Abstract
Carbaporphyrin ketals are porphyrinoid compounds in which a pyrrole ring of a typical porphyrin macrocycle has been replaced by a ketal-substituted indene ring. It was recently demonstrated that these compounds are effective in vitro against Leishmania tarentolae. Their in vitro effectiveness is increased when they are exposed to visible light; they act as photosensitizers capable of mediating the production of reactive oxygen species (ROS). Following on this evidence, the effectiveness and cytotoxicity of the dimethyl and diethyl carbaporphyrin ketals (CKOMe and CKOEt, respectively) were determined in vitro using pathogenic Leishmania species with and without exposure to visible light (2 and 4 h). The effectiveness against various pathogenic Leishmania species was determined to be in a micromolar range. Additionally, the effect of encapsulating the carbaporphyrin ketals in liposome formulations was tested. Liposomal delivery diminished their toxicity, while the effectiveness was enhanced upon exposure to visible light (photodynamic effect). The cytotoxicity levels for human U937 cells and hamster peritoneal macrophages were in the ranges of 0.3 to 9 μM and 7 to 330 μM, respectively. When tested in vivo, using a hamster (Mesocricetus auratus) model of cutaneous leishmaniasis, CKOMe was active even in the dark, suggesting that the compound, once metabolized in the animal tissue, produces an active ingredient that does not seem to be photosensitive. Reduction in lesion size, histopathologic analyses, and smears confirmed the in vivo effectiveness of the compound, since the parasitic load was diminished without noticeable toxic effects.
INTRODUCTION
Leishmaniasis is a parasitic endemic disease that is widely distributed around the world and affects a large economically disadvantaged population that lives in rural areas of tropical and subtropical countries (50). To date, conventional treatments include pentavalent antimonials, amphotericin B, pentamidine isothionate, and miltefosine. The utilization of these compounds in affected populations has some disadvantages related to high cost and duration of treatment, lack of patient adherence to treatment, and the development of resistant parasite strains to some of these medicines (7, 13, 33, 41, 44, 45). Additionally, pharmaceutical companies lack the economic motivation to invest in developing other medical treatments; therefore, leishmaniasis is considered a neglected disease by the World Health Organization (29). As a result, academic researchers have led the research and development of treatments which are deemed to be accessible, safe, effective at a low dose and a short length of treatment, easy to administer, and available at a reasonable cost. One of the strategies is based on the utilization of porphyrin derivatives with photosensitizing properties and a structural similarity to heme (2, 11, 42). In this strategy, a patient is treated with a porphyrin derivative which is accumulated by the parasite and then the affected tissue is exposed to visible light. Photoactivation of the compound induces the direct or indirect production of reactive oxygen species (ROS) via two proposed mechanisms (47). In one, the excited photosensitizer becomes an ion radical that reacts further with oxygen to produce superoxide and hydroxyl radicals. In the other, the excited photosensitizer transfers energy to oxygen, forming singlet oxygen (5, 10). These ROS are able to oxidize subcellular molecules inducing cellular apoptosis or necrosis (18, 23, 28). It has also been shown that porphyrins can be incorporated into liposomal delivery systems, diminishing their cytotoxicity without affecting the efficacy of the compounds during photodynamic treatment (17, 27).
Carbaporphyrin ketals (dimethyl and diethyl; CKOMe and CKOEt, respectively) (Fig. 1) are porphyrinoid compounds in which a pyrrole ring of the macrocycle characteristic of porphyrins has been replaced by a ketal-substituted indene ring (25, 31). These compounds are able to absorb visible and near infrared light, which makes them attractive as photodynamic therapy photosensitizers. Recently, it was demonstrated that the compounds are effective in vitro against promastigotes of Leishmania tarentolae and that their effectiveness is increased when exposed to visible light (34).
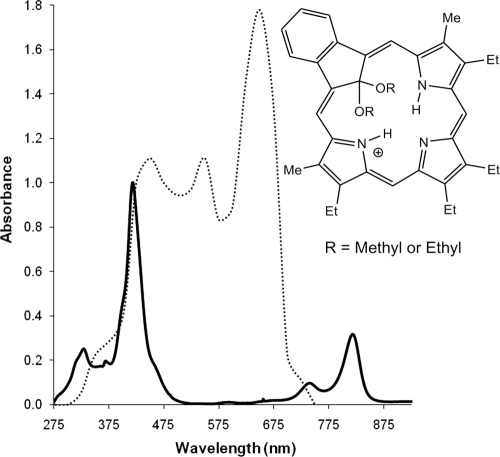
Fig. 1.
Absorption spectrum of dimethyl carbaporphyrin ketal (CKOMe, full line) overlaid with the emission spectrum of the lamp (dotted line, in arbitrary units). The spectrum of the diethyl carbaporphyrin ketal (CKOEt) is very similar to that of CKOMe and it is not shown.
Motivated by the previous results obtained using Leishmania tarentolae, we have determined the in vitro effectiveness of carbaporphyrin dimethyl and diethyl ketals, both without encapsulating and while encapsulated in a liposomal formulation, against axenic amastigotes of the human pathogenic strains Leishmania amazonensis, L. infantum, and L. panamensis. The in vitro effectiveness was also determined against intracellular amastigotes of L. amazonensis. The toxicity of the compounds was evaluated with the promonocytic U-937 cell line and peritoneal macrophages from hamsters (PMH). We also carried out studies on the in vivo toxicity and effectiveness of the compounds using the golden hamster (Mesocricetus auratus) animal model for cutaneous leishmaniasis. Acute dermal toxicity was evaluated using Wistar rats (Rattus norvegicus, Wistar strain).
MATERIALS AND METHODS
In vitro studies. (i) Carbaporphyrin dimethyl and diethyl ketals (CKOMe and CKOEt).
The compounds were synthesized as described by Lash and coworkers (31). Photophysical characterization (fluorescence yield, singlet oxygen yield, and production of superoxide) of the compounds in aqueous solution of ethanol (20% [vol/vol]) was carried out using standard techniques. Briefly, the relative fluorescence yield (φf) was obtained using a luminescence spectrophotometer (Perkin Elmer LS55) using acridine yellow (φf, 0.47) (36) dissolved in the same solvent as that used to dissolve the carbaporphyrin ketal. Singlet oxygen yield was determined by a trapping method (51). In this determination, a solution of the carbaporphyrin compound was mixed with an excess amount of the trapping compound 2,2,6,6-tetramethyl-4-piperidone (TEMP). The mixture was then illuminated with visible light (xenon lamp, 350- to 700-nm range, 75 W) for 70 min. Singlet oxygen reacts with the trap to produce the stable radical 2,2,6,6-tetramethyl-4-piperidone-N-oxyl (TEMPO), which was quantified using an electron paramagnetic resonance (EPR) spectrometer (Bruker EMX). Singlet oxygen yields (φD) were obtained relative to that of rose bengal dissolved in ethanol (φD, 0.76) (39). The production of superoxide in solution was verified via a colorimetric method. A solution of the carbaporphyrin ketal was mixed with nitroblue tetrazolium (NBT) and EDTA. This solution was illuminated with visible light (xenon lamp, 350 to 700 nm, 75 W) for 45 min. The superoxide generated oxidizes NBT to produce a colored formazan. Absorbance of the formazan was determined with a diode array UV-Vis spectrophotometer (Agilent 8452). Compounds were refrigerated and kept away from light before and during utilization. For biological assays, the compounds were dissolved in dimethyl sulfoxide (DMSO; Sigma), making sure that the final concentration of DMSO was below 0.5%, which is considered nontoxic for cells and parasites. Liposomal formulations of the compounds were prepared as described by Gardner et al. (24) and consisted of synthetic dimyristoyl phosphatidylcholine (DMPC), cholesterol (Ch), and distearoyl phosphatidylglycerol (DSPG), all from Avanti Lipids, in a DMPC/Ch/DSPG molar proportion of 2:1:0.8. The compounds were incorporated using a 1:500 compound/lipids molar proportion from chloroform stock solutions.
