Abstract
While it is well-known that adjunctive corticosteroid use improves the outcome of moderate-to-severe Pneumocystis jirovecii pneumonia (PcP) in patients with human immunodeficiency virus (HIV), there are limited data on its efficacy in non-HIV-infected patients with PcP. Patients undergoing fiber-optic bronchoscopy with bronchoalveolar lavage for suspected PcP from January 2007 through December 2010 were reviewed retrospectively. We compared demographics, clinical characteristics, and outcomes in 88 non-HIV-infected patients with moderate-to-severe PcP with (n = 59) and without (n = 29) adjunctive corticosteroid use. Outcomes of PcP were assessed by respiratory failure and 30-day and 90-day all-cause mortality. Survival curves were analyzed by the Kaplan-Meier method and estimated by the log rank test. All-cause mortality of moderate-to-severe PcP at 90 days was lower in the solid-organ transplant recipients than in all other patients (6/26 [23%] versus 34/62 [55%], respectively; P = 0.006), and mortality at 30 days was lower in patients with hematologic malignancies than in all other patients (4/26 [15%] versus 24/62 [39%], respectively; P = 0.03). The outcomes of PcP were not significantly different in moderate-to-severe PcP patients with and without adjunctive corticosteroid use, regardless of recent corticosteroid use. Survival analysis of PcP patients with and without corticosteroid use by the Kaplan-Meier method also did not reveal any difference (log rank test; P = 0.81). There again was no difference within the subgroup of PcP patients with solid-organ transplants. Adjunctive corticosteroid use may not improve the outcome of moderate-to-severe PcP in non-HIV-infected patients.
INTRODUCTION
Pneumocystis jirovecii pneumonia (PcP) remains an important opportunistic infection in the context of human immunodeficiency virus (HIV) infection as well as in other immunocompromised conditions (4, 14). Several important advances have greatly influenced the treatment of PcP in HIV-infected patients, notably the introduction of adjunctive corticosteroids (4, 14). In HIV-infected patients with PcP, further deterioration of oxygenation is often observed during the first 3 to 5 days of specific PcP therapy. Why therapy provokes a decline in pulmonary function is unclear, but one hypothesis is that the drug-induced death of microorganisms exacerbates pulmonary inflammation (1). Accordingly, early in the HIV epidemic, randomized trials evaluated the efficacy of adjunctive corticosteroid therapy in reducing morbidity and mortality in HIV-infected patients with moderate-to-severe PcP (3, 7, 11, 12). These trials demonstrated a survival benefit of corticosteroid administration. Because of this, corticosteroid should be given within 72 h of initiating antimicrobial therapy in an HIV-infected patient with PcP if the partial pressure of arterial oxygen (PaO2) is ≤70 mmHg or the alveolar-arterial oxygen difference (AaDO2) is ≥35 (9).
The clinical characteristics and outcomes of PcP differ in HIV-infected and non-HIV-infected patients (6, 10). However, there are very limited data on the efficacy of adjunctive corticosteroid in non-HIV-infected patients with PcP (5, 13), and no randomized studies have been conducted. In contrast to the data for HIV-infected patients, the data for non-HIV-infected PcP patients suggest that adjunctive corticosteroid might not improve outcomes (5, 13). The problems encountered in analyzing and interpreting these data are that the non-HIV-immunocompromised hosts were a nonhomogeneous group of patients and that most of them had been on corticosteroids at the time they developed PcP. Therefore, we evaluated the effects of adjunctive corticosteroid treatment on the outcomes of non-HIV-infected patients with moderate-to-severe PcP, using adjustments for recent use of corticosteroid and underlying disease.
MATERIALS AND METHODS
Study population.
This study was performed at the Asan Medical Center, a 2,700-bed tertiary care teaching hospital in Seoul, Republic of Korea. Patients undergoing fiber-optic bronchoscopy with bronchoalveolar lavage for suspected PcP from January 2007 through December 2010 were identified by examining bronchoscopy logs and laboratory databases. Only the first bronchoscopy in patients undergoing multiple bronchoscopies during a single clinical episode was analyzed. Patients under 16 years of age and HIV-infected patients were excluded from the analysis.
Microbiological methods.
