) [DOI] [PubMed] [Google Scholar]
- 11. Gilbert D. N., Moellering R. C., Jr., Eliopoulos G. M., Sande M. A. 2008. The Sanford guide to antimicrobial therapy 2008, 38th ed Antimicrobial Therapy, Inc., Sperryville, VA [Google Scholar]
- 12. Harbarth S., Samore M. H., Lichtenberg D., Carmeli Y. 2000. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation 101:2916–2921 [DOI] [PubMed] [Google Scholar]
- 13. Hermsen E. D., Smith Shull S., Puumala S. E., Rupp M. E. 2008. Improvement in prescribing habits and economic outcomes associated with the introduction of a standardized approach for surgical antimicrobial prophylaxis. Infect. Control Hosp. Epidemiol. 29:457–461 [DOI] [PubMed] [Google Scholar]
- 14. Horan T. C., Andrus M., Dudeck M. A. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 36:309–332 [DOI] [PubMed] [Google Scholar]
- 15. Hsu L. Y., et al. 2008. Reducing antimicrobial resistance through appropriate antibiotic usage in Singapore. Singapore Med. J. 49:749–755 [PubMed] [Google Scholar]
- 16. Kurz A., Sessler D. I., Lenhardt R. 1996. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N. Engl. J. Med. 334:1209–1215 [DOI] [PubMed] [Google Scholar]
- 17. Latham R., Lancaster A. D., Covington J. F., Pirolo J. S., Thomas C. S. 2001. The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect. Control Hosp. Epidemiol. 22:607–612 [DOI] [PubMed] [Google Scholar]
- 18. Liau K. H., et al. 2010. Outcome of a strategy to reduce surgical site infection in a tertiary-care hospital. Surg. Infect. (Larchmt.) 11:151–159 [DOI] [PubMed] [Google Scholar]
- 19. Mangram A. J., Horan T. C., Pearson M. L., Silver L. C., Jarvis W. R. 1999. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control. 27:97–132 (Quiz, 27:133–134; discussion, 27:196.) [PubMed] [Google Scholar]
- 20. Matthaiou D. K., Peppas G., Falagas M. E. 2009. Meta-analysis on surgical infections. Infect. Dis. Clin. North Am. 23:405–430 [DOI] [PubMed] [Google Scholar]
- 21. McConkey S. J., et al. 1999. Results of a comprehensive infection control program for reducing surgical-site infections in coronary artery bypass surgery. Infect. Control Hosp. Epidemiol. 20:533–538 [DOI] [PubMed] [Google Scholar]
- 22. McDonald M., Grabsch E., Marshall C., Forbes A. 1998. Single- versus multiple-dose antimicrobial prophylaxis for major surgery: a systematic review. Aust. N. Z. J. Surg. 68:388–396 [DOI] [PubMed] [Google Scholar]
- 23. Miliani K., L'Heriteau F., Astagneau P. 2009. Non-compliance with recommendations for the practice of antibiotic prophylaxis and risk of surgical site infection: results of a multilevel analysis from the INCISO Surveillance Network. J. Antimicrob. Chemother. 64:1307–1315 [DOI] [PubMed] [Google Scholar]
- 24. National Institute for Health and Clinical Excellence 2008. Prevention and treatment of surgical site infection, vol. CG74 National Institute for Health and Clinical Excellence, London, United Kingdom [Google Scholar]
- 25. Reason J. 2000. Human error: models and management. BMJ 320:768–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scottish Intercollegiate Guidelines Network 2008. Antibiotic prophylaxis in surgery, a national clinical guideline. (guideline no. 104). The Scottish Intercollegiate Guidelines Network (SIGN). SIGN publication, Edinburgh, Scotland [Google Scholar]
- 27. Simchen E., Rozin R., Wax Y. 1990. The Israeli Study of Surgical Infection of drains and the risk of wound infection in operations for hernia. Surg. Gynecol. Obstet. 170:331–337 [PubMed] [Google Scholar]
- 28. Song F., Glenny A. M. 1998. Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomized controlled trials. Br. J. Surg. 85:1232–1241 [DOI] [PubMed] [Google Scholar]
- 29. Steinberg J. P., et al. 2009. Timing of antimicrobial prophylaxis and the risk of surgical site infections: results from the Trial to Reduce Antimicrobial Prophylaxis Errors. Ann. Surg. 250:10–16 [DOI] [PubMed] [Google Scholar]
- 30. Stulberg J. J., et al. 2010. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA 303:2479–2485 [DOI] [PubMed] [Google Scholar]
- 31. Tanner J., Woodings D., Moncaster K. 2006. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst. Rev. 3:CD004122. [DOI] [PubMed] [Google Scholar]
- 32. van Kasteren M. E., et al. 2007. Antibiotic prophylaxis and the risk of surgical site infections following total hip arthroplasty: timely administration is the most important factor. Clin. Infect. Dis. 44:921–927 [DOI] [PubMed] [Google Scholar]
- 33. Wilson J., Ramboer I., Suetens C. 2007. Hospitals in Europe Link for Infection Control through Surveillance (HELICS). Inter-country comparison of rates of surgical site infection—opportunities and limitations. J. Hosp. Infect. 65(Suppl. 2):165–170 [DOI] [PubMed] [Google Scholar]
- 34. Zanetti G., Giardina R., Platt R. 2001. Intraoperative redosing of cefazolin and risk for surgical site infection in cardiac surgery. Emerg. Infect. Dis. 7:828–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zelenitsky S. A., Ariano R. E., Harding G. K., Silverman R. E. 2002. Antibiotic pharmacodynamics in surgical prophylaxis: an association between intraoperative antibiotic concentrations and efficacy. Antimicrob. Agents Chemother. 46:3026–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.