(ii) Parasites.
L. panamensis (MHOM/CO/87/UA140) was isolated from a Colombian patient infected with cutaneous leishmaniasis. L. amazonensis (LV78) engineered to produce β-lactamase (9) was kindly provided by F.S Buckner (Department of Medicine, University of Washington), and L. infantum (MCAN/ES/96/BCN150) is a reference strain from the World Health Organization (WHO).
(iii) In vitro cytotoxicity in mammalian cells.
The cytotoxic activity of compounds was assessed based on the viability of the human promonocytic cell line U937 (ATCC CRL-1593.2) and peritoneal macrophages obtained from hamsters (PMH). The cytotoxicity was evaluated by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT; Sigma) method as described previously (35, 40). Briefly, cells were grown in 96-well culture plates at a concentration of 105 cells/ml in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum (FBS; Invitrogen) and the corresponding concentration of the compounds using a serial dilution, starting at 250 μg/ml in duplicate (with and without encapsulation in liposomal formulation and with and without light exposure). Cells were incubated at 37°C with 5% CO2 for 96 h, and then the toxic effect was determined by adding 20 μl/well of MTT solution (0.5 mg/ml) and incubating at 37°C for 3 h. The reaction was stopped, and the quantity of formazan produced was measured with a Bio-Rad enzyme-linked immunosorbent assay (ELISA) reader set at 570 nm.
Cells cultured under the same conditions but in the absence of compounds provided a positive viability control. Both amphotericin B (Bristol Myers Squibb S.A.) and meglumine antimoniate (Sanofi Aventis) were used as cytotoxicity controls. The results are expressed as the 50% lethal concentration (LC50) calculated by the Probit method (22).
The light source was a fluorescent lamp in the 350- to 750-nm range, with peaks around 450, 550, and 650 nm (see Fig. 1). The lamp provides an average irradiance of 0.15 mW/cm2 (measured with a Digital Instruments LDX-1108 light meter) at a distance of 15 cm from the plates positioned perpendicular to the center of the lamp. Illumination times of 2 and 4 h provide energy fluences of 1.1 J/cm2 and 2.1 J/cm2, respectively.
(iv) Activity against axenic amastigotes.
The ability of the carbaporphyrin ketals to kill axenic amastigotes of L. panamensis, L. amazonensis, or L. infantum was determined based on the viability of the parasites evaluated by the MTT method as described previously (48). Briefly, amastigotes were cultivated in Schneider's medium (Sigma) at pH 5.4 supplemented with 20% FBS for 3 days at 32°C. They were harvested, washed, and resuspended (2 × 106 amastigotes/ml) in fresh medium. Wells of a 96-well plate were seeded with 100 μl of parasite suspension (in duplicate) and a volume of the test compound to provide serial dilutions starting at 100 μg/ml (with or without liposomal encapsulation and with or without light exposure). After 96 h of incubation at 32°C, the effect of drugs was determined by adding 40 μl/well of MTT and incubating at 32°C for 4 h. The reaction was stopped, and the quantity of formazan produced was measured with an ELISA reader set at 570 nm. Parasites cultivated in the absence of the compound but maintained under the same conditions were used as positive controls for viability. Results are reported as effective concentrations that kill 50% of intracellular parasites (EC50) calculated by the Probit method (22) and obtained as the averages from at least three independent experiments. The effectiveness of the compounds was compared with that obtained when using meglumine antimoniate and amphotericin B.
(v) Activity against intracellular amastigotes.
L. amazonensis (LV78) promastigotes, suspended in RMPI 1640 medium (Invitrogen) at a concentration of 5 × 106 promastigotes/ml, were used to infect U937 cells. U937 cells were maintained at a concentration of 3 × 105 cells/ml in RPMI 1640 medium at pH 7.2 and supplemented with 10% FBS, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine at 37°C and 5% CO2. After 2 to 3 days of growth, cells were washed with phosphate-buffered saline (PBS), centrifuged (70 × g), adjusted to a concentration of 5 × 105 cells/ml in RPMI, and incubated with 0.1 μg/ml of phorbol myristate acetate (PMA; Sigma). Aliquots (100 μl) of these cells were added to wells in a 96-well plate and incubated at 37°C under 5% CO2 for 72 h to allow adherence and differentiation into macrophages. Infection of macrophages was carried out by adding a 100-μl aliquot of promastigotes to the cells, followed by incubation at 34°C under 5% CO2 conditions for 24 h. Upon the change of medium, infected cells were incubated with the test compounds (with or without liposomal encapsulation and with or without light exposure) using six double serial dilutions from the LC50. Upon incubation for 48 h at 34°C under 5% CO2 conditions, an additional dose of the compounds and medium were added, followed by an additional incubation period of 48 h. The viability of the parasites inside the macrophages was measured using the colorimetric β-lactamase method (9) upon medium aspiration and the addition of 100 μl of Nitrocephin (100 μM) and Nonidet P-40 (0.1%) in PBS to each well. The absorbance of the metabolized substrate was read at 480 nm using an ELISA reader. The activity of the compounds was compared to that of a positive viability control consisting of infected cells that were not incubated with a test compound and grown under similar conditions. Results are reported as the EC50 as described above. The effectiveness of the compounds was compared with that obtained using meglumine antimoniate and amphotericin B.
Evaluation of in vivo toxicity and leishmanicidal activity.
The evaluation of the carbaporphyrin dimethyl ketal (CKOMe) was carried out with the hamster model for cutaneous leishmaniasis (26, 38). Golden hamsters (Mesocricetus auratus) were 6 weeks old with weights in the range of 100 to 140 g. The animals belonged to an endogamic colony raised and maintained in the animal facility at the Program for the Study and Control of Tropical Diseases (PECET) at Universidad de Antioquia. Animals were handled under the same micro- and macroenvironmental conditions (temperature of 24 to 28°C; 70 to 80% humidity), feeding (10% of body weight of Rodentia, with sterile water ad libitum), change of bedding material once a week, and grouping by gender and age range. Four experimental groups (n = 6 animals each) consisting of three males and three females, were named accordingly: (i) CKOMe—no light, (ii) CKOMe + 2 h illumination, (iii) CKOMe + 4 h illumination, and (iv) PBS (control). Additionally, two groups (n = 6 animals each) of animals were used to evaluate meglumine antimoniate as a control medicine at the curative (120 mg/kg of body weight day−1 for 10 days) and subcurative (80 mg/kg day−1 for 10 days) dosages.
Animals were infected in the dorsal skin by inoculation with promastigotes of L. amazonensis in early stationary growth phase that had been resuspended in PBS. The concentration of the inoculation dose was 1 × 107 per ml for females and 1.5 × 107 per ml for males. Animals were anesthetized previous to inoculation using a mixture of ketamine-xylazine (40 mg/kg and 5 mg/kg, respectively) via intraperitoneal injection under strict fasting conditions. Animals were checked on a weekly basis, with inspections of the area around the inoculation site and determinations of body weight and clinical condition. Treatment with CKOMe (0.5 mg/kg) was initiated immediately after typical skin lesions had developed (4 to 5 weeks postinfection). CKOMe doses (100 μl in PBS) were applied via intralesional inoculation every other day for a total of five doses. After each application of the CKOMe, animals that were exposed to visible light were immobilized and directly exposed to a fluorescent lamp (7 mW/cm2) for 2 or 4 h, in the same way as was used for the in vitro experiments. Meglumine antimoniate (100 μl, 120 or 80 mg/kg) was applied intramuscularly in the interior part of the leg. The effectiveness of each treatment was assessed by comparing the lesion sizes prior to and after treatments, using the following score system: cure (100% healing of the area and complete disappearance of the lesion), clinical improvement (a reduction of the size of the lesion by >10% in area), clinical failure (an increase in the size of the lesion), or relapse (a reactivation of the lesion after treatment). Animal condition was monitored for 100 days after treatment. After this period, animals were sacrificed humanely in a CO2 chamber, and a necropsy was carried out. Smears and samples for histopathology, blood chemistry, and parasitic load by limited dilution of the ganglia were obtained.