Bronchoalveolar lavage was performed through a fiber-optic bronchoscope using standard techniques (2). Diagnosis of PcP was based on a positive result in the direct immunofluorescence assay for P. jirovecii (Light Diagnostics Pneumocystis carinii DFA kit; Millipore, Billerica, MA). Additional microbiological investigations performed on bronchoalveolar lavage specimens included culture for conventional bacteria, mycobacteria, fungi, and viruses. Quantitative culture of bronchoalveolar lavage specimens was used to assay conventional bacteria, and the threshold value was ≥104 CFU/ml (8). Cytomegalovirus (CMV) was cultured by standard techniques; samples were inoculated into shell vial cultures of human fetal lung fibroblasts (Diagnostic Hybrids, Athens, OH), and growth was examined on days 1 and 7 by direct immunofluorescence staining. Viruses other than CMV, including respiratory syncytial virus, influenza virus, parainfluenza virus, and adenovirus, were detected by means of a direct immunofluorescence assay employing a respiratory virus screening and identification kit (Diagnostic Hybrids).
Clinical data.
All medical records were reviewed retrospectively. Based on the PaO2 while breathing room air, or the AaDO2, prior to the first bronchoscopy, patients were classified into those with mild (PaO2 > 70 mmHg or AaDO2 < 35), moderate (PaO2 ≤ 70 mmHg or AaDO2 ≥ 35), or severe (PaO2 < 60 mmHg or AaDO2 ≥ 45) PcP (14). We compared demographics, clinical characteristics, and outcomes in the moderate-to-severe PcP patients with and without the use of adjunctive corticosteroid. Use of adjunctive corticosteroid was defined as use started within 72 h of initiating specific PcP therapy and consisting of at least 40 mg prednisone twice daily for 5 days, regardless of the subsequent tapering schedule, and of use of corticosteroid prior to the onset of PcP (17). The outcome of PcP was assessed by respiratory failure and 30-day and 90-day all-cause mortality. Respiratory failure was defined as use of mechanical ventilation for more than 48 h. The reason for the choice of 48 h was to avoid analyzing instances of temporary use of mechanical ventilation for bronchoscopic examination.
To adjust for recent use of corticosteroid, we performed the same comparisons between moderate-to-severe PcP patients with and without the use of adjunctive corticosteroid after subdivision into those with and without recent corticosteroid use. Recent corticosteroid use was defined as use of more than 0.3 mg/kg body weight/day for over 3 weeks in the month preceding the onset of PcP. To adjust for underlying disease, the outcomes of the PcP patients with and without adjunctive corticosteroid use were compared within groups with a given underlying disease. Clinical decisions concerning treatment regimens for PcP were made by the physicians. Failure of initial treatment regimen was defined as clinical deterioration during the first 5 days of treatment or lack of improvement after 7 or more days of treatment (16).
Statistical analysis.
All statistics were calculated using SPSS version 12.0 (SPSS, Chicago, IL). Categorical variables were compared using the chi-square test or Fisher's exact test. Continuous variables were analyzed by the Mann-Whitney U test. Time-to-event analysis was performed using Kaplan-Meier estimates and the log rank test. All tests were two tailed, and differences were considered significant at P values of <0.05.
RESULTS
During the study period, 135 patients with PcP were identified. Among these, the following 24 patients were excluded: pediatric patients (n = 7) and HIV-infected patients (n = 17). Of the remaining 111 patients, 23 (20.7%) had mild PcP, 8 (7.2%) had moderate PcP, and 80 (72.1%) had severe PcP. Therefore, a total of 88 patients with moderate-to-severe PcP were included in the study. Of these, 26 (29.5%) were solid-organ transplant recipients (14 kidneys, 8 livers, 2 hearts, 2 pancreases), 26 (29.5%) had hematologic malignancies, including 5 (5.7%) hematopoietic stem cell transplant recipients, 12 (13.7%) had nonhematologic malignancies (7 had lung cancer, 3 colon cancer, 1 gastric cancer, 1 prostatic cancer), 9 (10.2%) had interstitial lung disease, and 7 (8.0%) had connective tissue disease. The majority of patients were treated with trimethoprim-sulfamethoxazole (98.9%). In the organ transplant recipients, the median interval from transplantation to the onset of PcP was 12 months (interquartile range, 9 to 16 months).
Outcome of moderate-to-severe PcP according to underlying disease.
