Abstract
Pneumonia due to methicillin-resistant Staphylococcus aureus (MRSA) often cannot be cured by vancomycin treatment. Poor lung tissue and intracellular penetration limits the ability to achieve effective bactericidal levels, particularly in alveolar macrophages, where MRSA can evade phagocytic killing. Compared to standard formulations, liposome encapsulation has been shown to enhance vancomycin intracellular killing of MRSA. In this murine pharmacokinetic and biodistribution study, PEGylated liposomal vancomycin, compared to standard and non-PEGylated formulations, significantly prolonged blood circulation time and increased deposition in lung, liver, and spleen and yet reduced accumulation in kidney tissue. As a result of optimizing antimicrobial targeting of infected lung tissue and limiting renal parenchymal exposure, administration of PEGylated liposomal vancomycin may improve the efficacy of treatment of MRSA pneumonia and reduce the risk of nephrotoxicity.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is a leading cause of hospital-acquired pneumonia (3, 25, 37). Vancomycin remains one of only two antimicrobial agents approved by the U.S. Food and Drug Administration (FDA) for treatment of MRSA pneumonia, but its clinical failure rates are high. Explanations for poor therapeutic outcomes include slow, time-dependent bactericidal activity; inadequate dosing; poor penetration into lung tissue and alveolar macrophages (23, 30, 33, 35, 43, 47); and, possibly, reduced susceptibility (9, 18, 46). S. aureus is an intracellular and extracellular pathogen (27, 44) that can survive within phagocytes and evade the immune system (15, 17).
Liposome encapsulation of antimicrobials potentially offers enhanced pharmacokinetics and pharmacodynamics and decreased toxicity compared to standard formulations (1, 12). Accumulating higher antimicrobial concentrations in infected tissues would allow increased uptake by activated tissue macrophages (5, 6) and presumably improve treatment efficacy. We therefore hypothesized that surface PEGylated liposome encapsulation, compared to standard vancomycin formulations, would more effectively deliver the “drug-to-bug” by depositing a higher concentration of vancomycin into lung tissue. To test this hypothesis, we prepared both surface PEGylated and conventional (i.e., lacking surface modification) liposomal vancomycin formulations and performed an in vivo study using healthy male CF-1 mice to compare their pharmacokinetic and biodistribution profiles with those of mice treated with the standard vancomycin solution.
MATERIALS AND METHODS
(i) Materials.
DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine) and MPEG-2000-DSPE (methylpolyethyleneglycol-1,2-distearoyl-phosphatidyl ethanolamine conjugate) were obtained from Genzyme Pharmaceuticals (Cambridge, MA). Cholesterol, vancomycin hydrochloride, sodium chloride, phosphate-buffered saline (PBS [pH 7.4]), trifluoroacetic acid, sucrose, and heparin sodium salt were obtained from Sigma Chemicals (St. Louis, MO). Norvancomycin was obtained from Northern China Pharmaceutical Corporation (Shijiazhuang, Hebei, China). Potassium chloride and dibasic sodium phosphate were obtained from Baker (Philipsburg, NJ). Monobasic potassium phosphate was procured from Fisher Scientific (Houston, TX). High-performance liquid chromatography (HPLC)-grade chloroform, acetonitrile, and methanol were obtained from EMD Chemicals (Gibbstown, NJ). Isoflurane was obtained from Piramal Health Care Limited (AP, India). Male CF-1 mice were obtained from Charles River Laboratories (Wilmington, MA).
(ii) Preparation of liposomal vancomycin formulations.
The liposomes were initially prepared using the thin-film hydration method (8) and the ammonium sulfate gradient method (20). Due to poor encapsulation efficiency and poor stability of the prepared formulations, we decided to prepare subsequent formulations using a modified dehydration-rehydration method (4). Both conventional and PEGylated liposomal vancomycin formulations were prepared using DSPC, cholesterol, and polyethylene glycol (PEG) in 3:1:0 and 3:1:0.02 molar ratios, respectively. The detailed procedure for the preparation of liposomal vancomycin formulations has been published recently (35).
(iii) Pharmacokinetic and biodistribution study.
Prior to our undertaking the study, the protocol was approved by the Institutional Animal Care and Use Committee at the Western University of Health Sciences. Vancomycin (5 mg/kg of body weight) in the form of a standard solution or conventional or PEGylated liposomes was injected into the tail vein of each mouse. Following drug administration, at each predetermined time point (5, 15, 30, and 45 min and 1, 2, 3, 4, 5, 6, 8, 12, 24, and 48 h), three mice were sacrificed by isoflurane inhalation and blood was collected. Liver, kidney, spleen, lung, and thigh muscle tissues were also collected at 1, 4, and 24 h for the biodistribution study.