Evaluation of acute dermal toxicity.
Acute dermal toxicity was evaluated according to the protocols described by the Organisation for Economic Co-operation and Development Guidelines for Testing of Chemicals (37). Five male (or female) Wistar rats (Rattus norvegicus, Wistar strain) with body weights of between 200 and 300 g were housed individually for at least 5 days before testing. Animals were weighed 24 h before administration of the compound in order to calculate dose size (6.45 mg/kg of body weight). A 2-cm by 2-cm area of fur was clipped, and a solution of CKOMe in DMSO was then applied topically as a fine and uniform film on the prepared skin, which was covered using a porous gauze bandage fixed with nonirritant tape. Care was taken in order to maintain the bandage away from the animal's mouth to avoid ingestion of the compound. Exposure to the compound was limited to 24 h. When the testing period was over, any residues of the compound were removed using water or an appropriate solvent. Observations were annotated systematically in individual registers. Each animal was checked frequently during day 1 and at least once a day for a minimum of 14 days. Additional actions were taken, such as refrigeration and necropsies of animals found dead and the quarantine and humane sacrifice of animals found to be weak or moribund. Observations recorded included changes in skin and fur, eyes and mucosal membranes, convulsions, salivation, diarrhea, lethargy, somnolence, coma, alterations of the respiratory, circulatory, central and autonomous nervous systems, motile activity, and behavioral patterns. Body weight was determined before applying the compound, weekly after application, and at the time of sacrifice or death. All animals tested were subjected to a necropsy to register any macroscopic pathological changes. The evaluation also included, if available, the ratio between the exposure of the animals to the substance and the incidence and severity of abnormalities, including clinical and behavioral, as well as the ratio to any macroscopic lesions, changes in body weight, mortality, and any other toxic effect.
Ethical aspects.
This project was approved by the Committee for the Ethical Utilization of Animals in Research at the Universidad de Antioquia (certificate no. 35, 29 November 2006).
Statistical analysis.
All statistical analyses were carried out using SAS v 9.0 (2002 version; SAS Institute, Inc., NC) using a P value of <0.05 as the significance level for all tests. The evaluation of cytotoxicity and effectiveness included multilevel factorial analysis with a fixed balanced effect in which different independent effects (light exposure, liposome encapsulation, cell types, and Leishmania species) were accounted for. Additionally, completely randomized designs with a balanced fixed effect were carried out in which only the effect of treatment was accounted for. The Tukey test was employed to assess the statistical significance between averages.
The in vivo efficacy of the compound was expressed in terms of the percentage of clinical healing, improvement, or failure and compared to the efficacy observed for the group of animals treated with meglumine antimoniate. Each value was estimated from the analysis of experimental data using sigmoidal models within the MSxlfit software. Parametric and nonparametric tests were carried out in order to compare percentages and frequencies. Variances are expressed as standard errors and the statistical significance was determined using the Student t test and analysis of variance (ANOVA) when comparing within a group and between groups, respectively. Additionally, the relationship between two variables or events was evaluated by the Pearson chi-square test of association.
RESULTS
Photophysical properties of CKOMe and CKOEt.
Both carbaporphyrin ketals are photosensitizers with a strong absorption band in the blue (Fig. 1.) (λmax, ∼420 nm) and a relatively strong absorption band in the near infrared (λmax, ∼800 nm) regions, which makes them attractive for photodynamic therapy. The compounds have very small fluorescence yields (φf, ∼0.002) when excited at 450 nm, with emission bands peaking at 490 nm and 830 nm. Both compounds produce singlet oxygen in ethanol solution with moderate yields (φD of 0.23 ± 0.06 for CKOMe and 0.18 ± 0.05 for CKOEt). They also produce superoxide in 20% ethanol solutions in proportion to the amount of CKOMe used (see the supplemental material). The production of these species induced by CKOMe inside promastigotes of Leishmania tarentolae was previously demonstrated by Morgenthaler et al. (34).
In vitro cytotoxicity of CKOMe and CKOEt.
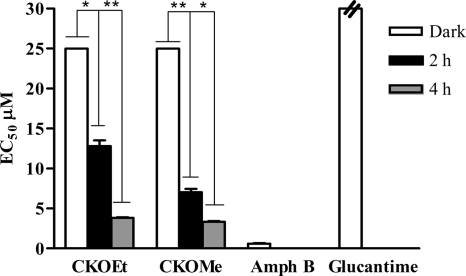
When the toxicity of the compounds (CKOMe and CKOEt) was evaluated in the promonocytic U937 cell line, it was observed that the compounds are highly toxic (Fig. 2). The LC50s for CKOEt and CKOMe in the absence of light were 4.16 ± 0.03 μM and 2.18 ± 0.01 μM, respectively. The effect was larger when the cells were exposed to visible light for 2 h (0.94 ± 0.02 μM for CKOEt and 0.35 ± 0.03 μM for CKOMe) and even more so when exposed to visible light for 4 h (0.33 ± 0.03 μM for CKOEt and 0.27 ± 0.05 μM for CKOMe). Both meglumine antimoniate and amphotericin B have lower toxicity than that of the tested compounds (28.5 ± 0.3 μM for amphotericin B and 896.8 ± 1.8 μM for meglumine antimoniate).
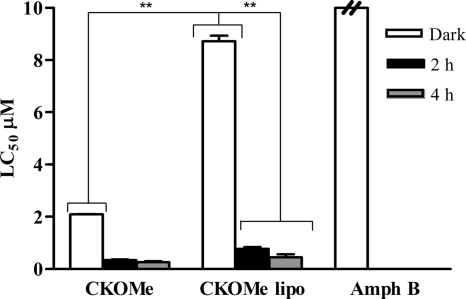
Fig. 2.
Dark and photoinduced (2-h and 4-h daily exposure to visible light) cytotoxicity of diethyl (CKOEt) and dimethyl (CKOMe) carbaporphyrin ketals on the promonocytic human cell line U937. Amphotericin B (Amph B) and meglumine antimoniate (Glucantime) are included for comparison. *, P < 0.05 (Tukey test).
The toxicity of CKOMe was diminished when formulated in liposomes (Fig. 3), with LC50s of 8.7 ± 0.3 μM when cells were not exposed to light, 0.77 ± 0.09 μM when exposed to 2 h of visible light, and 0.45 ± 0.16 μM when exposed to 4 h of light, although they were not at the cytotoxicity level of amphotericin B.
Fig. 3.
Dark and photoinduced (2-h and 4-h exposure to visible light) cytotoxicity of dimethyl carbaporphyrin ketal (CKOMe) in a liposomal (lipo) formulation on the promonocytic human cell line U937. Amphotericin B (Amph B) is included for comparison. **, 0.001 < P < 0.05 (Tukey test).