Table 1 shows the outcomes of moderate-to-severe PcP according to the underlying disease. All-cause mortality at 90 days was lower in the solid-organ transplant recipients than in all others (6/26 [23.1%] versus 34/62 [54.8%], respectively; P = 0.006), and mortality at 30 days was lower in patients with hematologic malignancies than in all others (4/26 [15.4%] versus 24/62 [38.7%], respectively; P = 0.03). However, there was no significant difference in the incidence of respiratory failure according to the underlying disease.
Table 1.
Outcomes of moderate-to-severe P. jirovecii pneumonia according to underlying diseasea
| Underlying disease | Respiratory failure | 30-day all-cause mortality | 90-day all-cause mortality |
|---|---|---|---|
| Solid-organ transplant recipient (n = 26) | 15 (57.7) | 5 (19.2) | 6 (23.1)b |
| Hematologic malignancy (n = 26) | 17 (65.4) | 4 (15.4)b | 10 (38.5) |
| Non-hematologic malignancy (n = 12) | 6 (50.0) | 5 (41.7) | 8 (66.7) |
| Interstitial lung disease (n = 9) | 6 (66.7) | 5 (55.6) | 7 (77.8) |
| Connective tissue disease (n = 7) | 6 (85.7) | 4 (57.1) | 4 (57.1) |
| Others (n = 8) | 4 (50.0) | 5 (62.5) | 5 (62.5) |
| Total (n = 88) | 54 (61.4) | 28 (31.8) | 40 (45.5) |
Data are numbers (%) of patients.
P values of <0.05 between patients with a given underlying disease and all other underlying diseases.
Comparison of moderate-to-severe PcP patients with and without adjunctive corticosteroid use.
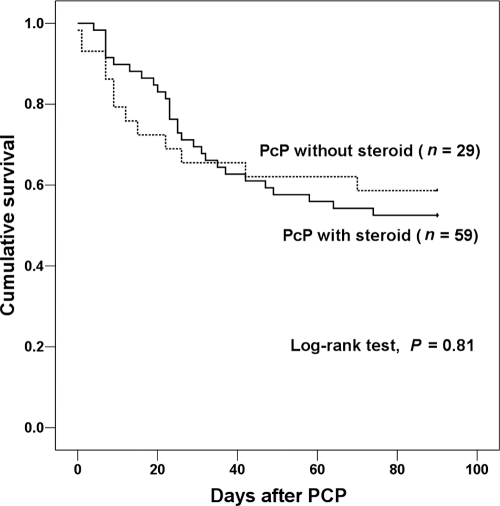
Table 2 presents the demographics, clinical characteristics, and outcomes in the patients with and without adjunctive corticosteroid use. Of the 88 patients, 59 (67%) received adjunctive corticosteroid therapy, and 29 (33%) did not. The PcP patients with adjunctive corticosteroid use were older than those without (59 versus 29 years old; P = 0.01). Those with adjunctive corticosteroid use also included fewer solid-organ transplant recipients (20.3% versus 48.3%, respectively; P = 0.007) and patients who received T-cell immunosuppressants (27.1% versus 51.7%, respectively; P = 0.02) compared to those without adjunctive corticosteroid. There were no significant differences in rates of pulmonary coinfection with bacteria, cytomegalovirus, and other viruses or in the rate of respiratory failure and 30-day and 90-day all-cause mortality between the two groups. Survival analysis by the Kaplan-Meier method also did not reveal a significant difference between the groups (log rank test, P = 0.81) (Fig. 1).
Table 2.