(iv) Plasma sample preparation and analysis.
Each collected blood sample was placed in a heparinized microcentrifuge tube, and plasma was separated by centrifugation. A simple protein precipitation procedure was used to extract vancomycin from the plasma sample. As an internal standard, 10 μl of a solution of norvancomycin (100-μg/ml) was added to 200 μl of plasma and the mixture was subjected to a vortex procedure for 1 min. Then, 250 μl of acetonitrile and 250 μl of methanol were added to the mixture, which was subjected to a vortex procedure for 1 min and centrifuged at 14,000 rpm for 10 min. Supernatant (400 μl) was then transferred into a new tube and subjected to evaporation to dryness by the use of a stream of filtered air for 1 to 2 h. The residue was reconstituted with 200 μl of nanopure water, and 20 μl of the resulting solution was analyzed to determine the vancomycin concentration by the use of a sensitive, validated HPLC method and a C18 column. The standard calibration curve was linear in the concentration range of 0.1 to 20 μg/ml, with a correlation coefficient (r) higher than 0.995. The lower limit of detection (LLOD; signal-to-noise ratio [S/N], 5) of vancomycin was 0.05 μg/ml, and the lower limit of quantitation (LLOQ; S/N, 10) was 0.1 μg/ml. The upper limit of quantitation was 20 μg/ml. The coefficients of variance ranged from 1.7 to 9.5% for intraday and 6.3 to 9.4% for interday precision.
(v) Tissue sample preparation and analysis.
Major organs (liver, kidney, lung, spleen, and muscle) were analyzed to determine the distribution of vancomycin in those tissues after intravenous administration of a 5-mg/kg dose of each of the formulations. Tissue samples were weighed and homogenized in PBS (0.5 mg/ml for liver, kidneys, lungs, and muscle; 0.2 mg/ml for spleen). After homogenization, 300 μl of the homogenate was placed in a 1.5-ml Eppendorf microcentrifuge tube. A 10-μl volume of a solution of norvancomycin (100 μg/ml) was added to each tube, and the mixture was subjected to a vortex procedure for 1 min. Then, 250 μl of acetonitrile and 250 μl of methanol were added to the mixture, which was subsequently subjected to a vortex procedure for 1 min and centrifuged at 14,000 rpm for 10 min. Supernatant (500 μl) was transferred into a test tube and evaporated to dryness. The residue was reconstituted with 200 μl of nanopure water, and 20 μl of the reconstituted sample was subjected to HPLC analysis using a method similar to that described above.
(vi) Pharmacokinetic data processing.
Noncompartmental analysis was used to calculate the pharmacokinetic parameters for total vancomycin in plasma and various tissues following the intravenous administration of the three vancomycin formulations. The peak concentration (Cmax) and the time to peak concentration (tmax) were directly obtained from the raw data sets. The area under the vancomycin concentration-time curve from 0 to 48 h (AUC0–48) for plasma and from 0 to 24 h (AUC0–24) for tissues was calculated using the linear trapezoidal method with the following equation (where i represents the index variable, time point):
| (1) |
Total body clearance (CL) was calculated as dose/AUC. Mean residence time (MRT) in tissue was calculated by dividing the area under the first moment curve from 0 to 24 h (AUMC0–24) by AUC0–24, whereas AUMC0–24 was calculated using the following equation:
| (2) |
(vii) Statistical analysis.
All data are shown as means ± standard deviations (SD). A two-way analysis of variance (ANOVA) followed by a Bonferroni post hoc analysis using Graphpad Prism software (La Jolla, CA) was performed to determine statistical significance for the tissue vancomycin levels. Pharmacokinetic parameters were compared statistically using the t test. A P value of ≤ 0.05 was considered significant.
RESULTS
(i) Pharmacokinetics.