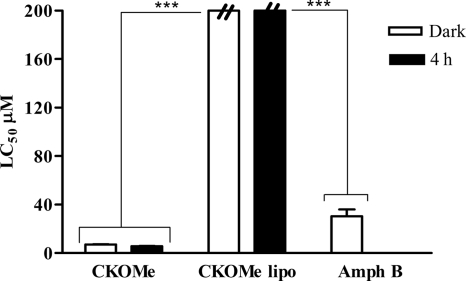
The toxicity of CKOMe on peritoneal macrophages from hamsters (PMH) was lower than that observed with the U937 cell line. For instance, the LC50 is 7.2 ± 0.2 μM without light exposure and 5.7 ± 0.2 μM when exposed to 4 h of visible light. There was a notable decrease of cytotoxicity when the compound was formulated in liposomes (Fig. 4) with LC50s superior (>334.4 μM without light exposure and 272.2 ± 2.6 μM when exposed to 4 h of light) to those measured for amphotericin B (30.4 ± 8.1 μM). It was also observed that when the PMH incubated with CKOMe were exposed to visible light for 4 h, there was an increase in the cytotoxicity of the compound, confirming the photosensitizing property of CKOMe.
Fig. 4.
Dark and photoinduced (4-h exposure to visible light) cytotoxicity of dimethyl carbaporphyrin ketal (CKOMe) and liposomal (lipo) dimethyl carbaporphyrin ketal (CKOMe lipo) on peritoneal macrophages from hamster (PMH). Amphotericin B (Amph B) is included for comparison. ***, P < 0.001 (Tukey test).
Effectiveness of CKOMe and CKOEt against axenic and intracellular amastigotes of pathogenic species of Leishmania.
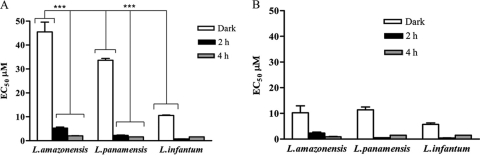
Both carbaporphyrin ketals were largely effective against axenic amastigotes of L. amazonensis, L. infantum, and L. panamensis (Fig. 5). For example, EC50s for CKOMe and CKOEt when applied to axenic amastigotes of L. amazonensis in the dark are 10.3 ± 3.8 μM and 46 ± 6 μM, respectively. The effectiveness is enhanced when the cells incubated with the compounds are exposed to 2 and 4 h of light. EC50s for CKOMe decrease to 2.3 ± 1.5 μM and 0.92 ± 0.17 μM, while those for CKOEt decrease to 5.3 ± 0.6 μM and 2.10 ± 0.3 μM upon 2 and 4 h of light exposure, respectively. The effectiveness of the compounds was larger than that of meglumine antimoniate (EC50, >2,730 μM). The EC50 for CKOMe without light exposure in L. amazonensis is of a magnitude similar to that for amphotericin B (EC50, 9.2 ± 2.1 μM), but CKOMe is more effective when the axenic amastigotes are exposed to visible light (2 or 4 h). CKOMe was more effective than amphotericin B against axenic amastigotes of L. panamensis and L. infantum when cultures were exposed to 2 h of visible light. CKOEt was, in general, less effective than amphotericin B against all Leishmania species tested, and it was only more effective than amphotericin B against L. infantum after exposing cultures to 2 h of visible light (Table 1).
Fig. 5.
Dark and photodynamic (2 h and 4 h exposure to visible light) efficacy of (A) diethyl carbaporphyrin ketal (CKOEt) and (B) dimethyl carbaporphyrin ketal (CKOMe) on axenic amastigotes of pathogenic Leishmania species. ***, P < 0.001 (Tukey test).
Table 1.
In vitro effectiveness of DMSO solutions of carbaporphyrin ketals against axenic amastigotes of Leishmania
| Compound | EC50 (μM) ± SD |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
L. amazonensis |
L. panamensis |
L. infantum |
|||||||
| Dark | Light, 2 h | Light, 4 h | Dark | Light, 2 h | Light, 4 h | Dark | Light, 2 h | Light, 4 h | |
| CKOMe | 10.3 ± 3.8 | 2.4 ± 0.5 | 0.9 ± 0.17 | 4.4 ± 1.5 | 0.50 ± 0.04 | <1.5 | 5.7 ± 0.8 | 0.40 ± 0.13 | <1.5 |
| CKOEt | 45.5 ± 5.8 | 5.3 ± 0.6 | 2.08 ± 0.03 | 33.6 ± 1.0 | 2.2 ± 0.2 | <1.6 | 10.9 ± 0.2 | 0.72 ± 0.05 | <1.6 |
| Amphotericin B | 9.2 ± 2.1 | ||||||||
| Meglumine antimoniate | >2,730 | ||||||||
Liposomal formulations increased the effectiveness of the compound against L. amazonensis (even in the dark) and L. panamensis. EC50s against L. amazonensis are <8.5 μM for both compounds when applied in the dark. The exposure to 2 h of visible light decreases the EC50 to 0.92 ± 0.06 μM for CKOMe and 1.13 ± 0.10 μM for CKOEt. Exposure to 4 h decreases the EC50 further, to <0.52 μM for CKOMe and 0.78 ± 0.10 μM for CKOEt. Thus, the compounds in liposome formulation are more effective than meglumine antimoniate, while they reach values of similar magnitude to the EC50 of amphotericin B upon 2 and 4 h of illumination with visible light, although such an effect was not observed for L. infantum (Table 2).
Table 2.
In vitro effectiveness of liposomal formulations of carbaporphyrin ketals against axenic amastigotes of Leishmania
| Compound | EC50 (μM) ± SD |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
L. amazonensis |
L. panamensis |
L. infantum |
|||||||
| Dark | Light, 2 h | Light, 4 h | Dark | Light, 2 h | Light, 4 h | Dark | Light, 2 h | Light, 4 h | |
| CKOMe | <8.4 | 0.92 ± 0.06 | <0.52 | 2.8 ± 0.2 | 0.59 ± 0.01 | 0.15 ± 0.01 | <8.4 | 4.3 ± 0.5 | 1.76 ± 0.05 |
| CKOEt | <8.0 | 1.13 ± 0.10 | 0.78 ± 0.10 | <8.0 | 1.07 ± 0.01 | 0.34 ± 0.10 | <8.0 | <8.0 | <10.4 |
The effectiveness of both compounds against intracellular amastigotes of L. amazonensis (Fig. 6) is larger than that of meglumine antimoniate (EC50, >683 μM) but did not surpass that of amphotericin B (EC50, 0.6 ± 0.1 μM) even after light exposure. The effectiveness of the compounds is, however, increased upon exposure to light. For instance, the EC50 for CKOMe decreases from 25 μM to 7.0 ± 0.6 μM upon exposure of cultures to 2 h of visible light and down to 3.35 ± 0.14 μM upon exposure to 4 h. Similar results were observed for CKOEt and with liposomal formulations (Table 3).
Fig. 6.
Dark and photodynamic (2 h and 4 h exposure to visible light) efficacy of diethyl carbaporphyrin ketal (CKOEt) and dimethyl carbaporphyrin ketal (CKOMe) on intracellular amastigotes of Leishmania amazonensis. Amphotericin B (Amph B) and meglumine antimoniate (Glucantime) are included for comparison. *, P < 0.05; **, 0.001 < P < 0.05 (Tukey test).
Table 3.