Demographics, clinical characteristics, and outcomes of moderate-to-severe P. jirovecii pneumonia with and without adjunctive steroid therapy in non-HIV-infected patientsa
| Characteristic | Adjunctive steroid therapy (n = 59) | No adjunctive steroid therapy (n = 29) | P value |
|---|---|---|---|
| Demographics | |||
| Age, median years (IQR)b | 59 (45–69) | 54 (39–60) | 0.01 |
| Male gender | 38 (64.4) | 18 (62.1) | 0.83 |
| Underlying disease | |||
| Solid-organ transplant recipient | 12 (20.3) | 14 (48.3) | 0.007 |
| Hematologic malignancy | 21 (35.6) | 5 (17.2) | 0.08 |
| Hematopoietic stem cell transplant recipient | 4 (6.8) | 1 (3.4) | 0.99 |
| Nonhematologic malignancy | 9 (15.3) | 3 (10.3) | 0.74 |
| Interstitial lung disease | 6 (10.2) | 3 (10.3) | 0.99 |
| Connective tissue disease | 6 (10.2) | 1 (3.4) | 0.42 |
| Others | 5 (8.5) | 3 (10.3) | 0.99 |
| Immunosuppressive agents use, previous month | |||
| Steroid | 23 (39.0) | 11 (37.9) | 0.92 |
| T-cell immunosuppressant | 16 (27.1) | 15 (51.7) | 0.02 |
| Anticancer agent | 27 (45.8) | 10 (34.5) | 0.31 |
| Time to BAL after admission, median days (IQR)c | 2 (1–5) | 6 (1–16) | 0.13 |
| Pulmonary coinfection | |||
| Bacteria | 0 (0) | 1 (3.4) | 0.33 |
| Cytomegalovirus | 14 (23.7) | 9 (31.0) | 0.46 |
| Virus other than cytomegalovirus | 0 (0) | 1 (3.4) | 0.33 |
| Treatment duration, median days (IQR) | 18 (14–24) | 16 (13–23) | 0.59 |
| Initial treatment regimen | |||
| Trimethoprim-sulfamethoxazole | 59 (100) | 28 (96.6) | 0.33 |
| Change to second-line regimen | 24 (40.7) | 7 (24.1) | 0.13 |
| Due to treatment failure | 19 (32.2) | 6 (20.7) | 0.26 |
| Due to adverse reaction | 5 (8.5) | 1 (3.4) | 0.66 |
| Respiratory failure | 37 (62.7) | 17 (58.6) | 0.71 |
| 30-day all-cause mortality | 18 (30.5) | 10 (34.5) | 0.71 |
| 90-day all-cause mortality | 28 (47.5) | 12 (41.4) | 0.59 |
Data are numbers (%) of patients, unless otherwise indicated.
IQR, interquartile range.
BAL, bronchoalveolar lavage.
Fig. 1.
Kaplan-Meier survival curves in patients with moderate-to-severe Pneumocystis jirovecii pneumonia (PcP) with or without adjunctive steroid therapy.
Using the median age (56 years old) of all the moderate-to-severe PcP patients as a reference point, we divided the patients into younger and older groups and compared the outcomes between patients with and without adjunctive corticosteroid according to age. Again there was no significant difference of outcomes (see Table S1 in the supplemental material), and the same was true when we first divided the patients into those receiving T-cell immunosuppressants and those not receiving them (see Table S2 in the supplemental material).
Again, when we first divided the patients into those with and without recent corticosteroid use and compared the outcomes between patients with and without adjunctive corticosteroid (Table 3), the outcomes in terms of respiratory failure rate and 30-day and 90-day mortality did not differ significantly, and this proved also to be the case when we adjusted for underlying disease by comparing the outcomes in moderate-to-severe PcP patients receiving solid-organ transplantation with (n = 12) and without (n = 14) adjunctive corticosteroid use (Table 4). Patients with other underlying diseases were not analyzed because of the small sample sizes.
Table 3.
Demographics, clinical characteristics, and outcomes of moderate-to-severe P. jirovecii pneumonia with and without adjunctive steroid therapy in non-HIV-infected patients with or without recent steroid usea
| Characteristic | Recent steroid use |