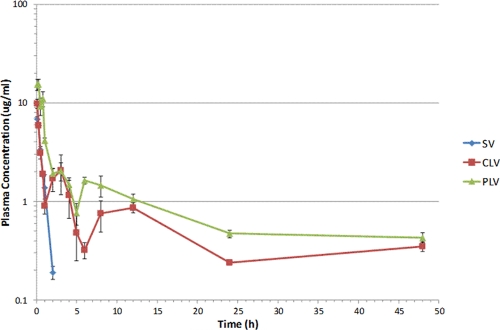
After the administration of a single dose of vancomycin (5 mg/kg) in standard solution and conventional and PEGylated liposomal formulations, plasma samples were analyzed using a validated HPLC assay to determine the levels of vancomycin. The pharmacokinetic profiles for all three formulations are shown in Fig. 1. Vancomycin plasma concentrations rapidly declined within the first 2 h of injection of the standard vancomycin formulation (half-life [t1/2], 22 min); after 2 h, no measurable amount could be found. However, following injection of conventional and PEGylated liposomal formulations, plasma vancomycin concentrations remained at >1 μg/ml until 4 h and 12 h, respectively. With both liposomal formulations, vancomycin was detectable in plasma samples at 48 h, when the experiment was terminated.
Fig. 1.
Pharmacokinetics of vancomycin following intravenous administration of a dose (5 mg/kg) of standard vancomycin solution (SV), conventional liposomal vancomycin (CLV), and PEGylated liposomal vancomycin (PLV) in mice. All values are reported as means ± SD.
Pharmacokinetic parameters were calculated using the noncompartmental method and are reported in Table 1. The peak vancomycin plasma concentrations at 15 min for the PEGylated liposomal formulation and at 5 min for the conventional liposomal formulation were 2.3- and 1.4-fold higher, respectively, than that at 5 min for the standard vancomycin formulation. The AUC for conventional liposomes was 4-fold higher than that of the standard vancomycin formulation, and the AUC of the PEGylated liposomes was a further 1.7-fold higher than that of conventional liposomes. The vancomycin concentrations were sustained when administered as liposomal formulations compared to the results seen with standard vancomycin. Clearance from plasma was decreased by liposomal encapsulation of vancomycin. Since plasma concentrations were determined from samples that included both released and still-encapsulated liposomal vancomycin, it was difficult to fit the data into a specific compartmental model and it was not possible to accurately determine all the pharmacokinetic parameters.
Table 1.
Main pharmacokinetic parameters of vancomycin in plasma after intravenous administration of a dose (5-mg/kg) of standard, conventional, and PEGylated liposomal vancomycin formulationsa
| Vancomycin formulation | Cmax (μg/ml) | AUC0-48 (μg · h/ml) | CL (ml/h) |
|---|---|---|---|
| SV | 6.8 ± 0.4 | 6.8 ± 0.3 | 18.3 ± 0.7 |
| CLV | 9.8 ± 1.1* | 25.9 ± 2.8*** | 4.9 ± 0.6*** |
| PLV | 15.5 ± 1.7** | 47.2 ± 2.4*** | 2.7 ± 0.1*** |
SV, standard formulation; CLV, conventional formulation; PLV, PEGylated formulation.
Symbols *, **, and *** denote P values of <0.05, 0.01, and 0.001, respectively, versus parameters for the standard solution.
(ii) Biodistribution.
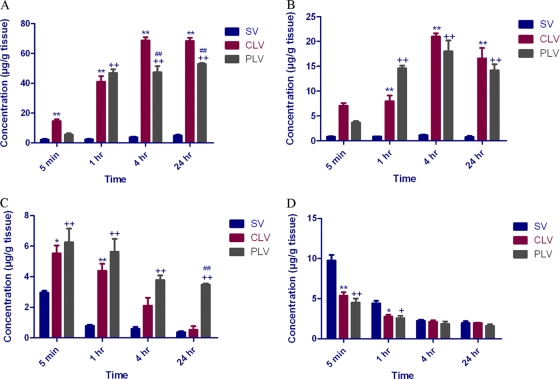
The biodistribution of vancomycin into major organs following administration of a single intravenous dose (5 mg/kg) of standard, conventional, and PEGylated liposomal vancomycin formulations was determined, and results from liver, spleen, lung, and kidney are presented in Fig. 2. No significant levels of vancomycin were detected in thigh muscle tissue treated with all three formulations. Liposome encapsulation enhanced reticuloendothelial system uptake, as evidenced by increased levels of vancomycin in spleen and liver tissues. However, compared to the levels of uptake seen with conventional liposomes, the uptake of vancomycin from the PEGylated liposomes at 4 h and 24 h was significantly less (P < 0.001). Maximum concentrations of vancomycin in liver from all three formulations were found at 4 h. The distribution of vancomycin from conventional and PEGylated liposomal formulations into liver tissue was significantly greater than that from standard vancomycin at 1, 4, and 24 h (P < 0.001). Remarkably, liposome encapsulation caused greater biodistribution of vancomycin into lung tissue compared to the level seen with standard vancomycin. PEGylated liposomes were distributed well compared to conventional liposomes at 5 min, 1 h, 4 h, and 24 h. Furthermore, compared to conventional liposome results, a statistically significant increase in vancomycin distribution from PEGylated liposomes was observed at the end of 24 h (P < 0.001). On the other hand, biodistribution of vancomycin into the kidneys was markedly reduced by liposome encapsulation and was most pronounced with the PEGylated formulation. A significant difference between the distribution of vancomycin into kidneys from the standard formulation and the distribution from both of the liposomal formulations was seen at 5 min (P < 0.001) and 1 h (P < 0.01). The AUC and MRT values for all three formulations in liver, kidneys, lungs, and spleen tissue were calculated and are reported in Table 2.