In vitro effectiveness of carbaporphyrin ketals against intracellular amastigotes of L. amazonensis
| Compound | EC50 (μM) ± SD (SI)a |
|||||
|---|---|---|---|---|---|---|
| Without liposomal formulation |
With liposomal formulation |
|||||
| Dark | Light, 2 h | Light, 4 h | Dark | Light, 2 h | Light, 4 h | |
| CKOMe | 25.0 ± 0.8 (0.288) | 7.0 ± 0.6 | 3.84 ± 0.11 (1.35) | <1.85 | <1.85 | <1.8 |
| CKOEt | 25.0 ± 0.8 | 12.8 ± 1.0 | 3.35 ± 0.14 | <2.6 | <2.6 | <3.2 |
| Amphotericin B | 0.61 ± 0.10 | |||||
| Meglumine antimoniate | >683 | |||||
SI, selectivity index, i.e., the LC50/EC50 relative to results with PMH cells.
In vivo effectiveness. (i) Reduction of lesion size.
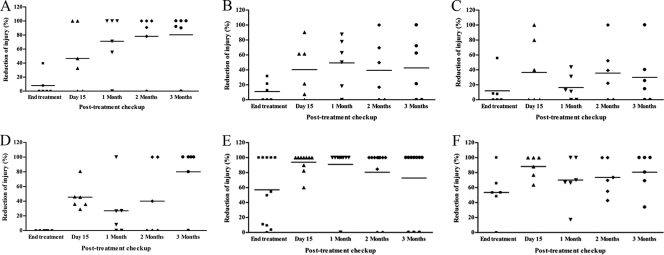
Treatment with CKOMe resulted in notable reduction of the size of skin lesions. Half of the animals (3 of 6) treated with the compound without light exposure showed 100% reduction in the size of the lesion 1 month after treatment. Size reduction increased to 80 to 100% for 5 out of 6 of the animals at the end of the study (100 days posttreatment). Animals treated with CKOMe and 2 and 4 h of light exposure also showed significant reduction of lesion size, oscillating between 50 and 100% from the first month posttreatment until the end of the study for 3 out of 6 animals in each group (Fig. 7).
Fig. 7.
Effect of treatment with CKOMe on cutaneous lesion size of hamsters infected with L. amazonensis in comparison with untreated animals and treatment with meglumine antimoniate: (A) without light exposure, (B) exposure to 2 h light, (C) exposure to 4 h light, (D) untreated, (E) treated with curative dose of meglumine antimoniate, (F) treated with subcurative dose of meglumine antimoniate.
The results obtained with animals in the control group (i.e., those treated with saline buffer) are interesting. One month after treatment, only 1 out of 6 animals had a lesion size reduction of 100%, while all other animals experienced lesion size reductions of below 20%. However, at the end of the study, 4 out of 6 animals had a lesion size reduction of 100% while one of the other 2 experienced a lesion size reduction of 80% and the other one did not show improvements. These results indicate that there is the possibility of spontaneous healing of the hamster lesion similar to what is observed for humans that are immunocompetent against cutaneous leishmaniasis (Fig. 7). Most of the animals treated with the control medicine (meglumine antimoniate) at the curative dose (120 mg/kg/day) showed 100% reduction of the lesion size from day 15 after treatment until the end of the study (100 days post treatment). However, only half of those treated with meglumine antimoniate at the subcurative dose (80 mg/kg/day) showed 100% reduction of the lesion size in the same time period (Fig. 7).
(ii) Relationship between therapeutic efficacy and clinical healing, improvement, and failure.
Treatment with CKOMe without light exposure was the most efficient, showing an efficacy of 33 to 50% between the first and third month after treatment and a 67% of definitive healing (Table 4). In contrast, treatment with CKOMe with exposure to 2 and 4 h of visible light shows 17% efficacy, with healing failure oscillating between 17 and 33% (Table 4). Interestingly, the compound is more effective in vivo when it is not exposed to light, which was unexpected on the basis of the in vitro experiments. One feasible explanation is that the in vivo conditions render a compound that is more active than the applied one and perhaps that the exposure to light inactivates CKOMe or its derivative produced in vivo. Interestingly, the results indicate that a longer exposure to light increases the effectiveness of treatment, which indicates that the photodynamic effect is still active. Although further studies will be required, there is also an indication (see the supplemental material for details) that CKOMe can be transalkoxylated or dealkoxylated upon light exposure when it is diluted in an aqueous solution of ethanol (80%). Mass spectrometry analyses reveal that 4 μM solutions of CKOMe or CKOEt exposed to visible light from a xenon lamp (75 W) experienced the replacement of an alkoxy group (-OCH3 or -OC2H5) by hydrogen, or in the case of the CKOMe, the replacement of a methoxy (-OCH3) group by an ethoxy (-OC2H5). A radical-mediated mechanism provides a plausible explanation for the light-induced reactivity of the compounds.
Table 4.
In vivo efficacy of treatment with CKOMe
| Treatment/compounda | Result of treatment at end of study (%) |
||
|---|---|---|---|
| Clinical cure | Failure | Clinical improvement | |
| CKOMe without light | 67 | 0 | 33 |
| CKOMe + 2 h of light | 67 | 33 | 0 |
| CKOMe + 4 h of light | 83 | 17 | 0 |
| Meglumine antimoniate, curative dose | 67 | 33 | 0 |
| Meglumine antimoniate, subcurative dose | 67 | 33 | 0 |
n = 6 animals per group.
The control treatment (saline) had an efficacy of 50% at the end of the study (100 days posttreatment) with 17% healing failure and definite healing of 33%. A curative dose of meglumine antimoniate (120 mg/kg/day) was 33 to 83% efficient, while a subcurative dose (80 mg/kg/day) was 50 to 67% efficient. Although 100% and 67% healing was initially observed for curative and subcurative doses, respectively, both treatments show failure of 33% 1 month after treatment (Table 4).
(iii) Body weight follow-up after treatment.
The body weights of animals treated with CKOMe did not differ significantly during the time in which animals were followed up after treatment (P was >0.05, ANOVA). No significant weight loss was observed for any animal treated at any time during the study (Fig. 8). In contrast, animals treated with meglumine antimoniate experienced a small amount of weight loss 1 month after treatment, with an increase in weight during the second month after treatment followed by a decrease at the time the study ended. Weights of animals in the control group were stable, with small increments between the second and third month posttreatment.
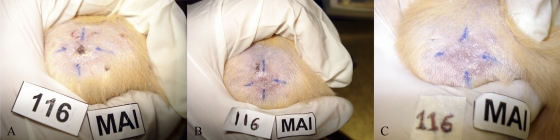
Fig. 8.
In vivo effect of carbaporphyrin dimethyl ketal (CKOMe). (A) Pretreatment photograph of lesion, (B) 15 days posttreatment and (C) 45 days posttreatment. Note the decrease in size and development of scar tissue. Improvement of lesion correlates well with the evaluation of parasitic loads.
(iv) Analyses of smears and parasitic load.
Smears from the liver, spleen, and ganglia of animals in the three groups treated with CKOMe (without light exposure, 2 and 4 h of light exposure) did not show the presence of parasites. Only one smear from a skin sample of an animal in the group of animals treated with CKOMe and 4 h of light showed amastigotes in a moderate amount. Evaluation of the parasitic load in the ganglia revealed no parasites upon treatment with CKOMe. In contrast, all samples from animals in the control group (treated with saline solution) were positive for parasitic load.
(v) Histopathological evaluations.