No recent steroid use |
||||
|---|---|---|---|---|---|---|
| Adjunctive steroid therapy (n = 23) | No adjunctive steroid therapy (n = 11) | P value | Adjunctive steroid therapy (n = 36) | No adjunctive steroid therapy (n = 18) | P value | |
| Demographics | ||||||
| Age, median years (IQR)b | 57 (40–70) | 55 (38–64) | 0.38 | 61 (45–69) | 54 (37–59) | 0.02 |
| Male gender | 15 (65.2) | 6 (54.5) | 0.71 | 23 (63.9) | 12 (66.7) | 0.84 |
| Underlying disease | ||||||
| Solid-organ transplant recipient | 2 (8.7) | 1 (9.1) | 0.99 | 10 (27.8) | 13 (72.2) | 0.002 |
| Hematologic malignancy | 3 (13.0) | 3 (27.3) | 0.36 | 18 (50.0) | 2 (11.1) | 0.005 |
| Hematopoietic stem cell transplant recipient | 1 (4.3) | 1 (9.1) | 0.99 | 3 (8.3) | 0 (0) | 0.54 |
| Nonhematologic malignancy | 3 (13.0) | 1 (9.1) | 0.99 | 6 (16.7) | 2 (11.1) | 0.70 |
| Interstitial lung disease | 6 (26.1) | 3 (27.3) | 0.99 | 0 (0) | 0 (0) | — |
| Connective tissue disease | 5 (21.7) | 1 (9.1) | 0.64 | 1 (2.8) | 0 (0) | 0.99 |
| Others | 4 (17.4) | 2 (18.2) | 0.99 | 1 (2.8) | 1 (5.6) | 0.99 |
| Immunosuppressive agents use, previous month | ||||||
| T-cell immunosuppressant | 5 (21.7) | 2 (18.2) | 0.99 | 11 (30.6) | 13 (72.2) | 0.004 |
| Anticancer agent | 7 (30.4) | 5 (45.5) | 0.46 | 20 (55.6) | 5 (27.8) | 0.054 |
| Time to BAL after admission, median days (IQR)c | 2 (1–6) | 6 (1–14) | 0.42 | 2 (1–5) | 6 (1–23) | 0.24 |
| Pulmonary coinfection | ||||||
| Bacteria | 0 (0) | 1 (9.1) | 0.32 | 0 (0) | 0 (0) | — |
| Cytomegalovirus | 9 (39.1) | 3 (27.3) | 0.70 | 5 (13.9) | 6 (33.3) | 0.15 |
| Virus other than cytomegalovirus | 0 (0) | 1 (9.1) | 0.32 | 0 (0) | 0 (0) | — |
| Treatment duration, median days (IQR) | 20 (14–23) | 14 (9–22) | 0.23 | 17 (13–24) | 19 (14–25) | 0.67 |
| Initial treatment regimen | ||||||
| Trimethoprim-sulfamethoxazole | 23 (100) | 10 (90.9) | 0.32 | 36 (100) | 18 (100) | — |
| Change to second-line regimen | 12 (52.2) | 3 (27.3) | 0.27 | 12 (33.3) | 4 (22.2) | 0.40 |
| Due to treatment failure | 9 (39.1) | 3 (27.3) | 0.71 | 10 (27.8) | 3 (16.7) | 0.51 |
| Due to adverse reaction | 3 (13.0) | 0 (0) | 0.54 | 2 (5.6) | 1 (5.6) | 0.99 |
| Respiratory failure | 15 (65.2) | 6 (54.5) | 0.71 | 22 (61.1) | 11 (61.1) | 0.99 |
| 30-day all-cause mortality | 11 (47.8) | 4 (36.4) | 0.72 | 7 (19.4) | 6 (33.3) | 0.32 |
| 90-day all-cause mortality | 12 (52.2) | 6 (54.5) | 0.90 | 16 (44.4) | 6 (33.3) | 0.43 |
Data are numbers (%) of patients, unless otherwise indicated.
IQR, interquartile range.
BAL, bronchoalveolar lavage.
Table 4.
Outcomes of moderate-to-severe P. jirovecii pneumonia with and without adjunctive steroid therapy in solid-organ transplant recipientsa
| Outcome | Adjunctive steroid therapy (n = 12) | No adjunctive steroid therapy (n = 14) | P value |
|---|---|---|---|
| Respiratory failure | 6 (50.0) | 9 (64.3) | 0.46 |
| 30-day all-cause mortality | 3 (25.0) | 2 (14.3) | 0.64 |
| 90-day all-cause mortality | 4 (33.3) | 2 (14.3) | 0.37 |
Data are numbers (%) of patients.
DISCUSSION
There have been many advances in the treatment of PcP in HIV-infected patients (4, 14). One is the use of adjunctive corticosteroid. Many studies have shown that adjunctive corticosteroid use prevents early deterioration after PcP treatment (3, 11) and decreases the rates of respiratory failure and mortality of HIV-infected moderate-to-severe PcP patients (1, 3, 7, 12, 17). Based on these results, adjunctive corticosteroid use within 72 h of initiating antimicrobial therapy is recommended in HIV-infected patients with PcP, if PaO2 is ≤70 mmHg or AaDO2 is ≥35 (9).