Fig. 2.
Distribution of total vancomycin in the tissues of CF-1 mice following intravenous administration of a dose (5 mg/kg) of standard vancomycin solution (SV), conventional liposomal vancomycin (CLV), and PEGylated liposomal vancomycin (PLV). (A) Spleen. **, P < 0.001 for conventional liposomes versus standard vancomycin at 5 min; ++, P < 0.001 for PEGylated liposomes versus standard vancomycin at 5 min; *, P < 0.01 for conventional liposomes versus standard vancomycin at 1 h; +, P < 0.01 for PEGylated liposomes versus standard vancomycin at 1 h. (B) Liver. **, P < 0.001 for conventional liposomes versus standard vancomycin at 5 min; ++, P < 0.001 for PEGylated liposomes versus standard vancomycin at 5 min; *, P < 0.01 for conventional liposomes versus standard vancomycin at 1 h; +, P < 0.01 for PEGylated liposomes versus standard vancomycin at 1 h. (C) Lung. **, P < 0.001 for conventional liposomes versus standard vancomycin at 5 min; ++, P < 0.001 for PEGylated liposomes versus standard vancomycin at 5 min; *, P < 0.01 for conventional liposomes versus standard vancomycin at 1 h; +, P < 0.01 for PEGylated liposomes versus standard vancomycin at 1 h. (D) Kidney. **, P < 0.001 for conventional liposomes versus standard vancomycin at 5 min; ++, P < 0.001 for PEGylated liposomes versus standard vancomycin at 5 min; *, P < 0.01 for conventional liposomes versus standard vancomycin at 1 h; +, P < 0.01 for PEGylated liposomes versus standard vancomycin at 1 h.
Table 2.
Biodistribution of vancomycin in liver, kidney, lung, and spleen tissue after intravenous administration of a dose (5-mg/kg) of standard, conventional, and PEGylated liposomal vancomycin formulationsa
| Vancomycin formulation | Liver |
Kidney |
Lung |
Spleen |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC0–24 (μg · h/ml) | Cmax (μg/ml) | MRT (h) | AUC0–24 (μg · h/ml) | Cmax (μg/ml) | MRT (h) | AUC0–24 (μg · h/ml) | Cmax (μg/ml) | MRT (h) | AUC0–24 (μg · h/ml) | Cmax (μg/ml) | MRT (h) | |
| SV | 21.9 ± 5.3 | 1.1 ± 0.2 | 10.5 ± 1.6 | 58.6 ± 5.7 | 9.8 ± 1.2 | 9.9 ± 0.9 | 13.5 ± 2.1 | 3.0 ± 0.2 | 8.8 ± 1.9 | 99.4 ± 18.7 | 4.9 ± 1.0 | 13.8 ± 0.5 |
| CLV | 426.3 ± 44.3*** | 21.0 ± 1.1*** | 11.7 ± 0.9 | 51.7 ± 40.1 | 5.4 ± 0.7** | 11.1 ± 0.8 | 40.7 ± 13.5* | 5.5 ± 0.9** | 5.7 ± 1.3 | 1,563.1 ± 69.6*** | 68.8 ± 3.7*** | 12.6 ± 0.4* |
| PLV | 378.9 ± 36.4*** | 18.0 ± 3.8*** | 11.2 ± 1.3 | 44.6 ± 3.9* | 4.5 ± 0.9** | 10.7 ± 2.1 | 92.2 ± 10.1*** | 6.3 ± 1.5* | 11.1 ± 0.6 | 1,169.4 ± 86.3*** | 53.0 ± 1.2*** | 12.8 ± 0.5 |
SV, standard formulation; CLV, conventional formulation; PLV, PEGylated formulation.