The evaluation of tissues from treated animals showed changes associated with the infection process by Leishmania similar to those observed in the control group (infected and untreated). Skin samples of 33% of hamsters showed a slight level of plasmocytes and multinuclear cells accompanied by moderate infiltration of lymphocytes and polymorphonuclear neutrophils (PMN) and a severe infiltration of macrophages in 17 to 83% of animals (see the supplemental material for details). These observations correspond to a diagnosis of granulomatous dermatitis, an inflammatory event that compromises the dermis and subjacent muscle. This is the main lesion associated with the infection, which is compatible with the severe manifestation of cutaneous leishmaniasis in 75% of the animals in the study. This association was statistically correlated (P was <0.05, Pearson chi-square test). Kidney samples showed moderate hyperplasia in the renal cortex and glomerules accompanied by slight atrophy of the kidneys in 75% of animals. These observations are compatible with membrane proliferative glomerulonephritis, a diagnosis which has been previously associated with Leishmania infection. Statistical association was large (P was <0.05, Pearson chi-square test). The livers of animals in all groups (see the supplemental material for details) showed slight to severe (untreated group) vacuolar changes in the hepatocytes, which may be associated with physiological processes such as hepatocytic accumulation of glycogen. These were, however, highly associated with Leishmania infection, because they correlate to elevated levels of alanine-amino transferase (ALT; see below). Other liver tissue abnormalities, such as fatty degeneration, fibrosis, and congestion (see the supplemental material for details), correspond to the infection process. In all the animals studied, there was vascular congestion in the spleen, although this is not related to the treatment of the infection. However, the moderate acute splenitis found in the majority of animals (80%) is associated with the systemic circulation of the infectious agent that induces the increase in the circulation of neutrophils. Other observations, such as vacuolar changes in hepatocytes, cardiomegalies, the presence of inclusions of intracytoplasmic eosinophils, as well as the vacuolization of cells in the renal tubes, are not statistically associated (P value of >0.05) with a toxic effect of the compound tested.
(vi) Toxicity upon treatment with CKOMe.
Chemical analysis of blood samples did not reflect changes in the normal levels of blood urea nitrogen (BUN) and creatinine (1.2 to 2.6 mg/ml and 0.04 to 0.10 mg/ml, respectively) (21) in any of the animals studied during the period of treatment and posttreatment. No significant statistical differences in the levels of these markers were found between treated and untreated animals. The blood levels of ALT were elevated in the treated animals compared to values reported in the literature (22 to 128 U/liter) (21) for golden hamsters raised in laboratories. However, elevated ALT levels were also observed for the control group (infected, untreated), indicating that hepatic dysfunction is caused by the infection and does not reflect any toxic influence of the treatment. Hemograms obtained pretreatment and 45 days after treatment reflected changes in the absolute and relative numbers of lymphocytes and neutrophils with a decrease in the cell count of lymphocytes and a notable increase in the count of polymorphonuclear neutrophils (PMN) (see the supplemental material). This process is part of the immunological inflammatory response to infection by Leishmania and is caused by the necessity of the infected organism to fight infection with the first response mechanism via phagocytes (such as neutrophils), thus incrementing the level of neutrophils in blood and tissue at the expense of lymphocytes. Other hematogram parameters, such as leukocyte, erythrocyte, and platelet counts, hemoglobin levels, and hematocrit, remained within normal levels.
(vii) Acute dermal toxicity.
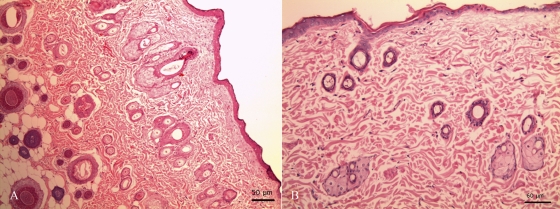
During evaluation of acute dermal toxicity, the subject animals did not experience either behavioral changes or alterations of the nervous, respiratory, and circulatory systems. The animals also did not experience changes in the conditions of their skin, fur, eyes and mucous membranes and did not experience notable changes in weight. None of them exhibited abnormal salivation, diarrhea, lethargy, excessive somnolence, or convulsions. Necropsies of sacrificed animals did not indicate macroscopic pathological alterations in the liver, kidneys, or heart. Only one animal showed a scratching lesion, which was totally resolved without any associated collateral effect. Histopathological analyses of skin samples did not reveal abnormalities and significant lesions due to treatment with CKOMe (Fig. 9).
Fig. 9.
Histopathological samples of rat skin (200× magnification). (A) Control sample (untreated). (B) Sample treated with CKOMe; histopathology was normal without significant alterations.
DISCUSSION
Both carbaporphyrin ketals were highly effective against axenic and intracellular amastigotes of the Leishmania species evaluated. In general, exposure of cultures to visible light (7 mW/cm2 for 2 or 4 h) increased the leishmanicidal activity of the compounds, confirming their photodynamic activity. Liposomal formulation of the compounds did not decrease their activity, and even without exposure to light, they were more active than when administered in DMSO solution against axenic and intracellular amastigotes of L. amazonensis. Similar results were previously observed when liposomal formulations of phthalocyanines were used against promastigotes of L. major (43). Both carbaporphyrin ketals were highly effective against axenic amastigotes of L. amazonensis, with EC50s of 2.1 μM and 0.92 μM for CKOEt and CKOMe, respectively. Effectiveness was improved when formulated in liposomes, with such formulations being even more effective than meglumine antimoniate and amphotericin B against axenic amastigotes of L. panamensis and L. amazonensis; EC50s were between 0.78 μM and 0.34 μM for CKOEt and 0.52 μM and 0.15 μM for CKOMe. The EC50s of the compounds when tested against intracellular amastigotes of L. amazonensis were between 1.8 μM and 25 μM, which are better than that measured for meglumine antimoniate although not superior to that shown by amphotericin B.
Carbaporphyrin ketals are effective against axenic and intracellular amastigotes at levels similar to those of compounds tested in other studies. For instance, zinc phthalocyanine and aluminum phthalocyanine chloride are active against promastigotes of L. infantum and L. panamensis in submicromolar doses upon red-light illumination at fluences of between 2.5 and 10 J/cm2 (20). Aluminum phthalocyanine chloride is also effective against intracellular amastigotes of L. amazonensis at doses in the 2 to 7 μM range and red-light fluences of between 1 and 3 J/cm2 (19). The effectiveness of the carbaporphyrin ketals is also comparable to that of cationic porphyrin derivates, such as meso-tetrakis(4-N,N,N-trimethyl-aniliniumyl) porphyrin tetrachloride, which was studied by Bristow et al. (8) against L. major. This compound is effective at doses of between 2 and 7 μM against promastigotes of this species, although it is toxic to macrophages but not to human keratinocytes.
Both CKOMe and CKOEt are toxic to in vitro cultures. A photodynamic effect of their toxic action on the human cell line U937 and peritoneal macrophages of hamster (PMH) was observed. Interestingly, the carbaporphyrin ketals were more toxic to U937 cells (0.3 to 0.5 μM) than to PMH cells. This indicates a selectivity of the compounds for these cell types. Such selectivity on U937 cells may be associated with the presence of polyunsaturated fatty acids (PFA) that are localized in the membrane of these tumoral cells, which constitute excellent targets for lipid peroxidation via photodynamic mechanisms (49).
There was a significant difference between the LC50s measured for these two cellular species for the compound, both in the liposomal formulation and when applied in DMSO solution. For instance, the LC50 of CKOMe when applied to PMH as a DMSO solution is 5.7 μM, in contrast to 272 μM when administered to these cells in liposomal formulation. The decrease of the cytotoxicity concurrent with an increase of the effectiveness of compounds when administered to cultures in liposomal formulations is expected based on previous literature reports (6, 15, 17).