Many clinicians have used adjunctive corticosteroid in this way in HIV-infected patients with PcP. However, there have been very few studies of the efficacy of adjunctive corticosteroid use in non-HIV-infected patients with PcP, but a number of retrospective studies have failed to show significant differences in morbidity and mortality in non-HIV-infected PcP patients with and without adjunctive corticosteroid use (5, 13, 15). In a previous study of non-HIV-infected PcP patients with a variety of underlying diseases, mortality of PcP patients with low-dose steroid use was not significantly different from that of PcP patients with high-dose steroid use (5/14 [36%] versus 7/16 [44%], respectively; P = 0.65) (13). Another study showed that the need for mechanical ventilation (10/23 [43%] versus 4/8 [50%]; P = 0.75) and the mortality rate (9/23 [39%] versus 4/8 [50%]; P = 0.59) were similar in the two groups (5). In a third study, of PcP patients with hematologic malignancies, there was even a trend toward increased mortality in the PcP patients with adjunctive corticosteroid compared to those without (15/35 [42.9%] versus 5/25 [20%], respectively; P = 0.06) (15). To the best of our knowledge, ours is the largest retrospective study aimed at determining the efficacy of adjunctive corticosteroid therapy in non-HIV-infected PcP patients. Our results support the previous smaller-sized reports indicating that adjunctive corticosteroid use may not improve the outcome of PcP in non-HIV-infected patients.
During the study period from January 2007 to December 2010, 135 PcP patients were identified. Among these, 7 pediatric patients were excluded. Of the remaining 128 patients, 111 (86%) patients were non-HIV-infected patients, and 17 (14%) were HIV-infected patients. It should be pointed out that the incidence of PcP in non-HIV-infected versus HIV-infected patients may not reflect the general population in Republic of Korea. During the study period, 2,353 cases of solid-organ transplantations (22% of nationwide cases) and 806 cases of hematologic stem cell transplantations were treated at the Asan Medical Center. On the other hand, only 291 HIV-infected patients were treated in our hospital.
It is well-known that the clinical characteristics and outcomes of PcP in HIV-infected patients are different from those in non-HIV-infected patients (6, 10). In addition, non-HIV-infected patients with PcP suffer from different underlying diseases, and these may affect the clinical outcomes of PcP. That is one of the reasons why there have been limited studies of the efficacy of adjunctive corticosteroid use in non-HIV-infected patients with PcP. To adjust for underlying diseases, we compared the outcomes of moderate-to-severe PcP in patients with and without adjunctive corticosteroid use according to underlying disease and found no significant difference between PcP patients receiving solid-organ transplantation with and without adjunctive corticosteroid use (Table 4). Unfortunately, we could not analyze PcP patients with other underlying diseases, such as hematologic malignancies, nonhematologic malignancies, interstitial lung disease, and connective tissues disease, due to the small sample sizes.
Another reason why it is difficult to analyze and interpret the efficacy of adjunctive corticosteroid use in non-HIV-infected PcP patients is that a considerable proportion of the patients have received corticosteroids previously for various reasons. In our study, 39% of the patients had received corticosteroid at a dose of more than 0.3 mg/kg/day for more than 3 weeks in the month prior to the onset of PcP. For this reason, we divided patients into two groups according to recent steroid use and found no significant difference of outcome (Table 3).
The current study showed a 6% difference (P = 0.59) in 90-day all-cause mortality between the group receiving adjunctive steroid therapy and the group not receiving adjunctive steroid therapy. It had 80% power to detect a 30% difference in 90-day all-cause mortality at a 5% significance level. It is possible that our sample size was too small to achieve statistical significance. Due to the limitation of statistical power, subgroup analysis by age, underlying disease analysis, and recent medication history have been conducted. The consistent results from the subgroup analysis support our result. We divided the patients into younger and older groups and compared the outcomes between patients with and without adjunctive corticosteroid separately for the ages, and there again was no significant difference of outcomes between those with and without adjunctive corticosteroid (see Table S1 in the supplemental material). There was also no significant difference of outcomes between PcP patients with and without adjunctive corticosteroid, regardless of the use/nonuse of T-cell immunosuppressants (see Table S2 in the supplemental material).
In conclusion, adjunctive corticosteroid use may not improve the outcome of moderate-to-severe PcP in non-HIV-infected patients, regardless of recent corticosteroid use. The current practice of using adjunctive corticosteroid in non-HIV-infected PcP patients as is done with HIV-infected PcP patients should be reconsidered. Well-designed prospective studies should be undertaken to reach a more reliable conclusion.