Symbols *, **, and *** denote P values of <0.05, 0.01, and 0.001, respectively, versus parameters for the standard solution.
DISCUSSION
Vancomycin remains an important part of a restricted treatment armamentarium for MRSA pneumonia, and yet concerns have been raised about its effectiveness. Retrospective analysis from two multinational, prospective, randomized, double-blind studies revealed that the clinical cure rate for vancomycin treatment of documented MRSA pneumonia was 36% compared to 59% for linezolid (52). Inherent pharmacokinetic limitations such as slow, time-dependent killing and poor penetration into lung tissue and alveolar macrophages (10, 23, 26, 30, 33, 35, 43, 47) have been blamed for high rates of vancomycin failure (28). Nevertheless, vancomycin is the comparator drug for randomized, double-blind, prospective trials evaluating novel agents (e.g., telavancin) for treatment of hospital-acquired pneumonia due to MRSA (38). Although vancomycin MICs ≤ 2 μg/ml are considered to represent susceptibility, treatment of invasive infections due to MRSA strains with drug MICs ranging from 1 to 2 μg/ml has had a lower success rate than treatment of infections due to strains with drug MICs ≤ 0.5 μg/ml (9, 41). Despite reports over the past several years of MRSA isolates displaying reduced vancomycin susceptibility (“MIC creep”) (9, 45, 46, 51), others have demonstrated MIC stability in the setting of low vancomycin consumption (2). More recently, data from a large U.S. multicenter study suggested that reports of MIC creep may be exaggerated and that it perhaps poses less of a widespread problem than the reports have indicated (40). Pharmacodynamic studies suggest that curative vancomycin treatment of MRSA infection requires achieving a ratio of the area under the concentration-time curve for 24 h to MIC (AUC24/MIC) ≥ 400 (30). Administration of higher-than-standard doses of vancomycin is needed to achieve an AUC24/MIC ≥ 400 (16). However, higher serum vancomycin levels increase the risk of nephrotoxicity (14, 31).
The vancomycin plasma concentration-time profile is complex and can be characterized in the form of one, two, and three compartment pharmacokinetic models (39), with 80% to 90% of the vancomycin recovered unchanged in the urine within 24 h after administration of a single dose (29). Vancomycin displays ∼45% protein binding to immunoglobulin A and albumin (48), and the concentration in alveolar lining fluid is only one-sixth that in plasma (26). In our study, the curves representing the plasma profiles for both liposomal formulations as shown in Fig. 1 were not smooth, with apparent rapid decreases between 2 h and 6 h followed by relative stabilization to 48 h. This phenomenon is intriguing and remains unexplained. One possibility is that, during the first 6 h of bloodstream circulation, liposome stability became compromised, thus increasing the ratio of unencapsulated to encapsulated vancomycin. Since we analyzed only total vancomycin concentrations, we could not directly determine the amount of vancomycin still trapped within liposomes.
Epithelial lining fluid (ELF) measurements have previously been used to reflect antibiotic activity for cases of pneumonia (7). However, ELF levels more specifically reflect extracellular concentrations that may be better suited for evaluation of upper respiratory tract infections or infections by pathogens whose activity is primarily extracellular (42). ELF measurements are typically performed via bronchoalveolar lavage (BAL), and data may be compromised by the presence of lavage fluid, cell contamination, protein binding, or incomplete lysis (22). Homogenized tissue has been used for measurement of pulmonary vancomycin levels (10) in humans; although others have criticized this technique, the correlation of vancomycin ELF concentrations with clinical outcomes has not been defined (42). Since BAL would have been technically difficult to perform in our mouse model and since lung tissue levels may be the most reliable predictor of treatment efficacy (21), we decided to utilize lung tissue homogenates in this study to determine the levels of biodistribution of the different formulations of vancomycin.