The selectivity index (SI) of the carbaporphyrin ketals is better than that previously reported for other compounds. Akilov et al. (3) showed that the incubation of promastigotes of L. major with 0.1 mM aminolevulinic acid (ALA) and ALA-protoporphyrin IX (ALA-PpIX) did not accomplish a significant decrease in the viability of the parasites. Also, inconclusive results were evident when these compounds were evaluated for intracellular parasites infecting the murine cell line J774.2. Indeed, the macrophages were more sensitive than the parasites to the compound's activity, suggesting that death of the host cells was likely to occur without complete eradication of the parasites. Similar results were observed by Kosaka et al. (30), who reported that in vitro therapy with ALA or ALA-PpIX at 0.1 μM and a light fluence of 10 J/cm2 required PpIX concentrations in the 100 to 1,000 μM range in order to significantly diminish the viability of parasites, although eradication of the parasites was not observed, even at fluences of 50 J/cm2.
Animal studies of the treatment of cutaneous leishmaniasis via photodynamic therapy are limited. Hasan and coworkers (3, 4, 30) evaluated the effect of photodynamic treatment with ALA on treating BALB/c mice infected with L. major. They observed that mice treated with a solution of ALA and light at a fluence of 50 J/cm2 4 h after topical application presented a significant reduction of parasitic load relative to results with a control group (untreated) and one group treated with ALA without light exposure.
The results of the in vivo experiments presented herein, although not conclusive, provide a closer look at the performance of a carbaporphyrin ketal and light exposure as an alternative treatment for cutaneous or mucocutaneous leishmaniasis. It is evident that CKOMe was more effective when the animal lesions were not exposed to visible light. This behavior might indicate that the compound is reacting via enzymatic action during the incubation period within the animal tissue producing an ingredient that may be active in the dark conditions but is sensitive to bleaching upon light exposure, which produces a less active (or inactive) compound (46). Although it is also possible that treatment with CKOMe also damages the host tissue, opening the possibility for recurrence of the infection.
As expected, there is no indication of systemic toxicity associated with the local application of the photosensitizer in the skin lesion caused by the infection. Histopathological results revealed changes in the skin, liver, kidney, and spleen associated with the infection by Leishmania (1, 12, 14, 16) but not as a result of a toxic effect of the compound on these organs. Skin analyses revealed direct action of the compounds on the parasite, with material phagocytized by large macrophages and the formation of granulomas around them in those samples with scarce numbers of parasites. Some skin smears from hamsters treated with CKOMe indicated the presence of parasites after the study even though the animal was considered clinically cured. This indicates that the treatment, although effective, does not eradicate the parasites completely. It is known that clinical cure does not guarantee the sterility of scars in terms of the parasite's persistence. It is therefore necessary to determine what the implications of parasite's persistence are after clinical cure. Future evaluations should focus on what happens from the point of view of the evolution of the infection, lesion reactivation, and infection transmission (32). The evaluation of biochemical parameters, such as blood urine nitrogen (BUN), creatinine, and alanine-amino transferase (ALT), as well as the hemogram, indicated alterations related to the infection by Leishmania. These data, in combination with the animal weight monitoring and the results obtained upon the evaluation of acute dermal toxicity in rats, indicate that CKOMe at the doses applied is nontoxic and has no adverse systemic and cutaneous effects associated with its application.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Colombian Science, Technology and Innovation Administrative Department (COLCIENCIAS) and the Center for Development of Products against Tropical Diseases (CIDEPRO), Universidad de Antioquia. V.M.T. appreciates the financial support received from COLCIENCIAS and CIDEPRO. D.L.C. and M.A.J. acknowledge funds from Illinois State University, College of Arts and Science via a Program of Excellence award. T.D.L. acknowledges support from the National Science Foundation under grant CHE-0911699.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 25 July 2011.
REFERENCES
- 1. Abreu-Silva A. L., et al. 2004. Histopathological studies of visceralized Leishmania (Leishmania) amazonensis in mice experimentally infected. Vet. Parasitol. 121:179–187 [DOI] [PubMed] [Google Scholar]
- 2. Akilov O. E., et al. 2006. The role of photosensitizer molecular charge and structure on the efficacy of photodynamic therapy against Leishmania parasites. Chem. Biol. 13:839–847 [DOI] [PubMed] [Google Scholar]
- 3. Akilov O. E., Kosaka S., O'Riordan K., Hasan T. 2007. Parasiticidal effect of delta-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis is indirect and mediated through the killing of the host cells. Exp. Dermatol. 16:651–660 [DOI] [PubMed] [Google Scholar]
- 4. Akilov O. E., Kosaka S., O'Riordan K., Hasan T. 2007. Photodynamic therapy for cutaneous leishmaniasis: the effectiveness of topical phenothiaziniums in parasite eradication and Th1 immune response stimulation. Photochem. Photobiol. Sci. 6:1067–1075 [DOI] [PubMed] [Google Scholar]
- 5. Allison R. R., et al. 2004. Photosensitizers in clinical PDT. Photodiagnosis Photodyn. Ther. 1:27–42 [DOI] [PubMed] [Google Scholar]
- 6. Alving C. R., et al. 1978. Therapy of leishmaniasis: superior efficacies of liposome-encapsulated drugs. Proc. Natl. Acad. Sci. U. S. A. 75:2959–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berman J. D. 2003. Current treatment approaches to leishmaniasis. Curr. Opin. Infect. Dis. 16:397–401 [DOI] [PubMed] [Google Scholar]
- 8. Bristow C.-A., Hudson R., Paget T. A., Boyle R. W. 2006. Potential of cationic porphyrins for treatment of cutaneous leishmaniasis. Photodiagnosis Photodyn. Ther. 3:162–167 [DOI] [PubMed] [Google Scholar]
- 9. Buckner F. S., Wilson A. J. 2005. Colorimetric assay for screening compounds against Leishmania amastigotes grown in macrophages. Am. J. Trop. Med. Hyg. 72:600–605 [PubMed] [Google Scholar]
- 10. Castano A. P., Demidova T. N., Hamblin M. R. 2005. Mechanisms in photodynamic therapy: part three—photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis Photodyn. Ther. 2:91–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang C. S., Chang K. P. 1985. Heme requirement and acquisition by extracellular and intracellular stages of Leishmania mexicana amazonensis. Mol. Biochem. Parasitol. 16:267–276 [DOI] [PubMed] [Google Scholar]
- 12. Coura S., et al. 2006. Comparison of paraffin embedded skin biopsies from different anatomical regions as sampling methods for detection of Leishmania infection in dogs using histological, immunohistological and PCR methods. BMC Vet. Res. 2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Croft S. L., Seifert K., Yardley V. 2006. Current scenario of drug development for leishmaniasis. Indian J. Med. Res. 123:399–410 [PubMed] [Google Scholar]
- 14. Dantas Cangussú S., et al. 2009. Histopatology of Leishmania major infection: revisiting L. major histopatology in the ear dermis infection model. Mem. Inst. Oswaldo Cruz 104:918–922 [DOI] [PubMed] [Google Scholar]