Supplementary Material
ACKNOWLEDGMENT
We have no potential conflicts of interest, and there is no financial support to report.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 25 July 2011.
REFERENCES
- 1. Anonymous 1990. Consensus statement on the use of corticosteroids as adjunctive therapy for pneumocystis pneumonia in the acquired immunodeficiency syndrome. The National Institutes of Health—University of California Expert Panel for Corticosteroids as Adjunctive Therapy for Pneumocystis Pneumonia. N. Engl. J. Med. 323:1500–1504 [DOI] [PubMed] [Google Scholar]
- 2. Anonymous 1989. Technical recommendations and guidelines for bronchoalveolar lavage (BAL). Report of the European Society of Pneumology Task Group. Eur. Respir. J. 2:561–585 [PubMed] [Google Scholar]
- 3. Bozzette S. A., et al. 1990. A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the AIDS. California Collaborative Treatment Group. N. Engl. J. Med. 323:1451–1457 [DOI] [PubMed] [Google Scholar]
- 4. Catherinot E., et al. 2010. Pneumocystis jirovecii Pneumonia. Infect. Dis. Clin. North Am. 24:107–138 [DOI] [PubMed] [Google Scholar]
- 5. Delclaux C., et al. 1999. Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in non-human immunodeficiency virus-infected patients: retrospective study of 31 patients. Clin. Infect. Dis. 29:670–672 [DOI] [PubMed] [Google Scholar]
- 6. Ewig S., et al. 1995. Clinical characteristics and outcome of Pneumocystis carinii pneumonia in HIV-infected and otherwise immunosuppressed patients. Eur. Respir. J. 8:1548–1553 [PubMed] [Google Scholar]
- 7. Gagnon S., et al. 1990. Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in the AIDS. A double-blind, placebo-controlled trial. N. Engl. J. Med. 323:1444–1450 [DOI] [PubMed] [Google Scholar]
- 8. Horan T. C., Andrus M., Dudeck M. A. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 36:309–332 [DOI] [PubMed] [Google Scholar]
- 9. Kaplan J. E., et al. 2009. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recommend. Rep. 58(RR-4):1–207 (Quiz, CE1–CE4.) [PubMed] [Google Scholar]
- 10. Mansharamani N. G., Garland R., Delaney D., Koziel H. 2000. Management and outcome patterns for adult Pneumocystis carinii pneumonia, 1985 to 1995: comparison of HIV-associated cases to other immunocompromised states. Chest 118:704–711 [DOI] [PubMed] [Google Scholar]
- 11. Montaner J. S., et al. 1990. Corticosteroids prevent early deterioration in patients with moderately severe Pneumocystis carinii pneumonia and the AIDS. Ann. Intern. Med. 113:14–20 [DOI] [PubMed] [Google Scholar]
- 12. Nielsen T. L., et al. 1992. Adjunctive corticosteroid therapy for Pneumocystis carinii pneumonia in AIDS: a randomized European multicenter open label study. J. Acquir. Immune Defic. Syndr. 5:726–731 [PubMed] [Google Scholar]
- 13. Pareja J. G., Garland R., Koziel H. 1998. Use of adjunctive corticosteroids in severe adult non-HIV Pneumocystis carinii pneumonia. Chest 113:1215–1224 [DOI] [PubMed] [Google Scholar]
- 14. Pfaller M. A., Anaissie E. J. 2009. Pneumocystis, p. 385–401 In Anaissie E. J., McGinnis M. R., Pfaller M. A. (ed.), Clinical mycology, 2nd ed Churchill Livingstone, Oxford, United Kingdom [Google Scholar]
- 15. Roblot F., et al. 2003. Pneumocystis carinii pneumonia in patients with hematologic malignancies: a descriptive study. J. Infect. 47:19–27 [DOI] [PubMed] [Google Scholar]
- 16. Smego R. A., Jr., Nagar S., Maloba B., Popara M. 2001. A meta-analysis of salvage therapy for Pneumocystis carinii pneumonia. Arch. Intern. Med. 161:1529–1533 [DOI] [PubMed] [Google Scholar]
- 17. Walmsley S., et al. 1995. A multicenter randomized double-blind placebo-controlled trial of adjunctive corticosteroids in the treatment of Pneumocystis carinii pneumonia complicating the AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 8:348–357 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.