Liposomes were first described in 1965 and have already been in use as effective drug delivery systems (8, 19). Similar in structure and composition to host cell membranes, liposomes are biodegradable and are low in toxicity and immunogenicity (49). Liposomes can carry both hydrophilic and lipophilic drugs through encapsulation within the aqueous core and lipid bilayer, respectively (19). Liposomes lacking surface modification (“conventional liposomes”) are rapidly engulfed by intravascular and hepatosplenic phagocytes of the monocyte-macrophage line (11). Conversely, surface modification through PEGylation creates “stealth” liposomes that evade opsonization and delay hepatosplenic clearance, thus allowing prolonged circulation time (34) and extravasation into infected tissues. S. aureus can survive inside neutrophils (50) and alveolar macrophages (13) and persist in phagolysosomes for 3 to 4 days before escaping into the cytoplasm, resulting in host cell lysis and dissemination (24). We recently confirmed earlier observations indicating that, compared to the standard formulation, conventional liposome encapsulation enhances vancomycin intracellular killing of MRSA (33, 35).
The present report demonstrates that, compared to the standard formulation, liposome encapsulation increases vancomycin distribution into lung, liver, and spleen tissue while decreasing accumulation within kidneys. This preferential distribution may lead to improved treatment efficacy and reduced renal toxicity. However, despite vancomycin not being historically known as a hepatotoxic agent, the marked liver distribution of the drug resulting from PEGylated liposome encapsulation raises concerns regarding the potential for an additional adverse effect. PEGylated liposomes, compared to conventional liposome encapsulation, further increase vancomycin lung tissue concentrations, perhaps due to a longer circulation time that allows greater release within the targeted tissue.
This in vivo model does present some important limitations. First, although overall lung tissue concentrations were measured, we did not determine the percentages of intracellular and interstitial vancomycin. Therefore, we can merely infer that drug deposited in the interstitium would ultimately gain entry into alveolar macrophages. Second, levels of biodistribution of liposomal vancomycin may differ in the setting of lung infection. The local tissue effects of edema, vasoconstriction, ischemia, and necrosis may variably and unpredictably alter the kinetics of drug delivery. Lung inflammation has been suggested to increase passive diffusion of (free and protein-bound) vancomycin, but as inflammation subsides with treatment of infection, tissue permeability may normalize (42). Third, we assume that persistent intracellular MRSA infection plays a prominent role in the potential for clinical relapse following a course of vancomycin treatment. Although this is a reasonable assumption, in reality, there are likely multiple factors that play a role affecting the clinical response to therapy. Furthermore, theoretical concerns are raised that MRSA pneumonia episodes complicated by ongoing bacteremia not due to intracellular parasitism may be inadequately treated with the administration of a “stealth” liposomal vancomycin formulation. Fourth, we opted to use a murine model for our biodistribution and pharmacokinetic studies on the basis of cost and space required for procurement and maintenance. Although murine models have been used for other antimicrobials (e.g., telavancin) targeting MRSA (36), concerns have been raised about the applicability to humans (32), particularly in cases of pneumococcal pneumonia.
Nevertheless, further studies are warranted to determine the in vivo efficacy of the use of liposomal vancomycin in treating MRSA lung infection. To address this topic, we are preparing to study these same formulations by the use of a murine model of pneumonia and methods published elsewhere (36) to measure outcome variables such as CFU counts, survival rates, and histopathological changes. We expect such an investigation to shed more light on the potential for development of PEGylated liposomal vancomycin as a novel delivery system that may improve treatment outcomes by targeting MRSA infections complicated by intracellular parasitism.
ACKNOWLEDGMENTS
J.W., G.B., and A.S.P. hold a Patent Cooperation Treaty (PCT) (international application PCT/US2011/041053; Novel formulation of PEGylated-lipsosome encapsulated glycopeptide antibiotics).
The funding source was an intramural grant from the Western University of Health Sciences.
Footnotes
Published ahead of print on 25 July 2011.