- 15. Davidson R. N., et al. 1996. Short-course treatment of visceral leishmaniasis with liposomal amphotericin B (AmBisome). Clin. Infect. Dis. 22:938–943 [DOI] [PubMed] [Google Scholar]
- 16. de Oliveira Cardoso F., et al. 2010. Immunopathological studies of Leishmania amazonensis infection in resistant and in susceptible mice. J. Infect. Dis. 201:1933–1940 [DOI] [PubMed] [Google Scholar]
- 17. Derycke A. S. L., de Witte P. A. M. 2004. Liposomes for photodynamic therapy. Adv. Drug Deliv. Rev. 56:17–30 [DOI] [PubMed] [Google Scholar]
- 18. Dolphin D. 1994. 1993 Syntex award lecture: photomedicine and photodynamic therapy. Can. J. Chem. 72:1005–1013 [Google Scholar]
- 19. Dutta S., Ray D., Kolli B. K., Chang K. P. 2005. Photodynamic sensitization of Leishmania amazonensis in both extracellular and intracellular stages with aluminum phtalocyanine chloride for photolysis in vitro. Antimicrob. Agents Chemother. 49:4474–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Escobar P., Hernández I. P., Rueda C. M., Martínez F., Páez E. 2006. Photodynamic activity of aluminum (III) and zinc (II) phthalocyanines in Leishmania promastigotes. Biomédica. 26:49–56 [PubMed] [Google Scholar]
- 21. Field K. J., Sibold A. 1999. The laboratory hamster and gerbil, p. 10–11 In Sibold A., Suckow M. (ed.), The laboratory animal pocket reference series, 1st ed CRC Press, Washington, DC [Google Scholar]
- 22. Finney J. D. 1978. Statistical method in biological assay, 3rd ed., p. 508 Academic Press, London, United Kingdom [Google Scholar]
- 23. Gagle M. P. 1997. Photodynamic therapy with porphyrins. DermWeb, University of British Columbia, Vancouver, Canada: http://www.dermatology.org/laser/pdt.html Accessed January 2004 [Google Scholar]
- 24. Gardner D. M., et al. 2010. Association of acenaphthoporphyrins with liposomes for the photodynamic treatment of leishmaniasis. Photochem. Photobiol. 86:645–652 [DOI] [PubMed] [Google Scholar]
- 25. Hayes M. J., Spence J. D., Lash T. D. 1998. Facile oxidation of a carbaporphyrin at the internal carbon atom: synthesis of novel benzo[18]annulene ketals. Chem. Commun. 1998:2409–2410 [Google Scholar]
- 26. Henao H. H., Osorio Y., Saravia N. G., Gómez A., Travi B. 2004. Efficacy and toxicity of pentavalent antimonials (Glucantime and Pentostam) in an American cutaneous leishmaniasis animal model: luminometry application. Biomédica 24:393–402 (In Spanish.) [PubMed] [Google Scholar]
- 27. Josefsen L., Boyle R. W. 2008. Photodynamic therapy: novel third-generation photosensitizers one step closer? Br. J. Pharmacol. 154:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim S. Y., Kim E. J., Park J. 2002. Control of singlet oxygen-induced oxidative in Escherichia coli. J. Biochem. Mol. Biol. 35:353–357 [DOI] [PubMed] [Google Scholar]
- 29. Kindhauser M. 2003. Communicable diseases 2002: global defence against the infectious disease threat. World Health Organization, Geneva, Switzerland [Google Scholar]
- 30. Kosaka S., Akilov O. E., O'Riordan K., Hasan T. 2007. A mechanistic study of delta-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis. J. Invest. Dermatol. 127:1546–1549 [DOI] [PubMed] [Google Scholar]
- 31. Lash T. D., et al. 2003. Regioselective oxidations of benzocarbaporphyrins with ferric chloride: a facile synthesis of bridged [18] annulene ketals with strong absorptions in the far red and an unexpected halogenation reaction at the interior carbon atom. J. Org. Chem. 68:8558–8570 [DOI] [PubMed] [Google Scholar]
- 32. Mendonça M. G., et al. 2004. Persistence of Leishmania parasites in scars after clinical cure of American cutaneous leishmaniasis: is there a sterile cure? J. Infect. Dis. 189:1018–1023 [DOI] [PubMed] [Google Scholar]
- 33. Minodier P., Parola P. 2007. Cutaneous leishmaniasis treatment. Travel Med. Infect. Dis. 5:150–158 [DOI] [PubMed] [Google Scholar]
- 34. Morgenthaler J. B., et al. 2008. Carbaporphyrin ketals as potential agents for a new photodynamic therapy treatment of leishmaniasis. Bioorg. Med. Chem. 16:7033–7038 [DOI] [PubMed] [Google Scholar]
- 35. Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55–63 [DOI] [PubMed] [Google Scholar]
- 36. Olmsted J. 1979. Calorimetric determinations of absolute fluorescence quantum yields. J. Phys. Chem. 83:2581–2584 [Google Scholar]
- 37. Organisation for Economic Co-operation and Development 1987. Test no. 402: acute dermal toxicity, section 4: health effects. OECD guidelines for the testing of chemicals. OECD Publishing, Paris, France: doi:10.1787/9789264070585-en [Google Scholar]
- 38. Travi B. L., Osorio Y. 1998. Failure of Albendazole as an alternative treatment of cutaneous leishmaniasis in the hamster model. Mem. Inst. Oswaldo Cruz 93:515. [DOI] [PubMed] [Google Scholar]
- 39. Redmond R. W., Gamlin J. N. 1999. A compilation of singlet oxygen yields from biologically relevant molecules. Photochem. Photobiol. 70:391–475 [PubMed] [Google Scholar]
- 40. Robledo S., et al. 2005. In vitro and in vivo cytotoxicities and antileishmanial activities of thymol and hemisynthetic derivatives. Antimicrob. Agents Chemother. 49:1652–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robledo S. M., Puerta J. A., Muñoz D. L., Guardo M., Vélez I. D. 2006. Efficacy and tolerance of pentamidine for treatment of cutaneous leishmaniasis caused by L. (V) panamensis in Colombia. Biomédica 26:188–193 [PubMed] [Google Scholar]
- 42. Sah J. F., et al. 2002. Genetic rescue of Leishmania deficiency in porphyrin biosynthesis creates mutants suitable for analysis of cellular events in uroporphyria and for photodynamic therapy. J. Biol. Chem. 277:14902–14909 [DOI] [PubMed] [Google Scholar]
- 43. Sazgarnia A., Bahreyni-Toosi M. H., Layegh P., Rajabi O. 2010. Liposomal zinc phthalocyanine as a potential agent for photodynamic therapy of leishmaniasis. Indian J. Dermatol. Venereol. Leprol. 76:417–418 [DOI] [PubMed] [Google Scholar]
- 44. Shyam S., Madhukar R. 2002. Advances in the treatment of leishmaniasis. Curr. Opin. Infect. Dis. 15:593–598 [DOI] [PubMed] [Google Scholar]
- 45. Soto J., Berman J. 2006. Treatment of New World cutaneous leishmaniasis with miltefosine. Trans. R. Soc. Trop. Med. Hyg. 100(Suppl. 1):S34–S40 [DOI] [PubMed] [Google Scholar]
- 46. Spikes J. D. 1992. Quantum yields and kinetics of the photobleaching of hematoporphyrin, Photofrin II, tetra(4-sulfonatophenyl)-porphine and uroporphyrin. Photochem. Photobiol. 55:797–808 [DOI] [PubMed] [Google Scholar]
- 47. Sternberg E. D., Brückner C., Dolphin D. 1998. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron Rep. 54:4151–4202 [Google Scholar]
- 48. Taylor V. M., et al. 2010. Leishmania tarentolae: utility as an in vitro model for screening of antileishmanial agents. Exp. Parasitol. 126:471–475 [DOI] [PubMed] [Google Scholar]
- 49. Voigt A., Agtha G., Zintl F. 2006. Polyunsaturated but not conjugated linoleic acid supplementation of leukemic U937 cells can act as an amplification factor for Photofrin-mediated photodynamic therapy. Photochem. Photobiol. 82:763–769 [DOI] [PubMed] [Google Scholar]
- 50. World Health Organization 2004. Scientific working group report on leishmaniasis. World Health Organization, Geneva, Switzerland: http://www.who.int/tdrold/publications/publications/swg_leish.htm [Google Scholar]
- 51. Yamakoshi Y., et al. 2003. Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2−• versus 1O2. J. Am. Chem. Soc. 125:12803–12809 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.