REFERENCES
- 1. Allen T. M. 1998. Liposomal drug formulations. Rationale for development and what we can expect for the future. Drugs 56:747–756 [DOI] [PubMed] [Google Scholar]
- 2. Alós J. I., Garcia-Canas A., Garcia-Hierro P., Rodriguez-Salvanes F. 2008. Vancomycin MICs did not creep in Staphylococcus aureus isolates from 2002 to 2006 in a setting with low vancomycin usage. J. Antimicrob. Chemother. 62:773–775 [DOI] [PubMed] [Google Scholar]
- 3. Am. J. Respir. Crit. Care Med 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388–416 [DOI] [PubMed] [Google Scholar]
- 4. Anderson K. E., Eliot L. A., Stevenson B. R., Rogers J. A. 2001. Formulation and evaluation of a folic acid receptor-targeted oral vancomycin liposomal dosage form. Pharm. Res. 18:316–322 [DOI] [PubMed] [Google Scholar]
- 5. Bakker-Woudenberg I. 2002. Long-circulating sterically stabilized liposomes as carriers of agents for treatment of infection or for imaging infectious foci. Int. J. Antimicrob. Agents 19:299–311 [DOI] [PubMed] [Google Scholar]
- 6. Bakker-Woudenberg I., Schiffelers R. M., Storm G., Becker M. J., Guo L. 2005. Long-circulating sterically stabilized liposomes in the treatment of infections. Methods Enzymol. 391:228–260 [DOI] [PubMed] [Google Scholar]
- 7. Baldwin D. R., Honeybourne D., Wise R. 1992. Pulmonary disposition of antimicrobial agents: methodological considerations. Antimicrob. Agents Chemother. 36:1171–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bangham A. D., Standish M. M., Watkins J. C. 1965. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 13:238–252 [DOI] [PubMed] [Google Scholar]
- 9. Choi E. Y., et al. 2011. Relationship between the MIC of vancomycin and clinical outcome in patients with MRSA nosocomial pneumonia. Intensive Care Med. 37:639–647 [DOI] [PubMed] [Google Scholar]
- 10. Cruciani M., et al. 1996. Penetration of vancomycin into human lung tissue. J. Antimicrob. Chemother. 38:865–869 [DOI] [PubMed] [Google Scholar]
- 11. de Steenwinkel J. E., et al. 2007. Targeted drug delivery to enhance efficacy and shorten treatment duration in disseminated Mycobacterium avium infection in mice. J. Antimicrob. Chemother. 60:1064–1073 [DOI] [PubMed] [Google Scholar]
- 12. Drulis-Kawa Z., Dorotkiewicz-Jach A. Liposomes as delivery systems for antibiotics. Int. J. Pharm. 387:187–198 [DOI] [PubMed] [Google Scholar]
- 13. Elliott G. R., et al. 1982. Influence of subinhibitory concentrations of penicillin, cephalothin, and clindamycin on Staphylococcus aureus growth in human phagocytic cells. Antimicrob. Agents Chemother. 22:781–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fanos V., Cataldi L. 2001. Renal transport of antibiotics and nephrotoxicity: a review. J. Chemother. 13:461–472 [DOI] [PubMed] [Google Scholar]
- 15. Foster T. J. 2005. Immune evasion by staphylococci. Nat. Rev. Microbiol. 3:948–958 [DOI] [PubMed] [Google Scholar]
- 16. Frymoyer A., Hersh A. L., Benet L. Z., Guglielmo B. J. 2009. Current recommended dosing of vancomycin for children with invasive methicillin-resistant Staphylococcus aureus infections is inadequate. Pediatr. Infect. Dis. J. 28:398–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garzoni C., Kelley W. L. 2009. Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol. 17:59–65 [DOI] [PubMed] [Google Scholar]
- 18. Gould I. M. 2008. Clinical relevance of increasing glycopeptide MICs against Staphylococcus aureus. Int. J. Antimicrob. Agents 31(Suppl. 2):1–9 [DOI] [PubMed] [Google Scholar]
- 19. Gregoriadis G. 1995. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 13:527–537 [DOI] [PubMed] [Google Scholar]
- 20. Jung S. H., Seong H., Cho S. H., Jeong K. S., Shin B. C. 2009. Polyethylene glycol-complexed cationic liposome for enhanced cellular uptake and anticancer activity. Int. J. Pharm. 382:254–261 [DOI] [PubMed] [Google Scholar]
- 21. Kalil A. C., et al. 2010. Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: a systematic review and meta-analysis. Crit. Care Med. 38:1802–1808 [DOI] [PubMed] [Google Scholar]
- 22. Kiem S., Schentag J. J. 2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob. Agents Chemother. 52:24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kollef M. H. 2007. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin. Infect. Dis. 45:S191–S195 [DOI] [PubMed] [Google Scholar]
- 24. Kubica M., et al. 2008. A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS One 3:e1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuehnert M. J., et al. 2005. Methicillin-resistant Staphylococcus aureus hospitalizations, United States. Emerg. Infect. Dis. 11:868–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lamer C., et al. 1993. Analysis of vancomycin entry into pulmonary lining fluid by bronchoalveolar lavage in critically ill patients. Antimicrob. Agents Chemother. 37:281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lowy F. D. 2000. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 8:341–343 [DOI] [PubMed] [Google Scholar]
- 28. Maclayton D. O., Hall R. G., II 2007. Pharmacologic treatment options for nosocomial pneumonia involving methicillin-resistant Staphylococcus aureus. Ann. Pharmacother 41:235–244 [DOI] [PubMed] [Google Scholar]
- 29. Matzke G. R., Zhanel G. G., Guay D. R. P. 1986. Clinical pharmacokinetics of vancomycin. Clin. Pharmacokinet. 11:257–282 [DOI] [PubMed] [Google Scholar]
- 30. Moise-Broder P. A., Forrest A., Birmingham M. C., Schentag J. J. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 43:925–942 [DOI] [PubMed] [Google Scholar]
- 31. Nakamura T., Takano M., Yasuhara M., Inui K. 1996. In-vivo clearance study of vancomycin in rats. J. Pharm. Pharmacol. 48:1197–1200 [DOI] [PubMed] [Google Scholar]
- 32. Nuermberger E. 2005. Murine models of pneumococcal pneumonia and their applicability to the study of tissue-directed antimicrobials. Pharmacotherapy 25:134S–139S [DOI] [PubMed] [Google Scholar]
- 33. Onyeji C. O., Nightingale C. H., Marangos M. N. 1994. Enhanced killing of methicillin-resistant Staphylococcus aureus in human macrophages by liposome-entrapped vancomycin and teicoplanin. Infection 22:338–342 [DOI] [PubMed] [Google Scholar]
- 34. Reference deleted.
- 35. Pumerantz A., et al. 2011. Preparation of liposomal vancomycin and intracellular killing of meticillin-resistant Staphylococcus aureus (MRSA). Int. J. Antimicrob. Agents 37:140–144 [DOI] [PubMed] [Google Scholar]
- 36. Reyes N., et al. 2005. Efficacy of telavancin (TD-6424), a rapidly bactericidal lipoglycopeptide with multiple mechanisms of action, in a murine model of pneumonia induced by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:4344–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rubinstein E., Kollef M. H., Nathwani D. 2008. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 46:S378–S385 [DOI] [PubMed] [Google Scholar]
- 38. Rubinstein E., et al. 2011. Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin. Infect. Dis. 52:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rybak M. J., et al. 2009. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 29:1275–1279 [DOI] [PubMed] [Google Scholar]
- 40. Sader H. S., et al. 2009. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob. Agents Chemother. 53:4127–4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sakoulas G., et al. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 42:2398–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scheetz M. H., Wunderink R. G., Postelnick M. J., Noskin G. A. 2006. Potential impact of vancomycin pulmonary distribution on treatment outcomes in patients with methicillin-resistant Staphylococcus aureus pneumonia. Pharmacotherapy 26:539–550 [DOI] [PubMed] [Google Scholar]
- 43. Shulman L. M., et al. 2006. Subjective report versus objective measurement of activities of daily living in Parkinson's disease. Mov. Disord. 21:794–799 [DOI] [PubMed] [Google Scholar]
- 44. Sinha B., Fraunholz M. 2010. Staphylococcus aureus host cell invasion and post-invasion events. Int. J. Med. Microbiol. 300:170–175 [DOI] [PubMed] [Google Scholar]
- 45. Soriano A., et al. 2008. Influence of vancomycin MIC on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46:193–200 [DOI] [PubMed] [Google Scholar]
- 46. Steinkraus G., White R., Friedrich L. 2007. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001-05. J. Antimicrob. Chemother. 60:788–794 [DOI] [PubMed] [Google Scholar]
- 47. Stevens D. L. 2006. The role of vancomycin in the treatment paradigm. Clin. Infect. Dis. 42(Suppl. 1):S51–S57 [DOI] [PubMed] [Google Scholar]
- 48. Sun H., Maderazo E. G., Krusell A. R. 1993. Serum protein-binding characteristics of vancomycin. Antimicrob. Agents Chemother. 37:1132–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Voinea M., Simionescu M. 2002. Designing of ‘intelligent’ liposomes for efficient delivery of drugs. J. Cell. Mol. Med. 6:465–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Voyich J. A., et al. 2005. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175:3907–3919 [DOI] [PubMed] [Google Scholar]
- 51. Wang G., Hindler J. F., Ward K. W., Bruckner D. A. 2006. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J. Clin. Microbiol. 44:3883–3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wunderink R. G., Rello J., Cammarata S. K., Croos-Dabrera R. V., Kollef M. H. 2003. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 124:1789–1797 [PubMed] [Google Scholar]